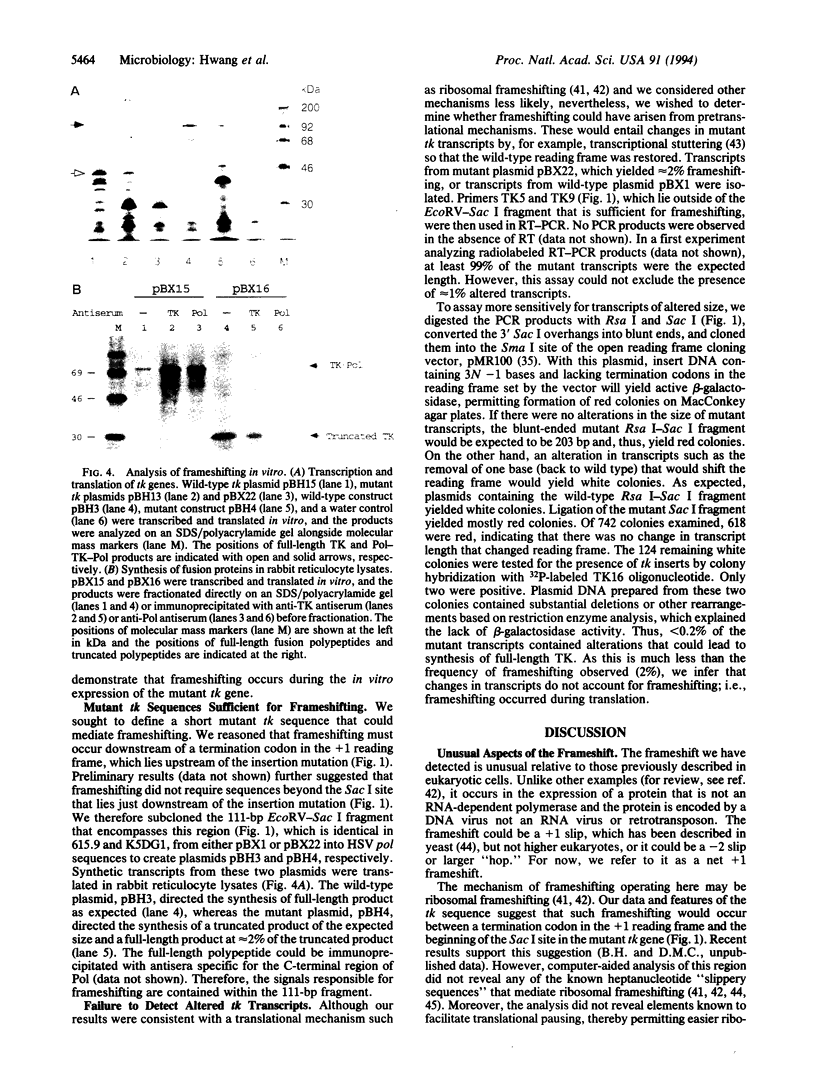
Abstract
Clinical resistance to antiviral drugs requires that a virus evade drug therapy yet retain pathogenicity. Thymidine kinase (TK)-negative mutants of herpes simplex virus are resistant to the drug, acyclovir, but are attenuated for pathogenicity in animal models. However, numerous cases of clinical resistance to acyclovir have been associated with viruses that were reported to express no TK activity. We studied an acyclovir-resistant clinical mutant that contains a single-base insertion in its tk gene, predicting the synthesis of a truncated TK polypeptide with no TK activity. Nevertheless, the mutant retained some TK activity and the ability to reactivate from latent infections of mouse trigeminal ganglia. The mutant expressed both the predicted truncated polypeptide and a low level of a polypeptide that comigrated with full-length TK on polyacrylamide gels and reacted with anti-TK antiserum, providing evidence for a frameshifting mechanism. In vitro transcription and translation of mutant tk genes, including constructs in which reporter epitopes could be expressed only if frameshifting occurred, also gave rise to truncated and full-length polypeptides. Reverse transcriptase-polymerase chain reaction analysis coupled with open reading frame cloning failed to detect alterations in tk transcripts that could account for the synthesis of full-length polypeptide. Thus, synthesis of full-length TK was due to an unusual net +1 frameshift during translation, a phenomenon hitherto confined in eukaryotic cells to certain RNA viruses and retrotransposons. Utilization of cellular frameshifting mechanisms may permit an otherwise TK-negative virus to exhibit clinical acyclovir resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Weiss R. B., Thompson S., Gesteland R. F. Towards a genetic dissection of the basis of triplet decoding, and its natural subversion: programmed reading frame shifts and hops. Annu Rev Genet. 1991;25:201–228. doi: 10.1146/annurev.ge.25.120191.001221. [DOI] [PubMed] [Google Scholar]
- Belcourt M. F., Farabaugh P. J. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990 Jul 27;62(2):339–352. doi: 10.1016/0092-8674(90)90371-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch C. J., Tachedjian G., Doherty R. R., Hayes K., Gust I. D. Altered sensitivity to antiviral drugs of herpes simplex virus isolates from a patient with the acquired immunodeficiency syndrome. J Infect Dis. 1990 Sep;162(3):731–734. doi: 10.1093/infdis/162.3.731. [DOI] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S. C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989 May 19;57(4):537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Crumpacker C. S. Analysis of the thymidine kinase gene from clinically isolated acyclovir-resistant herpes simplex viruses. Virology. 1991 Feb;180(2):793–797. doi: 10.1016/0042-6822(91)90093-q. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Miller C. H., Schrager L. E., Crumpacker C. S. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):297–300. doi: 10.1056/NEJM198902023200507. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Fleming H. E., Jr, Leslie L. K., Retondo M. J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985 Feb;53(2):477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Weinheimer S. P., McKnight S. L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986 Oct 3;234(4772):53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- Digard P., Bebrin W. R., Weisshart K., Coen D. M. The extreme C terminus of herpes simplex virus DNA polymerase is crucial for functional interaction with processivity factor UL42 and for viral replication. J Virol. 1993 Jan;67(1):398–406. doi: 10.1128/jvi.67.1.398-406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digard P., Coen D. M. A novel functional domain of an alpha-like DNA polymerase. The binding site on the herpes simplex virus polymerase for the viral UL42 protein. J Biol Chem. 1990 Oct 15;265(29):17393–17396. [PubMed] [Google Scholar]
- Efstathiou S., Kemp S., Darby G., Minson A. C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989 Apr;70(Pt 4):869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Gateley A., Gander R. M., Johnson P. C., Kit S., Otsuka H., Kohl S. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J Infect Dis. 1990 Apr;161(4):711–715. doi: 10.1093/infdis/161.4.711. [DOI] [PubMed] [Google Scholar]
- Gordon Y., Gilden D. H., Shtram Y., Asher Y., Tabor E., Wellish M., Devlin M., Snipper D., Hadar J., Becker Y. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76(1):39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Schooley R. T. Resistance to antiviral drugs: the end of innocence. N Engl J Med. 1989 Feb 2;320(5):313–314. doi: 10.1056/NEJM198902023200510. [DOI] [PubMed] [Google Scholar]
- Irmiere A. F., Manos M. M., Jacobson J. G., Gibbs J. S., Coen D. M. Effect of an amber mutation in the herpes simplex virus thymidine kinase gene on polypeptide synthesis and stability. Virology. 1989 Feb;168(2):210–220. doi: 10.1016/0042-6822(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Izumi K. M., Stevens J. G. Two thymidine kinase deficient herpes simplex viruses exhibit unexpected virulence properties. Microb Pathog. 1988 Feb;4(2):145–153. doi: 10.1016/0882-4010(88)90056-3. [DOI] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Ruffner K. L., Kosz-Vnenchak M., Hwang C. B., Wobbe K. K., Knipe D. M., Coen D. M. Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J Virol. 1993 Nov;67(11):6903–6908. doi: 10.1128/jvi.67.11.6903-6908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. P., Bodin E. T., Coen D. M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990 Sep;64(9):4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Sheppard M., Ichimura H., Nusinoff-Lehrman S., Ellis M. N., Fyfe J. A., Otsuka H. Nucleotide sequence changes in thymidine kinase gene of herpes simplex virus type 2 clones from an isolate of a patient treated with acyclovir. Antimicrob Agents Chemother. 1987 Oct;31(10):1483–1490. doi: 10.1128/aac.31.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D. A., Nadeau K. C., Rundle S. A., Schaffer P. A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P., Ellis M. N., Hackman R. C., Shepp D. H., Meyers J. D. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis. 1990 Jul;162(1):244–248. doi: 10.1093/infdis/162.1.244. [DOI] [PubMed] [Google Scholar]
- Marcy A. I., Yager D. R., Coen D. M. Isolation and characterization of herpes simplex virus mutants containing engineered mutations at the DNA polymerase locus. J Virol. 1990 May;64(5):2208–2216. doi: 10.1128/jvi.64.5.2208-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Ellis M. N., Keller P. M., Biron K. K., Lehrman S. N., Barry D. W., Furman P. A. Plaque autoradiography assay for the detection and quantitation of thymidine kinase-deficient and thymidine kinase-altered mutants of herpes simplex virus in clinical isolates. Antimicrob Agents Chemother. 1985 Aug;28(2):181–187. doi: 10.1128/aac.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir K. M., French D. C., Dube D. K., Loeb L. A. Permissible amino acid substitutions within the putative nucleoside binding site of herpes simplex virus type 1 encoded thymidine kinase established by random sequence mutagenesis [corrected]. J Biol Chem. 1992 Apr 5;267(10):6584–6589. [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Safrin S., Crumpacker C., Chatis P., Davis R., Hafner R., Rush J., Kessler H. A., Landry B., Mills J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1991 Aug 22;325(8):551–555. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., del Bene V., Scott R. T., Dudley-Thorpe J. B. Characterization of acyclovir-resistant and -sensitive herpes simplex viruses isolated from a patient with an acquired immune deficiency. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):127–134. doi: 10.1093/jac/18.supplement_b.127. [DOI] [PubMed] [Google Scholar]
- Sears A. E., Meignier B., Roizman B. Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol. 1985 Aug;55(2):410–416. doi: 10.1128/jvi.55.2.410-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipley J., Goldman E. Increased ribosomal accuracy increases a programmed translational frameshift in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2315–2319. doi: 10.1073/pnas.90.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990 Jun;9(6):2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshart K., Knopf C. W. The herpes simplex virus type I DNA polymerase. Polypeptide structure and antigenic domains. Eur J Biochem. 1988 Jul 1;174(4):707–716. doi: 10.1111/j.1432-1033.1988.tb14155.x. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Youle M. M., Hawkins D. A., Collins P., Shanson D. C., Evans R., Oliver N., Lawrence A. Acyclovir-resistant herpes in AIDS treated with foscarnet. Lancet. 1988 Aug 6;2(8606):341–342. doi: 10.1016/s0140-6736(88)92402-6. [DOI] [PubMed] [Google Scholar]