Abstract
GATA transcription factors are transcriptional regulatory proteins that contain a characteristic type-IV zinc finger DNA-binding domain and recognize the conserved GATA motif in the promoter sequence of target genes. Previous studies demonstrated that plant GATA factors possess critical functions in developmental control and responses to the environment. To date, the GATA factors in soybean (Glycine max) have yet to be characterized. Thus, this study identified 64 putative GATA factors from the entire soybean genomic sequence. The chromosomal distributions, gene structures, duplication patterns, phylogenetic tree, tissue expression patterns, and response to low nitrogen stress of the 64 GATA factors in soybean were analyzed to further investigate the functions of these factors. Results indicated that segmental duplication predominantly contributed to the expansion of the GATA factor gene family in soybean. These GATA proteins were phylogenetically clustered into four distinct subfamilies, wherein their gene structure and motif compositions were considerably conserved. A comparative phylogenetic analysis of the GATA factor zinc finger domain sequences in soybean, Arabidopsis (Arabidopsis thaliana), and rice (Oryza sativa) revealed four major classes. The GATA factors in soybean exhibited expression diversity among different tissues; some of these factors showed tissue-specific expression patterns. Numerous GATA factors displayed upregulation or downregulation in soybean leaf in response to low nitrogen stress, and two GATA factors GATA44 and GATA58 were likely to be involved in the regulation of nitrogen metabolism in soybean. Overexpression of GmGATA44 complemented the reduced chlorophyll phenotype of the Arabidopsis ortholog AtGATA21 mutant, implying that GmGATA44 played an important role in modulating chlorophyll biosynthesis. Overall, our study provides useful information for the further analysis of the biological functions of GATA factors in soybean and other crops.
Introduction
GATA transcription factors are a group of regulators that contain the highly conserved type-IV zinc finger motif. These factors bind to the consensus DNA sequence (A/T)GATA(A/G) and are also designated as GATA factors [1]. They were originally identified and characterized in animals and fungi, and typically encoded by multi-gene families. Most proteins include one or two zinc fingers fitting the consensus sequence CX2CX17–18CX2C, followed by a basic region. Animal GATA factors typically contain two CX2CX17CX2C zinc finger domains, and only the C-terminal finger is involved in DNA binding [1–2]. Most fungal GATA factors contain a single CX2CX17CX2C or CX2CX18CX2C domain, which is highly similar to the carboxyl terminal finger of animal GATA factors [3–4]. The first plant GATA factor gene NTL1 (NIT2-like) was identified from tobacco (Nicotiana tabacum) [5]. This finding revealed the presence of GATA factors in higher plants. Previous studies predicted 30 and 29 GATA transcription factors in the Arabidopsis and rice genomes, respectively [6–7]. Most plant GATA factors contain a single CX2CX18CX2C domain, but some also contain either zinc finger loops of 20 residues or more than two zinc finger domains [6].
The biological functions of GATA factors have been broadly studied in animals and fungi. Animal GATA factors have critical functions in development, differentiation, and cell proliferation [2]. Fungal GATA factors are involved in the regulation of nitrogen metabolism, light induction, siderophore biosynthesis, and mating-type switching [4]. Substantial evidence indicated that plant GATA factors are involved in different biological functions. In general, plant GATA factors regulate light-mediated and circadian-regulated gene expression [8–14]. Several Arabidopsis GATA factors are DNA-binding proteins that interact with light-responsive promoters [15–16]. GATA2 (At2g45050) has been identified as a key transcriptional regulator that mediates the crosstalk between brassinosteroid and light signaling pathways [17]. Some plant GATA factors also serve vital functions in some developmental processes. Several Arabidopsis GATA factors have been reported to regulate inflorescence and flower development [18–19], shoot apical meristem development [19], hypocotyl and petiole elongation [20], organ differentiation [21], and seed germination [22]. In addition, GATA factors are involved in the regulation of plant nitrogen metabolism. Previous experiments showed that NIT2, the major nitrogen regulatory protein of Neurospora crassa [23], specifically binds to two fragments of the nitrate reductase gene of tomato in vitro [24]. The regions of the spinach NiR (nitrite reductase) promoter are involved in nitrogen regulation, and footprinting results suggested that GATA factors function in NiR gene regulation [25]. Recent studies have proven that GNC (GATA factor, Nitrate-inducible, Carbon metabolism-involved) and CGA1/GNL (Cytokinin-responsive GATA1/GNC-Like) serve important functions in chlorophyll synthesis and potentially regulate carbon and nitrogen metabolism [7, 26]. Similarly, Cga1 (Cytokinin-responsive GATA transcription factor1) reportedly regulates chloroplast development in rice. OsCga1 overexpression maintains chloroplast development under reduced nitrogen conditions, leading to an increased harvest index despite reduced plant size [27]. Several GATA factors have been functionally characterized in Arabidopsis and rice. However, the biological functions of most GATA factor family members remain poorly understood.
Soybean (Glycine max) is an important food and oil crop that serves as an important protein source for both human consumption and animal feed [28]. To date, few data are available about the GATA factor gene family in soybean. To our knowledge, limited reports exist on the biological functions of soybean GATA factors; one GATA factor (Glyma03g27250) and two GATA factors (Glyma13g00200.1 and Glyma14g10830.1) are involved in soybean nodule development and seed development, respectively [29–30]. The complete soybean genomic sequence has been released and facilitated studies of gene discovery and function [31]. We initially conducted a genome-wide survey of GATA factor-related sequences in soybean to elucidate the functions of GATA proteins in soybean. We identified 64 soybean GATA genes. Detailed analyses of phylogenetic relationships, gene structures, chromosomal distribution, duplication patterns, and conserved motifs of all soybean GATA factors were performed. Subsequently, evolutionary relationships among the GATA family in soybean, Arabidopsis, and rice, and the expression profiles of all soybean GATA genes in various tissues were analyzed. The expression patterns of these GATA genes in response to different nitrate conditions were also conducted to investigate the potential functions of soybean GATA factors involved in the regulation of nitrogen metabolism. Our genome-wide systematic analysis of GATA factors in soybean provides a basis for further investigation on the evolution and functions of GATA factors.
Materials and Methods
Database searches for the identification of GATA factor family members in soybean
We conducted BLAST and keyword searches to collect all potential soybean proteins containing GATA zinc finger. BLASTP search against the soybean genome was carried out at the National Center for Biological Information (NCBI; http://blast.ncbi.nlm.nih.gov/Blast) using the amino acid sequence of four GATA factors from different origins [Arabidopsis AtGATA1 (CAA73999), Aspergillus nidulans AreA (P17429), N. crassa WC1 (Q01371), and chicken GATA1 (AAA49055)] as queries as previously described [6]. All sequences with an E-value below 1.0 were collected. A keyword search was conducted at the Phytozome (v9.0) database (http://www.phytozome.net) for putative soybean GATA factors by searching ontologies with the term (PF00320) of GATA domain. If more than one transcript existed, the primary transcript was selected as representative. These collected putative GATA factor genes were confirmed using the Pfam (http://pfam.sanger.ac.uk/) and InterPro (https://www.ebi.ac.uk/interpro/) databases. Soybean expressed sequence tag (EST) sequences were searched by blastn program in the Gene Indices at DFCI (http://compbio.dfci.harvard.edu/tgi/) using the transcript sequences of the identified putative soybean GATA factors as queries.
Phylogenetic tree constructions
Phylogenetic analysis was performed using MEGA5 software [32]. ClustalW was used to conduct multiple alignments of the full-length deduced amino acid sequences of soybean GATA factors or the conserved GATA zinc finger domain sequences of the GATA factors in soybean, Arabidopsis, and rice. Then, a phylogenetic tree was constructed by the neighbor-joining method with the Poisson substitution model, uniform rates, and pairwise deletion. A total of 1000 bootstrap replicates were carried out to identify the phylogeny.
Gene structure and chromosomal location
For exon/intron structural analysis, the genomic DNA and cDNA sequences corresponding to each predicted soybean GATA factor gene were downloaded from the Glyma (v1.1) of Phytozome or NCBI database. Their exon/intron structures were analyzed using the gene structure display server program (http://gsds.cbi.pku.edu.cn) [33]. The chromosomal location of soybean GATA genes was generated using Chromosome Visualization Tool (CViT) at the Legume Information System (http://comparative-legumes.org/) [34]. The presence of soybean GATA factor genes in segmental duplication blocks was investigated using CViT and synteny viewer as previously described [35].
Identification of conserved motifs in soybean GATA proteins
The conserved motifs of 64 soybean GATA protein sequences were analyzed by the Multiple Em for Motif Elicitation (MEME) program (http://meme.nbcr.net/meme/cgi-bin/meme.cgi) [36]. We set the distribution of a single motif among the sequences as “any number of repetitions”, the maximum number of motifs as 30, and the width of each motif as 6 to 100. The functional annotation of the identified motifs was performed using the Pfam and InterPro databases.
Plant materials and treatments
Soybean (G. max L.) low nitrogen-tolerant variety “No. 116” [37] was used as the plant material. Soybean seeds were germinated and grown in a greenhouse. Roots, stems, young leaves, mature flowers, and immature seeds were collected from adult plants for gene expression analysis. Low nitrogen stress treatment was performed at 10 d after germination as follows. Soybean seedlings with cut-off cotyledons were transferred to half Hoagland solution for 4 d and then transferred to low nitrogen (10% of the normal nitrogen concentration) half Hoagland solution when the primary leaves unfolded. The half Hoagland hydroponic solution (pH 6.0) contained 2 mM Ca(NO3)2·4H2O, 2.5 mM KNO3, 0.5 mM NH4NO3, 0.5 mM KH2PO4, 1 mM MgSO4·7H2O, 0.05 mM Fe-EDTA, 0.005 mM KI, 0.1 mM H3BO3, 0.1 mM MnSO4·H2O, 0.03 mM ZnSO4·7H2O, 0.0001 mM CuSO4·5H2O, 0.001 mM Na2MO4·2H2O, and 0.0001 mM CoCl2·6H2O. To compensate the concentration of Ca2+ and K+, the low nitrogen solution was prepared by replacing Ca(NO3)2·4H2O and KNO3 with CaSO4 and K2SO4, respectively. The culture solution was changed every 3 d. After 4 h, 3 d, and 6 d of low nitrogen stress treatment, the leaves and roots were harvested separately, with three biological replicates per sample. Untreated seedlings in half Hoagland solution were used as controls for all samples. The collected plant materials were immediately frozen in liquid nitrogen and stored at −80°C for RNA isolation.
The Arabidopsis thaliana seeds of Columbia ecotype and a mutant were surface-sterilized with 10% (w/v) NaClO and thoroughly washed three times with sterile water. After stratification at 4°C for 3 days in darkness, seeds were sown on Murashige and Skoog (MS) medium containing 3% sucrose and 0.8% agar in the illuminated incubator. Seedlings were transplanted to soil 10 days after germination in the growth chamber. The illuminated incubator and growth chamber were both controlled at 23°C with 16/8 h (light/dark) photoperiod. The mutant of AtGATA21 (gnc, SALK_001778) was obtained from the Arabidopsis Biological Resource Center (ABRC).
Vector construction and Arabidopsis transformation
To generate the 35S::GmGATA44 overexpression construct, the coding sequence of GmGATA44 was amplified using the primers 5′-ATGATTCCAGCCTATCGCC-3′ and 5′-TCAATGAACAAGGCCATAAGATA-3′. Then it was cloned into the pGWC vector and recombined into the pB2GW7 using the LR recombinase reaction (Invitrogen, USA). The recombinant construct containing the 35S::GmGATA44 cassette was introduced into Agrobacterium tumefaciens strain GV3101 by freeze-thaw method and then transformed into the Arabidopsis homozygous mutant gnc via floral dip method [38]. The gnc mutant has a T-DNA insertion in the gene locus At5g56860, encoding a GATA protein AtGATA21. The transgenic plants were screened on MS medium containing 3% (w/v) sucrose and 20 mg/L Basta and confirmed by PCR analyses. The transcript levels of GmGATA44 and AtGNC were determined by semi-quantitative reverse transcriptase (RT)-PCR, and UBQ10 (At4g05320) was used as a reference control. In addition, chlorophyll contents in transgenic Arabidopsis leaves were measured as previously described [39].
RNA extraction, semi-quantitative RT-PCR and quantitative real-time PCR
Total RNA was extracted from the roots, stems, leaves, flowers, and seeds of soybean plants using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instruction. The quality of the RNA was assessed by agarose gel electrophoresis, and the concentration was measured by an Epoch microplate spectrophotometer (BioTek, USA). RNA samples were treated with RNase-free DNase I (Thermo Scientific, USA) to avoid DNA contamination. First-strand cDNA was synthesized from 2 μg RNA using M-MLV reverse transcriptase (Promega, USA) according to the supplier’s protocol. Semi-quantitative RT-PCR for gene expression in Arabidopsis plants was carried out using the following program: an initial denaturation of 94°C for 5 min, followed by 31 cycles of 94°C for 30 s, 56°C for 30s, and 72°C for 30s, and a final extension at 72°C for 10 min. PCR products were detected by 1% agarose gel. Quantitative real-time PCR for gene expression in soybean and Arabidopsis plants was performed on the Rotor-Gene Q (Qiagen, Germany) using SYBR Green SuperReal Premix (Tiangen, China). Real-time PCR primers were designed using Primer 5.0 software. Primer specificity was verified using the BLAST tool from the NCBI database. The housekeeping genes ACT11 (Glyma18g52780) and GAPDH (At3g26650) were used as the endogenous control to normalize the samples of soybean and Arabidopsis, respectively. The thermal cycling conditions were as follows: 95°C for 15 min; 40 cycles of 95°C for 10 s, 60°C for 15s, and 72°C for 20s. All reactions were performed at least in triplicate. Relative gene expression was analyzed using the 2−ddCt method. All primers for semi-quantitative RT-PCR and quantitative real-time PCR were listed in S1–S3 Tables.
Results and Discussion
GATA factor family in soybean
BLASTP searches in the soybean database of NCBI using Arabidopsis full-length GATA1 protein sequences, as well as sequences from A. nidulans AreA, N. crassa WC1, and chicken GATA1, yielded 56 sequences. Keyword search in the phytozome soybean genome database using the GATA domain (PF00320) yielded 63 candidate sequences. Finally, 64 different soybean loci encoding GATA proteins were identified by removing redundant sequences and different transcripts of the same gene. All these putative GATA protein sequences contained the conserved GATA zinc finger domain, which was confirmed by Pfam and InterPro. Soybean had relatively more GATA factors than Arabidopsis and rice, with 30 and 29, respectively [6–7]. The members of the GATA factor family in soybean were 2.1- and 2.2-times those in Arabidopsis and rice, respectively.
The 64 soybean GATA factors were named GmGATA1 to GmGATA64 according to their chromosomal positions. Table 1 provides detailed information on soybean GATA genes. The nucleotide and amino acid sequences of these soybean GATA factors are available in S1 Text. The identified soybean GATA factors encoded peptides ranging from 80 to 551 amino acids with the isoelectric point (pI) varying from 4.63 to 9.66 and the molecular weight (Mw) varying from 9.1 kD to 60.8 kD. All GmGATA genes contained the full-length coding sequence (CDS), except for GmGATA48. Analysis of the soybean EST databases indicated that partial cDNA sequences were reported for 53 of the 64 GmGATA factor genes (Table 1).
Table 1. GATA gene family in soybean.
| Gene name | Locus name | GenBank Accession No | Genome Location | Peptide Length | Subfamily | Number of EST | pI | Mw (KD) |
|---|---|---|---|---|---|---|---|---|
| GmGATA1 | Glyma01g10390 | XM_006573963 | 1 (13444507–13445181) | 154 | I | 10 | 8.16 | 17.9 |
| GmGATA2 | Glyma01g37450 | NM_001255324 | 1 (49804698–49806831) | 352 | I | 16 | 5.58 | 39.3 |
| GmGATA3 | Glyma01g41370 | XM_003517352 | 1 (52875919–52881926) | 551 | IV | 6 | 6.38 | 60.7 |
| GmGATA4 | Glyma02g05710 | XM_003519830 | 2 (4571075–4572829) | 351 | I | 5 | 6.97 | 38.8 |
| GmGATA5 | Glyma02g06320 | XM_003519850 | 2 (5032247–5034308) | 252 | II | 0 | 7.66 | 28.2 |
| GmGATA6 | Glyma02g07850 | XM_003518166 | 2 (6203860–6206352) | 280 | I | 11 | 8.3 | 31.3 |
| GmGATA7 | Glyma02g08145 | XM_006574699 | 2 (6375823–6381916) | 333 | I | 0 | 5.67 | 36.1 |
| GmGATA8 | Glyma02g37980 | XM_003518220 | 2 (43271674–43276335) | 310 | III | 10 | 6.08 | 33.3 |
| GmGATA9 | Glyma03g27250 | XM_003521041 | 3 (34875366–34876853) | 226 | I | 4 | 7.23 | 25.5 |
| GmGATA10 | Glyma03g39220 | XM_006577154 | 3 (45384711–45385677) | 80 | II | 2 | 9.54 | 9.1 |
| GmGATA11 | Glyma04g01090 | XM_003522812 | 4 (648001–649711) | 305 | I | 13 | 6.61 | 33.9 |
| GmGATA12 | Glyma04g05431 | XM_006578015 | 4 (4112757–4114427) | 292 | II | 0 | 9.15 | 32.5 |
| GmGATA13 | Glyma04g08990 | XM_003523698 | 4 (7123655–7125290) | 305 | I | 3 | 5.82 | 33.9 |
| GmGATA14 | Glyma04g10330 | XM_003523746 | 4 (8555417–8560793) | 309 | III | 0 | 5.75 | 33.8 |
| GmGATA15 | Glyma04g10340 | XM_003522723 | 4 (8563510–8577349) | 350 | III | 8 | 4.63 | 37.9 |
| GmGATA16 | Glyma05g05320 | XM_003524080 | 5 (4651466–4657300) | 542 | IV | 2 | 6.31 | 60.2 |
| GmGATA17 | Glyma05g30385 | XM_006580167 | 5 (35775012–35778754) | 164 | IV | 0 | 8.21 | 18.7 |
| GmGATA18 | Glyma05g30390 | 5 (35782101–35784464) | 151 | IV | 0 | 9.63 | 17.4 | |
| GmGATA19 | Glyma06g01110 | XM_003527087 | 6 (662860–664553) | 294 | I | 23 | 6.67 | 32.9 |
| GmGATA20 | Glyma06g09080 | XM_003527795 | 6 (6665494–6667093) | 326 | I | 0 | 5.93 | 36.0 |
| GmGATA21 | Glyma06g10280 | XM_006580925 | 6 (7764041–7769818) | 304 | III | 13 | 5.7 | 33.4 |
| GmGATA22 | Glyma06g10290 | NM_001255457 | 6 (7772054–7779091) | 351 | III | 12 | 4.67 | 38.1 |
| GmGATA23 | Glyma07g01960 | XM_003530066 | 7 (1354648–1356366) | 409 | I | 1 | 7.06 | 45.2 |
| GmGATA24 | Glyma07g14750 | XM_003530126 | 7 (14558214–14560706) | 237 | I | 5 | 6.75 | 26.5 |
| GmGATA25 | Glyma07g30140 | XM_006583711 | 7 (35186721–35192803) | 355 | III | 0 | 4.91 | 39.6 |
| GmGATA26 | Glyma07g37190 | NM_001248912 | 7 (42333282–42334247) | 130 | II | 28 | 9.59 | 13.7 |
| GmGATA27 | Glyma08g07170 | XM_003532550 | 8 (5156192–5161740) | 358 | III | 1 | 5.24 | 39.9 |
| GmGATA28 | Glyma08g15061 | XM_006585246 | 8 (10938066–10939051) | 136 | II | 1 | 6.42 | 15.5 |
| GmGATA29 | Glyma08g19681 | XM_006585408 | 8 (14863972–14869093) | 192 | IV | 0 | 9.24 | 21.5 |
| GmGATA30 | Glyma08g21630 | XM_003531606 | 8 (16457753–164593338) | 347 | I | 2 | 6.7 | 38.2 |
| GmGATA31 | Glyma08g23720 | XM_003531711 | 8 (18071626–18076907) | 300 | III | 10 | 6.19 | 33.0 |
| GmGATA32 | Glyma08g45835 | XM_003530797 | 8 (45105002–45109921) | 282 | IV | 1 | 5.94 | 31.2 |
| GmGATA33 | Glyma09g07090 | XM_003534865 | 9 (5932308–5935017) | 337 | II | 6 | 9.73 | 38.2 |
| GmGATA34 | Glyma10g25480 | NM_001255531 | 10 (33374598–33378291) | 245 | I | 1 | 8.1 | 27.0 |
| GmGATA35 | Glyma10g35470 | XM_003536302 | 10 (43685975–43689694) | 347 | I | 2 | 5.65 | 37.3 |
| GmGATA36 | Glyma11g04060 | XM_003539037 | 11 (2712320–2718194) | 551 | IV | 1 | 6.23 | 60.8 |
| GmGATA37 | Glyma11g07350 | XM_006590627 | 11 (5150063–5151830) | 245 | II | 11 | 8.3 | 27.3 |
| GmGATA38 | Glyma11g11930 | XM_003538957 | 11 (8506932–8508989) | 299 | I | 1 | 6.03 | 33.5 |
| GmGATA39 | Glyma11g20480 | XM_003538144 | 11 (17278618–17283515) | 305 | I | 26 | 9.25 | 34.5 |
| GmGATA40 | Glyma11g25375 | XM_003539243 | 11 (24001667–24002360) | 156 | II | 0 | 9.01 | 17.6 |
| GmGATA41 | Glyma12g04180 | XM_003540620 | 12 (2731228–2733212) | 289 | I | 1 | 5.32 | 31.9 |
| GmGATA42 | Glyma12g08131 | XM_003539719 | 12 (5856841–5863863) | 304 | I | 2 | 8.56 | 34.6 |
| GmGATA43 | Glyma12g29730 | XM_003540138 | 12 (33187595–33192408) | 326 | I | 1 | 8.7 | 36.1 |
| GmGATA44 | Glyma13g00200 | XM_003543677 | 13 (8613–10819) | 314 | II | 3 | 9.26 | 35.3 |
| GmGATA45 | Glyma13g40020 | XM_003543431 | 13 (40579059–40583715) | 327 | I | 0 | 8.74 | 36.1 |
| GmGATA46 | Glyma14g10830 | XM_003545327 | 14 (9019823–9023050) | 306 | II | 3 | 9.54 | 34.3 |
| GmGATA47 | Glyma14g22460 | XM_003545560 | 14 (26583812–26586274) | 383 | I | 3 | 6.18 | 42.5 |
| GmGATA48 | Glyma14g24201 | 14 (28955340–28956098) | 183* | I | 0 | |||
| GmGATA49 | Glyma14g36150 | XM_003544786 | 14 (45351002–45356038) | 307 | III | 22 | 6.23 | 33.3 |
| GmGATA50 | Glyma15g05065 | XM_006598234 | 15 (3636636–3637215) | 193 | II | 0 | 6.43 | 21.4 |
| GmGATA51 | Glyma15g18380 | XM_003546407 | 15 (15112589–15114772) | 315 | II | 4 | 9.23 | 35.4 |
| GmGATA52 | Glyma16g04670 | XM_003548710 | 16 (3926537–3928215) | 281 | I | 0 | 6.25 | 31.5 |
| GmGATA53 | Glyma16g24381 | XM_006599914 | 16 (28259082–28263005) | 225 | I | 0 | 9.62 | 24.8 |
| GmGATA54 | Glyma16g25370 | XM_003548840 | 16 (29320916–29322986) | 251 | II | 1 | 6.66 | 28.1 |
| GmGATA55 | Glyma16g26870 | XM_003548012 | 16 (30961775–30963809) | 279 | I | 23 | 8.29 | 31.1 |
| GmGATA56 | Glyma16g27171 | XM_003548024 | 16 (31186968–31192884) | 333 | I | 5 | 5.62 | 35.9 |
| GmGATA57 | Glyma17g03410 | XM_003550478 | 17 (2280211–2281440) | 140 | II | 5 | 9.62 | 14.4 |
| GmGATA58 | Glyma17g06290 | XM_003550586 | 17 (4473612–4475846) | 322 | II | 6 | 9.49 | 36.2 |
| GmGATA59 | Glyma17g15610 | XM_003549894 | 17 (12356334–12362270) | 544 | IV | 0 | 6.59 | 60.3 |
| GmGATA60 | Glyma17g27110 | XM_003550072 | 17 (28534119–28536348) | 366 | I | 5 | 6.13 | 40.8 |
| GmGATA61 | Glyma17g34670 | XM_006601169 | 17 (38656842–38660298) | 306 | II | 2 | 9.39 | 34.0 |
| GmGATA62 | Glyma19g28650 | XM_003553957 | 19 (36220313–36221912) | 274 | I | 18 | 6.01 | 30.6 |
| GmGATA63 | Glyma19g41780 | XM_003554576 | 19 (47959000–47959480) | 96 | II | 0 | 9.66 | 11.0 |
| GmGATA64 | Glyma20g32050 | XM_003556186 | 20 (40684758–40688188) | 348 | I | 9 | 5.39 | 37.6 |
Asterisk indicates that the sequence is partial.
All soybean GATA factors contain a single zinc finger. To further investigate the features of the GATA zinc finger domain, the conserved GATA zinc finger domains consisting of approximately 55 residues from 64 soybean GATA factors were aligned (S1 Fig). Except the two pairs of Cys residues, Thr-15, Pro-16, Arg-19, Gly-21, Pro-22, and the amino acid around the second pair of Cys residues (LCNACG) were conserved in almost all the sequences. These highly conserved residues are similar to the GATA factors of Arabidopsis and rice [6]. Most GmGATA genes encode GATA factors with 18 residues in the zinc finger loop (CX2CX18CX2C), and nine GmGATA genes encode GATA factors with 20 residues in the zinc finger loop (CX2CX20CX2C). Similar to Arabidopsis and rice, soybean does not contain the animal- and fungal-type CX2CX17CX2C zinc finger domains.
Notably, three GmGATA genes have an atypical GATA zinc finger. GmGATA50 presented four rather than two residues between the first and the second Cys residues of the zinc finger (CTNFYC). A similar irregularity has been found in the Caenorhabditis elegans GATA factor END-1 and Arabidopsis GATA factor AtGATA29, which may function in recognizing GATA DNA motifs [40]. Meanwhile, the GATA factors GmGATA28 and GmGATA48 only have half GATA motif (CANCDTTSTPLWRNAP for GmGATA28 and TPQWRVKPLGPKTLCKAC for GmGATA48). These sequences may be the remains of an ancestral entire zinc finger. The half GATA motif has also been found in the rice GATA factor OsGATA24 [6].
Phylogenetic relationships and gene structures of the GATA factor family genes in soybean
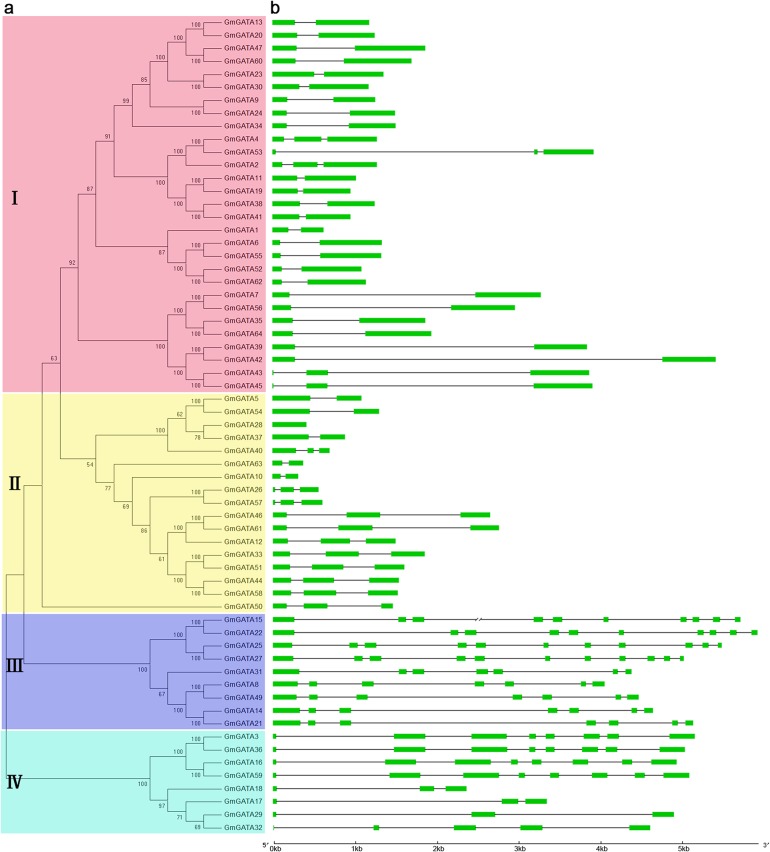
To determine the phylogenetic relationships among the different members of the GATA factor family in soybean, a phylogenetic analysis based on alignments of the 63 full-length GATA protein sequences was performed, except GmGATA48. As shown in Fig 1A, the neighbor-joining phylogenetic tree divided 63 GmGATA genes into four clades. Previous reports classified seven subfamilies (I, II, III, IV, V, VI, and VII) of GATA factors from Arabidopsis and rice GATA factor gene families [6]. Subfamilies I, II, III, and IV were present in soybean. The gene structures of the corresponding genes are shown in Fig 1B. The members within each subfamily showed similar exon/intron structures.
Fig 1. Phylogenetic analysis and gene structure of soybean GATA factors.
(a) Phylogenetic tree construction of soybean GATA factors based on the full-length deduced amino acid sequences using MEGA 5.0 by the neighbor-joining method with 1000 bootstrap replicates. Bootstrap values are shown as percentages (>50%) on the branches. GmGATA48 was not presented in this tree because its sequence is partial. The tree showed four major phylogenetic subfamilies (subfamilies I to IV) indicated with different colored backgrounds.(b) Exon/intron structures of GmGATA genes. Green boxes represent exons, and black lines indicate introns. GmGATA48 was not displayed in this figure because its sequence is partial. The 7 kb length base pair was represented with slash–slash. The sizes of exons and introns can be estimated using the scale at the bottom.
Subfamily I comprised 29 members (the largest number of members) with two or three exons. Subfamily II was formed by 17 members with two or three exons, except GmGATA28, which has one exon. Subfamily III was formed by 9 members with seven, ten, or eleven exons. Subfamily IV constituted of eight members with three, five, or eight exons. These gene structures of GATA factors are similar to those of Arabidopsis and rice [6]. GmGATA genes contained exons ranging from two to eleven in their CDS. The large variation in structures of soybean GATA factor family members could indicate that the soybean genome has changed significantly during its long evolutionary history. Several pairs of GATA proteins have a high degree of homology in the terminal nodes of each subfamily, suggesting that they are putative paralogous pairs. A total of 25 putative paralogous pairs were identified, with sequence identity ranging from 73% to 96% (S4 Table).
For the number of residues in the GATA zinc finger loop, most GmGATA genes encoded GATA factors with 18 residues (CX2CX18CX2C) that belonged to subfamilies I, II, and IV, whereas some encoded GATA factors with 20 residues (CX2CX20CX2C) that belonged to subfamily III. In addition, the zinc finger of the GmGATA genes of subfamilies I, II, and III was located at the carboxyl-terminal end of the protein, whereas that of subfamily IV was located at the amino-terminal end. These results are consistent with those in Arabidopsis and rice [6].
Similar to Arabidopsis, soybean contains subfamilies I, II, III, and IV but not rice-specific subfamilies V, VI, and VII. This result further confirmed the hypothesis proposed by [6] that subfamilies I, II, III, and IV appeared before the divergence between monocot and dicot, and that subfamilies V, VI, and VII evolved after the divergence between monocot and dicot or disappeared in dicot.
Genome distribution and duplication of soybean GATA genes
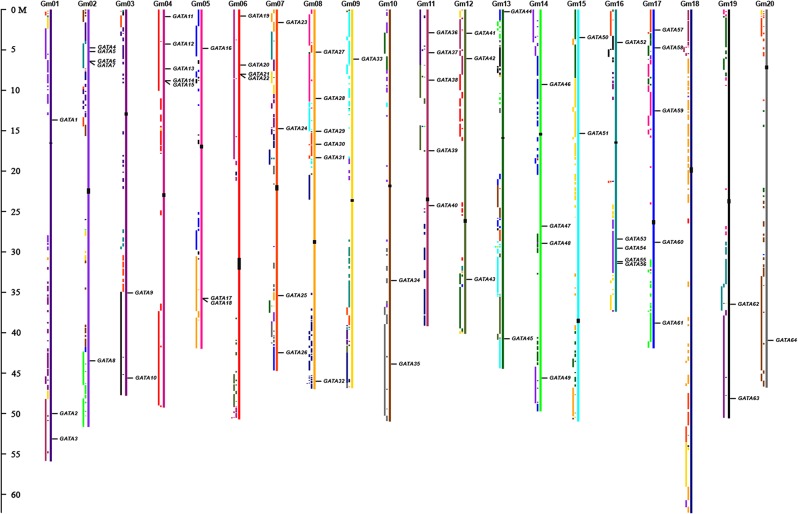
The physical locations of the GATA genes on soybean chromosomes are shown in Fig 2. Sixty-four soybean GATA genes were unevenly distributed on all 20 chromosomes, except for chromosome 18. Among these chromosomes, chromosome 8 had the largest number of GATA genes with six, followed by chromosomes 2, 4, 11, 16, and 17 with five. By contrast, chromosomes 3, 10, 13, 15, and 19 had two GATA genes, and chromosomes 9 and 20 only contained one. Some clustering of GATA genes occurred on several chromosomes. For example, GmGATA14 and GmGATA15 were located in a 2.7-kb segment on chromosome 4, GmGATA17 and GmGATA18 were located in a 3.6-kb segment on chromosome 5, and GmGATA21 and GmGATA22 were located in a 2.2-kb segment on chromosome 6.
Fig 2. Chromosomal location and region duplication of soybean GATA factor genes.
The schematic diagram of genome-wide chromosome organization and segmental duplication was made from the CViT genome search and synteny viewer at the Legume Information System (http://comparative-legumes.org). Colored blocks to the left of each chromosome show duplications with chromosomes of the same color. For example, the black blocks at the bottom of Gm03 correspond with regions on the black Gm19, and vice versa. Locations of centromeric repeats are shown as black rectangles over the chromosomes. The scale on the left represents the length of the chromosome.
Gene duplication events are important for gene family expansion. Gene duplication may arise through several patterns, including segmental duplication, tandem duplication, retroposition, and transposition events [41]. Paralogous pairs located on the same chromosome either adjacent or separated by five or fewer genes were considered to be duplicated by tandem duplication. Paralogous pairs within known genomic duplication blocks were assigned as duplicates through segmental duplication [35]. A previous study showed that the soybean genome has undergone two rounds of whole genome duplication, including an ancient duplication prior to the divergence of papilionoid (58 Mya to 60 Mya) and a Glycine-specific duplication (13 Mya) [31]. The GmGATA genes were mapped to the duplicated blocks through CViT and synteny viewer at the Legume Information System (http://comparative-legumes.org/) to analyze the potential duplicate patterns of these genes during genome evolution. The distributions of soybean GATA genes relative to the corresponding duplicated genomic blocks are shown in Fig 2. Of the 25 putative paralogous pairs of GmGATA genes, 23 were located in segmental duplication blocks. Another two putative paralogous pairs (GmGATA9/24 and GmGATA47/60) lacked the corresponding duplicates and were not located in the same chromosome. Therefore, no tandem duplication was found in the identified GmGATA genes. Nearly 72% of the 64 GmGATA genes were involved in the segmental duplication. This result suggested that segmental duplication significantly contributed to the expansion of the soybean GATA factor gene family.
Conserved motifs outside the GATA domain
To further reveal the diversification of GATA genes in soybean, putative conserved motifs were predicted by the program MEME, and 30 distinct motifs were identified in all 64 GATA proteins. The schematic distribution of the 30 motifs among the different gene subfamilies is shown in Fig 3, and the identified multilevel consensus sequence for the motifs is shown in S5 Table. Motif 1 present in 54 GmGATA proteins and motif 4 present in the other nine GmGATA proteins were the conserved GATA zinc finger domains CX2CX18CX2C and CX2CX20CX2C, respectively. The conserved GATA zinc finger domain was not found in GmGATA28 by MEME, which may be attributed to the small half GATA motif in GmGATA28. As expected, most of the closely related members in the same subfamily had common motif compositions. Motifs 2 and 5 appeared in nearly all members of subfamily I. Motif 21 was the conserved motif in subfamily II. Motifs 3 and 8 were specific to subfamily III. Motif 3 was annotated as the CCT domain. It was first discovered in transcription factor TOC1 and CONSTANS proteins, which are involved in plant photoperiodic signaling, and the CCT domain was implicated in mediating protein-protein interactions [42–43]. Motif 8 was annotated as the TIFY domain, which may be involved in jasmonic acid-related stress response and developmental processes [44]. The CCT and TIFY motifs are also conserved in the GATA factor members of subfamily III in Arabidopsis and rice. In subfamily IV, four closely related members contain motifs 9, 6, 14, 24, 30, 26, and 7. These similarities in motif patterns suggest the similar functions of the GATA factors in the same subfamily. The differences in motif distribution in the different subfamilies of GATA factors indicated the functional divergence of the GATA factors over evolutionary history.
Fig 3. Schematic distribution of the conserved motifs in soybean GATA factors by MEME.
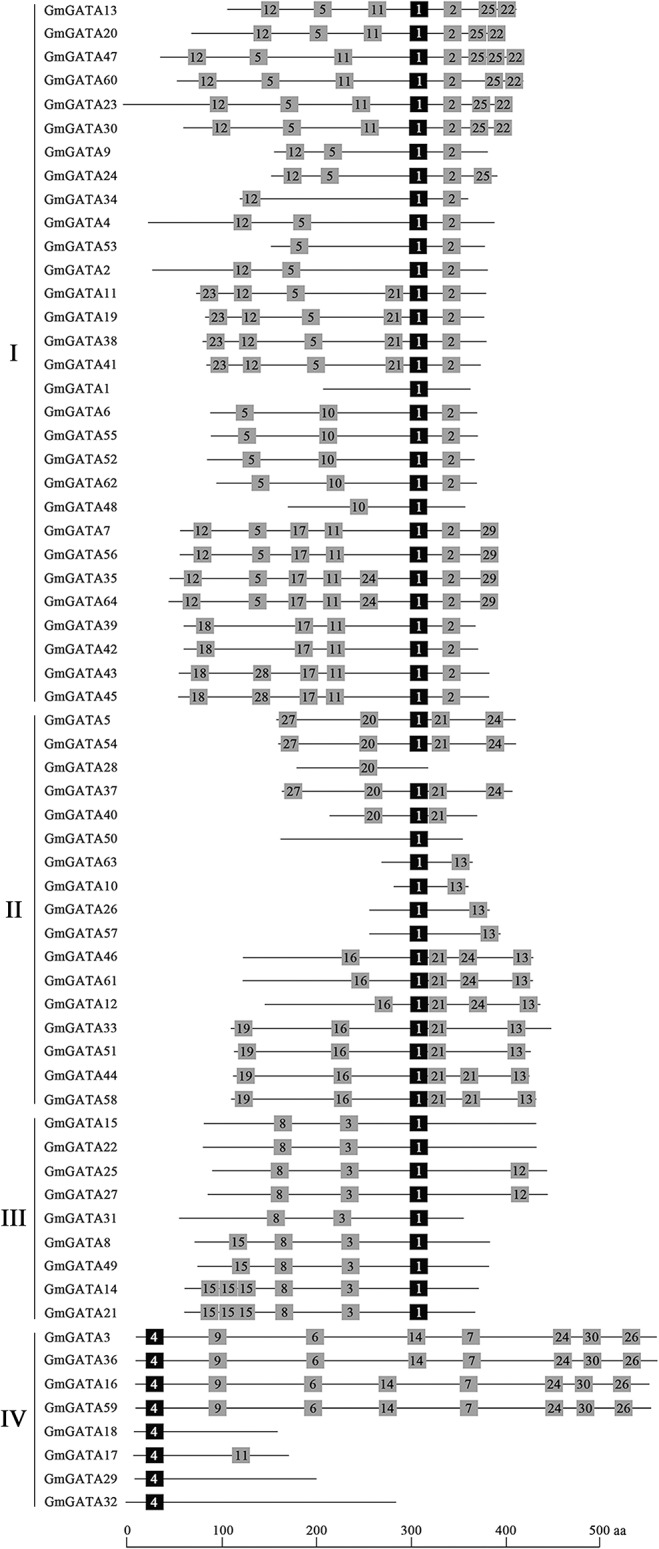
Each numbered box represents a conserved motif in the protein. Motifs 1 and 4 represent the conserved GATA zinc finger motifs CX2CX18CX2 and CX2CX20CX2, respectively. Multilevel consensus sequences for the MEME-defined motifs are listed in S5 Table. The length of the protein can be estimated using the scale at the bottom.
Evolutionary relationships among the GATA family in Arabidopsis, rice, and soybean
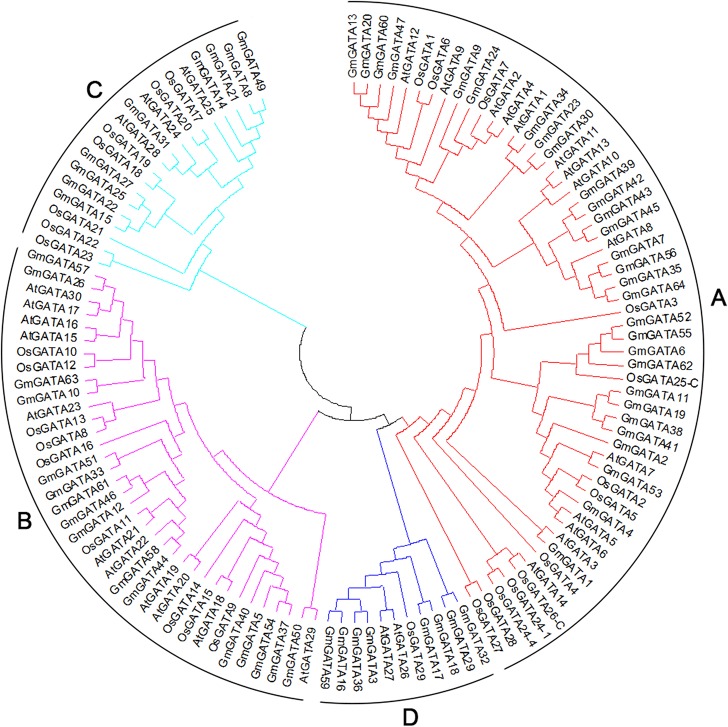
Given the high degree of diversity among the full-length GATA protein sequences, we analyzed the phylogenetic relationship of the GATA proteins in soybean, Arabidopsis, and rice on the alignment of the conserved GATA zinc finger domain, a region of approximately 55 residues (from amino acid −2 to residue +53 with respect to the first Cys) [45]. The amino acid sequences and subfamily information of Arabidopsis and rice GATA factors are available in S6 Table. For rice GATA factors OsGATA25 and OsGATA26 with two GATA domains, the N-domain is denoted by OsGATA25-N or OsGATA26-N, and the C-domain is denoted by OsGATA25-C or OsGATA26-C as previously described [6]. For rice GATA factors OsGATA24 with four GATA domains, the different domains are numbered from the amino- to the carboxy terminus (OsGATA24-1, OsGATA24-2, OsGATA24-3, and OsGATA24-4) [6]. GmGATA28, GmGATA48, OsGATA25-N, OsGATA26-N, OsGATA24-2, and OsGATA24-3 were excluded in the phylogenetic relationship analysis in this study because of the divergent domain.
The phylogenetic tree showed that all the GATA zinc finger sequences from the three higher plants were divided into four major clades (Classes A, B, C, and D) (Fig 4). This result is similar to that previously reported for Arabidopsis and rice [6]. Among these classes, Class A constituted the largest clade, containing 56 members and accounting for 46% of the total GATA zinc finger sequences, Class B formed the second largest clade containing 36 members and accounting for 29% of the total GATA zinc finger sequences, and the other two clades contained 19 (Class C) and 11 (Class D) members, respectively. The zinc fingers of the soybean GATA proteins from subfamilies I, II, III, and IV belonged to Classes A, B, C, and D, respectively. Similar results were obtained in Arabidopsis [6]. The GATA zinc fingers from three higher plants distributed interspersedly in all classes, suggesting that the expansion of GATA zinc fingers occurred before the divergence of soybean, Arabidopsis, and rice. Some putative orthologs, namely, AtGATA1/GmGATA34, AtGATA7/GmGATA53, GmGATA1/AtGATA3, GmGATA31/AtGATA28, and OsGATA11/AtGATA21, were proposed based on the phylogenetic tree.
Fig 4. Phylogenetic tree of the amino acid sequences of zinc finger domains from soybean, Arabidopsis, and rice.
The tree was conducted based on the zinc finger amino acid sequences using MEGA 5.0 by the neighbor-joining method with 1000 bootstrap replicates. The tree shows four major phylogenetic classes (Classes A to D) indicated with different colors.
In general, the GATA factors in the same clade may have similar functions. In Class A, nine soybean GATA factors (GmGATA13/20/60/47/9/24/34/23/30) clustered with the Arabidopsis GATA factors AtGATA1, AtGATA2, and AtGATA4, which are reportedly involved in the light regulation of gene expression and photomorphogenesis [16–17]. Eight soybean GATA factors (GmGATA39/42/43/45/7/56/35/64) clustered with the Arabidopsis GATA factor AtGATA8 (BME3, Blue Micropylar End3), which functions as a positive regulator of seed germination [22]. In Class B, seven soybean GATA factors (GmGATA51/33/58/44/12/61/46) clustered with the Arabidopsis GATA factors AtGATA21 (GNC) and AtGATA22 (GNL/CGA1) and rice GATA factor OsGATA11 (Cga1); these factors regulate chloroplast development, chlorophyll biosynthesis, starch production, plant architecture, and carbon and nitrogen metabolism [7, 27, 46–47]. Four soybean GATA factors (GmGATA40/37/5/54) clustered with the Arabidopsis GATA factor AtGATA18 (HAN, HANABU TARANU) and rice GATA factor OsGATA15 (NL1, NECK LEAF1); these factors are involved in regulating flower and shoot apical meristem development and organ differentiation during reproductive development [19, 21]. In Class C, nine soybean GATA factors (GmGATA27/25/22/15/49/8/21/14/31) clustered with the Arabidopsis GATA factor AtGATA25 (ZIM, Zinc-finger protein expressed in Inflorescence Meristem); this factor is involved in hypocotyl and petiole elongation [20]. Understanding the phylogenetic relationship of GATA factors from soybean, Arabidopsis, and rice enables us to investigate the potential biological functions of soybean GATA factors.
Tissue expression profiles of soybean GATA genes
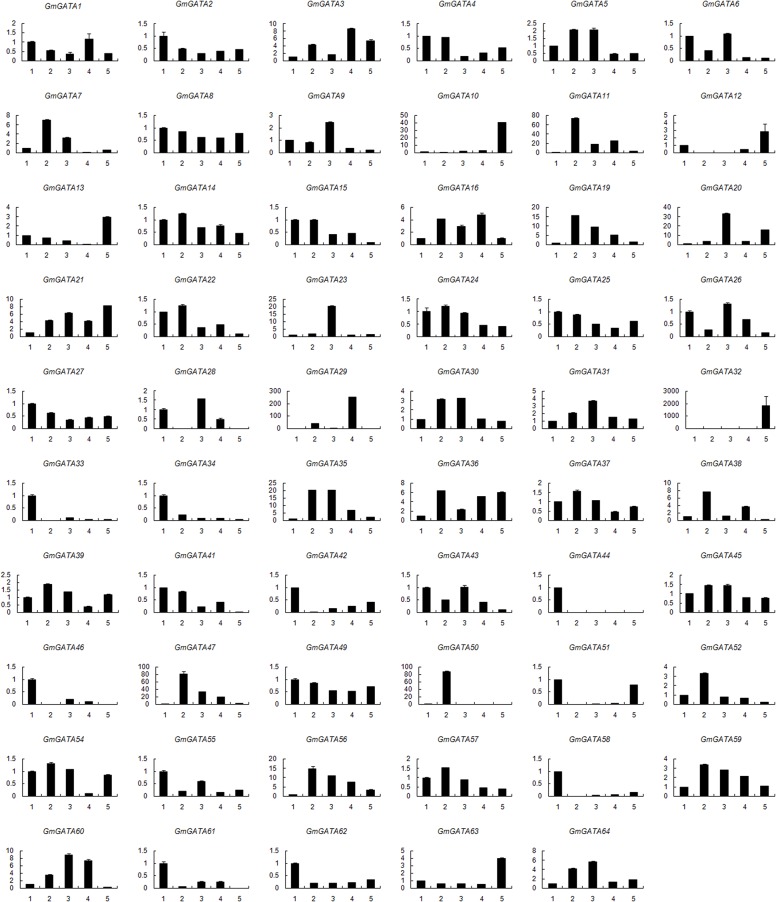
To identify the tissue expression patterns of GmGATA genes in soybean, specific primers were designed for each of the GATA factor genes (S1 Table), and the expression profiles of the 64 GmGATA genes were investigated in various tissues, including root, stem, young leaf, flower, and immature seed, by real-time PCR. Results showed that the soybean GATA genes were expressed in distinct patterns (Fig 5). The GmGATA8, GmGATA45, and GmGATA49 genes showed less than twofold expression variation in different tissues, suggesting that they are not developmentally regulated at the transcription level. Some GmGATA genes were constitutively expressed in different tissues, but with preferential expression in certain tissues. For example, GmGATA33/34/42/46/58/62 were predominantly expressed in young leaf; GmGATA7/11/38/47/52 in root; GmGATA9, GmGATA20, and GmGATA23 in stem; and GmGATA10, GmGATA13, and GmGATA63 in immature seed. Moreover, GmGATA29, GmGATA32, GmGATA44, and GmGATA50 exhibited a highly tissue-specific expression pattern in flower, immature seed, young leaf, and root, respectively. Among these four genes, GmGATA44 having maximum similarity with the Arabidopsis GATA gene AtGATA22 based on GATA zinc finger sequences (Fig 4) shared a highly similar expression pattern to AtGATA22 [14], a regulator of chloroplast development and chlorophyll biosynthesis [7, 46]. The GATA genes highly expressed in specific organs of plants are crucial for the functioning or development of a specific organ.
Fig 5. Relative expression profiles of soybean GATA genes in various organs.
Data were obtained by real-time PCR normalized against the reference gene ACT11 and shown as a percentage of expression in leaf. Numbers on the x-axis indicate various tissues: 1 (young leaf), 2 (root), 3 (stem), 4 (flower), and 5 (immature seed).
In addition, four GmGATA genes showed no expression in one or two tissues. GmGATA12 was undetectable in root and stem but highly expressed in seed; GmGATA28 was not expressed in root and seed but moderately expressed in stem; GmGATA29 and GmGATA61 were not expressed in seed but highly expressed in flower and young leaf, respectively. Five GmGATA genes GmGATA17/18/40/48/53 were not detected in any examined tissues. This result is consistent with the fact that no EST sequences corresponding with the five GmGATA genes were found in the Gene Indices at DFCI (Table 1). This result may be attributed to the insufficient sampling or the presence of untranscribed pseudogenes in the family. Genes within the same segmental duplicated pair usually have similar expression profiles. GmGATA3/36, GmGATA6/55, GmGATA8/49, GmGATA10/63, GmGATA11/19, GmGATA15/22, GmGATA16/59, GmGATA25/27, GmGATA35/64, GmGATA44/58, and GmGATA46/61 were expressed at similar profiles, implying redundant functions. In addition, other segmental duplicated gene pairs (e.g., GmGATA13/20, GmGATA23/30, and GmGATA33/51) showed significantly different tissue expression profiles, implying divergent functions. Some members in the same subfamily shared a highly similar expression profile. For example, GmGATA4/2/11/19/38/41 from the same clade in subfamily I showed predominant expression in leaf or root, and GmGATA46/61/33/44/58 from the same clade in subfamily II had predominant expression in leaf. All these expression profiles suggest redundancy and divergence in the biological functions of soybean GATA factor genes during plant growth and development.
Expression profiles of soybean GATA genes under low nitrogen stress condition
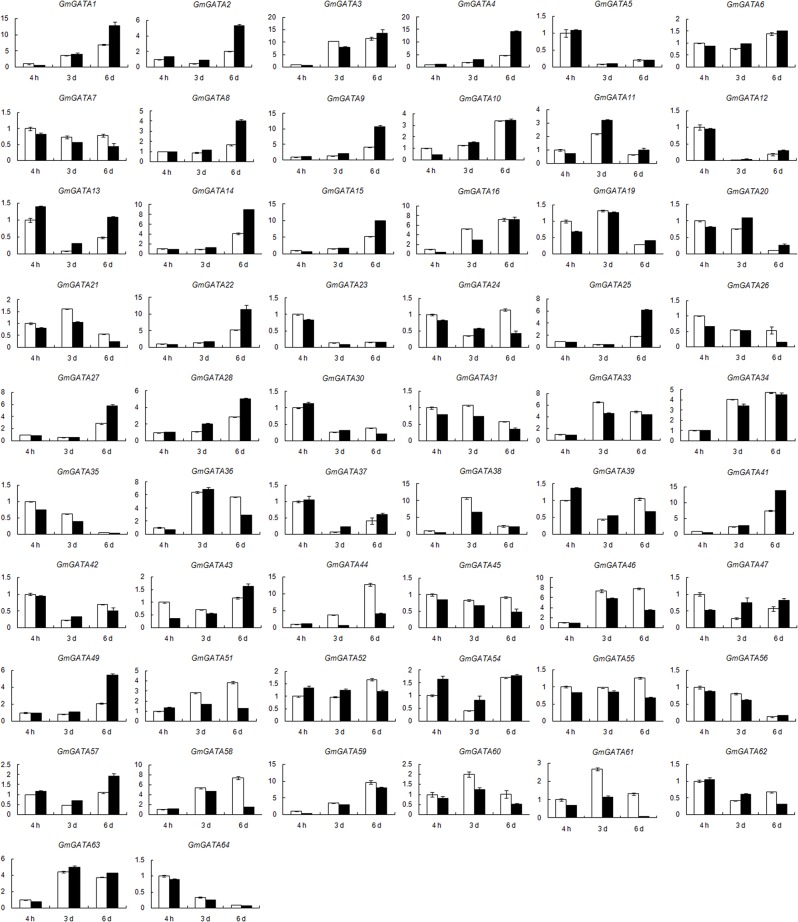
Previous studies showed that some members of the plant GATA factor gene family are involved in nitrogen response [7, 27, 48]. Therefore, we analyzed transcript abundance from low nitrogen solution-grown and half Hoagland solution-grown soybean seedlings by real-time PCR to determine whether or not the soybean GATA factor genes are nitrogen regulated. The expression data in leaf and root are shown in Figs 6 and 7, respectively. We compared the expression levels of GmGATA genes in these seedlings at 4 h, 3 d, and 6 d after treatment.
Fig 6. Expression of soybean GATA genes in leaves in response to low nitrogen stress.
Data were obtained by real-time PCR normalized against the reference gene ACT11 and shown as a percentage of expression in control leaves at 4 h. White column represents the expression under normal nitrogen condition, and black column represents the expression under limited nitrogen condition. Eight genes (GmGATA17/18/29/32/40/48/50/53) not expressed in soybean leaf under normal condition were not induced under low nitrogen stress and not present in this figure.
Fig 7. Expression of soybean GATA genes in roots in response to low nitrogen stress.
Data were obtained by real-time PCR normalized against the reference gene ACT11 and shown as a percentage of expression in control roots at 4 h. White column represents the expression under normal nitrogen condition, and black column represents the expression under limited nitrogen condition. Fourteen genes (GmGATA12/17/18/28/29/32/33/40/44/46/48/51/53/58) not expressed in soybean root under normal condition were not induced under low nitrogen stress and not present in this figure.
As shown in Fig 6, 26 soybean GATA genes were differentially expressed in the leaves of low nitrogen-treated seedlings compared with those of the untreated control seedlings, and most of them showed different expression levels at 6 d after treatment. A total of 12 genes showed significantly higher expression in the leaves of low nitrogen-treated seedlings than in those of the untreated control seedlings (Fig 6). The greatest differences were observed for GmGATA25 (increased by 2.36-fold at 6 d after treatment), GmGATA4 (increased by 2.05-fold at 6 d after treatment), and GmGATA13 (increased by 2.64-fold at 3 d after treatment). Among the 12 differentially expressed GATA factor genes, six (GmGATA2/4/9/13/20/47) belonged to one clade of subfamily I, and the other six (GmGATA8/14/22/25/27/49) belonged to subfamily III. By contrast, 14 genes showed lower expression in the leaves of low nitrogen-treated seedlings than in those of the untreated control seedlings (Fig 6). The greatest differences were observed for GmGATA61 (decreased by 58% and 95% at 3 and 6 d after treatment, respectively), GmGATA44 (decreased by 81% and 67% at 3 and 6 d after treatment, respectively), GmGATA58 (decreased by 79% at 6 d after treatment), and GmGATA26 (decreased by 74% at 6 d after treatment). Among these 14 genes, half of them (GmGATA10/26/44/46/51/58/61) belonged to one clade of subfamily II, four (GmGATA24/35/43/62) belonged to subfamily I, one (GmGATA21) belonged to subfamily III, and two (GmGATA16/59) belonged to subfamily IV.
Some segmental duplicated gene pairs, such as GmGATA8/49, GmGATA16/59, and GmGATA25/27, shared similar expression change in leaves in response to low nitrogen stress. However, some pairs showed different expression profiles. For example, for GmGATA14/21, the expression of GmGATA14 increased by 1.17-fold in low nitrogen-treated leaves compared with the control at 6 d after treatment, whereas GmGATA21 decreased by 57%. For GmGATA33/51, GmGATA51 decreased by 68% in low nitrogen-treated leaves compared with the control at 6 d after treatment, whereas GmGATA33 showed no expression change in response to low nitrogen stress. Similar results were also obtained for GmGATA26/57, GmGATA10/63, GmGATA43/45, GmGATA35/64, and GmGATA52/62. These findings suggest redundancy and divergence in the biological functions of soybean GATA factor genes in response to low nitrogen stress.
Fewer differentially expressed GATA factor genes were found in soybean roots than in soybean leaves. Seven GATA genes (GmGATA10/24/52/62/16/50/60) showed significantly different expression levels between the roots of low nitrogen-treated and untreated control seedlings (Fig 7). The greatest differences were observed for GmGATA52 (increased by 1.52-fold at 6 d after treatment compared with the control) and GmGATA50 (decreased by 79% at 6 d after treatment compared with the control). Among these seven genes, four (GmGATA24/52/62/60) belonged to subfamily I, two (GmGATA10/50) belonged to subfamily II, one (GmGATA16) belonged to subfamily IV, and none belonged to subfamily III. Four GATA genes (GmGATA10/16/24/62) exhibited different expression levels in both leaves and roots compared with the control.
To further analyze the correlation between the differentially expressed GATA factors and nitrogen metabolism-related genes in soybean roots in response to low nitrogen, a total of seven genes involved in nodulation (ENOD40 [49]), preliminary nitrogen reduction (INR1 [50], INR2 [50] and NiR [51]), nitrogen transport (NRT1-2 and NRT2 [52]), and nitrogen assimilation (GS1 [53]) were selected for real-time PCR assay. Results showed that the expression levels of ENOD40 and GS1 were not altered significantly in low nitrogen-treated roots compared with the control (Fig 8). The results indicated that the differentially expressed GATA factors were not associated with the nodulation specific gene ENOD40. INR1, INR2 and NiR were all down-regulated after low nitrogen treatment, and NRT1-2 and NRT2 were both up-regulated (Fig 8). The correlation analysis between these soybean nitrogen metabolism-related genes and the differentially expressed GATA factors indicated that NRT1-2 was co-expressed with GATA52 in low nitrogen condition, as they were both up-regulated at 6 d after low nitrogen treatment. Moreover, NRT1-2 contained the GATA binding domain in its promoter region (S2 Text). Whether GATA52 could interact with the promoter of NRT1-2 and regulate its expression will be analyzed in the future. Additionally, INR2 and NRT2 also contained the GATA binding domain in their promoter regions (S2 Text). Whether some other GATA factors interact with the promoters of INR2 and NRT2 will be analyzed in our future study.
Fig 8. Expression of soybean nodulation and nitrogen metabolism-related genes in roots in response to low nitrogen stress.
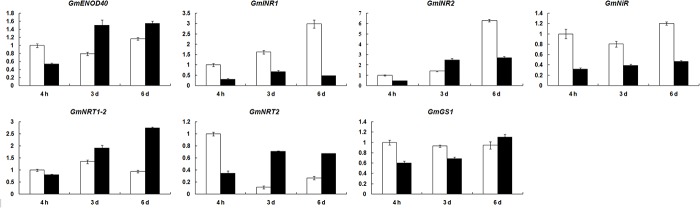
Data were obtained by real-time PCR normalized against the reference gene ACT11 and shown as a percentage of expression in control roots at 4 h. White column represents the expression under normal nitrogen condition, and black column represents the expression under limited nitrogen condition.
GmGATA44 modulates chlorophyll content
As previously mentioned, the expression patterns of GmGATA44 and GmGATA58 were similar to those of the Arabidopsis orthologs AtGATA21 and AtGATA22 and the rice ortholog OsGATA11. They are all inducible by nitrate [27, 48] and exhibit the strongest expression in green leaf tissues [14, 27, 47]. These findings indicate the functional conservation among soybean, Arabidopsis, and rice. AtGATA21, AtGATA22, and OsGATA11 are involved in regulating chlorophyll synthesis and nitrogen metabolism [7, 27].
The Arabidopsis gnc mutant has a T-DNA insertion in the exon of AtGATA21 gene, leading to the reduced chlorophyll phenotype. To confirm whether GmGATA44 had similar biological functions of the orthologous gene AtGATA21, overexpression of GmGATA44 under the control of CaMV 35S promoter was carried out in the gnc mutant background to complement this mutant. A total of 50 GmGATA44 overexpressing (OX) transgenic plants were obtained, and two lines (OX31 and OX43) were chose for further analysis. Semi-quantitative RT-PCR results showed that the exogenous GmGATA44 was abundantly expressed in both OX31 and OX43 lines, and the endogenous AtGNC was expressed in wild-type Arabidopsis rather not in the gnc mutant and two transgenic lines (Fig 9A). Both OX31 and OX43 lines restored pale green leaves of the gnc mutant to green and even greener leaves than that of wild-type plants (Fig 9B). The results of chlorophyll content in leaves also corresponded to this complementation. The chlorophyll accumulation was improved significantly in both OX31 and OX43 lines, compared to the gnc mutant, even more than that of wild-type plants (Fig 9C). In addition, strong accumulation of chlorophyll was also obviously observed in the seedling hypocotyls of both OX31 and OX43 lines (Fig 9B).
Fig 9. GmGATA44 modulates chlorophyll content.
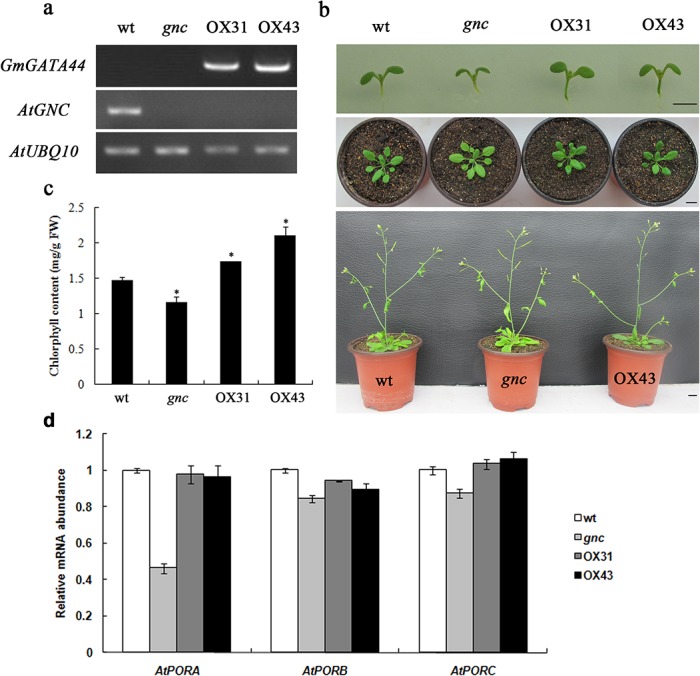
(a) Expression levels of GmGATA44 and AtGNC in the wild-type Arabidopsis (wt), the gnc mutant and two GmGATA44 overexpressing transgenic lines (OX31 and OX43) using semi-quantitative RT-PCR from 3 week old rosette leaf tissue.(b) Images of the wild-type plant, the gnc mutant and GmGATA44 overexpressing transgenic plants at one week (upper panel), 3 weeks (middle panel) and 5 weeks (bottom panel) post germination. Bars = 1 cm.(c) Chlorophyll content of the wild-type plants, the gnc mutant and two GmGATA44 overexpressing transgenic lines at 3 weeks post germination. Data are presented as mean ± SD (N = 10) from triplicate independent measurements. Data analysis was performed using SAS software, and significant differences were calculated using the Student’s t-test at 95% confidence limit. Asterisk indicates significant differences from the wild-type plant.(d) Relative expression levels of AtPORA, AtPORB and AtPORC in the wild-type plant, the gnc mutant and two GmGATA44 overexpressing transgenic lines by real-time PCR from 3 week old rosette leaf tissue. Data were obtained by real-time PCR normalized against the reference gene GAPDH and shown as a percentage of expression in the wild-type plant.
Changes in chlorophyll contents indicated that genes involved in chlorophyll biosynthesis might be altered. Consistent with the previous report [54], the expression levels of AtPORA, AtPORB and AtPORC were reduced in the gnc mutant compared with the wild-type plants (Fig 9D), which had been suggested to be the molecular cause for the greening defect of the gnc mutant [54]. Overexpression of GmGATA44 in the gnc mutant led to the up-regulation of these POR genes, especially for AtPORA. Moreover, it should be noted that the expression level of AtPORC was increased slightly more than that in the wild-type plants. Additionally, other 14 genes involved in tetrapyrrole pathway [55] and two key genes (AtDXS and AtDXR) in methylerythritol phosphate pathway [56] for chlorophyll biosynthesis were also analyzed, and they were not found to be altered significantly in the two overexpressing lines compared with the gnc mutant (S2 Fig).
These results suggested that GmGATA44 played an important role in modulating chlorophyll biosynthesis, similar to the function of the ortholog AtGATA21. Chlorophyll level is often used as a reflection of nitrogen status. The response of transgenic plants to low nitrogen stress will be analyzed in the further study.
Conclusion
We identified 64 GATA genes in soybean through a genome-wide analysis. The soybean genome had more GATA genes than the Arabidopsis or rice genome. The great expansion of the soybean GATA factor gene family was likely due to segmental duplication during the evolutionary history. An overview of the soybean GATA factor gene family was revealed through the comprehensive investigation of their chromosomal distributions, gene structures, duplication patterns, phylogenetic tree, and conserved motifs. A comparative analysis of the GATA factor gene family across soybean, Arabidopsis, and rice helped us facilitate further gene function analysis of soybean GATA genes. Our results also provided useful information by identifying candidate tissue-specific and low nitrogen stress responsive soybean GATA genes. The preliminary function analysis showed GmGATA44 had the similar function in modulating chlorophyll biosynthesis with its orthologs in Arabidopsis and rice. These investigations and analyses could increase knowledge on the functions of soybean GATA genes in the regulation of soybean growth and nitrogen metabolism.
Supporting Information
The sequences are retrieved from the Phytozome or NCBI database.
(DOC)
(DOC)
The 55-amino acid regions of 63 soybean GATA domains and the 29-amino acid regions containing the half GATA domain of GmGATA28 were aligned. Residues conserved in all or most of the soybean GATA domains are highlighted. Asterisks indicate the conserved cysteine residues (Cys) in the GATA domain.
(TIF)
Data were obtained by real-time PCR normalized against the reference gene GAPDH and shown as a percentage of expression in the wild-type plants.
(TIF)
(DOC)
(DOC)
(DOC)
Pairwise identities and amino acid sequence alignments of the 25 homologous pairs identified from the soybean GATA family.
(XLS)
The motif numbers correspond to those described in Fig 3.
(XLS)
The GATA factor sequences of Arabidopsis and rice were obtained from the NCBI and rice genome annotation databases (http://rice.plantbiology.msu.edu/; release 7.0), respectively. The nomenclature is according to previous reports [6, 14].
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the National Transgenic Project (No. 2014ZX08004-005), Agricultural Science and Technology Innovation Program, and National Natural Science Foundation of China (No. 31201153). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lowry JA, Atchley WR. Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. J Mol Evol. 2000;50(2):103–15. [DOI] [PubMed] [Google Scholar]
- 2. Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates). Curr Opin Genet Dev. 2002;12(4):416–22. [DOI] [PubMed] [Google Scholar]
- 3. Teakle GR, Gilmartin PM. Two forms of type IV zinc-finger motif and their kingdom-specific distribution between the flora, fauna and fungi. Trends Biochem Sci. 1998;23(3):100–2. [DOI] [PubMed] [Google Scholar]
- 4. Scazzocchio C. The fungal GATA factors. Curr Opin Microbiol. 2000;3(2):126–31. [DOI] [PubMed] [Google Scholar]
- 5. Daniel-Vedele F, Caboche M. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Mol Gen Genet. 1993;240(3):365–73. [DOI] [PubMed] [Google Scholar]
- 6. Reyes JC, Muro-Pastor MI, Florencio FJ. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004;134(4):1718–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bi YM, Zhang Y, Signorelli T, Zhao R, Zhu T, Rothstein S. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J. 2005;44(4):680–92. [DOI] [PubMed] [Google Scholar]
- 8. Buzby JS, Yamada T, Tobin EM. A light-regulated DNA-binding activity interacts with a conserved region of a Lemna gibba rbcS promoter. Plant Cell. 1990;2(8):805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carre IA, Kay SA. Multiple DNA-protein complexes at a circadian-regulated promoter element. Plant Cell. 1995;7(12):2039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castresana C, Garcia-Luque I, Alonso E, Malik V, Cashmore A. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia . EMBO J. 1988;7(7):1929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnik PA, Cashmore AR. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA. 1988;85(19):7089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam E, Kano-Murakami Y, Gilmartin P, Niner B, Chua NH. A metal-dependent DNA-binding protein interacts with a constitutive element of a light-responsive promoter. Plant Cell. 1990;2(9):857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teakle GR, Kay SA. The GATA-binding protein CGF-1 is closely related to GT-1. Plant Mol Biol. 1995;29(6):1253–66. [DOI] [PubMed] [Google Scholar]
- 14. Manfield IW, Devlin PF, Jen CH, Westhead DR, Gilmartin PM. Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiol. 2007;143(2):941–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teakle GR, Manfield IW, Graham JF, Gilmartin PM. Arabidopsis thaliana GATA factors: organisation, expression and DNA-binding characteristics. Plant Mol Biol. 2002;50(1):43–57. [DOI] [PubMed] [Google Scholar]
- 16. Jeong MJ, Shih MC. Interaction of a GATA factor with cis-acting elements involved in light regulation of nuclear genes encoding chloroplast glyceraldehyde-3-phosphate dehydrogenase in Arabidopsis. Biochem Biophys Res Commun. 2003;300(2):555–62. [DOI] [PubMed] [Google Scholar]
- 17. Luo XM, Lin WH, Zhu S, Zhu JY, Sun Y, Fan XY, et al. Integration of light and brassinosteroid-signaling pathways by a GATA Transcription Factor in Arabidopsis . Dev Cell. 2010;19(6):872–83. 10.1016/j.devcel.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishii A, Takemura M, Fujita H, Shikata M, Yokota A, Kohchi T. Characterization of a novel gene encoding a putative single zinc-finger protein, ZIM, expressed during the reproductive phase in Arabidopsis thaliana . Biosci, Biotechnol, Biochem. 2000;64(7):1402–9. [DOI] [PubMed] [Google Scholar]
- 19. Zhao Y, Medrano L, Ohashi K, Fletcher JC, Yu H, Sakai H, et al. HANABA TARANU is a GATA transcription factor that regulates shoot apical meristem and flower development in Arabidopsis. Plant Cell. 2004;16(10):2586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shikata M, Matsuda Y, Ando K, Nishii A, Takemura M, Yokota A, et al. Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J Exp Bot. 2004;55(397):631–9. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Yin H, Qian Q, Yang J, Huang C, Hu X, et al. NECK LEAF 1, a GATA type transcription factor, modulates organogenesis by regulating the expression of multiple regulatory genes during reproductive development in rice. Cell Res. 2009;19(5):598–611. 10.1038/cr.2009.36 [DOI] [PubMed] [Google Scholar]
- 22. Liu PP, Koizuka N, Martin RC, Nonogaki H. The BME3 (Blue Micropylar End 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J. 2005;44(6):960–71. [DOI] [PubMed] [Google Scholar]
- 23. Fu YH, Marzluf GA. nit-2, the major nitrogen regulatory gene of Neurospora crassa, encodes a protein with a putative zinc finger DNA-binding domain. Mol Cell Biol. 1990;10(3):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarai G, Truong HN, Daniel-Vedele F, Marzluf GA. NIT2, the nitrogen regulatory protein of Neurospora crassa, binds upstream of nia, the tomato nitrate reductase gene, in vitro. Curr Genet. 1992;21(1):37–41. [DOI] [PubMed] [Google Scholar]
- 25. Rastogi R, Bate NJ, Sivasankar S, Rothstein SJ. Footprinting of the spinach nitrite reductase gene promoter reveals the preservation of nitrate regulatory elements between fungi and higher plants. Plant Mol Biol. 1997;34(3):465–76. [DOI] [PubMed] [Google Scholar]
- 26. Hudson D, Guevara D, Yaish MW, Hannam C, Long N, Clarke JD, et al. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis . PLoS One. 2011;6(11):e26765 10.1371/journal.pone.0026765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hudson D, Guevara DR, Hand AJ, Xu Z, Hao L, Chen X, et al. Rice cytokinin GATA transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiol. 2013;162(1):132–44. 10.1104/pp.113.217265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kereszt A, Li D, Indrasumunar A, Nguyen CD, Nontachaiyapoom S, Kinkema M, et al. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc. 2007;2(4):948–52. [DOI] [PubMed] [Google Scholar]
- 29. Libault M, Joshi T, Takahashi K, Hurley-Sommer A, Puricelli K, Blake S, et al. Large-scale analysis of putative soybean regulatory gene expression identifies a Myb gene involved in soybean nodule development. Plant Physiol. 2009;151(3):1207–20. 10.1104/pp.109.144030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer LJ, Gao J, Xu D, Thelen JJ. Phosphoproteomic analysis of seed maturation in Arabidopsis, rapeseed, and soybean. Plant Physiol. 2012;159(1):517–28. 10.1104/pp.111.191700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–83. 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- 32. Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30(5):1229–35. 10.1093/molbev/mst012 [DOI] [PubMed] [Google Scholar]
- 33. Guo AY, Zhu QH, Chen X, Luo JC. GSDS: a gene structure display server. Yi Chuan. 2007;29(8):1023–6. [PubMed] [Google Scholar]
- 34. Cannon EK, Cannon SB. Chromosome visualization tool: a whole genome viewer. Int J Plant Genomics. 2011;2011:373875 10.1155/2011/373875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo Y, Qiu LJ. Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS One. 2013;8(9):e76809 10.1371/journal.pone.0076809 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Bailey TL, Williams N, Misleh C, Li WW. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34(suppl 2):369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hao QN, Zhou XA, Sha AH, Wang C, Zhou R, Chen SL. Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes. BMC Genomics. 2011;12:525 10.1186/471-2164-12-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–6. [DOI] [PubMed] [Google Scholar]
- 39. Wellburn R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144(3):307–13. [Google Scholar]
- 40. Shoichet SA, Malik TH, Rothman JH, Shivdasani RA. Action of the Caenorhabditis elegans GATA factor END-1 in Xenopus suggests that similar mechanisms initiate endoderm development in ecdysozoa and vertebrates. Proc Natl Acad Sci USA. 2000;97(8):4076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, dePamphilis CW. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J. 2007;50(5):873–85. [DOI] [PubMed] [Google Scholar]
- 42. Robson F, Costa MMR, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, et al. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28(6):619–31. [DOI] [PubMed] [Google Scholar]
- 43. Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Más P, et al. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289(5480):768–71. [DOI] [PubMed] [Google Scholar]
- 44. Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. The tify family previously known as ZIM. Trends Plant Sci. 2007;12(6):239–44. [DOI] [PubMed] [Google Scholar]
- 45. Omichinski JG, Clore GM, Schaad O, Felsenfeld G, Trainor C, Appella E, et al. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993;261(5120):438–46. [DOI] [PubMed] [Google Scholar]
- 46. Chiang YH, Zubo YO, Tapken W, Kim HJ, Lavanway AM, Howard L, et al. Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiol. 2012;160(1):332–48. 10.1104/pp.112.198705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mara CD, Irish VF. Two GATA transcription factors are downstream effectors of floral homeotic gene action in Arabidopsis. Plant Physiol. 2008;147(2):707–18. 10.1104/pp.107.115634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, et al. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004;136(1):2483–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang WC, Katinakis P, Hendriks P, Smolders A, de Vries F, Spee J, et al. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 1993;3(4):573–85. [DOI] [PubMed] [Google Scholar]
- 50. Wu S, Lu Q, Kriz AL, Harper JE. Identification of cDNA clones corresponding to two inducible nitrate reductase genes in soybean: analysis in wild-type and nr1 mutant. Plant Mol Biol. 1995;29(3):491–506. [DOI] [PubMed] [Google Scholar]
- 51. Li X, Zhao J, Walk TC, Liao H. Characterization of soybean beta-expansin genes and their expression responses to symbiosis, nutrient deficiency, and hormone treatment. Appl Microbiol Biotechnol. 2014;98(6):2805–17. 10.1007/s00253-013-5240-z [DOI] [PubMed] [Google Scholar]
- 52. Amarasinghe BH, de Bruxelles GL, Braddon M, Onyeocha I, Forde BG, Udvardi MK. Regulation of GmNRT2 expression and nitrate transport activity in roots of soybean (Glycine max). Planta. 1998;206(1):44–52. [DOI] [PubMed] [Google Scholar]
- 53. Ortega JL, Temple SJ, Sengupta-Gopalan C. Constitutive overexpression of cytosolic glutamine synthetase (GS1) gene in transgenic alfalfa demonstrates that GS1 may be regulated at the level of RNA stability and protein turnover. Plant Physiol. 2001;126(1):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Richter R, Behringer C, Muller IK, Schwechheimer C. The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev. 2010;24(18):2093–104. 10.1101/gad.594910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eckhardt U, Grimm B, Hortensteiner S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol Biol. 2004;56(1):1–14. [DOI] [PubMed] [Google Scholar]
- 56. Kim S, Schlicke H, Van Ree K, Karvonen K, Subramaniam A, Richter A, et al. Arabidopsis chlorophyll biosynthesis: an essential balance between the methylerythritol phosphate and tetrapyrrole pathways. Plant Cell. 2013;25(12):4984–93. 10.1105/tpc.113.119172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The sequences are retrieved from the Phytozome or NCBI database.
(DOC)
(DOC)
The 55-amino acid regions of 63 soybean GATA domains and the 29-amino acid regions containing the half GATA domain of GmGATA28 were aligned. Residues conserved in all or most of the soybean GATA domains are highlighted. Asterisks indicate the conserved cysteine residues (Cys) in the GATA domain.
(TIF)
Data were obtained by real-time PCR normalized against the reference gene GAPDH and shown as a percentage of expression in the wild-type plants.
(TIF)
(DOC)
(DOC)
(DOC)
Pairwise identities and amino acid sequence alignments of the 25 homologous pairs identified from the soybean GATA family.
(XLS)
The motif numbers correspond to those described in Fig 3.
(XLS)
The GATA factor sequences of Arabidopsis and rice were obtained from the NCBI and rice genome annotation databases (http://rice.plantbiology.msu.edu/; release 7.0), respectively. The nomenclature is according to previous reports [6, 14].
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.