Abstract
Cadmium (Cd) pollution is an environmental problem worldwide. Phytoremediation is a convenient method of removing Cd from both soil and water, but its efficiency is still low, especially in aquatic environments. Scientists have been trying to improve the ability of plants to absorb and accumulate Cd based on interactions between plants and Cd, especially the mechanism by which plants resist Cd. Eichhornia crassipes and Pistia stratiotes are aquatic plants commonly used in the phytoremediation of heavy metals. In the present study, we conducted physiological and biochemical analyses to compare the resistance of these two species to Cd stress at 100 mg/L. E. crassipes showed stronger resistance and was therefore used for subsequent comparative proteomics to explore the potential mechanism of E. crassipes tolerance to Cd stress at the protein level. The expression patterns of proteins in different functional categories revealed that the physiological activities and metabolic processes of E. crassipes were affected by exposure to Cd stress. However, when some proteins related to these processes were negatively inhibited, some analogous proteins were induced to compensate for the corresponding functions. As a result, E. crassipes could maintain more stable physiological parameters than P. stratiotes. Many stress-resistance substances and proteins, such as proline and heat shock proteins (HSPs) and post translational modifications, were found to be involved in the protection and repair of functional proteins. In addition, antioxidant enzymes played important roles in ROS detoxification. These findings will facilitate further understanding of the potential mechanism of plant response to Cd stress at the protein level.
Introduction
Cadmium (Cd), which is one of the most common heavy metal pollutants [1], is easily absorbed by plants and enriched in other organisms through the food chain [2,3]. Cd causes diverse biotoxic effects and diseases [2,4] that can threaten the growth, development and survival of an organism. Cd pollution has become an environmental problem worldwide because of its unseen, long-term and irreversible characteristics [5]. Thus, various methods and techniques have been developed to remove Cd and other heavy metals from the environment [6]. Among them, phytoremediation is a simple, economic and clean method that has attracted a great deal of attention [6,7].
Exposure to Cd pollution influences the development of plants in various ways. Cd can damage the cell structure and division [8], inhibit photosynthesis and transpiration [9,10], and induce oxidative stress [11,12]. However, plants can resist Cd stress through a variety of approaches. For example, they can control the absorption and use of Cd [13]. Additionally, many plants can detoxicate Cd by forming compounds with the help of phytochelatins (PCs) [14–16]. Plants can also coordinate synthesis and consumption of PCs and other thiols to form a metabolic equilibrium to accumulate Cd and combat its toxicity [17]. PCs are also considered to be important to the maintenance of glutathione and other antioxidant systems needed for plant survival [18]. Another strategy employed by plants to reduce Cd concentrations in the cytoplasm is compartmentalization [19,20], in which Cd is sequestered in vacuoles. In addition, plants can also improve antioxidant systems to respond to increased oxidative stress caused by Cd stress [21,22]. Based on these detoxification functions, some plants become hyperaccumulator species and are applied to remove Cd [23]. However, hyperaccumulator plants account for only a small part of the plant kingdom, and most plants cannot well resist Cd toxicity because of their inherent genetic basis. Nevertheless, genetic modification has been demonstrated to improve the resistance of plants to Cd [20], which may reduce Cd toxicity to plants and improve their efficiency for phytoremediation of Cd contamination. Proteomics, transcriptomics and metabolomics are able to provide accurate information regarding the molecular mechanisms of interactions between organisms and the environment [24–26]. Over the last decade, proteomic techniques have been applied to explore proteome changes induced by Cd stress in many model plants and hyperaccumulators [27–29]. Several types of functional proteins, including those involved in photosynthesis [27,30–33], energy and carbohydrate metabolism [27,29–32,34,35], transcription and translation [32,33,35], oxidation and reduction [27,29,30,33], and stress-response proteins [29,34,35], have shown common changes in most studied plants. In addition, some special proteomics have been used to investigate the targeted processes or proteins. For example, Schneider et al. [36] applied a quantitative proteomics approach to evaluate the contribution of vacuolar transporters to Cd detoxification in barley and identified several important transporters that might be potential candidates for further investigation. Alvarez et al. [37] implemented two quantitative proteomics approaches, fluorescence two-dimensional difference gel electrophoresis and multiplexed isobaria tagging technology, to demonstrate the involvement of many enzymes that played essential roles in the Cd hyperaccumation and tolerance of Brassica juncea. These studies helped us to better understand how plants resisted Cd or other heavy metals.
However, although hyperaccumulator plants have occasionally been discovered and the molecular mechanism of plant resistance to Cd stress has been gradually revealed, most studies conducted to date have focused on terrestrial plants [23,28], while there have been few relevant investigations of aquatic plants [23,28]; therefore, the resources used for phytoremediation of Cd-polluted water are still largely limited. It is well known that Cd can easily spread in aquatic environments as the water flows, which will lead to difficulties in management and remediation of Cd pollution in water. Thus, it is essential to identify many more aquatic Cd hyperaccumulators and improve the removal ability of Cd in common aquatic species. Eichhornia crassipes and Pistia stratiotes are two common submersed plants that have been widely applied to the remediation of sewage to reduce eutrophication and heavy metals pollution [38,39]. These two plants are known to have the attributes of rapid growth, strong resistance to pollution and being convenient for salvage [40]. Previous studies have shown that these species underwent differential accumulation effects under varying Cd concentrations [39]. However, the difference in tolerance to Cd stress between E. crassipes and P. stratiotes and the physiological and molecular mechanism through which it occurs are still unknown. In addition, previous studies were usually performed using relatively low Cd concentrations [28], while ignoring the short-term effects of high Cd concentrations on plants. Therefore, in the present study, we measured physiological and biochemical reactions to compare the resistance of E. crassipes and P. stratiotes to Cd stress at 100 mg/L. Because E. crassipes showed stronger resistance, we conducted comparative proteomics to explore the potential mechanism of E. crassipes tolerance to Cd stress. The results of this study will enhance our understanding of interactions between aquatic plants and Cd, which will improve Cd phytoremediation.
Results
Changes in morphology
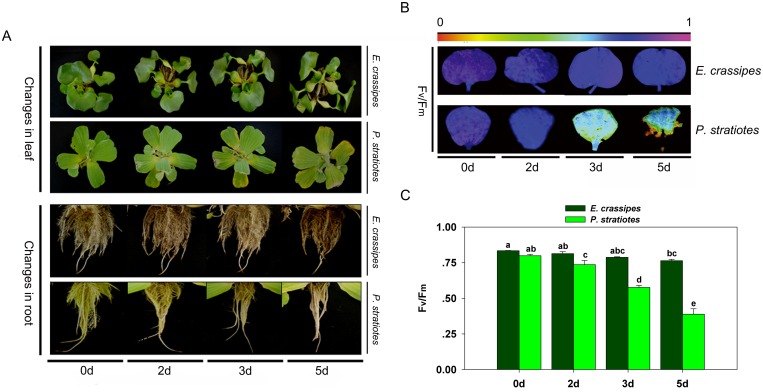
We first observed the morphological change to see the different resistance to high-concentration Cd between E. crassipes and P. stratiotes. Under Cd stress, the leaves of E. crassipes began to droop, but showed no discoloration or withering with increased treatment time (Fig 1A). However, the leaves of P. stratiotes turned yellow and withered from the leaf edges as the treatment time increased, and they began to fall off after 5 d of treatment (Fig 1A). Greater differences were observed in the roots relative to the leaves. Specifically, the roots of E. crassipes showed no obvious changes in response to Cd exposure until 5 d of treatment (Fig 1A), at which point the lateral roots began to fall. However, the roots of P. stratiotes started falling after Cd exposure for 2 d (Fig 1A), and they became rotten after 5 d of treatment (Fig 1A).
Fig 1. Changes in morphology and chlorophyll fluorescence of E. crassipes and P. stratiotes exposed to 100 mg/L Cd for different times.
A: Changes in leaf and root morphology. B: Fv/Fm images. The pseudocolor code depicted at the top of the image ranges from 0 (red) to 1 (purple). C: Average Fv/Fm values. Data are the means ± SE. Different letters following mean values indicate significant differences (Tukey’s test, P<0.05).
Changes in photosynthesis characteristics
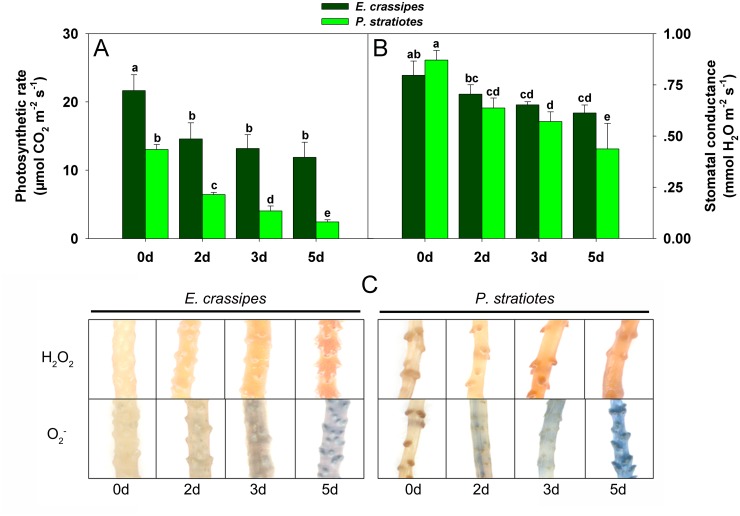
To investigate the differential response to Cd stress between E. crassipes and P. stratiotes from the physiological level, we measured the maximum quantum yield (ratio of variable to maximum fluorescence; Fv/Fm) of photosystem II (PS II). In the present study, Fv/Fm decreased in response to Cd treatment in both species (Fig 1B and 1C), but there were significant differences in the changes between species. Specifically, the Fv/Fm of E. crassipes decreased by 2.5%, 5.7%, and 8.4% after 2, 3, and 5 d of treatment, respectively, whereas that of P. stratiotes decreased by 8.5%, 27.9%, and 51.4% relative to the corresponding controls (Fig 1C). Similarly, photosynthesis showed different reductions between species. Specifically, the photosynthetic rate and stomatal conductance were reduced in response to Cd stress in E. crassipes (Fig 2A and 2B), but no differences were observed from 2 to 5 d of treatment (Fig 2A and 2B). However, both the photosynthetic rate and stomatal conductance decreased sharply in P. stratiotes following Cd exposure, with significant differences being observed at different time points (Fig 2A and 2B).
Fig 2. Changes in photosynthetic characteristics and reactive oxygen species (ROS) of E. crassipes and P. stratiotes exposed to 100 mg/L Cd for different times.
A: Photosynthetic rate change. B: Stomatal conductance change. C: In situ detection of H2O2 and O2 -. Data are presented as mean ± standard error. Different letters following mean values indicate significant differences (Tukey’s test, P<0.05).
Reactive oxygen species (ROS), malondialdehyde (MDA) and proline accumulation
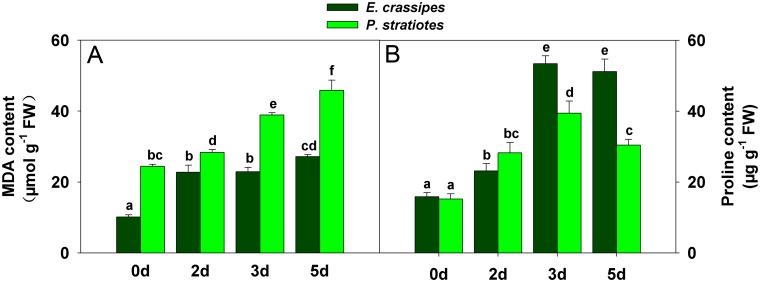
To understand the change of ROS metabolism in plants under Cd stress, we detected the accumulation of ROS (H2O2 and O2 -) and MDA. H2O2 and O2 - both increased gradually with increasing treatment time in E. crassipes and P. stratiotes (Fig 2C). P. stratiotes obviously produced more H2O2 and O2 - after the same treatment time when compared with E. crassipes (Fig 2C). Similarly, the level of MDA increased gradually in both E. crassipes and P. stratiotes with increasing Cd exposure duration, but with different accumulation levels between species (Fig 3A). The MDA content in E. crassipes increased rapidly at first, then continued to increase slightly, whereas it increased rapidly throughout the experimental period in P. stratiotes (Fig 3A).
Fig 3. Changes in malondialdehyde (MDA) and proline content in E. crassipes and P. stratiotes exposed to 100 mg/L Cd for different times.
A: MDA content change. B: Proline content change. Data are presented as mean ± standard error. Different letters following mean values indicate significant differences (Tukey’s test, P<0.05).
The proline content was measured to explore the potential role of proline in response to Cd stress. In our study, the proline content differed between E. crassipes and P. stratiotes (Fig 3B). Although the initial proline content in E. crassipes was less than in P. stratiotes, it continued to increase with exposure time (Fig 3B). Conversely, the proline content in P. stratiotes increased from 2 to 3 d of treatment, but decreased at 5 d (Fig 3B).
Dynamics of antioxidant enzyme activities
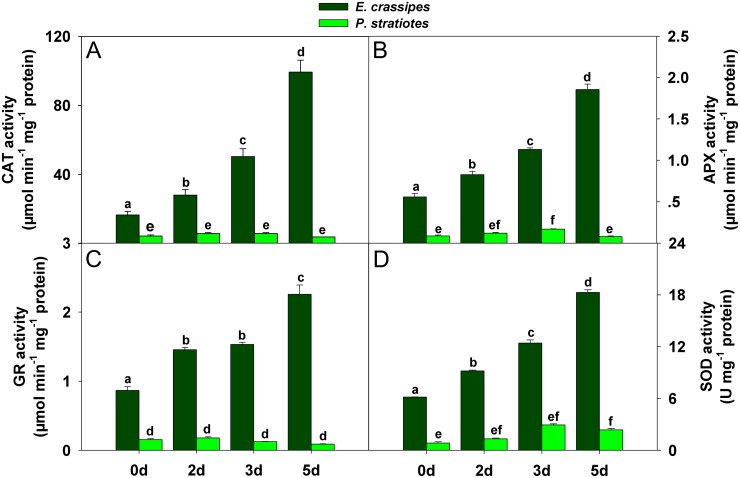
To investigate the role of antioxidant system in regulating ROS accumulation, we measured the activities of four common antioxidant enzymes. The activities of catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.8.1.7), and superoxide dismutase (SOD; EC 1.15.1.1) in E. crassipes were consistently much higher than in P. stratiotes (Fig 4), and they all increased significantly with increasing treatment time in E. crassipes (Fig 4). However, antioxidant enzyme activities first increased, then decreased in P. stratiotes (Fig 4). The maximum CAT, APX, and SOD activities were observed at 3 d, after which they began to decrease (Fig 4A, 4B and 4D). The highest GR activity was observed following exposure to Cd stress for 2 d (Fig 4C).
Fig 4. Changes in antioxidant enzyme activity in E. crassipes and P. stratiotes exposed to 100 mg/L Cd for different times.
A: CAT activity. B: APX activity. C: GR activity. D: SOD activity. Data are presented as mean ± standard error. Different letters following mean values indicate significant differences (Tukey’s test, P<0.05).
Dynamic change in expression of differential proteins
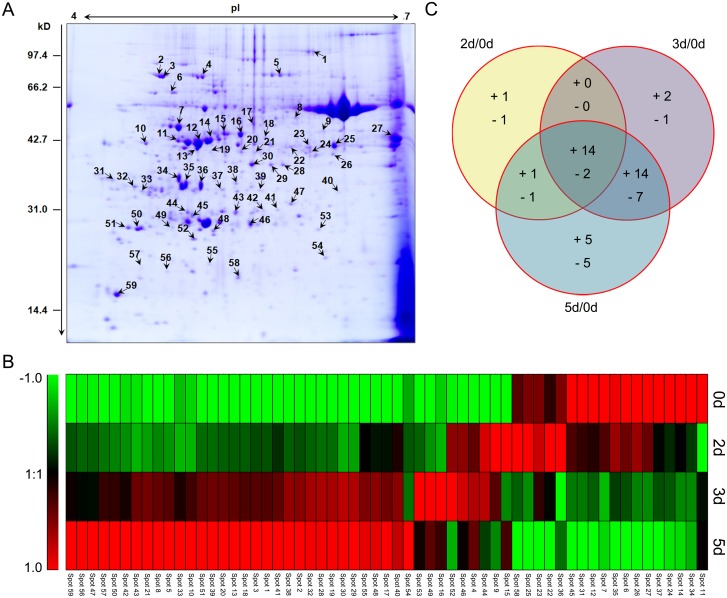
To further explore the underlying mechanism of E. crassipes tolerance toward Cd stress, the leaf proteomes of E. crassipes samples were evaluated by two-dimensional electrophoresis (2-DE). Each sample was replicated three times (S1, S2 and S3 Figs), and more than 500 protein spots were detected within each sample after staining. Of these, 87 showed increased expression (>1.50) or decreased expression (<0.67) in treated samples (2–5d) relative to the control (0d). Ultimately, 59 differentially expressed proteins (Fig 5A) were successfully identified by MALDI-TOF-MS/MS analysis and the National Center for Biotechnology Information (NCBI) nonredundant protein database (Table 1). Hierarchical cluster analysis was conducted to categorize the identified proteins that showed differential expression profiles in response to Cd stress (Fig 5B). Venn diagram analysis was used to reflect change patterns in proteins from treated samples (2–5d) relative to the control (0d) (Fig 5C). The results showed that up-regulation was much greater than down-regulation (Fig 5C), and that proteins were mainly affected during the later stage (3 or 5d) of Cd stress (Fig 5C).
Fig 5. Comparative proteomics analyses of four E. crassipes samples treated with 100 mg/L Cd for different times.
A: Representative 2-DE (the control sample of the first set of gel images) showing spot numbers of identified proteins. B: Hierarchical clustering of the identified protein expression profiles of different samples. Different colors correspond to the protein log-transformed fold-change ratios depicted in the bar on the left of the figure. C: Venn diagram analysis of differentially expressed proteins of each treated sample compared with the control sample (0d). “+” and “-” represent up-regulated and down-regulated proteins, respectively.
Table 1. Identification and analysis of differentially expressed proteins in leaves of Eichhornia crassipes treated by Cd stress for different times.
| Spot | Protein name | Acc. No. a | Theo. Mw/pI b | Exp. Mw/pI c | SC d | Score | Organism | Ratio e | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 2d/0d | 3d/0d | 5d/0d | ||||||||
| Photosynthesis | ||||||||||
| 6 | putative rubisco subunit binding-protein alpha subunit precursor (60 kDa chaperonin alpha subunit) | gi|31193919 | 61.36/5.36 | 68.71/5.05 | 29.11 | 118 | Oryza sativa Japonica Group | 0.87 | 0.72 | 0.54 |
| 7 | predicted ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic-like | gi|359481752 | 52.19/5.69 | 57.14/5.11 | 14.77 | 394 | Vitis vinifera | 0.75 | 0.48 | 0.45 |
| 12 | ribulose-1,5-bisphosphate carboxylase/oxygenase activase 1 | gi|12620881 | 48.19/5.54 | 38.71/5.30 | 19.63 | 334 | Gossypium hirsutum | 0.75 | 0.64 | 0.49 |
| 14 | RuBisCO activase | gi|445628 | 42.95/5.50 | 40.17/5.43 | 12.79 | 115 | Nicotiana tabacum | 0.70 | 0.59 | 0.52 |
| 24 | fructose-bisphosphate aldolase, chloroplast precursor, putative, expressed | gi|108864048 | 41.81/6.07 | 39.74/6.34 | 24.22 | 370 | Oryza sativa Japonica Group | 0.60 | 0.56 | 0.42 |
| 26 | chloroplast stem-loop binding protein-41 | gi|15229384 | 44.07/8.54 | 38.46/6.54 | 14.25 | 141 | Arabidopsis thaliana | 0.90 | 0.66 | 0.52 |
| 34 | chloroplast photosynthetic water oxidation complex 33kDa subunit precursor | gi|152143640 | 28.48/5.48 | 41.99/5.12 | 22.64 | 159 | Morus nigra | 0.44 | 0.34 | 0.28 |
| 35 | OEE1 | gi|302595735 | 34.49/5.40 | 39.98/5.19 | 30.86 | 435 | Helianthus annuus | 0.92 | 0.72 | 0.48 |
| 45 | predicted carbonic anhydrase, chloroplastic-like | gi|357130587 | 51.29/8.90 | 34.35/5.67 | 21.57 | 57 | Brachypodium distachyon | 0.82 | 0.66 | 0.55 |
| 52 | thylakoid luminal 19 kDa protein | gi|357457687 | 26.38/5.82 | 27.09/5.27 | 16.94 | 96 | Medicago truncatula | 1.17 | 1.55 | 1.03 |
| 59 | ribulose bisphosphate carboxylase | gi|119720808 | 18.71/8.23 | 17.89/6.84 | 37.65 | 99 | Brassica rapa | 1.47 | 1.72 | 2.56 |
| Growth and development | ||||||||||
| 5 | maturase K | gi|197257987 | 57.74/9.86 | 78.01/6.02 | 17.55 | 40 | Siphocodon spartioides | 1.12 | 1.39 | 1.66 |
| 15 | actin | gi|218533930 | 41.88/5.31 | 42.44/5.55 | 55.97 | 801 | Caragana korshinskii | 2.22 | 1.25 | 1.69 |
| 18 | old-yellow-enzyme homolog | gi|2232254 | 42.13/5.90 | 41.23/5.94 | 11.08 | 132 | Catharanthus roseus | 1.70 | 2.30 | 3.11 |
| Metabolism processes | ||||||||||
| Biosynthesis and degradation | ||||||||||
| 16 | glutamine synthetase | gi|15238559 | 47.78/6.43 | 41.86/5.72 | 15.35 | 191 | Arabidopsis thaliana | 1.10 | 1.53 | 2.04 |
| 22 | beta-cyanoalanine synthase | gi|30840956 | 38.26/6.38 | 40.62/6.13 | 6.53 | 75 | Betula pendula | 1.14 | 0.88 | 0.66 |
| 23 | malate dehydrogenase | gi|320449084 | 35.79/5.76 | 39.95/6.30 | 25.9 | 176 | Zea mays | 1.06 | 0.95 | 0.64 |
| 27 | predicted aminomethyl transferase, mitochondrial-like | gi|356555678 | 44.72/8.77 | 43.51/6.97 | 28.01 | 85 | Glycine max | 0.97 | 0.76 | 0.53 |
| 37 | xyloglucantransglusylase/hydrolase 1 | gi|304273280 | 32.13/6.06 | 39.46/5.53 | 3.93 | 69 | Gladiolus grandiflorus | 0.88 | 0.77 | 0.62 |
| 44 | cytosolic triosephosphate isomerase | gi|310768740 | 27.26/5.04 | 34.51/5.21 | 13.04 | 67 | Pteris vittata | 1.31 | 1.82 | 2.32 |
| 47 | triosephosphate isomerase, cytosolic | gi|226495391 | 27.24/5.52 | 35.11/6.24 | 29.25 | 87 | Zea mays | 1.17 | 1.27 | 1.70 |
| 58 | granule-bound starch synthase precursor | gi|4588607 | 63.39/7.86 | 15.78/5.80 | 51.68 | 772 | Triticum aestivum | 1.28 | 0.77 | 0.52 |
| Energy related | ||||||||||
| 11 | AMP deaminase family protein | gi|566209963 | 90.88/6.30 | 40.03/5.11 | 10.36 | 51 | Populus trichocarpa | 0.61 | 0.79 | 0.81 |
| 40 | ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit | gi|148787961 | 48.36/6.34 | 40.02/6.62 | 45.6 | 685 | Eichhornia crassipes | 2.33 | 2.70 | 2.88 |
| 41 | predicted probable ATP synthase 24 kDa subunit, mitochondrial | gi|225438529 | 27.67/9.26 | 36.26/6.05 | 17.01 | 90 | Vitis vinifera | 2.05 | 2.60 | 3.27 |
| 56 | ATP synthase CF1 epsilon subunit (chloroplast) | gi|374257035 | 14.69/4.95 | 20.26/5.03 | 53.73 | 466 | Japonolirion osense | 1.17 | 1.28 | 1.71 |
| Oxidation-reduction process | ||||||||||
| 1 | glycine dehydrogenase, putative | gi|255550796 | 115.78/6.57 | 108.69/6.37 | 8.8 | 126 | Ricinus communis | 1.74 | 2.29 | 3.04 |
| 8 | glycine dehydrogenase, putative | gi|255580957 | 42.88/6.05 | 45.19/6.23 | 36.97 | 611 | Ricinus communis | 1.28 | 1.77 | 2.19 |
| 19 | plastidic aldolase | gi|164470331 | 43.16/6.38 | 38.24/5.43 | 35.52 | 229 | Solanum tuberosum | 2.16 | 3.80 | 4.30 |
| 20 | plastidic aldolase family protein | gi|224094919 | 42.72/6.85 | 38.56/5.72 | 37.37 | 67 | Populus trichocarpa | 1.50 | 1.99 | 2.44 |
| 25 | predicted glyceraldehyde-3-phosphate dehydrogenase A, chloroplastic-like | gi|357163943 | 43.11/7.01 | 41.09/6.55 | 8.44 | 100 | Brachypodium distachyon | 1.25 | 0.61 | 0.43 |
| 28 | isoflavone reductase-like protein | gi|373939378 | 33.29/5.74 | 35.81/6.10 | 4.25 | 88 | Daucus carota | 1.73 | 2.78 | 3.06 |
| 29 | phenylcoumaran benzylic ether reductase | gi|3114899 | 33.99/5.66 | 35.62/5.99 | 14.94 | 86 | Populus trichocarpa | 2.77 | 3.43 | 3.91 |
| 30 | pterocarpan reductase | gi|116077986 | 33.97/5.94 | 45.60/5.82 | 6.13 | 89 | Lotus japonicus | 1.36 | 2.93 | 3.20 |
| 36 | oxidoreductase, aldo/keto reductase family protein, expressed isoform 1 | gi|590718087 | 39.48/7.56 | 39.74/5.33 | 27.24 | 52 | Theobroma cacao | 1.16 | 0.57 | 0.76 |
| 46 | peptide methionine sulfoxide reductase | gi|357494493 | 22.92/5.84 | 32.97/5.82 | 17.82 | 95 | Medicago truncatula | 1.77 | 1.88 | 1.57 |
| Defense response | ||||||||||
| 2 | chloroplast heat shock protein 70–1 | gi|15233779 | 76.58/5.07 | 76.57/4.93 | 14.35 | 281 | Arabidopsis thaliana | 1.55 | 2.05 | 2.34 |
| 3 | heat shock protein, putative | gi|255570990 | 75.43/5.35 | 76.45/4.97 | 15.79 | 612 | Ricinus communis | 1.64 | 2.10 | 2.67 |
| 4 | 70 kDa heat shock cognate protein 2 | gi|45331283 | 71.58/5.14 | 76.65/5.34 | 34.41 | 622 | Vigna radiata | 2.03 | 2.20 | 2.04 |
| 33 | 14-3-3 family protein | gi|55375985 | 29.79/4.75 | 40.02/4.75 | 29.77 | 167 | Malus x domestica | 1.02 | 1.61 | 2.48 |
| 42 | 2-oxoglutarate-iron(II) dependent oxygenase | gi|302815609 | 36.85/5.77 | 35.96/5.93 | 17.43 | 39 | Selaginella moellendorffii | 1.17 | 1.52 | 2.19 |
| Antioxidant enzymes | ||||||||||
| 43 | cytosolic ascorbate peroxidase | gi|153799884 | 27.95/5.16 | 35.19/5.67 | 22.71 | 189 | Dimocarpus longan | 1.13 | 1.86 | 2.37 |
| 51 | 2-cys-peroxiredoxin | gi|327422155 | 22.21/4.92 | 30.89/4.59 | 18.59 | 303 | Vigna unguiculata | 1.23 | 1.51 | 1.75 |
| 55 | chloroplast copper/zinc superoxide dismutase | gi|304651504 | 20.38/5.31 | 20.15/5.38 | 13.93 | 191 | Hordeum vulgare | 2.07 | 2.44 | 2.76 |
| Ion transport and regulation | ||||||||||
| 21 | cation efflux family protein isoform 2 | gi|590613599 | 45.81/5.76 | 39.07/5.88 | 33.25 | 41 | Theobroma cacao | 1.07 | 1.28 | 1.42 |
| 48 | calcineurin B-like protein | gi|357437489 | 28.33/4.68 | 31.18/5.46 | 6.25 | 46 | Medicago truncatula | 1.46 | 1.68 | 1.81 |
| Transcription and translation | ||||||||||
| 9 | elongation factor tu, putative | gi|255567660 | 49.29/6.62 | 43.25/6.46 | 23.83 | 120 | Ricinus communis | 1.69 | 1.42 | 1.15 |
| 10 | peptidyl-prolyl cis-trans isomerase, putative | gi|255552604 | 51.55/4.97 | 39.73/4.78 | 17.63 | 71 | Ricinus communis | 1.12 | 1.91 | 2.79 |
| 17 | chloroplast translational elongation factor Tu | gi|6525065 | 50.55/6.05 | 42.76/5.84 | 27.62 | 370 | Oryza sativa Japonica Group | 1.47 | 1.66 | 1.85 |
| 31 | nucleic acid binding protein1 | gi|162463757 | 33.15/4.60 | 40.55/4.33 | 21.77 | 170 | Zea mays | 0.84 | 0.74 | 0.66 |
| 32 | putative elongation factor | gi|90704791 | 24.69/4.56 | 41.07/4.65 | 10.62 | 112 | Cryptomeria japonica | 1.34 | 1.76 | 1.89 |
| 39 | DNA-binding storekeeper protein-related transcriptional regulator | gi|18411272 | 34.04/5.84 | 36.23/5.85 | 18.45 | 42 | Arabidopsis thaliana | 1.21 | 1.45 | 1.66 |
| 54 | small ribosomal protein subunit 4 | gi|67035885 | 21.91/9.91 | 23.29/6.21 | 21.52 | 61 | Pterogonidium pulchellum | 1.04 | 1.02 | 1.51 |
| Protein post-translational modification | ||||||||||
| 38 | predicted phosphoglycolate phosphatase-like | gi|357164381 | 39.01/5.76 | 40.94/5.66 | 14.21 | 107 | Brachypodium distachyon | 1.47 | 1.99 | 2.23 |
| 49 | predicted methyltransferase-like protein 23-like isoform X1 | gi|568825272 | 27.17/5.06 | 31.59/5.02 | 6.87 | 41 | Citrus sinensis | 1.10 | 1.50 | 1.31 |
| 57 | ubiquitin-like superfamily protein | gi|145360542 | 27.71/6.33 | 22.99/4.74 | 23.67 | 46 | Arabidopsis thaliana | 1.14 | 1.30 | 1.56 |
| Others | ||||||||||
| 13 | ALA-interacting subunit 5 | gi|42572169 | 32.05/9.28 | 35.54/5.32 | 22.26 | 49 | Arabidopsis thaliana | 1.34 | 1.65 | 1.98 |
| 50 | zinc knuckle family protein | gi|357498441 | 41.47/8.56 | 30.54/4.68 | 48.5 | 43 | Medicago truncatula | 1.12 | 1.29 | 1.57 |
| 53 | AP3-2 type 1 | gi|27990434 | 22.45/8.98 | 26.09/6.48 | 27.98 | 58 | Berberis gilgiana | 1.22 | 1.83 | 1.38 |
aAcc. No., database accession numbers according to NCBInr;
bTheo. Mw/pI, theoretical Mw/pI;
cExp. Mw/pI, experimental Mw/pI;
dSC, sequence coverage;
eRatio, different protein spot intensity ratios of samples after 2 d, 3 d and 5 d exposure relative to the control (0d).
Functional classification of identified proteins
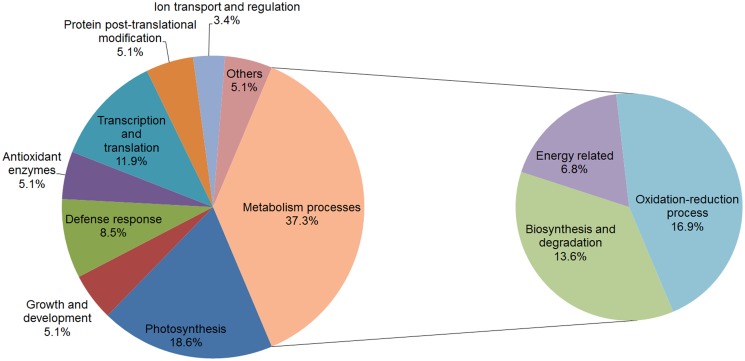
The identified proteins could be classified into nine functional groups: photosynthesis [putative rubisco subunit binding-protein alpha subunit precursor (spot 6), predicted ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic-like (spot 7), ribulose-1,5-bisphosphate carboxylase/oxygenase activase 1 (spot 12), RuBisCO activase (spot 14), fructose-bisphosphate aldolase, chloroplast precursor, putative, expressed (spot 24), chloroplast stem-loop binding protein-41 (spot 26), chloroplast photosynthetic water oxidation complex 33kDa subunit precursor (spot 34), OEE1 (spot 35), predicted carbonic anhydrase, chloroplastic-like (spot 45), ribulose bisphosphate carboxylase (spot 59), and thylakoid luminal 19 kDa protein (spot 52)], growth and development [maturase K (spot 5), actin (spot 15), and old-yellow-enzyme homolog (spot 18)], metabolism processes, defense response [chloroplast heat shock protein 70–1 (spot 2), putative heat shock protein (spot 3), and 70 kDa heat shock cognate protein 2 (spot 4), 14-3-3 family protein (spot 33), and 2-oxoglutarate-iron(II)-dependent oxygenase (spot 42)], antioxidant enzymes [cytosolic ascorbate peroxidase (spot 43), 2-cys-peroxiredoxin (spot 51), and chloroplast copper/zinc superoxide dismutase (spot 55)], ion transport and regulation [cation efflux family protein isoform 2 (spot 21) and calcineurin B-like protein (spot 48)], transcription and translation [elongation factor tu, putative (spot 9), putative peptidyl-prolyl cis-trans isomerase (spot 10), chloroplast translational elongation factor Tu (spot 17), nucleic acid binding protein1 (spot 31), putative elongation factor (spot 32), DNA-binding storekeeper protein-related transcriptional regulator (spot 39), and small ribosomal protein subunit 4 (spot 54)], protein post-translational modification [predicted phosphoglycolate phosphatase-like (spot 38), predicted methyltransferase-like protein 23-like isoform X1 (spot 49), and ubiquitin-like superfamily protein (spot 57)] and proteins with other functions [ALA-interacting subunit 5 (spot 13), zinc knuckle family protein (spot 50), and AP3-2 type 1 (spot 53)] (Table 1). In particular, the proteins involved in metabolism processes could be further divided into three categories: biosynthesis and degradation [Glutamine synthetase (spot 16), beta-cyanoalanine synthase (spot 22), malate dehydrogenase (spot 23), predicted aminomethyl transferase, mitochondrial-like (spot 27), xyloglucantransglusylase/hydrolase 1 (spot 37), cytosolic triosephosphate isomerase (spot 44), triosephosphate isomerase, cytosolic (spot 47), and granule-bound starch synthase precursor (spot 58)], energy related [AMP deaminase family protein (spot 11), ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (spot 40), predicted probable ATP synthase 24 kDa subunit, mitochondrial (spot 41), and ATP synthase CF1 epsilon subunit (chloroplast) (spot 56)] and oxidation-reduction process [glycine dehydrogenase, putative (spot 1), glycine dehydrogenase, putative (spot 8), plastidic aldolase (spot 19), plastidic aldolase family protein (spot 20), predicted glyceraldehyde-3-phosphate dehydrogenase A, chloroplastic-like (spot 25), isoflavone reductase-like protein (spot 28), phenylcoumaran benzylic ether reductase (spot 29), pterocarpan reductase (spot 30), oxidoreductase, aldo/keto reductase family protein, expressed isoform 1 (spot 36), and peptide methionine sulfoxide reductase (spot 46)] (Table 1). Among all the identified proteins, the functional group of metabolism processes (37.3%) accounted for the largest number of differentially expressed proteins (Fig 6). In addition, proteins related to photosynthesis (18.6%), transcription and translation (11.9%), and defense response (8.5%) also constituted larger proportions of the differential proteins (Fig 6).
Fig 6. Functional classification of the identified proteins.
Discussion
Differences in tolerance of Cd between E. crassipes and P. stratiotes
E. crassipes and P. stratiotes are often used together in studies of heavy metals pollution [39,41], and these organisms commonly exhibit different accumulation effects when exposed to Cd2+, Zn2+, and Cu2+ [39]. However, less work has focused on the toxicity of Cd toward these plants, or on their Cd resistance. In this study, we compared the tolerance of Cd stress between E. crassipes and P. stratiotes based on morphological and physiological aspects. When exposed to Cd for the same time period, the leaves and roots of P. stratiotes were more seriously damaged than those of E. crassipes, with significant yellowing of the leaves and shedding of roots occurring (Fig 1A). Physiological detection showed that photosynthesis was greatly inhibited in P. stratiotes relative to E. crassipes (Fig 2A). P. stratiotes also suffered more severe oxidative stress or damage owing to a higher generation of ROS (Fig 2C) and their oxidation product, MDA (Fig 3A). These results demonstrated that E. crassipes was much more tolerant to Cd than P. stratiotes.
E. crassipes has long been regarded as one of the best plants for sewage purification and is widely applied in ecological restoration engineering [42] based on its strong tolerance of sewage and excellent growth characteristics [40]. In this study, E. crassipes appeared to be able to normalize its physiological functions, even after being subjected to 100 mg/L Cd, indicating its strong resistance to contamination and potential for application in removal of Cd from water. To further understand the underlying mechanisms of Cd tolerance, proteomics analysis of E. crassipes was conducted in conjunction with biochemical analyses during exposure to Cd.
Proteins involved in photosynthesis
As shown in Figs 1B, 1C and 2A, inhibited photosynthesis is one of the most obvious phenomena in plants stressed by Cd [10,43]. It is generally believed that this occurs in response to blockage of photosynthetic pigment synthesis [43] and destruction of chloroplasts [8]. These effects could result in leaf chlorosis and wither, which were shown in our study (Fig 1A). Many studies have shown that proteins related to photosynthesis were commonly influenced by Cd stress [27,30–33]. In the present study, the photosystem efficiency of both E. crassipes and P. stratiotes decreased (Figs 1C, 2A and 2B) as observed in other plants exposed to Cd [30,31]. Accordingly, most proteins involved in photosynthesis (spots 6, 7, 12, 14, 24, 26, 34, 35, and 45) were down-regulated in E. crassipes with increasing treatment time (Fig 5B and Table 1). The down-regulation of these proteins has often been observed in other studies of the effects of Cd stress on plants [33]. For example, Rubiso, a key enzyme involved in CO2 assimilation during the Calvin—Benson cycle, has been reported to be compromised by Cd in both non-hyperaccumulator [31,44] and hyperaccumulator plants [33,45]. The results of the present study indicated that the efficiency of CO2-fixation decreased under Cd stress, which confirmed the reduction in photosynthesis. However, several proteins were induced by Cd exposure. For example, a photosynthetic enzymes, ribulose bisphosphate carboxylase (spot 59), showed continuously increasing expression, while thylakoid luminal 19 kDa protein (spot 52) was up-regulated during the early stage (3 d) and then decreased to the control level (5 d) (Fig 5B and Table 1). These results suggested that the stimulation of some proteins played essential roles in maintenance of the photosynthesis when other proteins were inhibited.
Proteins involved in metabolism processes
Relevant proteomics results have indicated that exposure to Cd resulted in the alteration of proteins related to biosynthesis and degradation [27,31,34,35]. The proteins involved in different metabolic pathways usually exhibit diverse expression patterns during Cd treatment [27,31,34,35]. In the present study, two proteins related to biosynthesis (spots 27 and 37) were continuously down-regulated as the treatment time increased (Fig 5B and Table 1). Additionally, three related proteins (spots 22, 23, and 58) were not down-regulated until exposure to Cd for 3 d (Fig 5B and Table 1). However, some enzymes in metabolic pathways often showed up-regulation under Cd stress. Glutamine synthetase (GS) is involved in the synthesis of glutathione (GSH) via the glutamate biosynthesis pathway [32], and GSH can synthesize PCs via PC synthase, which can form complexes with Cd in cytosol and then be transported into vacuoles [46]. Triosephosphate isomerase, a key enzyme in glycolysis, plays an important role in efficient energy production [27]. These enzymes showed increased abundance in studies of exposure to Cd in soybean [27] and B. juncea [30], which was consistent with the results of the present study. Glutamine synthetase (spot 16), cytosolic triosephosphate isomerase (spot 44) and triosephosphate isomerase, cytosolic (spot 47) showed increasing expression during exposure to Cd stress in our study (Fig 5B and Table 1). Overall, the results indicated that E. crassipes had an active response to Cd stress, even if some biosynthesis pathways were restrained.
Proteins involved in energy metabolism have been confirmed to play important roles in plant response to abiotic stresses [47–49]. When exposed to Cd stress, plants were shown to increase energy demand, so proteins related to energy production, such as ATP synthetase, usually showed enhanced abundance [27,31,33]. In the present study, we identified four proteins related to energy metabolism (Table 1). Three of these proteins (spots 40, 41, and 56) exhibited increasing expression, while the expression of only one protein (spot 11) decreased as the treatment time increased (Fig 5B and Table 1). These results demonstrate the important role of energy in plant response to stress.
In addition, ten proteins related to the oxidation-reduction process (spots 1, 8, 19, 20, 25, 28, 29, 30, 36, and 46) exhibited various expression patterns among samples exposed to Cd for different lengths of time (Fig 5B and Table 1). The differential expression of some of these proteins has been reported in previous studies [28], while that of others is reported here for the first time. Their diverse expression indicated that they actively coped with or were passively affected by Cd stress.
Protein synthesis and modification
Dramatic changes in a number of proteins associated with transcription and translation have been observed in numerous plants under Cd stress [33,35]. Different from some previous studies [33,35], most proteins involved in transcription and translation (spots 10, 17, 32, 39, and 54) were gradually up-regulated in the present study. One protein (spot 31) was down-regulated and one protein (spot 9) was up-regulated in the early stage and down-regulated in the later stage during application of Cd stress (Fig 5B and Table 1). The various expression patterns suggested that proteins related to transcription and translation played diverse roles in the treatment of Cd stress.
Protein post-translation modifications such as ubiquitination, phosphorylation, and methylation play very important roles in organisms [50]. These changes can result in the protein structure becoming more complex and the function being improved, which results in more precise adjustments and more specific effects [50]. One important physiological function of cells regulated by protein post-translation modifications is the cellular response to environmental conditions [50]. For example, protein phosphorylation is considered to be closely related to the interaction between Kobresia pygmaea and the environment with increasing elevation [47]. A proteomics study of the hyperaccumulator plant Phytolacca Americana by Zhao et al. [33] indicated that post-translational modifications such as phosphorylation might have occurred during Cd treatment. In the present study, we found that predicted phosphoglycolate phosphatase-like (spot 38), predicted methyltransferase-like protein 23-like isoform X1 (spot 49), and ubiquitin-like superfamily protein (spot 57), which are related to phosphorylation, methylation, and ubiquitination, respectively, showed consistent up-regulation under Cd stress (Fig 5B and Table 1). These findings indicated that post-translation modifications might participate in the regulation of E. crassipes resistance to Cd stress.
Antioxidant enzymes and related proteins
An obvious response of plants to Cd exposure is oxidative stress caused by ROS [27,29,30,33]. Many studies have demonstrated that plant antioxidant systems can be induced to eliminate excessive ROS and prevent oxidation [21,22]. However, previous studies have also revealed that the antioxidant systems in different plant species are usually quite different [49], and high-concentration Cd stress may inhibit plant antioxidant systems [11,51]. Similar results were observed upon comparison of E. crassipes and P. stratiotes (Fig 4). The differences in activities of CAT, APX, GR and SOD between E. crassipes and P. stratiotes showed that E. crassipes had stronger antioxidant ability than P. stratiotes. Thus, higher levels of ROS (H2O2 and O2 -) accumulated in P. stratiotes than E. crassipes with increasing Cd exposure time (Fig 2C), which led to the generation of high concentrations of MDA (Fig 3A). The enzymes involved in oxidative stress defenses also show dynamic expression in plants under Cd stress [28]. Proteomic-related studies revealed that the enzymes involved in peroxide detoxification [31,37,52,53] and peroxiredoxins [53–55] were usually upregulated by Cd in plants. Similarly, cytosolic ascorbate peroxidase (spot 43) and 2-cys-peroxiredoxin (spot 51) were differentially up-regulated in E. crassipes with increasing Cd exposure time (Fig 5B and Table 1). Cu/Zn SOD were downregulated in several plants under Cd stress [31,37,56], but the chloroplast copper/zinc superoxide dismutase (spot 55) in our study showed increased abundance during Cd treatment. In summary, common and unique changes in the expression of enzymes related to ROS detoxification were observed in E. crassipes when compared with other plants, indicating their important roles in protecting cell structure and function.
Proline and proteins involved in defense response
Proline can protect plant cells against several stresses during various stages of accumulation [57], which helps plants avoid oxidative damage [58] by mediating osmotic adjustment and stabilizing macromolecules [59]. Proline also has been reported to be induced by Cd in Silene vulgaris [60], and the accumulation was proposed to be a consequence of metal-induced water deficit [60]. Based on the previous studies, we measured the proline content in our study. The results clearly demonstrated that proline could be induced by Cd; however, the accumulation mechanism requires further study. The different change in proline content between E. crassipes and P. stratiotes revealed differential resistance to Cd treatment by these two species.
Stress-related proteins have been shown to play an essential role in plant resistance to Cd stress [29,34,35]. HSPs are an important group of protective proteins that can protect other proteins from damage or repair damaged proteins [61]. HSPs can accumulate when plants are exposed to various stresses, including Cd treatment [31,53,54,62,63]. In the present study, three HSPs in E. crassipes, chloroplast heat shock protein 70–1 (spot 2), putative heat shock protein (spot 3), and 70 kDa heat shock cognate protein 2 (spot 4), were all obviously up-regulated as the treatment time of Cd stress increased (Fig 5B and Table 1), indicating that they played significant roles in tolerance to Cd stress. 14-3-3 proteins are known to participate in the regulation of plant development and stress responses in higher plants [64]. For example, a 14-3-3 protein in tomato modulates H+ efflux, basipetal auxin transport, and the PKS5-J3 pathway during root growth following alkaline stress [65]. The results of the present study showed that a 14-3-3 family protein (spot 33) was up-regulated with increased treatment time (Fig 5B and Table 1), indicating its importance to the response to Cd stress. This is first study to report induction of this protein in response to Cd stress in plants. Interestingly, we also found that a 14-3-3 protein showed induced expression in E. crassipes cultured in the eutrophic water [66]. These findings appeared to indicate the unique function of 14-3-3 proteins in response to sewage in E. crassipes. In addition, another protein (spot 42) was also up-regulated during exposure to Cd stress (Fig 5B and Table 1). Taken together, these findings suggest that the metabolites and proteins involved in resistance to stress helped E. crassipes tolerate high levels of Cd.
Proteins involved in ion transport and regulation
The absorption, transportation, or discharge of Cd2+ in plants is a complex sequence of processes regulated by various transporters [67–70]. Schneider et al. [36] specifically employed quantitative proteomics to elucidate the contribution of vacuolar transporters in barley subjected to Cd treatment. Ultimately, they identified 56 vacuolar transporters with various expression patterns and demonstrated that some played important roles in Cd detoxification [36]. In the present study, two proteins related to ion transport (spots 21 and 48) were up-regulated in E. crassipes with increasing Cd exposure (Fig 5B and Table 1), suggesting that they played a significant role in ameliorating Cd stress.
Conclusion
In the present study, E. crassipes exhibited stronger tolerance to high-concentration Cd stress than P. stratiotes at the morphological and physiological level; therefore, we performed comparative proteomics to explore the internal mechanism of the E. crassipes response to Cd stress. Based on the functional categories and expression patterns of 59 differential proteins, we identified a series of complex regulation processes during the response to Cd stress. While some proteins involved in life activities were negatively restrained, analogous proteins were up-regulated to compensate for the corresponding functions. Thus, E. crassipes could still maintain a higher physiological status relative to P. stratiotes. At the same time, several stress-resistance substances and proteins including proline and HSPs, as well as protein post-translational modifications, were found to be involved in the protection and renovation of functional proteins. In addition, antioxidant enzymes played important roles in the removal of excess ROS to reduce oxidative stress. These findings will lead to improved understanding of the potential mechanism of plant responses to Cd stress at the protein level.
Materials and Methods
Ethics statement
Plant materials used in this study were collected from Lake Dianchi (N 25°01′38″, E 102°40′21″) in Kunming, Yunnan Province, China. No specific collecting permits were required for this location. We confirm that the plants we used are neither endangered nor protected species.
Material collection and treatment
Both E. crassipes and P. stratiotes plantlets were collected from Lake Dianchi (N 25°01′38″, E 102°40′21″) in May 2013 during the clonal reproduction period. Samples were collected from the same population so that they would have a similar genetic background. The plantlets were acclimated in a greenhouse (sunlight; 25–28°C/18–20°C, 12h-day/12h-night) for 15 d using Hoagland’s nutrient solution (HNS) [71] as the planting water. Isometric plantlets of E. crassipes and P. stratiotes were then placed into water boxes (30 cm × 20 cm × 20 cm). Each plantlet was treated in a single box with 10 L HNS water added by 100 mg/L CdCl2 in the greenhouse (sunlight; 25–28°C/18–20°C, 12h-day/12h-night). The plantlets were then photographed and collected for subsequent measurement and analysis at 0, 2, 3, and 5 d, respectively. There were three replicates for each time point sample.
Chlorophyll fluorescence and photosynthetic measurement
Chlorophyll fluorescence was analyzed as previously described [49] using a pulse-amplitude modulation chlorophyll fluorometer (Heinz Walz GmbH, Effeltrich, Germany). Briefly, E. crassipes were dark-adapted for 30 min at the time of sampling to measure the maximum quantum yield (Fv/Fm) of photosystem II (PSII) by analyzing a whole leaf. The maximum fluorescence (Fm) was recorded by a 0.8-s pulsed light of 4,000 μmol s-1 m-2, while the minimal fluorescence (Fo) was recorded during the weak measuring pulses.
A portable photosynthesis system (Li-6400; Li-Cor Inc., Lincoln, NE, USA) was used to measure the net photosynthetic rate and stomatal conductance of leaves. During the measurements, the water vapor pressure deficit was set to about 1.0 kPa and the atmospheric CO2 concentration was 400 μmol mol-1. The leaf was illuminated by either a quartz halogen light source or a red light-emitting diode (Li-6400-02, Li-Cor Inc.) under a light intensity of 1,000 μmol photons m-2 s-1.
In situ H2O2 and O2 - detection
The in situ detection of H2O2 and O2 - was performed as previously described, with minor modification [72]. H2O2 in the roots was detected with 1 mg ml-1 of diaminobenzidine (DAB), while O2 - was measured using 10-2 M nitro-blue tetrazolium (NBT). For analysis, three roots from each sample were vacuum-infiltrated in 10 ml of solution for 2 h, after which they were cleared in boiling ethanol (95%) for 10 min. The samples were then stored and examined in 95% ethanol.
MDA and proline content measurement
The MDA content was determined as previously described [47]. Briefly, approximately 0.5 g of fresh leaves were homogenized in 10 ml of 10% trichloroacetic acid (TCA) and then centrifuged at 12,000 ×g for 10 min. Next, 2 ml of 0.6% thiobarbituric acid in 10% TCA were added to 2 ml of the supernatant. The mixture was subsequently heated in boiling water for 30 min, then quickly cooled in an ice bath. After centrifugation at 10,000 ×g for 10 min, the absorbance of the supernatant at 450, 532, and 600 nm was determined. The MDA concentration was reported as nmol g-1 fresh weight (FW).
Proline content was measured as previously reported [73], with slight modification. Briefly, approximately 0.2 g of fresh leaves was homogenized in 10 ml of 3% aqueous sulphosalicylic acid, after which the homogenate was centrifuged at 2,000 ×g for 10 min. Next, 2 ml of the extract was incubated with 2 ml of acidic-ninhydrine and 2 ml of glacial acetic acid for 1 h in boiling water, after which the reaction was terminated in an ice bath. The reaction mixture was then extracted with 4 ml toluene and mixed vigorously with a test tube stirrer for 15–20 s. The chromophore containing toluene was subsequently aspirated from the aqueous phase and warmed to room temperature, after which the absorbance was read at 520 nm using toluene as a blank. The proline concentration was determined from a standard curve and calculated as μg g-1 FW.
Antioxidant enzyme activity determination
Antioxidant enzymes were extracted using a previously described method [48]. The activities of CAT, APX, GR, and SOD were measured spectrophotometrically by monitoring the change of absorbance at 240, 290, 340 and 560 nm, respectively [74,75].
Total protein extraction
Total proteins were extracted from E. cerassipes using a previously described method [76], with slight modification. Briefly, approximately 1 g powder of fresh leaves was homogenized with 5 mL TRIzol at 25°C for 5 min. Next, 1 mL chloroform was added and the mixtures were allowed to stand at −20°C for 5 min. Following centrifugation at 4°C and 12,000 ×g for 10 min, the supernatants were removed and the lower phases were mixed with isometric isopropanol and allowed to stand at −20°C for 2 h. The mixtures were then centrifuged at 4°C and 12,000 ×g for 10 min, after which the supernatants were removed. Next, the precipitates were washed three times with isopropanol and dried at 25°C, after which they were dissolved in denaturation buffer (7 M urea, 2 M thiourea, 4% (w/v) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate, and 60 mM DTT) for 1 h with intermittent shaking.
Protein 2-DE
Protein 2-DE was performed as previously described [47]. A total of 1,200 μg of proteins extracted from each sample were first separated by isoelectric focusing (IEF) using gel strips with a pH gradient of 4 to 7 (Immobiline Dry Strip, pH 4–7 NL, 17 cm; BioRad, Hercules, CA, USA). The strips were rehydrated for 14 h in 320 ml of dehydration buffer and then focused at 20°C for a total of 64 kV-h with a PROTEAN IEF Cell system (Bio-Rad). After IEF, the strips were equilibrated for 20 min, first in equilibration buffer I [6 M urea, 0.375 M Tris (pH 8.8), 2% (w/v) SDS, 20% (v/v) glycerol, and 2% (w/v) DTT) and then in equilibration buffer II [6 M urea, 0.375 M Tris (pH 8.8), 2% (w/v) SDS, 20% (v/v) glycerol, and 2% (w/v) iodoacetamide]. The equilibrated strips were then placed over 12.5% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for 2-DE at 25 mA for 5 h. The 2-De gels were stained with colloidal CBB.
Spot digestion and protein identification for mass spectrometry analyses
Protein spot digestion and protein identification were performed as previously described [49]. Protein spots displaying significant changes in abundance following plant exposure to Cd stress were excised manually from colloidal CBB-stained 2-DE gels using sterile pipette tips. Spots were transferred to 1.5-ml sterile tubes, destained with 50 mM NH4HCO3 for 1 h at 40°C, reduced with 10 mM DTT in 100 mM NH4HCO3 for 1 h at 60°C, and incubated with 40 mM iodoacetamide in 100 mM NH4HCO3 for 30 min. Gels were then minced, air-dried, and rehydrated in 12.5 ng μl-1 sequencing-grade modified trypsin (Promega, Fitchburg, WI, USA) in 25 mM NH4HCO3 overnight at 37°C. Tryptic peptides were extracted three times from the gel grains using 0.1% trifluoroacetic acid (TFA) in 50% acetonitrile. Supernatants were concentrated to approximately 10 ml using a SpeedVac (Thermo Fisher, Waltham, MA, USA) and then desalted using reversed-phase ZipTip pipette tips (C18, P10; Millipore, Billerica, MA, USA). Peptides were eluted with 50% acetonitrile and 0.1% TFA.
Lyophilized peptide samples were dissolved in 0.1% TFA, and MS analysis was conducted using a 4800 Plus MALDI-TOF/TOF Proteomics Analyzer (Applied Biosystems, Foster City, CA, USA). MS acquisition and processing parameters were set to reflector-positive mode and an 800–3,500-Da acquisition mass range, respectively. The laser frequency was 50 Hz, and each sample spectrum was acquired over 700 laser pulses. For secondary MS analysis, four to six ion peaks with signal-to-noise ratios exceeding 100 were selected from each sample as precursors. TOF/TOF signal data for each precursor were then accumulated from 2,000 laser pulses. Primary and secondary mass spectra were transferred to Excel files and compared against a non-redundant NCBI protein database restricted to Viridiplantae (i.e., green plants) using the MASCOT search engine (www.matrixscience.com). The following search parameters were used: no molecular weight restriction, one missed trypsin cleavage allowed, iodoacetamide-treated cysteine, oxidation of methionine, a peptide tolerance of 100 ppm, and an MS/MS tolerance of 0.25 Da. Protein identifications were validated manually based on at least three matching peptides. Keratin contamination was removed, and the MOWSE threshold was set above 40 (P<0.05). Only significant hits in the MASCOT probability analysis were accepted as protein identifications.
Expression analysis and functional classification
After staining with Coomassie Brilliant Blue, the 2-DE gels were scanned and the images were used to analyse the proteins expression using the PDQuest 2D analysis software (BioRad) based on their relative volumes. The volume of each spot was normalized [77] to compensate for subtle differences in sample loading or gel staining/destaining during individual experiments. Proteins with expressions that varied by at least 1.5-fold were regarded as differentially expressed. Functional classification of differentially expressed proteins was conducted according to Blast2Go [78].
Statistical analysis
Statistical analyses were performed using SPSS version 18.0. ANOVA was used to identify differences among treatments (Tukey’s test, P<0.05).
Supporting Information
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Martins LL, Mourato MP, Cardoso AI, Pinto AP, Mota AM, Goncalves MDS, et al. (2011) Oxidative stress induced by cadmium in Nicotiana tabacum L.: effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiologiae Plantarum 33: 1375–1383. [Google Scholar]
- 2. Gardea-Torresdey JL, de la Rosa G, Peralta-Videa JR, Montes M, Cruz-Jimenez G, Cano-Aguilera I (2005) Differential uptake and transport of trivalent and flexavalent chromium by tumbleweed (Salsola kali). Archives of Environmental Contamination and Toxicology 48: 225–232. [DOI] [PubMed] [Google Scholar]
- 3. Kulik A, Anielska-Mazur A, Bucholc M, Koen E, Szymanska K, Zmienko A, et al. (2012) SNF1-Related Protein Kinases Type 2 Are Involved in Plant Responses to Cadmium Stress. Plant Physiology 160: 868–883. 10.1104/pp.112.194472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tschuschke S, Schmitt-Wrede HP, Greven H, Wunderlich F (2002) Cadmium resistance conferred to yeast by a non-metallothioneinencoding gene of the earthworm Enchytraeus. Journal of Biological Chemistry 277: 5120–5125. [DOI] [PubMed] [Google Scholar]
- 5. Waalkes MP (2000) Cadmium carcinogenesis in review. Journal of Inorganic Biochemistry 79: 241–244. [DOI] [PubMed] [Google Scholar]
- 6. Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: Using plants to remove pollutants from the environment. Current Opinion in Biotechnology 8: 221–226. [DOI] [PubMed] [Google Scholar]
- 7. Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, et al. (1997) Phytoremediation of soil metals. Current Opinion in Biotechnology 8: 279–284. [DOI] [PubMed] [Google Scholar]
- 8. Kesseler A, Brand MD (1995) The Mechanism of the Stimulation of State-4 Respiration by Cadmium in Potato-Tuber (Solanum-Tuberosum) Mitochondria. Plant Physiology and Biochemistry 33: 519–528. [Google Scholar]
- 9. Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant Journal 32: 539–548. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki N, Koizumi N, Sano H (2001) Screening of cadmium-responsive genes in Arabidopsis thaliana . Plant Cell and Environment 24: 1177–1188. [Google Scholar]
- 11. Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. Journal of Experimental Botany 52: 2115–2126. [DOI] [PubMed] [Google Scholar]
- 12. Stohs SJ, Bagchi D (1995) Oxidative Mechanisms in the Toxicity of Metal-Ions. Free Radical Biology and Medicine 18: 321–336. [DOI] [PubMed] [Google Scholar]
- 13. Chardonnens AN, ten Bookum WM, Kuijper LDJ, Verkleij JAC, Ernst WHO (1998) Distribution of cadmium in leaves of cadmium tolerant and sensitive ecotypes of Silene vulgaris . Physiologia Plantarum 104: 75–80. [Google Scholar]
- 14. Curie C, Cassin G, Couch D, Divol F, Higuchi K, Jean M, et al. (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Annals of Botany 103: 1–11. 10.1093/aob/mcn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo WJ, Meetam M, Goldsbrough PB (2008) Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiology 146: 1697–1706. 10.1104/pp.108.115782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. International Journal of Molecular Sciences 13: 3145–3175. 10.3390/ijms13033145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mishra S, Tripathi RD, Srivastava S, Dwivedi S, Trivedi PK, Dhankher OP, et al. (2009) Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresource Technology 100: 2155–2161. 10.1016/j.biortech.2008.10.041 [DOI] [PubMed] [Google Scholar]
- 18. Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiology and Biochemistry 44: 25–37. [DOI] [PubMed] [Google Scholar]
- 19. Ueno D, Koyama E, Kono I, Ando T, Yano M, Ma JF (2009) Identification of a Novel Major Quantitative Trait Locus Controlling Distribution of Cd Between Roots and Shoots in Rice. Plant and Cell Physiology 50: 2223–2233. 10.1093/pcp/pcp160 [DOI] [PubMed] [Google Scholar]
- 20. Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, et al. (2010) Gene limiting cadmium accumulation in rice. Proceedings of the National Academy of Sciences of the United States of America 107: 16500–16505. 10.1073/pnas.1005396107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iannelli MA, Pietrini F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites australis plants. Plant Physiology and Biochemistry 40: 977–982. [Google Scholar]
- 22. Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Science 161: 1135–1144. [Google Scholar]
- 23. Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction—the Use of Plants to Remove Heavy-Metals from Soils. Environmental Science & Technology 29: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 24. Barros E, Lezar S, Anttonen MJ, van Dijk JP, Rohlig RM, Kok EJ, et al. (2010) Comparison of two GM maize varieties with a near-isogenic non-GM variety using transcriptomics, proteomics and metabolomics. Plant Biotechnology Journal 8: 436–451. 10.1111/j.1467-7652.2009.00487.x [DOI] [PubMed] [Google Scholar]
- 25. Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, et al. (2008) Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. Journal of Proteome Research 7: 2980–2998. 10.1021/pr800128q [DOI] [PubMed] [Google Scholar]
- 26. Colmsee C, Mascher M, Czauderna T, Hartmann A, Schluter U, Zellerhoff N, et al. (2012) OPTIMAS-DW: A comprehensive transcriptomics, metabolomics, ionomics, proteomics and phenomics data resource for maize. Bmc Plant Biology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hossain Z, Hajika M, Komatsu S (2012) Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids 43: 2393–2416. 10.1007/s00726-012-1319-6 [DOI] [PubMed] [Google Scholar]
- 28. Villiers F, Ducruix C, Hugouvieux V, Jarno N, Ezan E, Garin J, et al. (2011) Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 11: 1650–1663. 10.1002/pmic.201000645 [DOI] [PubMed] [Google Scholar]
- 29. Zeng XW, Qiu RL, Ying RR, Tang YT, Tang L, Fang XH (2011) The differentially-expressed proteome in Zn/Cd hyperaccumulator Arabis paniculata Franch. in response to Zn and Cd. Chemosphere 82: 321–328. 10.1016/j.chemosphere.2010.10.030 [DOI] [PubMed] [Google Scholar]
- 30. D'Alessandro A, Taamalli M, Gevi F, Timperio AM, Zolla L, Ghnaya T, et al. (2013) Cadmium Stress Responses in Brassica juncea: Hints from Proteomics and Metabolomics. Journal of Proteome Research 12: 4979–4997. 10.1021/pr400793e [DOI] [PubMed] [Google Scholar]
- 31. Kieffer P, Planchon S, Oufir M, Ziebel J, Dommes J, Hoffmann L, et al. (2009) Combining Proteomics and Metabolite Analyses To Unravel Cadmium Stress-Response in Poplar Leaves. Journal of Proteome Research 8: 400–417. 10.1021/pr800561r [DOI] [PubMed] [Google Scholar]
- 32. Semane B, Dupae J, Cuypers A, Noben JP, Tuomainen M, Tervahauta A, et al. (2010) Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. Journal of Plant Physiology 167: 247–254. 10.1016/j.jplph.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 33. Zhao L, Sun YL, Cui SX, Chen M, Yang HM, Liu HM, et al. (2011) Cd-induced changes in leaf proteome of the hyperaccumulator plant Phytolacca americana. Chemosphere 85: 56–66. 10.1016/j.chemosphere.2011.06.029 [DOI] [PubMed] [Google Scholar]
- 34. Marmiroli M, Imperiale D, Maestri E, Marmiroli N (2013) The response of Populus spp. to cadmium stress: Chemical, morphological and proteomics study. Chemosphere 93: 1333–1344. 10.1016/j.chemosphere.2013.07.065 [DOI] [PubMed] [Google Scholar]
- 35. Muneer S, Hakeem KR, Mohamed R, Lee JH (2014) Cadmium Toxicity Induced Alterations in the Root Proteome of Green Gram in Contrasting Response towards Iron Supplement. International Journal of Molecular Sciences 15: 6343–6355. 10.3390/ijms15046343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider T, Schellenberg M, Meyer S, Keller F, Gehrig P, Riedel K, et al. (2009) Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics 9: 2668–2677. 10.1002/pmic.200800806 [DOI] [PubMed] [Google Scholar]
- 37. Alvarez S, Berla BM, Sheffield J, Cahoon RE, Jez JM, Hicks LM, et al. (2009) Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. Proteomics 9: 2419–2431. 10.1002/pmic.200800478 [DOI] [PubMed] [Google Scholar]
- 38. Liu P, Song C, Zhu H, Zhang QJ, Jia CX. (2011) Studies on eutrophicated water quality improvement by three kinds of hydrophytes. Journal of Hydroecology 32: 69–74. (In Chinese with English abstract) [Google Scholar]
- 39. Tian GT, Duan DX, Du XH, XuGJ, Zhang JL, Zhang ML, et al. (2014) Accumulation and removal effect of compound pollution of heavy metals in water body of Eichhornia crasipes and Pistia stratiotes L. Journal of Yangze University (Nat Sci Edit) 11: 54–59. (In Chinese with English abstract) [Google Scholar]
- 40. Wang Z, Zhang ZY, Zhang JQ, Zhang YY, Liu HQ, Yan SH, et al. (2012) Large-scale utilization of water hyacinth for nutrient removal in Lake Dianchi in China: The effects on the water quality, macrozoobenthos and zooplankton. Chemosphere 89: 1255–1261. 10.1016/j.chemosphere.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 41. Yan Z, Xie SB, Li SY, Tang DS, Liu YJ. (2012) Physiological and biochemical responses of Eichhornia crassipes and Pistia stratiotes induced at uranium stress. Journal of Safety and Environment 12; 1–5. (In Chinese with English abstract) [Google Scholar]
- 42. Hu MH, Yuan JH, Chang HQ, Yang XE. (2009) In situ remediation of eutrophic water bodies by the combination of water hyacinth and immobilized nitrogen cycle bacteria. Chinese Journal of Environmental Engineering 3: 2163–2169. (In Chinese with English abstract) [Google Scholar]
- 43. Plekhanov SE, Chemeris YK (2003) Early toxic effects of zinc, cobalt, and cadmium on photosynthetic activity of the green alga Chlorella pyrenoidosa Chick S-39. Biology Bulletin 30: 506–511. [PubMed] [Google Scholar]
- 44. Hajduch M, Rakwal R, Agrawal GK, Yonekura M, Pretova A (2001) High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: Drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis 22: 2824–2831. [DOI] [PubMed] [Google Scholar]
- 45. Tuomainen MH, Nunan N, Lehesranta SJ, Tervahauta AI, Hassinen VH, Schat H, et al. (2006) Multivariate analysis of protein profiles of metal hyperaccumulator Thlaspi caerulescens accessions. Proteomics 6: 3696–3706. [DOI] [PubMed] [Google Scholar]
- 46. Grill E, Loffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the Heavy-Metal-Binding Peptides of Plants, Are Synthesized from Glutathione by a Specific Gamma-Glutamylcysteine Dipeptidyl Transpeptidase (Phytochelatin Synthase). Proceedings of the National Academy of Sciences of the United States of America 86: 6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li X, Yang YQ, Ma L, Sun XD, Yang SH, Kong XX, et al. (2014) Comparative Proteomics Analyses of Kobresia pygmaea Adaptation to Environment along an Elevational Gradient on the Central Tibetan Plateau. Plos One 9: e98410 10.1371/journal.pone.0098410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Yang YQ; Sun XD, Lin HM, Chen JH, Ren J, et al. (2014) Comparative Physiological and Proteomic Analyses of Poplar (Populus yunnanensis) Plantlets Exposed to High Temperature and Drought. Plos One 9: e107605 10.1371/journal.pone.0107605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang YQ, Chen JH, Liu Q, Ben C, Todd CD, Shi JS, et al. (2012) Comparative Proteomic Analysis of the Thermotolerant Plant Portulaca oleracea Acclimation to Combined High Temperature and Humidity Stress. Journal of Proteome Research 11: 3605–3623. 10.1021/pr300027a [DOI] [PubMed] [Google Scholar]
- 50. Hu J, Guo YT, Li YM. (2005) Research progress of protein post-translational modofication. Chinese Science Bulletin 50: 1061–1072. (In Chinese with English abstract) [Google Scholar]
- 51. Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). Journal of Experimental Botany 52: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 52. Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HC, et al. (2010) Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. Journal of Plant Physiology 167: 161–168. 10.1016/j.jplph.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 53. Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, et al. (2006) The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 6: 2180–2198. [DOI] [PubMed] [Google Scholar]
- 54. Ahsan N, Lee SH, Lee DG, Lee H, Lee SW, Bahk JD, et al. (2007) Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. Comptes Rendus Biologies 330: 735–746. [DOI] [PubMed] [Google Scholar]
- 55. Durand TC, Sergeant K, Planchon S, Carpin S, Label P, Morabito D, et al. (2010) Acute metal stress in Populus tremula x P. alba (717-1B4 genotype): Leaf and cambial proteome changes induced by cadmium(2+). Proteomics 10: 349–368. 10.1002/pmic.200900484 [DOI] [PubMed] [Google Scholar]
- 56. Kieffer P, Dommes J, Hoffmann L, Hausman JF, Renaut J (2008) Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics 8: 2514–2530. 10.1002/pmic.200701110 [DOI] [PubMed] [Google Scholar]
- 57. Kuznetsov VV, Shevyakova NI (1997) Stress responses of tobacco cells to high temperature and salinity. Proline accumulation and phosphorylation of polypeptides. Physiologia Plantarum 100: 320–326. [Google Scholar]
- 58. Mihailova G, Petkova S, Buchel C, Georgieva K (2011) Desiccation of the resurrection plant Haberlea rhodopensis at high temperature. Photosynthesis Research 108: 5–13. 10.1007/s11120-011-9644-2 [DOI] [PubMed] [Google Scholar]
- 59. Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany 59: 206–216. [Google Scholar]
- 60. Schat H, Sharma SS, Vooijs R (1997) Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris . Physiologia Plantarum 101: 477–482. [Google Scholar]
- 61. Kato S, Yamagishi K, Tatsuzawa F, Suzuki K, Takano S, Eguchi M, et al. (1993) Identification of Cytoplasmic and Nuclear Low-Molecular-Weight Heat-Shock Proteins in Tomato Fruit. Plant and Cell Physiology 34: 367–370. [PubMed] [Google Scholar]
- 62. Hradilova J, Rehulka P, Rehulkova H, Vrbova M, Griga M, Brzobohaty B (2010) Comparative analysis of proteomic changes in contrasting flax cultivars upon cadmium exposure. Electrophoresis 31: 421–431. 10.1002/elps.200900477 [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez-Celma J, Rellan-Alvarez R, Abadia A, Abadia J, Lopez-Millan AF (2010) Changes induced by two levels of cadmium toxicity in the 2-DE protein profile of tomato roots. Journal of Proteomics 73: 1694–1706. 10.1016/j.jprot.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 64. Mayfield JD, Paul AL, Ferl RJ (2012) The 14-3-3 proteins of Arabidopsis regulate root growth and chloroplast development as components of the photosensory system. Journal of Experimental Botany 63: 3061–3070. 10.1093/jxb/ers022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu WF, Jia LG, Shi WM, Baluska F, Kronzucker HJ, Liang JS, et al. (2013) The Tomato 14-3-3 Protein TFT4 Modulates H+ Efflux, Basipetal Auxin Transport, and the PKS5-J3 Pathway in the Root Growth Response to Alkaline Stress. Plant Physiology 163: 1817–1828. 10.1104/pp.113.224758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li X, Xi HC, Sun XD, Yang YQ, Yang SH, Zhou YL, et al. (2015) Comparative proteomics exploring the molecular mechanism of eutrophic water purification using water hyacinth (Eichhornia crassipes). Environ Sci Pollut Res Int 10.1007/s11356-014-4020-3 [DOI] [PubMed] [Google Scholar]
- 67. Wagner A, Donaldson L, Kim H, Phillips L, Flint H, Steward D, et al. (2009) Suppression of 4-Coumarate-CoA Ligase in the Coniferous Gymnosperm Pinus radiata . Plant Physiology 149: 370–383. 10.1104/pp.108.125765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Papoyan A, Kochian LV (2004) Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiology 136: 3814–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mills RF, Krijger GC, Baccarini PJ, Hall JL, Williams LE (2003) Functional expression of AtHMA4, a P-1B-type ATPase of the Zn/Co/Cd/Pb subclass. Plant Journal 35: 164–176. [DOI] [PubMed] [Google Scholar]
- 70. Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP (2002) Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens . Plant Physiology 128: 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hothem SD, Marley KA, Larson RA (2003) Photochemistry in Hoagland's nutrient solution. Journal of Plant Nutrition 26: 845–854. [Google Scholar]
- 72. Able AJ (2003) Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 221: 137–143. [DOI] [PubMed] [Google Scholar]
- 73. Bates LS, Waldren RP, Teare ID (1973) Rapid Determination of Free Proline for Water-Stress Studies. Plant and Soil 39: 205–207. [Google Scholar]
- 74. Jiang MY, Zhang JH (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology 42: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 75. Nakano Y, Asada K (1981) Hydrogen-Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach-Chloroplasts. Plant and Cell Physiology 22: 867–880. [Google Scholar]
- 76. Young C, Truman P (2012) Proteins isolated with TRIzol are compatible with two-dimensional electrophoresis and mass spectrometry analyses. Analytical Biochemistry 421: 330–332. 10.1016/j.ab.2011.10.045 [DOI] [PubMed] [Google Scholar]
- 77. Wan XY, Liu JY (2008) Comparative proteomics analysis reveals an intimate protein network provoked by hydrogen peroxide stress in rice seedling leaves. Molecular & Cellular Proteomics 7: 1469–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.