Abstract
Novel antitumor therapies against the PI3K-AKT-mTOR pathway are increasingly used to treat cancer, either as single agents or in combination with chemotherapy or other targeted therapies. Although these agents are not known to be myelosuppressive, an increased risk of infection has been reported with rapamycin analogs. However, the risk of infection with new inhibitors of this pathway such as PI3K, AKT, mTORC 1/2 or multi-kinase inhibitors is unknown.
Methods
In this retrospective case-control study, we determined the incidence of infection in a group of 432 patients who were treated on 15 phase I clinical trials involving PI3K-AKT-mTOR pathway inhibitors (cases) vs a group of 100 patients on 10 phase I clinical trials of single agent non-PI3K-AKT-mTOR pathway inhibitors (controls) which did not involve conventional cytotoxic agents. We also collected data from 42 patients who were treated with phase I trials of combinations of PI3K-AKT-mTOR inhibitors and MEK inhibitors and 24 patients with combinations of PI3K-AKT-mTOR inhibitors and cytotoxic chemotherapies.
Results
The incidence of all grade infection was significantly higher with all single agent PI3K-AKT-mTOR inhibitors compared to the control group (27% vs 8% respectively, OR: 4.26, 95% CI: 1.9-9.1, p=0.0001). The incidence of grade 3 and 4 infection was also significantly higher with PI3K-AKT-mTOR inhibitors compared to the control group (10.3% vs 3%, OR: 3.74, 95% CI: 1.1-12.4, p=0.02). Also the combination of PI3K-AKT-mTOR inhibitors and chemotherapy was associated with a significantly higher incidence of all grade (OR: 4.79, 95% CI: 2.0-11.2, p=0.0001) and high grade (OR: 2.87, 95% CI: 1.0-7.6, p=0.03) infection when compared with single agent PI3K-AKT-mTOR inhibitors.
Conclusion
Inhibitors of the PI3K-AKT-mTOR pathway can be associated with a higher risk of infection. Combinations of PI3K-AKT-mTOR inhibitors and cytotoxic chemotherapy significantly increase the risk of infection. This should be taken into consideration during the design and conduct of trials involving PI3K-AKT-mTOR pathway inhibitors, particularly when combined with chemotherapy or myelosuppressive agents.
Keywords: Infection, PI3K inhibitors, AKT inhibitors, mTOR inhibitors, TORC1/2 inhibitors, PI3K-AKT-mTOR pathway, PI3 kinase pathway, infections
INTRODUCTION
The phosphatidylinositol 3-kinase (PI3K)-AKT-mTOR pathway is a critical signaling pathway which is frequently altered in human cancer (1). Aberrant activation of this pathway is associated with tumor growth, angiogenesis and survival. Mutations of the catalytic isoform p110α of the PI3K gene (PIK3CA) are amongst the most frequent mutations in human cancer (1). Also, loss of function of the tumor suppressor gene PTEN is frequently seen in human tumors and leads to activation of this pathway (2). Additionally, aberrations of RAS and AKT may lead to activation of this pathway and make it an attractive target for treating cancer (3, 4). Clinical benefit of mTOR inhibition with rapalogs in renal cell carcinoma and other cancers has provided robust evidence that this pathway can be successfully targeted for the treatment of cancer (5-9). This has led to further clinical development of compounds targeting other key components of this pathway such as PI3K, AKT and mTORC 1/2.
While the PI3K-AKT-mTOR pathway is vital to the survival of cancer cells, its importance in the homeostatsis of ‘normal’ non-cancer cells cannot be overstated. As well as its role in normal cell growth, regulation of blood glucose homeostasis and lipid metabolism (10-14), the PI3K-AKT-mTOR pathway plays an important role in regulating the immune system and cytokines production by immune cells (15). Emerging data indicate that the inhibition of PI3K signaling reduces the generation and suppresses the secretion of pro-inflammatory cytokines and leads to an attenuated immune response (15-18). It is known that mTOR inhibitors regulate the immune function and are licensed for use in renal transplantation (19, 20). Though not classically myelosuppressive, they do have qualitative effects on the immune system and have been shown to increase the risk of infection (21, 22).
Several novel targeted agents are currently being investigated in phase I clinical trials targeting one or more nodes on the PI3K-AKT-mTOR pathway. In addition to clinical trials exploring the activity and tolerability of these compounds as single agents in cancer therapeutics, these drugs are also being tested in combination with other targeted agents or in combination with cytotoxic chemotherapeutics, some at the later phases of development. It is anticipated that there will be an increase in the number of such compounds entering clinical practice in the next few years. However, these agents are associated with clinically relevant toxicities. While some of the toxicities of these agents such as hyperglycemia, rash and diarrhea are well recognized and described in the literature (23-28), little is known about the possible risk of infection with such drugs, either as single agents or in combination with cytotoxic chemotherapy or other targeted therapies. In this study, we therefore sought to investigate the risk of infection in patients treated with drugs that inhibit the PI3K-AKT-mTOR pathway in a phase I setting.
PATIENTS AND METHODS
This is a retrospective case-control study of 532 patients with metastatic solid cancers treated on phase I clinical trials at the Drug Development Unit at the Royal Marsden Hospital, United Kingdom between 2008 and 2013. Study subjects consisted of 366 patients with advanced metastatic solid cancer who were treated on 12 phase I clinical trials involving single agent PI3K-AKT-mTOR inhibitors (cases) and 100 patients with advanced metastatic solid cancer who were treated on 10 phase I clinical trials involving single agents with mechanisms of action not primarily targeting the PI3K-AKT-mTOR pathway (controls) (Table 1). This sample size provided 80% power to detect a difference of at least 15% in the rate of infection between the cases and the control group. In addition, we also collected data on 42 patients with advanced cancers who were treated on two phase I clinical trials involving combinations of PI3K-AKT-mTOR inhibitors and MEK inhibitors (PAMi + MEKi) and 24 patients who were treated on three phase I clinical trials involving combination of PI3K-AKT-mTOR inhibitors and cytotoxic chemotherapies (PAMi + chemo) (Table 2). Approval for this study was obtained from the Institutional Audit Committee (DDU058).
Table 1. Baseline characteristics in cases and controls.
Baseline characteristics such as age at the time of recruitment, primary tumor type, prior lines of chemotherapy, performance status were balanced between cases and controls.
| Cases | Controls | ||
|---|---|---|---|
| Study subjects (single agent therapy) | 366 | 100 | |
| Age, Year Median (Range) | 56.7 (22-81) | 54.3 (17-88) | |
| Gender | Male | 179 | 53 |
| Female | 187 | 47 | |
| BMI, Kg/m2 Median (Range) | 25.9 (16.9-43) | 26.8 (18.3- 38) | |
| Tumor type (All cases, single agents and combinations) | Lung & Mesothelioma | 49 (13%) | 11 (11%) |
| Breast | 34 (9.3%) | 11 (11%) | |
| Colorectal | 95 (26%) | 16 (16%) | |
| Gynecological | 61 (16.6%) | 6 (6%) | |
| RCC | 18 (5%) | 3 (3%) | |
| Prostate | 22 (6.8%) | 10 (10%) | |
| Others | 87 (23.7%) | 43 (43%) | |
| No of prior lines of chemotherapy | 0-2 | 183 (50%) | 52 (52%) |
| 3-5 | 148 (40.5%) | 36 (36%) | |
| >6 | 20 (5.5%) | 4 (4%) | |
| Unknown | 15 (4%) | 8 (8%) | |
| Performance Status | 0 | 88 (24%) | 26 (26%) |
| 1 | 276 (75%) | 72 (72%) | |
| 2 | 2 (0.5%) | 2 (2%) | |
| RM Score * | 0 | 68 (18.5%) | 22 (22%) |
| 1 | 129 (35%) | 30 (30%) | |
| 2 | 106 (29%) | 32 (32%) | |
| 3 | 52 (14.5%) | 16 (16%) | |
| Unknown | 11 (3%) | - | |
| Time on trial (days) | 84 | 109 | |
RM score (reference): Albumin + No. metastatic sites + LDH
Table 2. Phase I clinical trials in cases and controls.
Phase I clinical trials in the case group comprised of 12 phase I trial of single agent PI3K-AKT-mTOR inhibitors, 3 trials of combination of PI3K-AKT-mTOR inhibitors and chemotherapy and 2 trials of combination of PI3K-AKT-mTOR inhibitors and MEKi.
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| Target | No. of trials | No. of patients | Target | No. of trials | No. of patients | |
| Single agent | PI3K | 4 | 80 | VEGF | 2 | 22 |
| mTORC | 4 | 141 | EGFR | 1 | 22 | |
| AKT | 3 | 104 | MEK | 1 | 12 | |
| Dual PI3K/mTORC inhibitors | 1 | 41 | c-MET | 1 | 12 | |
| Total | 12 | 366 | HDAC | 2 | 11 | |
| Combination with chemotherapy | 3 | 24 | HSP90 | 1 | 11 | |
| Combination with MEK inhibitors | 2 | 42 | Integrin | 1 | 5 | |
| Rock | 1 | 4 | ||||
| IGF-1R | 1 | 1 | ||||
| Total | 17 | 432 | Total | 10 | 100 | |
Clinical trials in the control group comprised of 10 trials with agents not known to predominantly inhibit PI3K-AKT-mTOR directly.
VEGF: Vascular Endothelial Growth Factor; EGFR: Epidermal Growth Factor Receptor; MEK: MAP Kinase; HDAC: Histone deacetylase; HSP 90: Heat Shock Protein 90; IGF-1 R: Insulin-like growth factor 1 Receptor; Rock: Rho-associated Kinase
Patients in the case group consisted of all patients who were treated with PI3K-AKT-mTOR inhibitors for which the results of the study either had been published or presented at a scientific meeting. In order to reduce the risk of selection bias, for the control group we used a sealed envelope method to randomly select 10 phase I clinical trials from a list of trials not involving PI3K-AKT-mTOR inhibitors, DNA damaging agents (chemotherapy) or DNA repair targeting agents (for example, PARP inhibitors). Subsequently, we randomly selected 100 patients from the above 10 trials using the random table number method.
Patient demographics, tumor, drug-related and laboratory data were collected from hospital electronic patients’ records, anonymized and entered into a database. Infection related data consisted of events with infection-related symptoms for which antibiotics had been prescribed at the discretion of the treating physician, with or without a positive laboratory culture. Some patients had been treated with antibiotics due to clinical concerns that their symptoms represent an infective event whereas no positive laboratory cultures have been available for these patients. Every infective event across all cycles of treatment during the conduct of phase I trials was collected. The source of infection, microbiological data, antibiotic types, mode of administration and duration, body temperature, laboratory data including neutrophil and lymphocyte counts and CRP were collected where available. Tumor and patients’ baseline characteristics were balanced between both study groups (Table 1).
The clinical trials of single agent PI3K-AKT-mTOR inhibitors consisted of 12 phase I clinical trials including 4 PI3K inhibitors (2 pan-PI3K, 1 PIK3α subunit and 1 PIK3β subunit specific inhibitors), 3 AKT inhibitors, 4 mTORC1/2 inhibitors and one dual pan-PI3K-mTORC1/2 inhibitor. Combination trials included three phase I clinical trials of chemotherapy combination (2 AKT inhibitors, one mTORC1/2 inhibitor and chemotherapy) and 2 phase I clinical trials of a MEKi combination (1 AKTi and MEKi trial, and 1 PI3Ki and MEKi trial), as shown in Table 2. Clinical trials in the control group consisted of 10 randomly selected phase I clinical trials with single agent targeted compounds including VEGF, EGFR, MEK, c-MET, HDAC, HSP90, Integrin, ROCK and IGFR-1 inhibitors.
Statistics
Data were entered into an Access database (Microsoft, USA) and exported into SPSS (IBM Corp., Version 20.0, Armonk, NY) for data analysis. The Chi-square test and Fisher’s exact test were used to describe the difference in incidence of infection between cases and controls. Odds ratio and confidence intervals were used to describe the risk of infection between cases and controls. Univariate and multivariate logistic regression tests were used to compare the risk of infection between different inhibitors of the PI3K-AKT-mTOR pathway. Multivariate logistic regression models were used for association studies between baseline characteristics and the risk of developing an infection.
RESULTS
Patient characteristics
Baseline characteristics were compared between cases on single agent PI3K-AKT-mTOR pathway inhibitors and controls on single agent targeted therapies not involving the PI3K-AKT-mTOR pathway and were well balanced between the two groups (Table 1). Study subjects in both cohorts reflect the typical phase I patients as previously described from our institution (29, 30).
Median age at the time of recruitment to the phase I clinical trials was 56.7 years for the cases and 54.3 years for the control group. Baseline characteristics such as number of prior lines of chemotherapy, performance status and the Royal Marsden (RM) prognostic score (31) were balanced between cases and controls. Both groups were well balanced for most primary cancers except for gynecological and colorectal cancers where these malignancies were 10% more prevalent in the case group compared to the control group. Study subjects on both cohorts reflects typical phase I patients as previously described from our institution. Median duration of time on trials was 84 days for the cases and 109 days for the control group.
Increased risk of infection with single agent PI3K-AKT-mTOR inhibitors
In this retrospective case-control study, the incidence of all grade infective events requiring antibiotic treatments during the course of their treatment was 27% (99/366 patients) in the case group and 8% (8/100 patients) in the control group (Table 3). The risk of all grade infective events was significantly higher in patients treated with single agent PI3K-AKT-mTOR inhibitor trials compared to the control group (OR: 4.26, 95% CI: 1.9-9.1, p=0.0001). We also identified 38 patients (10.3%) in the case group and 3 patients (3%) in the control group who had developed grade 3 and 4 infective events requiring hospitalization and treatment with intravenous antibiotics during the course of their treatment. Patients on the single agent PI3K-AKT-mTOR inhibitor trials also had a higher risk of developing high grade infective events compared to the control group (OR: 3.74, 95% CI: 1.1-12.4, p=0.02). Infective events with single agent PI3K-AKT-mTOR inhibitors were not dose-dependent and occurred during all cycles of treatment. Data for the timing of occurrence of the first infective event were available for 81 patients in the case group, of which 25 patients (30.8%) had developed infection during the first cycle, 13 patients (16%) during the second cycle and 20 patients (25%) during the third cycle of treatment. The incidence of infection was lower in subsequent cycles (5 patients (6%) during the 4th cycle, 3 patients (3.7%) during the 5th cycle and 13 patients (16%) during all other subsequent cycles). This indicates that this is less likely to be cumulative, however, it is difficult to draw a definite conclusion in this retrospective dataset.
Table 3. Incidence and risk of infection between cases and controls and between single agent PI3K-AKT-mTOR and combination therapies.
| All grade infection | Grade 3/4 infection | |||||||
|---|---|---|---|---|---|---|---|---|
| With infection | Without infection | OR (95% CI) | p | With infection | Without infection | OR (95% CI) | p | |
| Controls | 8 (8%) | 92 (92%) | - | - | 3 (3%) | 97 (97%) | - | - |
| Single agent PAMi | 99 (27%) | 267(73%) | 4.26* (1.9-9.1) | 0.0001 | 38 (10%) | 328 (90%) | 3.74* (1.1-12.4) | 0.02 |
| PAMi + Chemotherapy | 16 (62%) | 9 (38%) | 4.79** (2.0- 11.2) | 0.0001 | 6 (25%) | 18 (75%) | 2.87** (1.0-7.6) | 0.03 |
| PAMi + MEKi | 26 (62%) | 16 (38%) | 4.38** (2.2-8.5) | <0.0001 | 3 (7%) | 39 (93%) | 0.66** (0.1-2.2) | 0.5 |
Odds ratio calculated between single agent PI3K-AKT-mTOR inhibitors and control group
Odds ratio calculated between single agent PI3K-AKT-mTOR inhibitors and PI3K-AKT-mTOR inhibitors combination therapies (PAMi+chemotherapy and PAMi+MEKi)
Increased risk of infection with a combination of ‘PI3K-AKT-mTOR inhibitors and chemotherapy’ and ‘PI3K-AKT-mTOR inhibitors and MEK inhibitors’
In addition to 366 cases treated with single agent PI3K-AKT-mTOR inhibitors, we also collected data from 24 patients on three PI3K-AKT-mTOR inhibitor trials combined with chemotherapy, and 42 patients on two trials of PI3K-AKT-mTOR inhibitors combined with MEK inhibitors (MEKi). The incidence of all grade infective events was 62.5% (15/24 patients) in patients treated with PI3K-AKT-mTOR inhibitors and chemotherapy combination, and 62% (26/42 patients) in the PI3K-AKT-mTOR inhibitors and MEKi combination cohort (Table 3). The risk of all grade infection in patients who received the combination of PI3K-AKT-mTOR inhibitors and chemotherapy was significantly higher than those who received single agent PI3K-AKT-mTOR inhibitors (OR: 4.79, CI: 2.0-11.2, p=0.0001). Also, the risk of all grade infection was significantly higher with the combination of PI3K-AKT-mTOR inhibitors and MEKi compared to single agent PI3K-AKT-mTOR inhibitors (OR: 4.38, CI: 2.2-8.5, p<0.0001) or single agent MEKi (OR: 4.87, CI: 1.1-20.7, p= 0.03).
The incidence of grade 3 and 4 infective events were 16.6% (4/24 patients) in the PI3K-AKT-mTOR inhibitors and chemotherapy combination, and 7.1% (3/42 patients) in the PI3K-AKT-mTOR inhibitors and MEKi combination cohort. The risk of high-grade infective events was significantly higher in patients who received the combination of PI3K-AKT-mTOR inhibitors and chemotherapy (OR: 2.87, CI: 1.0-7.6, p=0.03). However, this risk was not statistically significant in patients on PAMi + MEKi combination trials (OR: 0.66, CI: 0.19-2.2, p=0.5), probably due to the small sample size. We did not identify any patients who had high grade infection with a single agent MEKi trial.
Difference between different classes of single agent PI3K-AKT-mTOR inhibitors
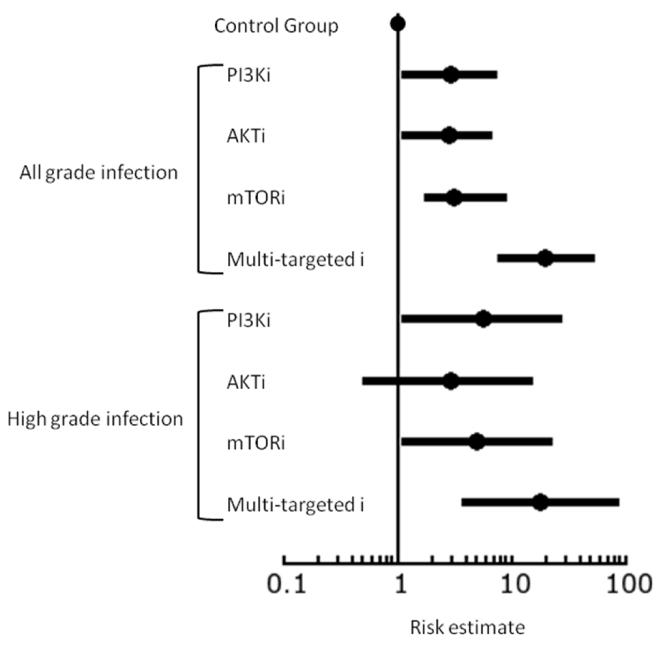
We performed the Chi-square test, univariate and multivariate logistic regression to investigate the difference in the risk of all grade and high grade infection between different classes of PI3K-AKT-mTOR pathway inhibitors. The risk of all grade and high grade infection with each inhibitor has been shown in Table 4 and the forest plot in Figure 1. There is an increased risk of all grade and high grade infection with all inhibitors of the PI3K-AKT-mTOR. In univariate and multivariate logistic regression analysis, dual PI3K/mTOR inhibitors were associated with significantly higher risk of infection compared to PI3K, AKT or mTOR inhibitors.
Table 4. Incidence and risk of infection between different inhibitors of PI3K-AKT-mTOR pathway.
| All grade infection | Grade 3/4 infection | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | OR (95% CI) | p | No | Yes | OR (95% CI) | p | |
| Controls | 92 (92%) | 8 (8%) | - | - | 98 (98%) | 2 (2%) | - | - |
| mTORCi | 105 (74.5%) | 36 (25.5%) | 3.9 (1.7-8.9) | 0.001 | 128 (90.7%) | 13 (9.3%) | 4.9 (1.0-22.5) | 0.03 |
| PI3Ki | 62 (79.5%) | 16 (20.5%) | 2.9 (1.1-7.3) | 0.019 | 70 (89.7%) | 8 (10.3%) | 5.6 (1.1-27.1) | 0.03 |
| Multi kinase PI3K/mTORC inhibitors | 15 (36.6%) | 26 (63.4%) | 19.9 (7.6-52.1) | <0.0001 | 30 (73.2%) | 11(26.8%) | 17.9 (3.7-85.6) | 0.0003 |
| AKTi | 85 (80.2%) | 21 (19.8%) | 2.8 (1.1-6.7) | 0.018 | 100 (94.4%) | 6 (5.6%) | 2.9 (0.5-14.9) | 0.19 |
Figure 1.
Forest plot of risk estimates of all grade and high grade infection between different inhibitors of PI3K-AKT-mTOR pathway
Types of infections
Of the 140 patients with infection in the case group (99 patients on single agent PI3K-AKT-mTOR inhibitors and 41 patients on combination treatments), 138 patients (98.5%) had bacterial infections and were started on antibacterial treatments. Only two patients (1.5%) had grade 1 viral infection with herpes zoster virus - one patient on single agent mTORC1/2 inhibitor and one patient on a PI3K-AKT-mTOR inhibitor and MEKi combination trial. Both patients received oral antiviral treatments and responded well to the treatment. We did not identify any other patient with viral exacerbations, protozoan, fungal or mycobacterial infections in the case group. Similarly, all 8 patients in the control group had bacterial infection and responded to antibacterial treatments.
In the case group 101 patients (72%) had one episode of infection and 39 patients (28%) had two or more episodes of infection during their treatment. Diagnosis of infection in all patients was made by the treating physicians based on infection-related symptoms at the time of presentation. All patients received appropriate antimicrobial treatment according to the hospital guidelines. Compliance with the local antibiotics policy was 98%. Urinary tract infections (38%, 54 patients) and respiratory tract infections (28%, 39 patients) were the most common causes of infection followed by gastroenteritis, sepsis, cellulitis, and line infections in 47 (33%) of patients.
We also collected microbiological culture data from patients at the time of infection. Microbiological culture data was available for 73 patients, of which 49 patients had positive and 24 patients had negative cultures. Of 49 patients with positive cultures, 34 patients (70%) had positive urine culture, 7 patients (14%) positive wound culture, 6 patients (12%) positive blood culture, one patient (2%) positive sputum culture and one patient (2%) positive faeces culture. Escherichia coli (48%) and Pseudomonas (22%) were the most grown bacteria in the culture followed by other enterococci and pneumococci (28%).
Full blood count data at the time of infection was available for 127 patients; 13 patients had presented to their local hospital and their blood count was not available. The average neutrophil count at the time of infection for the case group was 6.6 × 109/l (range: 0.03-22.1 ×109/l). Nine patients (6.4%) in the case group were found to have neutropenia. All 9 patients but one were on combination of AKTi and chemotherapy. The remaining one patient was on single agent AKTi with a normal baseline neutrophil count. Six out of nine patients with neutropenia were admitted and received treatment for neutropenic sepsis according to hospital neutropenic sepsis and antibiotic guidelines. One patient died due to sepsis but we could not establish the neutrophil counts at the time of infection from the admitting local hospital. This patient was on a dose level above the maximum tolerated dose of the drug and immediate dose reduction was initiated for the remainder of the patients on the respective trial. No patient in the control group was found to have neutropenia.
Also, 43 patients (30%) were found to have lymphopenia at the time of presentation with infection, while the rest had a normal lymphocyte count. Average lymphocyte counts at the time of infection for the case group was 1.2 × 109/l (range: 0.2-3.1 × 109/l).
The respective clinical trial had to be stopped in 6 patients due to grade 3 - 4 infection (3 patients with urinary tract infection, two patients with sepsis and one patient with pneumonia). One patient who had been treated with a dose level above the maximum tolerated dose of a dual Pi3K/mTOR inhibitor died as a result of sepsis.
Predictive markers of infection with PI3K-AKT-mTOR inhibitors
We looked at various baseline characteristics to establish if any clinical or laboratory variables at baseline were predictors for developing infection with PI3K-AKT-mTOR inhibitors. In univariate logistic regression analyses, age, baseline performance status, albumin, LDH, WCC, neutrophil and lymphocyte counts and history of type 1 or type 2 diabetes did not significantly predict the risk of infection. The only predictor of infection in univariate logistic regression was the number of cycles of treatment (Table 5). This finding was also confirmed with the logistic regression model. The number of cycles of treatment was also confirmed as a predictor of the risk of infection in the multivariate logistic mixed model (OR: 1.109, 95% CI: 1.02-1.19, p=0.008).
Table 5. Evaluation of baseline characteristics as predictive factor of infection in patients treated with single agent PI3K-AKT-mTOR inhibitors.
| Infection indicators | Odds Ratio | P | 95% Lower CI. | 95% Upper CI. |
|---|---|---|---|---|
| Age | 1.01 | 0.524 | 0.99 | 1.02 |
| PS | 1.38 | 0.23 | 0.81 | 2.35 |
| RM Score | 0.82 | 0.127 | 0.64 | 1.06 |
| BMI | 1 | 0.991 | 0.99 | 1.01 |
| Baseline albumin | 1 | 0.878 | 0.95 | 1.05 |
| Baseline LHD | 1 | 0.057 | 1 | 1 |
| Baseline white cell counts | 1 | 0.854 | 0.94 | 1.05 |
| Baseline neutrophils count | 0.98 | 0.702 | 0.91 | 1.07 |
| Baseline lymphocytes count | 1.19 | 0.198 | 0.92 | 1.54 |
| Baseline N/L ratio | 0.98 | 0.617 | 0.9 | 1.07 |
| Number of metastatic sites | 0.88 | 0.633 | 0.52 | 1.48 |
| Number of cycles received | 1.0880 | 0.012 | 1.01 | 1.16 |
| History of Type 1 DM | 9.91e-07 | 0.985 | 0 | - |
| History of Type 2 DM | 0.64 | 0.68 | 0.07 | 5.4359 |
The only predictive factor of infection with PI3K-AKT-mTOR inhibitors is the number of cycles of treatment. Patients who were on the treatment for longer time were more likely to develop infection.
DISCUSSION
mTOR inhibitors have long been used as immune-suppressants in transplant patients and infection is a well-described adverse event reported in patients treated with such drugs (19, 20). There is evidence that rapalogs such as temsirolimus and everolimus are associated with an increased risk of infection (22). In a meta-analysis involving 1924 patients with renal cell carcinoma treated on phase II and phase III randomized clinical trials involving temsirolimus and everolimus compared with 1256 patients on respective control arms, Kaymakcalan and colleagues reported a 2-fold increase in the risk of all grade infection and 2.6-fold increase risk of high grade infection associated with mTOR inhibitors (22). Also, in a recent meta-analysis of 10 randomized clinical trials involving 1911 cancer patients on temsirolimus and everolimus and 1624 patients on the control arm, Garcia and co-workers reported a 1.86-fold increase in the risk of all grade infection and 2.86-fold rise in the risk of high grade infection in patients treated with these agents (21).
To our knowledge, the risk of infection with novel agents against critical components of this pathway such as PI3K, AKT, TORC1/2, or multi-kinase inhibitors, has not been described. This is the first study to show that the risk of infection is significantly increased with new inhibitors against the PI3K-AKT-mTOR pathway in cancer patients. We show that the risk of all grade and high-grade infection with single agent PI3K-AKT-mTOR inhibitors is 3.8 and 5.3 times higher, respectively, compared to other non-myelosuppressive targeted therapies, (p=0.0005 and p=0.005, respectively). We also show that the risk of all grade and high-grade infection is even more prominent when these agents are combined with cytotoxic chemotherapy compared to single agent PI3K-AKT-mTOR inhibitors (OR: 4.49 and 2.87 respectively, p=0.0006 and 0.03, respectively). We report that the risk of infection is increased with all inhibitors of PI3K-AKT-mTOR pathway, although this risk was significantly higher with dual PI3K/mTOR inhibitors compared to PI3K, AKT or mTOR inhibitors.
In this study, we collected data from patients who had developed infective-related symptoms during the course of their treatment with PI3K-AKT-mTOR inhibitors. Some patients had been treated with antibiotics due to clinical concerns that their symptoms could represent an infective event in the absence of positive laboratory cultures to confirm the diagnosis of infection. Whilst we excluded patients who had other diagnoses, such as drug-induced pneumonitis and rash, we acknowledge that in the absence of positive laboratory culture it is difficult to prove that all the infective events captured in this study are, in fact, purely due to infection; disease-related factors could be a contributing reason for the infection. Whilst the majority of baseline characteristics were well balanced between cases and control groups, it is worth noting that there was a 10% higher prevalence of patients with gynecological and colorectal cancer in the case group compared to the control group. Although this slight imbalance is unlikely to explain the significant difference in the risk of infection between cases and controls, disease-related causes of infection could be a confounding factor to this study. Also, the PI3K-AKT-mTOR pathway interacts with different nodes in signal transduction networks and it is difficult to exclude minor interactions of the drugs in the control group with the PI3K-AKT-mTOR pathway. Short term toxicities (often 28 days) are often reported in phase I trials with 6-12 patients in pharmacologically relevant doses. This often underplays toxicities seen in later phase clinical trials and leads to pool data collection from multiple clinical trials of drugs on the same pathway. The findings from this study will need to be validated in future phase II and phase III studies but we feel this body of work gives a preview of the risk of infection that may occur when larger groups of patients are treated with these inhibitors in the next few years.
Although we have not compared the risk of infection between the combination of PI3K-AKT-mTOR inhibitors and chemotherapy and chemotherapy alone, the rate of all grade infection with the PI3K-AKT-mTOR chemotherapy combination (62.5%) is higher than the rate of infection known for most cytotoxic agents and highlights the possibility that co-administration of PI3K-AKT-mTOR inhibitors and chemotherapy may synergistically contribute to this increased risk and warrants further investigations in studies with larger cohorts.
It is well recognized that mTOR inhibitors such as temsirolimus and everolimus have immune-suppressive effects. The mechanism of infection with the new classes of PI3K-AKT-mTOR inhibitors is not fully understood, however, there is evidence that the PI3K-AKT-mTOR signaling pathway regulates the production of cytokines in innate immune cells (15). Further, T-cells are dependent on this signaling pathway for their differentiation, activation, maintenance and trafficking (32-34). In our series, only a minority of patients had leukopenia and the incidence of neutropenia was rare; therefore, it seems that defects in the function of immune response rather than the number of immune cells are the likely cause of infection with these drugs.
The potential immunosuppressive effects of PI3K-AKT-mTOR inhibitors need further investigations not only in relation to the increased risk of infection with these agents but also to investigate if such potential immunosuppression might increase the risk of cancer progression. While some recent studies suggest that the risk of developing cancer increases in patients who receive immunosuppression after organ transplant (35-37), there is no evidence that patients who are on post-transplant immunosuppressive therapy with mTOR inhibitors have a higher risk of developing malignancies; there is evidence that immunosuppression with mTOR inhibitors in transplant patients reduces the risk of cancer when compared with other immunosuppressive agents (38, 39).
The use of antitumor agents targeting the PI3K-AKT-mTOR pathway as a therapeutic strategy for treating cancer has increased in the past few years. A number of these agents are currently being investigated in later phase clinical trials. Therapeutic strategies using these inhibitors include single agent therapy (35%), and combinations with chemotherapy (15%), MEK inhibitors (14%) and other agents (36%) (40). Whilst the combination therapy strategy is likely to be pursued in future due to enhanced antitumor activity associated with the inhibition of multiple signaling pathways, the results from our study highlight the important factors that need to be taken into consideration when designing and interpreting future trials involving PI3K-AKT-mTOR pathway inhibitors.
Overall, this study suggests that the risk of infection (27% all grade and 10% high grade infection) across all PI3K-AKT-mTOR targeting drugs is substantial. Although this is not in the range of the incidences observed with chemotherapy (30-70% depending on the type of cytotoxic chemotherapy (41, 42)), it does add to the morbidity of patients with advanced cancer. In addition, while the high risk of infection with chemotherapy combinations is not unexpected, the increased risk of infection (62%) when combined with MEK inhibitors, which are not known to increase the risk of infective complications (43-46), is worth noting. Finally, although there was a degree of leukopenia in our series of patients (30%), the incidence of neutropenia was low (6.4%), suggesting a qualitative rather than quantitative effect on the immune system. Although most cancer centers have antibiotic policies, which are dependent on neutrophil counts and symptoms, these data suggest an unmet need to formulate a ‘non-neutropenic’ antibiotic policy that takes into account the infective risks of patients on PI3K-AKT-mTOR pathway inhibitors.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Multiple agents targeting the PI3K-AKT-mTOR pathway are licensed for use in cancer (mTOR inhibitors) or are being currently evaluated in clinical trials. The immunosuppressive function of m-TOR inhibitors is known and they have been used in transplant medicine. However, little is known about the immunosuppressive function of new inhibitors of the PI3K-AKT-mTOR pathway. This study shows a significant overall increase in the incidence of infection in patients treated with PI3K-AKT-mTOR pathway inhibitors compared to targeted agents that do not directly target this pathway, in phase I clinical trials. Combined targeting of the PI3K-AKT-mTOR and other signal transduction pathways or chemotherapy further increases the risk of infection. Currently, there are well defined algorithms to manage neutropenic sepsis. The increased incidence of infections not associated with neutropenia in patients treated with PI3K-AKT-mTOR pathway inhibitors calls for awareness and the need for development of new guidelines for the treatment of infections in this setting.
Acknowledgements
This work was supported by Cancer Research UK [grant numbers C347/A15403, C309/A11566], and an Experimental Cancer Medicine Centre Network award to The Institute of Cancer Research (joint initiative, Cancer Research UK & Department of Health for England) [program grant C12540/A15573]). All authors acknowledge National Institute for Health Research funding to the NIHR Biomedical Research Centre (jointly to The Institute of Cancer Research and The Royal Marsden).
REFERENCES
- 1.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 2.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 4.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–9. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. New Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–81. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, et al. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Pro Natl Acad Sci USA. 2008;105:9250–5. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman GA. The integral role of mTOR in lipid metabolism. Cell Cycle. 2011;10:861–2. doi: 10.4161/cc.10.6.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie S, Chen M, Yan B, He X, Chen X, Li D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS One. 2014;9:e94496. doi: 10.1371/journal.pone.0094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin CF, Cloutier A, Ear T, Sylvain-Prevost S, Mayer TZ, Bouchelaghem R, et al. A class IA PI3K controls inflammatory cytokine production in human neutrophils. Eur J Immunol. 2011;41:1709–19. doi: 10.1002/eji.201040945. [DOI] [PubMed] [Google Scholar]
- 17.Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells. J Biol Chem. 2014;289:7747–62. doi: 10.1074/jbc.M113.519686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–13. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardinger KL, Koch MJ, Brennan DC. Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy. 2004;24:1159–76. doi: 10.1592/phco.24.13.1159.38094. [DOI] [PubMed] [Google Scholar]
- 20.Kelly PA, Gruber SA, Behbod F, Kahan BD. Sirolimus, a new, potent immunosuppressive agent. Pharmacotherapy. 1997;17:1148–56. [PubMed] [Google Scholar]
- 21.Garcia C, Wu S. Risk of serious infection with mTOR inhibitors everolimus and temsirolimus in the treatment of cancer: A meta-analysis of randomized controlled trials. J Clin Oncol. 2014;32:5s. [Google Scholar]
- 22.Kaymakcalan MD, Je Y, Sonpavde G, Galsky M, Nguyen PL, Heng DY, et al. Risk of infections in renal cell carcinoma (RCC) and non-RCC patients treated with mammalian target of rapamycin inhibitors. Br J Cancer. 2013;108:2478–84. doi: 10.1038/bjc.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerji U, Dean E, Gonzalez M, et al. First-in-human phase I trial of the dual mTORC1 and mTORC2 inhibitor AZD2014 in solid tumors. J Clin Oncol. 2012:30. [Google Scholar]
- 24.Banerji U, Ranson M, Schellens J. Results of two phase I multicenter trials of AZD5363, an inhibitor of AKT1, 2 and 3: Biomarker and early clinical evaluation in Western and Japanese patients with advanced solid tumors. Cancer Res. 2013:73. [Google Scholar]
- 25.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316–25. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- 28.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 29.Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–6. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 30.Molife LR, Alam S, Olmos D, Puglisi M, Shah K, Fehrmann R, et al. Defining the risk of toxicity in phase I oncology trials of novel molecularly targeted agents: a single centre experience. Ann Oncol. 2012;23:1968–73. doi: 10.1093/annonc/mds030. [DOI] [PubMed] [Google Scholar]
- 31.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–33. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–53. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engels EA, Pfeiffer RM, Fraumeni JF, Jr., Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher MP, Kelly PJ, Jardine M, Perkovic V, Cass A, Craig JC, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21:852–8. doi: 10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167–98. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–9. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 39.Valantine H. Is there a role for proliferation signal/mTOR inhibitors in the prevention and treatment of de novo malignancies after heart transplantation? Lessons learned from renal transplantation and oncology. J Heart Lung Transplant. 2007;26:557–64. doi: 10.1016/j.healun.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–53. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 41.Bow EJ. Infection risk and cancer chemotherapy: the impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. J Antimicrob Chemother. 1998;41(Suppl D):1–5. doi: 10.1093/jac/41.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 42.Glisson BS, Murphy BA, Frenette G, Khuri FR, Forastiere AA. Phase II Trial of docetaxel and cisplatin combination chemotherapy in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2002;20:1593–9. doi: 10.1200/JCO.2002.20.6.1593. [DOI] [PubMed] [Google Scholar]
- 43.Ascierto PA. MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:1364. doi: 10.1056/NEJMc1209663. author reply 5. [DOI] [PubMed] [Google Scholar]
- 44.Catalanotti F, Solit DB, Pulitzer MP, Berger MF, Scott SN, Iyriboz T, et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013;19:2257–64. doi: 10.1158/1078-0432.CCR-12-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 46.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncology. 2013;31:482–9. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.