Abstract
Tolman proposed that complex animal behavior is mediated by the cognitive map, an integrative learning system that allows animals to reconfigure previous experience in order to compute predictions about the future. The discovery of place cells in the rodent hippocampus immediately suggested a plausible neural mechanism to fulfill the “map” component of Tolman’s theory. Recent work examining hippocampal representations occurring at fast time scales suggests that these sequences might be important for supporting the inferential mental operations associated with cognitive map function. New findings that hippocampal sequences play an important causal role in mediating adaptive behavior on a moment-by-moment basis suggest specific neural processes that may underlie Tolman’s cognitive map framework.
Introduction
A long-standing conjecture in the study of animal learning, memory, and decision making is that adaptive behavior is supported by a cognitive map, a flexible, generative learning system that allows animals to reconfigure previous experience to make inferences about the future and plan forthcoming behavior [1–3]. While the spatially-tuned firing patterns of hippocampal pyramidal neurons (place cells) provide an intuitive neural substrate for the mapping component of the cognitive map [4–6], understanding how hippocampal computations relate to the more cognitive aspects of Tolman’s construct has proven challenging. Work in human and non-human species suggests that the hippocampus underlies both retrospective, mnemonic processes as well as prospective, future-oriented mental abilities [7–11], raising an interesting question: how can place cell representations, which undoubtedly form a reliable representation of the animal’s actual location in an environment (e.g. [12]), also support the more complicated cognitive processes associated with the cognitive map? Recent work suggests that the answer lies in the sequential activation patterns of hippocampal pyramidal neurons.
Hippocampal network states
The information represented within hippocampal sequences differs as a function of the hippocampal network state. The computations performed by hippocampus change in the presence and absence of neuromodulators [13–15] and state-input processes [4]. These network states are reflected in patterns of local field potential (LFP) oscillations, which are often divided into theta and non-theta states [4, 16–19]. The theta state accompanies active behavior or attentive processes, during which the LFP exhibits prominent oscillations in the theta frequency band (6–12 Hz). Theta oscillations organize the spiking of place cells. Within each theta cycle, place cells fire in a sequential order: cells with place fields behind the animal fire first and cells with place fields farther ahead of the animal fire later. Consequently, over the course of the theta cycle, place cells trace out an ensemble representation of spatial trajectories near the animal. During slow wave sleep and awake quiescence (e.g. grooming, food consumption), the hippocampal LFP is less orderly; instead of regular oscillations, broad band voltage fluctuations typify the large, irregular activity (LIA) state. In LIA, place cells activate in fast sequences during sharp-wave ripple (SWR) complexes, so named for the characteristic high-frequency ripple waveforms that punctuate the otherwise irregular LFP. Place cell spiking during SWRs does not necessarily represent the animal’s current location in space. Instead, ensemble firing sequences trace out trajectories that may traverse regions of space the animal does not currently occupy [20, 21].
Ensemble sequence representations
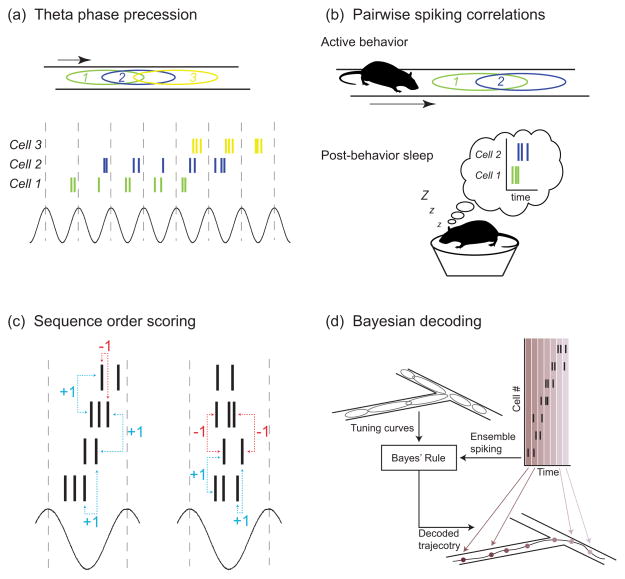
Hippocampal sequences were first identified through changes in the firing of place cells relative to the theta rhythm (fig. 1a). When running through a place field, place cells were observed to precess through phases of theta, so that on entry, spikes occurred late in the cycle, but, on exit, they occurred early in the cycle [22, 23]. It was immediately recognized that this phase precession implied a sequential firing representation along the path of the animal [23, 24]; it has since become clear that the phase precession is a consequence of sequences changing as an animal progresses through a task [25, 26]. Although hippocampal sequences were originally characterized by assessing pairwise correlations in spiking of place cells [27, 28] (fig. 1b), sequences are fundamentally an ensemble phenomenon, and the recent advances in our ability to observe, measure, and detect sequences derive from the ability to record many place cells simultaneously and look for higher-order structure in their firing patterns (fig. 1, c&d). The ensemble approach has several advantages over analyses predicated on single cells or pairs of neurons.
Figure 1. Methods of sequence extraction.
A variety of analytical approaches have been developed to identify and quantify temporally-structured spiking in hippocampal ensembles, ranging from techniques that examine one or a few neurons (a&b) to approaches that leverage ensemble activity (c&d). (a) Early work [22, 23] identified that hippocampal place cell spiking phase precesses against the theta cycle. This phase precession implies the existence of orderly, temporal patterns within theta cycles; however, certain types of sequences might not result in phase precession [26]. (b) Other early work (e.g. [27]) noted that correlations that were established between place cell spiking during exploration persisted during subsequent sleep, suggesting that place cell firing patterns recurred during offline states. (c) Sequence scoring methods like the one developed by Lee and Wilson [76] or Gupta and colleagues [47, 58] quantify the temporal structure of neural activity in a given time window. Pairs of spikes with an order of activation matching the order in which the animal passes through their fields during behavior are scored +1, while pairs of spikes whose activation order is opposite their ordering in space are scored -1. Summing across all spike pairs results in a metric of both the direction of the sequence (sign of the sequence score) and the temporal precision of the sequence (magnitude of the sequence score). The activity in the right panel exhibits temporal structure characteristic of theta sequences; the ordering of many spike pairs is consistent with a forward-directed representation. Unpatterned ensemble spiking (right panel) is consistent with both forward- and backward-ordered representations equally often, resulting in a net sequence score near zero. (d) Bayesian decoding methods [77] use the tuning curves of hippocampal neurons measured during active behavior (i.e. place fields) to estimate which positions in space are most likely being represented by ensemble spiking activity. Decoding hippocampal sequence representations reveals the spatial trajectories that sequence events encode.
First, ensemble analyses offer the ability to interpret neural activity on single trials. Instead of recording the activity of one neuron over many instances of the same behavior and averaging its response, ensemble analyses allow for meaningful interpretation of neural activity on single trials and at fast time scales, both of which are advantageous for understanding cognition, as mental operations are often transient, can unfold quickly, and are likely to vary on a trial-by-trial basis [29]. Single trial analyses avoid uncertainty over how multiple events should be averaged or aligned, as cognitive processes are unlikely to unfold consistently over a fixed duration and may not be reliably timed relative to either behavior or external features of the environment.
Ensemble approaches also offer a means of explicitly testing models of how representations are encoded by neural systems [30, 31]. With a defined tuning model, it is possible to test how well representations like hippocampal sequences conform to the expected patterns of activity predicted from those observed tuning functions. Thus, ensemble approaches have allowed researchers to assess how hippocampal representations of space mentally simulate trajectories that represent previous behaviors [32–34], possible future actions [33, 35, 36**], and even imagined experiences, such as paths that animals have never actually traversed [37, 38*] or within environments they do not currently occupy [39, 40].
LIA sequences for memory, planning, and inference
LIA sequences were first studied during sleep, and quickly rose to prominence as a potential mechanism of memory consolidation [41–43]. Consistent with a role for LIA sequences occurring during sleep in memory consolidation, disrupting SWR-associated sequences with electrical stimulation following performance of memory-guided behaviors impairs task learning [44–46]. Recent work examining LIA sequences during awake states, however, hints at a broader functional role for these representations.
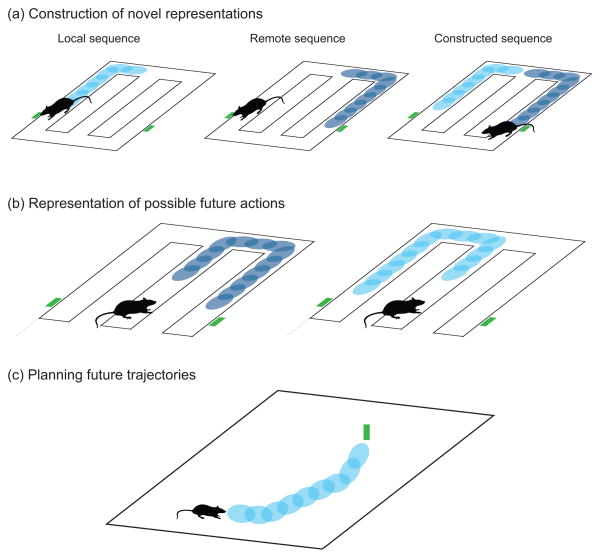
Gupta and colleagues [47] recorded LIA sequences as rats performed a multiple-T decision making task, and observed that the content of representations did not necessarily favor paths leading to reward. In fact, in some cases the authors observed an over-representation of paths traversing the non-rewarded arm of the T-maze, a finding that is difficult to reconcile with a consolidation function, as the represented trajectories did not match the animals’ cumulative behavioral experience. The authors further observed that in some cases, LIA sequences synthesized de novo paths never traversed by the animal. These constructed trajectories plotted “shortcut” paths between reward sites (fig. 2a). In a similar vein, recent reports of “pre-play”, LIA sequences representing regions of space that animals could view, but not directly experience, further suggest that LIA sequences actively synthesize spatial representations, rather than simply consolidating actual experience [37, 38*]. In light of neuropsychological and functional imaging evidence suggesting that the human hippocampus is involved in similar synthetic, generative processes [48–51], these data suggest that the rodent hippocampus might manipulate spatial representations during LIA sequences to underpin similar mental operations.
Figure 2. Diverse functional roles for LIA sequences.
(a) Gupta and colleagues [47] showed that sequence representations of paths actually traversed by rats (e.g. left and right loops on the T-shaped decision maze) were recombined in a novel fashion to represent shortcut paths between potential food delivery sites. (b) LIA sequences expressed prior to initiation of a new behavior could represent the set of possible actions available to the animal in a given situation. Sequence representations simulating potential future actions (in this case left or right turns at the choice point) could be evaluated by other neural structures to guide behavior. Figure constructed after Singer and colleagues [52]. (c) Pfeiffer and Foster [36] found that as rats performed a goal-directed navigation task in a large, open area, LIA sequences depicted paths between the rat’s current location and its eventual spatial goal.
Two recent studies establish a tight correlation between awake LIA sequences and rats’ immediately forthcoming behavior. Singer and colleagues [52**] recorded awake LIA sequences from rats performing a memory-guided W-maze task. The authors found that the degree of ensemble coordination within sequence representations predicted the successful completion of the next task trial, suggesting that LIA sequences facilitate accurate task performance. Interestingly, the content of representations (i.e. which parts of the maze the sequences represented) did not differ between sequences that occurred before correct and incorrect trials. Instead, it appears that the quality of sequences, regardless of where they represented, was the principle determinant of successful choice. This finding favors a model in which sequences represent the space of potential actions available to the animal (fig. 2b), and are then evaluated by downstream reward processing structures [53, 54].
In work that offers an interesting contrast, Pfeiffer and Foster [36**] observed that awake LIA sequence content clearly reflected rats’ future behavior as they performed a goal directed decision making task. Prior to navigating to a goal location in a large, two-dimensional environment, sequences represented paths that began near the animal and ended in the region of space the rat would next visit (fig. 2c). An interesting possibility is that the nature of sequence expression may depend on the precise demands of the behavioral task. In Pfeiffer and Foster’s [36**] task, representations of paths leading to non-rewarded locations would have less utility than in Singer et al.’s [52**] binary choice task, where knowing with certainty either the correct or incorrect choice would suffice for accurate decisions. Future work comparing SWR representations in animals trained to perform both binary and multi-alternative decision making tasks could elucidate how awake LIA sequences vary to support different behavioral challenges.
In elegant work suggesting a causal role for LIA sequences in decision making, Jadhav and colleagues [55**] showed that electrical disruption of awake SWRs impaired rats’ decision making performance on a memory-guided behavioral task. This manipulation affected behavior during the initial acquisition of the hippocampus-dependent task, and also degraded choice accuracy in animals pre-trained to asymptotic performance, implicating awake LIA sequences in both decision making during early learning phases and the long-term maintenance of stable behavioral performance.
The different effects of SWR disruption during awake states [55**] and during sleep states [44–46] suggests that there are functional differences between these SWR components. Differences have also been found in representations during wake and sleep states [56], with wake states showing flexible sequences [32, 34, 47] more likely to be involved in some sort of analytical processing than consolidation, but with sleep states being more consistent with a consolidation hypothesis.
Theta sequences and online planning
Theta sequences have historically been the focus of less research than LIA sequences, as it was originally thought that they arose passively from the independent phase precession of individual place cells. Recent findings, however, challenge that notion [25, 26]. For example, spike time correlations between place cells are more precise than the correlation between the animal’s position and the theta phase of spikes that results from phase precession [28], and theta sequences are patterned with greater precision than would be expected if phase precession alone structured place cell spiking [57]. Furthermore, phase precession depends on motion, while theta sequences do not [26].
These data suggest that sequences are the primary organizing structure of spikes within theta cycles, and that theta sequence representations are coordinated at an ensemble level and imbued with richer structure than previously appreciated [25]. Gupta and colleagues [58**] examined theta sequences on a cycle-by-cycle basis, and found that sequences within theta cycles actively parsed the environment in a way that could not result from phase precession alone. This processing by theta sequences resulted in a cognitively “chunked” representation of space (fig. 3), effectively achieving a sort of information compression that might be useful behaviorally.
Figure 3. Spatial chunking by theta sequences.
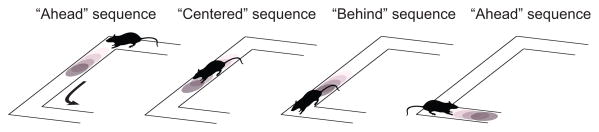
Gupta and colleagues [58] found that the content of theta sequences varies around salient landmarks in the environment in a manner that gives rise to a partitioned representation of space. On rounding the first maze corner, sequences are forward-shifted, preferentially representing space in front of the animal. Between the two turns, sequences tend to be centered around the animal’s actual location. As the rat draws near to the second maze corner, sequences lag behind the animal, extending asymmetrically backward. Once past the final corner, representations are again forward-shifted.
Work by Johnson and Redish [35*] showed that theta-state spiking might contribute to decision making. As rats paused at the choice point of a multiple-T decision making task, hippocampal ensembles traced out forward-directed paths corresponding to possible future actions, suggesting a mechanism for deliberation between concurrently-available choices. These forward-directed paths remained structured within theta cycles, suggesting that they were a similar form of theta sequences. Interestingly, much like the Singer and Frank [52**] LIA result reviewed above, Johnson and Redish [35*] were unable to find a relationship between the sequences and the actual decision made, suggesting that the hippocampus may be playing a similar constructive role, identifying the space of possibilities. Johnson and Redish [35*] did find that these extended theta sequences became stereotyped and then vanished as behavior became more automated. Like Singer and Frank’s [52**] W-task, Johnson and Redish’s [35*] T-task was also a binary choice. It remains unknown whether these results would change in the light of a more open task such as used by Pfeiffer and Foster [36**].
Do theta sequence representations play a causal role in decision making that extends beyond the more general tuning properties of place cells? Cannabinoid agonists offer an intriguing manipulation for dissociating the influence of ensemble spiking sequences and other place cell properties on decision making by abolishing ensemble coordination of place cell spiking while minimally affecting cells’ spatial tuning or firing rates. Robbe and colleagues [59, 60] found that administration of cannabinoid agonists disrupted accurate decision making in rats performing a memory-guided spatial task. Importantly, cannabinoid agonism caused task performance to fall to chance levels even in well trained animals, suggesting that temporal coordination within theta cycles likely plays a role in the moment-to-moment selection of behavior. Together, these data suggest that theta sequences might be an important brain mechanism for deliberative decision making.
Conclusions and future directions
It is increasingly apparent that sequences play a more active and complex role in information processing than encoding veridical experience. Their role in flexibly manipulating and permuting representations of space to generate novel paths that might aid action selection meshes well with the cognitive map envisioned by Tolman [2, 3]; however, important questions about the function of hippocampal sequences remain unanswered.
Foremost, the interaction between LIA representations and sequences during the theta network state remains largely untested. Although there is good evidence that both are causally involved in planning and decision making [55**, 59, 60], it is unclear precisely which cognitive functions each are responsible for, whether they are interdependent, or whether the absence of one type of sequence might induce compensatory changes in the expression of the other. Future work that manipulates theta and LIA sequences independently could help reveal the relationship between hippocampal sequential representations that occur during different network states.
We know little of how hippocampal sequence representations affect other brain regions, although several lines of evidence hint at interplay between the hippocampus and extra-hippocampal structures during both theta [61–66] and non-theta [67–72] states. Simultaneously measuring neural activity in multiple brain regions as animals are engaged in complex behaviors will further our understanding of how the hippocampus and other brain regions interact to support cognition.
Finally, it is unclear whether the mnemonic and planning functions of LIA sequences are separable. Interestingly, causal manipulations clearly implicate sleep [44, 45] and awake [55**] LIA representations in both memory consolidation and decision making, respectively. One possibility is that the animal’s behavioral state (asleep vs. awake) determines which function prevails. The reduced sensory input associated with sleep states might allow internal hippocampal dynamics to dominate information processing, favoring memory consolidation processes, while waking states and their accompanying stream of information about the external world might shift hippocampal processing to memory recall or planning functions [73]. This arrangement would suggest, however, that consolidation of learning could not take place online, in waking states, as experience occurs. If awake consolidation is indeed possible, sequences dedicated to mnemonic and planning processes might be distinguishable, either by the content of representations or by some other neural signal, such as the LFP, components of which have been shown to vary with the quality of sequence representations [74], or even by the activity of other brain structures (such as pre-limbic cortex, [75]). Selective interruption of different functional classes of LIA sequences could lead to more precise understanding of how hippocampal sequences contribute to cognitive processes.
Highlights.
Sequences of spiking are the dominant organization principle of hippocampal activity.
New ensemble techniques allow observation and detection of hippocampal sequences.
Sharp-wave sequences are involved in both consolidation and choosing future behavior.
Theta sequences support online planning to guide behavior in real time.
Acknowledgments
This work was supported by a University of Minnesota Doctoral Dissertation Fellowship (AMW) and National Institutes of Health grant R01-MH-080318 (ADR).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tolman EC. Purposive Behavior in Animals and Men. Appleton-Century-Crofts; New York: 1932. [Google Scholar]
- 2.Tolman EC. Cognitive maps in rats and men. Psychological Review. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 3.Johnson A, Crowe D. Revisiting Tolman: Theories and cognitive maps. Cognitive Critique. 2009;1:43–72. [Google Scholar]
- 4.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon Press; Oxford: 1978. [Google Scholar]
- 5.Redish AD. Beyond the Cognitive Map: From Place Cells to Episodic Memory. MIT Press; Cambridge MA: 1999. [Google Scholar]
- 6.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nature Reviews Neuroscience. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 7.Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- 8.Schacter D, Addis D. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1245. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassabis D, Maguire E. The construction system of the brain. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner R. The role of the hippocampus in prediction and imagination. Annual Review of Psychology. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 11.Addis D, Cheng T, Roberts R, Schacter D. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011;21:1045–1052. doi: 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 13.Hasselmo ME, Bower JM. Acetylcholine and memory. Trends in Neurosciences. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- 14.Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- 15.Amemiya S, Noji T, Kubota N, Nishijima T, Kita I. Noradrenergic modulation of vicarious trial-and-error behavior during a spatial decision-making task in rats. Neuroscience. 2014;265:291–301. doi: 10.1016/j.neuroscience.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Green J, Arduini A. Hippocampal electrical activity in arousal. Journal of Neurophysiology. 1954;17:531–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 17.Vanderwolf C. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 18.Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Research. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 19.Carr M, Frank L. A single microcircuit with multiple functions: state dependent information processing in the hippocampus. Current Opinion in Neurobiology. 2012;22:704–708. doi: 10.1016/j.conb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature Neuroscience. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buhry L, Azizi AH, Cheng S. Reactivation, replay and preplay: how it might all fit together. Neural Plasticity. 2011;2011:1–11. doi: 10.1155/2011/203462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Keefe J, Recce M. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 23.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–173. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Maurer AP, McNaughton BL. Network and intrinsic cellular mechanisms underlying theta phase precession of hippocampal neurons. Trends in Neurosciences. 2007;30:325–333. doi: 10.1016/j.tins.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philosophical Transactions of the Royal Society B Biological Sciences. 2009;364:1193–1201. doi: 10.1098/rstb.2008.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redish AD, Ekstrom AD. Hippocampus and related areas: what the place cell literature tells us about cognitive maps in rats and humans. In: Waller DA, Nadel L, editors. Handbook of Spatial Cognition. APA; in press. [Google Scholar]
- 27.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 28.Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Johnson A, Fenton AA, Kentros C, Redish AD. Looking for cognition in the structure in the noise. Trends in Cognitive Sciences. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson JC, Redish AD. Detecting dynamical changes within a simulated neural ensemble using a measure of representational quality. Network: Computation in Neural Systems. 2003;14:629–645. [PubMed] [Google Scholar]
- 31.Johnson A, Jackson J, Redish AD. Measuring distributed properties of neural representations beyond the decoding of local variables implications for cognition. In: Hölscher C, Munk MHJ, editors. Mechanisms of information processing in the Brain: Encoding of information in neural populations and networks. Cambridge University Press; 2008. pp. 95–119. [Google Scholar]
- 32.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 33.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. Journal of Neuroscience. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. In one of the first studies to use a neural decoding approach to investigate cognitive representations within hippocampal ensembles, the authors report forward-directed representations as animals contemplate their next course of action while paused at the choice point of a decision making task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Pfeiffer B, Foster D. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. By recording very large ensembles of hippocampal place cells, the authors were able to decode awake LIA sequence trajectories in a complex, two-dimensional environment. They show that SWR representations preceding navigations trials were biased to the locations that animals were about to travel to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dragoi G, Tonegawa S. Distinct preplay of multiple novel spatial experiences in the rat. Proceedings of the National Academy of Sciences, USA. 2013;110:9100–9105. doi: 10.1073/pnas.1306031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. In this work (and the preceding reference), the authors demonstrate that hippocampal ensembles represent trajectories through a never-experienced region of space during LIA sequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson JC, Johnson A, Redish AD. Hippocampal sharp waves and reactivation during awake states depend on repeated sequential experience. Journal of Neuroscience. 2006;26:12415–12426. doi: 10.1523/JNEUROSCI.4118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nature Neuroscience. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzsáki G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 42.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. Journal of Neuroscience. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 44.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nature Neuroscience. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 45.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Current Opinion in Neurobiology. 2011;21:452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barron H, Dolan R, Behrens T. Online evaluation of novel choices by simultaneous representation of multiple memories. Nature neuroscience. 2013;16:1492–1498. doi: 10.1038/nn.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bornstein AM, Daw ND. Cortical and hippocampal correlates of deliberation during model-based decisions for rewards in humans. PLoS computational biology. 2013;9:e1003387. doi: 10.1371/journal.pcbi.1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Singer A, Carr M, Karlsson M, Frank L. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. Here, the authors demonstrate a clear relationship between accurate decision making and ensemble coordination within awake SWR sequences immediately preceding a choice trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson A, van der Meer MAA, Redish AD. Integrating hippocampus and striatum in decision-making. Current Opinion in Neurobiology. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Meer MAA, Redish AD. Expectancies in decision making, reinforcement learning, and ventral striatum. Frontiers in Neuroscience. 2010 doi: 10.3389/neuro.01.006.2010. [DOI] [PMC free article] [PubMed]
- 55**.Jadhav S, Kemere C, German P, Frank L. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2013;336:1454–1458. doi: 10.1126/science.1217230. The authors used electrical stimulation triggered on hippocampal SWRs to disrupt awake LIA sequences as rats performed a hippocampus-dependent decision making task. Sequence disruption impaired task learning, and also degraded performance in well-trained animals, arguing for a causal role for awake LIA sequences in decision making. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wikenheiser AM, Redish AD. The balance of forward and backward hippocampal sequences shifts across behavioral states. Hippocampus. 2013;23:22–29. doi: 10.1002/hipo.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster DJ, Wilson MA. Hippocampal theta sequences. Hippocampus. 2007;17:1093–1099. doi: 10.1002/hipo.20345. [DOI] [PubMed] [Google Scholar]
- 58**.Gupta A, van der Meer M, Touretzky D, Redish A. Segmentation of spatial experience by hippocampal θ sequences. Nature Neuroscience. 2012;15:1032–1039. doi: 10.1038/nn.3138. In one of the first studies to examine spiking sequence representations within individual theta cycles, the authors demonstrate that theta sequence dynamics were modulated by environmental features, resulting in a cognitively-parsed, “chunked” representation of space. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature Neuroscience. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 60.Robbe D, Buzsaki G. Alteration of Theta Timescale Dynamics of Hippocampal Place Cells by a Cannabinoid Is Associated with Memory Impairment. J Neurosci. 2009;29:12597–12605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 62.Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- 63.Benchenane K, Peyrache A, Khamassi M, Tierny PL, Gioanni Y, Battaglia FP, Wiener S. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in integrative neuroscience. 2010:4. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Meer MAA, Redish AD. Covert expectation-of-reward in rat ventral striatum at decision points. Frontiers in Integrative Neuroscience. 2009;3:1–15. doi: 10.3389/neuro.07.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Meer MAA, Redish AD. Theta phase precession in rat ventral striatum links place and reward information. Journal of Neuroscience. 2011;31:2843–2854. doi: 10.1523/JNEUROSCI.4869-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann KL, McNaughton BL. Coordinated reactivation of distributed nemory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- 69.Lansink CS, Goltstein PM, Lankelma JV, Joosten RNJMA, McNaughton BL, Pennartz CMA. Preferential Reactivation of Motivationally Relevant Information in the Ventral Striatum. J Neurosci. 2008;28:6372–6382. doi: 10.1523/JNEUROSCI.1054-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CMA. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule learning related neural patterns in the prefrontal cortex during sleep. Nature Neuroscience. 2009;12:919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- 72.Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard H, Besserve M, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 73.Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Computation. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- 74.Carr M, Karlsson M, Frank L. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang JX, Cohen NJ, Voss JL. Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiology of Learning and Memory. doi: 10.1016/j.nlm.2014.04.003. in press. [DOI] [PMC free article] [PubMed]
- 76.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow-wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ. Interpreting neuronal population activity by reconstruction: Unified framework with application to hippocampal place cells. Journal of Neurophysiology. 1998;79:1017–1044. doi: 10.1152/jn.1998.79.2.1017. [DOI] [PubMed] [Google Scholar]