Abstract
“Orthobiologics” represents an important category of therapeutics for the regeneration of bone defects caused by injuries or diseases, and bone growth factors are a particularly rapidly growing sub-category. Clinical application of bone growth factors has accelerated in the last two decades with the introduction of BMPs into clinical bone repair. Optimal use of growth factor-mediated treatments heavily relies on controlled delivery, which can substantially influence the local growth factor dose, release kinetics, and biological activity. The characteristics of the surrounding environment, or “context”, during delivery can dictate growth factor loading efficiency, release and biological activity. This review discusses the influence of the surrounding environment on therapeutic delivery of bone growth factors. We specifically focus on pathophysiological components, including soluble components and cells, and how they can actively influence the therapeutic delivery and perhaps efficacy of bone growth factors.
Keywords: Orthobiologics, Growth factors, Pathophysiological microenvironment, Growth factor delivery, Bone regeneration
1. Introduction
Regeneration of injured or diseased bone tissue represents a tremendous clinical need. With an estimated number of over 1 million fractures each year in the United States at a cost of $10 billion; the field of bone tissue engineering is aiming at developing new technologies with the goal of meeting the clinical need [1]. Therapeutic strategies often rely on the delivery of “orthobiologics”, which include bone growth factors (GFs), small molecules, pro-osteogenic cell types, and polynucleotides (e.g. RNA, DNA). As an emerging class of therapeutic agents, orthobiologics have generated a high level of interest for clinical orthopedic applications [2–4]. In this review we focus on bone growth factors, a subset of orthobiologics, as they have been widely explored in controlled release applications and have a significant recent history in clinical applications. This class of orthobiologics emerged after Urist and co-workers first demonstrated the potential of demineralized bone matrix (DBM) to induce ectopic bone formation in animal muscle pouches [5]. In subsequent studies, the investigators identified a family of bone morphogenetic proteins (BMPs), members of the transforming growth factor-β (TGF-β) superfamily, as the bone growth factors present in this matrix and responsible for inducing bone formation.
1.1. Importance of biologics delivery in orthopedic and related applications
Clinical translation of BMPs for orthopedic applications has progressed substantially over the past 30 years [2–4]. There are an extensive number of preclinical studies that have demonstrated the effectiveness of applying BMPs, and have led to the clinical introduction of rhBMP-2 and rhBMP-7 within absorbable collagen sponges for spinal fusion, open tibial fractures and oral maxillofacial applications since 2002. Specific examples include Medtronic’s INFUSE® product (rhBMP-2 in collagen sponge) (Fig. 1) and Stryker’s OP-1 product (rhBMP-7 in collagen sponge). Several clinical studies demonstrate the pro-osteogenic capabilities of these BMPs. For example, the use of INFUSE® in lumbar spine fusion resulted in higher fusion rates, with results that were superior to autologous bone grafts (Fig. 1-D) [6,7]. During this span, rhBMP-7 (in Stryker’s “OP-1” product) and rhBMP-2 (in Medtronic’s INFUSE® product) represented the most extensively investigated molecules [8–10]. Common bone defect sites where the use of rhBMPs has improved healing in clinical orthopedic applications include diaphyseal tibial nonunions [4], open fractures [11], and lumbar spine fusions [2,4,8,9, 12,13].
Fig. 1.
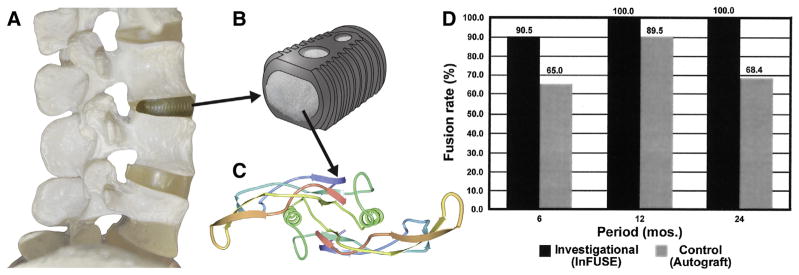
Application of InFUSE™ bone grafts: A) Implantation of a lumbar interbody fusion device for spinal fusion B) a lumbar interbody tapered fusion cage representation of the InFUSE™ bone graft from Medtronic C) Crystal structure of BMP-2 (modified from www.pdb.org) D) Comparison of postoperative fusion outcomes in the investigational group (InFUSE™ Bone Graft) and the control group (iliac crest autograft) [6]. Reproduced with permission.
1.2. Purpose and scope of review
Several approaches have demonstrated an ability to control bone growth factor delivery in vitro. However, the characteristics of the in vivo environment can substantially influence the delivery kinetics as well as the efficacy of a bone growth factor delivery strategy. The surrounding environment has a significant impact on the ability to load, release and maintain the biological activity of bone growth factors. Ideal delivery systems would not only maintain local biological activity of bone growth factors, but also deliver growth factors in response to physiological requirements, having the capacity to sense changes in the tissue environment and alter protein release accordingly. This review focuses on describing the influence of the surrounding environment on therapeutic delivery of bone growth factors. We introduce the promise and challenges of orthobiologics delivery, then review the characteristics of the in vivo environments that receive bone growth factors. Finally, we describe the impact of dynamic in vivo environments on bone growth factor delivery, and discuss how the environmental “context” may influence the efficacy of bone growth factors and other emerging orthobiologics.
2. Bone growth factors as orthobiologics
Since the original discovery of BMPs, several growth factors have shown potential for bone regeneration. These include multiple members of the TGFβ superfamily, insulin like growth factor 1 (IGF-1), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs) and vascular endothelial growth factor (VEGF). Interest in these growth factors is supported by biological studies of fracture healing [14]. Specifically, fracture examination during the different phases of defect healing has identified the required presence of TGF-β superfamily members, IGF-1, FGFs, PDGFs, and VEGF for proper new bone formation to occur [8,14,15]. Their presence at controlled concentration, timing, and spatial location within defect site suggests they serve an essential role in the fracture healing mechanism [16]. The generalized biological mechanism of bone growth factor-mediated defect repair begins with their secretion from resident cells as an injury response within the fracture. The secreted growth factor can then bind to the cognate receptor on a resident precursor cell. This results in the activation of the intracellular kinase domain, followed by a phosphorylation cascade that ends with translocation of second messenger to the nucleus, where a key transcription factor activation upregulates the expression of pro-osteogenic genes [10,14].
The details of the bone growth factor signal-transduction mechanisms that relate to osteogenesis are an actively studied area, and have been reviewed elsewhere [10,14]. Briefly, pro-osteogenic BMP signal-transduction occurs via the type II serine-threonine kinase family of TGF-β receptors, and the associated downstream SMAD signaling. The lack of tissue specificity of the BMP ligand–receptor interaction, susceptibility of the growth factor to denaturation and proteolytic degradation, and the need to maintain the appropriate therapeutic concentration result in a series of therapeutic delivery challenges. While BMP delivery provides the most well characterized example of the promise and complexity of bone growth factors, it is not a unique example. Other bone growth factors are similarly capable of inducing bone formation, but suffer poor in vivo stability as well as the potential for significant side effects, suggesting a general need to control bone growth factor dosages and delivery kinetics. As a result, many recent studies have focused on developing localized, sustained release systems capable of controlling bone growth factor delivery to bone defect sites [17,18].
2.1. Unique challenges in delivery of bone growth factors
The concept of appropriate local and sustained dosages is particularly challenging for growth factor orthobiologics. Not only do growth factors need to reach the intended site of interest with maintained bioactivity and at an efficacious concentration, but the delivery mechanism must also be appropriate for the growth factor’s therapeutic index. The therapeutic index is defined as the ratio of the maximal nontoxic system concentration to the minimal does that elicits a positive therapeutic response [19]. Unfortunately, for biologically active compounds such as rhBMP-2 this index can be quite small [20,21]. This is due in part to rhBMP-2’s poor solubility, short biological half-life, and rapid local clearance under physiological conditions in vivo. As a result, large BMP dosages are required. Indeed, there is more exogenous BMP-2 in a single dose of the INFUSE® product than would be present in 1000 human bone defects, raising concerns about safety and cost [22]. High rhBMP-2 dosages have recently been directly associated with serious side effects in clinical studies (including heterotopic bone formation [23,24], edema [25,26], and retrograde ejaculation [27]). In essence, the delivery of bone growth factors poses unique drug delivery challenges due to the high doses required due in part to poor pharmacokinetics, the low doses required to limit off-target effects, and the narrow therapeutic range of efficacy at the actual defect site. Therefore, controlled local delivery is an important property that biomaterial-mediated delivery of growth factors can offer [28].
3. Dynamic microenvironments during bone growth factor delivery
The microenvironment is a highly diverse and complex milieu composed of cells, extracellular matrix (ECM), and soluble factors. The crosstalk between these components provides the surrounding cells with regulatory signals which lead to the development of desirable cells, tissues and organs. More importantly, the microenvironment for resident cells is a highly dynamic niche, in which biophysical and biochemical cues are presented to the cells in a dynamic fashion [29]. The dynamic changes in the ECM can not only influence the immobilization and liberation of affiliated growth factors, but also modulate the physiochemical properties of the local microenvironment, which can further affect growth factor activity [30]. Although cells do not tend to directly affect growth factor release, their interaction with the ECM under certain physiological and pathological conditions could influence growth factor delivery. This effect has been clearly demonstrated by the action of many cell types such as platelets, macrophages and osteoclasts during bone healing. Furthermore, a variety of environmental factors such as pH, drug carrier, mechanical stimuli and serum also tend to change dynamically during the delivery of bone growth factors [31]. Table 1 summarizes the dynamics of the microenvironment during bone healing.
Table 1.
Summary of various dynamic factors influencing growth factor delivery in pathophysiological contexts.
| Factor | Components | Influence on GFs delivery | Reference |
|---|---|---|---|
| ECMs | Proteoglycans, fibrin, collagen, fibronectin. | Directly binds to GFs via affinity interactions; augment GFs activity through synergistic effect with ECM; cooperatively work with integrins for activation signaling pathways | [33,45–47,57,58] |
| pH | Natural environment, engineering environment, pH responsive delivery systems | Change GFs release kinetics by changing carrier degradation; influence GFs bioactivity (protein aggregates) | [77,78,97] |
| GF carrier | Carrier materials, carrier degradation, enzyme-mediated degradation | Primary mechanism to control GF release rate; immobilize GF on carriers; cause damage to GFs due to the degradation byproducts | [85,105,111,116] |
| Surrounding cells | Macrophages, platelets, osteoclasts | Clearance of GF carriers via phagocytosis; formation of hematoma; participate in carrier degradation and resorption | [48,146,155,159,163] |
| Biomechanics | Mechanical stimuli to tissues and GF carrier systems | Mechanical loading increases GF release during healing; deformation of GF carrier by pressing causes GF release | [167,169,171] |
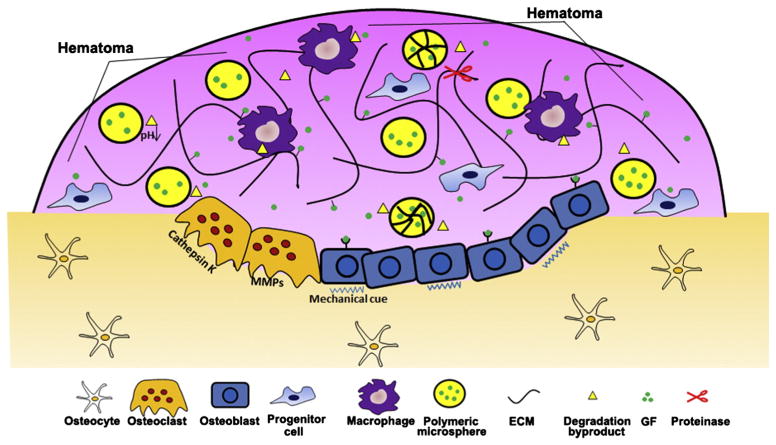
Bone healing is a tightly regulated process accompanied by a series of physicochemical and biological events which can influence growth factor delivery [32]. During bone tissue formation, regular inflammatory cells, including macrophages and neutrophils, are involved in creating the local environment for GF delivery. In addition, bone specific cells including osteoblasts, osteoclasts, and osteocytes actively regulate binding and release of GFs from ECM and other drug carriers [33]. For example, GFs embedded within bone matrix are liberated during osteoclast-mediated bone remodeling [34]. Growth factor bioactivity is also closely associated with local ECM components present during bone healing. For instance, basic FGF (bFGF) sequestering on fibrin fibers within a fracture hematoma stabilized its bioactivity, and augmented its impact on bone healing [35]. Therefore, a variety of environmental factors during bone healing influence the delivery of GFs in multiple ways including GF binding/release, activity (i.e. protein stabilization), and synergistic effects between GFs and the ECM, and all these factors can affect the eventual outcomes of bone healing. While the impact of the dynamic microenvironment on regulation of cell behavior has been extensively reviewed elsewhere [36,37], how it influences the delivery of growth factors has not been systematically reviewed. In view of the importance of bone growth factor delivery, several aspects of the dynamic microenvironment that influence growth factor release and efficacy (Fig. 2) are discussed in the following sections.
Fig. 2.
Various aspects of dynamic microenvironment can influence the delivery of bone growth factor during bone healing. Osteoblasts and osteoclast directly participate in the catabolic and anabolic processes of bone formation. The carrier of the delivery system can directly control the release kinetics of the growth factors. pH at the bone defect sites can affect growth factor release by changing the degradation rate of the carrier. The presentation of growth factors to target cells is also modulated by ECM while ECM components is consistently remodeled by inflammatory and other types of cells via secretion of proteases such as MMPs. Besides, mechanical stimulus also play an importantly during the whole bone regeneration process.
3.1. The extracellular matrix
The ECM is an assembled network composed of structural and functional components secreted and deposited by cells, and providing mechanical strength, cell adhesive ligands and growth factor sequestering ligands [38]. The interactions between the ECM, cells and growth factors result in mutual influence and dynamic reciprocity. The ECM can serve as a storage reservoir through its direct interaction with a growth factor. It can also indirectly influence growth factor activity by controlling cell adhesion via the presentation of adhesive ligands [39]. Additionally, the abnormalities caused by various physiological or pathological conditions can alter the interaction between the ECM and growth factors, thereby altering the course of tissue healing [40,41]. The impact of the ECM as an environmental factor on bone growth factor delivery is highlighted in the following sections.
3.1.1. Direct interaction between growth factors and the ECM
The ECM can locally sequester and release growth factors, and therefore serves as a repository. Many ECM components contain growth factor binding sites with high affinity for certain types of growth factors, and are able to bind to these molecules through non-covalent interactions (e.g. H-bonds, Van der Waals interactions) [33]. For example, proteoglycans (PGs), possess extraordinary capacity to bind to a variety of growth factors. PGs harboring glycosaminoglycan (GAGs) such as heparin and heparan sulfate contain high concentrations of anionic sulfate and glucuronic acid, which enable them to non-covalently bind to cationic heparin-binding domains on growth factors [42]. The sequestering of growth factors by the ECM can localize the growth factor and protect a bound growth factor from degradation. For example, it was found that bFGF could only act as mitogen when bound to heparan sulfate at the cell surface [43]. The bound bFGF also stimulated plasminogen activator production by endothelial cells in a more sustained manner over time when compared to free bFGF [44]. ECM proteins such as fibrin, collagen, fibronectin and vitronectin can also bind to a number of growth factors either indirectly via their heparin-binding domains or more directly via their growth factor-binding domains [45–47]. Fibrin is of particular interest for orthobiologics delivery, as it not only provides a scaffold for cell infiltration, but can also concentrate growth factors at wound sites during the inflammatory phase of healing. A set of pro-angiogenic factors such as bFGF, VEGF and interleukin-1 (IL-1) was sequestered in fibrin and used to induce endothelial cell proliferation, which ultimately led to angiogenesis [35,48]. Collagens, the most abundant class of ECM proteins in tissues, can also bind to a variety of growth factors such as VEGF, PDGF, and TGF-β1 [49]. Reports have found that PDGFs and TGF-β1 could retain their activity when bound on collagen IV [50,51].
In contrast, certain ECM components can also down-regulate growth factor activity [52]. For instance, the binding between TGF-β and decorin can keep TGF-β away from its cognate receptor and decrease its bioavailability during development and regeneration [52]. Another ECM component, SPARC (secreted protein, acidic, and rich in cysteine), was also shown to impair the mitogenic effect of PDGF-AA and -BB on human smooth muscle cells upon binding. Some inhibitory effects of ECM proteins are dependent on the context in which GFs are sequestered. For example, collagen has the ability to retain activity of GFs bound on its surface, but collagen also reduced the biological activity of VEGF when endothelial cells were adhered to collagen I [53]. A similar inhibitory result was found on other ECM components such as heparan sulfate and chondroitin sulfate [54,55]. Together, these results have demonstrated that certain types of ECM can inhibit the activity of certain growth factors. This may have particular relevance when choosing a carrier for delivery of bone GFs, as an ECM-derived carrier with inhibitory capabilities would decrease their bioactivity.
3.1.2. Indirect interactions between growth factors and the ECM
The ECM can indirectly regulate the cellular response to growth factors via signaling through a variety of cellular adhesion ligands [56]. This is mainly achieved via supramolecular complexes of integrin receptors and growth factor receptors (GFR), initiated by presentation of cellular adhesion ligands to the cells [57,58]. Thus, the integrin binding domains on ECM proteins such as fibronectin, vitronectin, laminin and collagen not only present as cellular adhesion ligands, but also actively participate in growth factor signaling. For example, VEGF dependent endothelial cell proliferation was significantly faster on substrates coated with fibronectin when compared to bovine serum albumin (BSA) coated substrates. This was mainly attributed to the activation of VEGFR-2 through binding to integrin subunit β3, which was elevated by the presence of cellular adhesive ligands [59]. A similar mechanism has been reported for multiple growth factors including VEGF, EGF and TGF-β1 [60–62]. The synergistic cooperation between integrins and GFRs can sensitize the GFR, which may allow for activation of signal transduction with a lower concentration of the growth factor. Therefore, this synergy may be useful to potentiate the growth factor activity. For example, the cooperation of αvβ3 integrin together with TGF-β1 remarkably enhanced fibroblast proliferation, while blocking of αvβ3 integrin abolished the synergistic effect. Interestingly, αvβ3 integrin expression was co-localized with TGFβIIR under TGF-β1 exposure, indicating that these receptors clustered during signal transduction [63]. Similarly, the biological activity of EGF and TGF-β1 was also enhanced when their corresponding receptor was influenced by ECM components such as collagen, fibronectin and vitronectin [61,64]. Furthermore, the impact of cell adhesion on growth factor signaling influenced cell motility. Griffith and co-workers reported that various aspects of fibroblast migration, such as locomotion speed, membrane extension and retraction activity were actively regulated by either fibronectin or nanoscale RGD clustering under EGF stimulation [65,66]. Taken together, these studies highlight the importance of cell-ECM adhesion as a regulator of GF signaling; and may provide further information to instruct design of GF carriers in orthopedic applications.
3.1.3. ECM under various conditions
ECM-growth factor interactions can be disrupted by the abnormalities of ECM caused by various physiological and pathological conditions [67]. The ECM composition, stiffness, and pH are influenced by disease, trauma, and aging, and changes in the ECM affect the response of cells to growth factors. Fracture healing provides an illustrative example, in which a hematoma rich in fibrin is formed immediately after the disruption of tissue and blood vessel integration [68]. The fibrin based hematoma is critical for bone healing, partly due to its ability to bind to multiple growth factors involved in bone regeneration [69,70]. In contrast, the absence of a fibrin clot during chronic wound healing severely interrupts the process of tissue regeneration and leads to formation of non-healing wounds [71]. The ECM abnormalities of non-healing wounds can also include protease-inhibitor imbalance, high local pH and excessive scar formation which could affect growth factor activity locally [72]. For example, PDGF activation triggered by cell adhesion to fibronectin has been interrupted by high local pH at wound sites [73, 74]. Excess level of matrix metalloproteinases (MMPs) has caused loss of ECM, leading to the lack of adhesive sites for cell anchorage [40]. Cancer can also substantially change the ECM stiffness, pH value and composition, and influence the cellular response to growth factors [75]. For instance, Asthagiri et al. reported that matrix stiffing significantly sensitized cells to EGF and resulted in the loss of contact inhibition during proliferation [76].
3.2. pH
Microenvironmental pH has been considered an important factor influencing a variety of aspects of growth factor delivery, including release kinetics, and stability [77,78]. Most growth factor delivery studies are performed at near physiological pH, which is 7.35. However, the local pH at delivery sites in vivo can drastically change due to various physiological and pathological conditions [79–81]. For example, the acidic milieu created by osteoclasts can result in a local pH of 4.50 during bone remodeling [82]. Such pH changes could have a significant impact on both the carrier material and the growth factors to be delivered. Various reports have shown that the degradation rate of many carrier materials, including polyesters, calcium phosphates, and natural polymers was a function of solution pH [83–85]. Since carrier degradation is often directly correlated to GF release kinetics, the pH could substantially affect a GF release strategy. Proteins like GFs are often highly sensitive to pH variation due to their structural fragility. Exposure of proteins to extreme pH could be deleterious to their bioactivity, which could further affect the biological activity of GF delivery [86,87]. Here, we discuss the impact of pH on growth factor delivery from both natural and synthetic carriers. We also briefly discuss pH responsive drug delivery systems, as an example of a physiologically responsive “smart” drug delivery strategy.
3.2.1. Impact of pH on orthobiologics delivery in natural environments
Although the pH of natural environments is usually maintained in the range of 7.35, many disease or injury conditions may substantially change this value. Inflammation of tissues is often associated with acidic pH caused by the accumulation of metabolic acid generated during inflammatory cell activation [80]. The low local pH observed in a fracture hematoma originates from similar sources, as inflammation is an early event after a fracture takes place [68]. Extremely low pH in natural microenvironments is also found near the milieu where osteoclasts are actively involved in bone remodeling. This acidic pH (~pH 4.5) is generated by secretion of hydrochloric acid (HCl) by osteoclasts [82]. The low pH environment created by osteoclasts may be of particular relevance to calcium phosphate, calcium sulfate, and calcium carbonate based growth factor delivery systems, as these minerals degrade faster in acidic pH [88]. Liu et al. identified that osteoclast-mediated degradation of calcium phosphate carriers as one of the major GF release mechanisms. Specifically, the release of BMP-2 from calcium phosphate coated implants was closely associated with local osteoclast activity [34,89]. On the other hand, pathological environments such as chronic wounds can cause pH increases up to 8.0, which affects the activity of MMPs [41]. In turn, the presence of active MMPs can degrade the growth factors delivered to the wounds and inhibit wound healing [90]. Local pH changes can also affect the growth factor binding to ECM components. For example, Nugent et al. found that acidic pH could increase the binding of VEGF to endothelial cells and fibronectin [91]. Therefore, variation of pH in natural environments can affect the delivery of orthobiologics in multiple ways suggesting that design of delivery systems must fit the “microclimate” pH of the intended delivery site.
3.2.2. Impact of pH on drug delivery in synthetic microenvironment
Synthetic microenvironments within implants and drug carriers also affect growth factor release and bioactivity. pH changes in synthetic microenvironments are often caused by polymer degradation [92]. For instance, Schwendeman and colleagues have identified that an acidic microenvironment was one of the most important sources of irreversible inactivation of proteins encapsulated in polymeric carriers [93–97]. A study by Fu et al. showed that the minimum microenvironmental pH within poly(lactic-co-glycolic acid) (PLGA) microspheres could be as low as 1.50 [87]. Such strong acidity can cause protein unfolding which eventually led to protein aggregation. The low pH also promoted peptide bond hydrolysis, further disrupting bioactivity of the proteins [86,98]. Modulation of local pH within controlled release systems has become an attractive strategy to improve the stability of protein during delivery. For example, Zhu et al. successfully neutralized the acidity from polymer degradation byproducts by incorporating poorly soluble basic salts into the delivery devices. This method successfully prevented degradation of proteins such as BSA, bFGF and BMP-2 for up to one month [97]. In another approach, the use of bioresorbable polyphosphazenes eliminated the generation of acidic byproducts during degradation [99]. Interestingly, the acidic microenvironment could also be leveraged to stabilize proteins that are unstable at neutral pH. Camptothecin, an anticancer drug that undergoes rapid hydrolysis at pH = 7.40, was stabilized by encapsulating into PLGA microspheres due to the low pH generated during the degradation of the microspheres [100].
3.2.3. pH-responsive drug delivery systems
The variation of local pH in physiological environments has inspired the development of a series of environmental responsive drug delivery systems, which has been comprehensively summarized elsewhere [101–105]. Here we will focus on their application in the field of orthopedics. Akashi et al. developed a poly (γ-glutamic acid)-sulfonate matrix for pH-controlled release of bFGF in which bFGF release was successfully controlled by switching pH between 7.40 and acidic values [106]. Researchers have also designed stimulus responsive delivery systems which could respond to the pH of the wound sites and trigger release of therapeutic agents such as VEGF and EGF [91,104]. For example, Maiti et al. reported that the environmental pH within a healing wound initiated release of VEGF and bFGF encapsulated in a poly (NIPAAm-co-AAc) hydrogel without loss of bioactivity [107]. More recently, Farokhzad et al. developed a novel strategy leveraging the pH at the site of an infection to target antibiotic delivery to the bacteria cell wall. Vancomycin loaded in PLGA-PLH-PEG nanoparticles could rapidly bind to both Gram-positive and Gram-negative bacteria through the selective protonation of the imidazole groups of PLH at acidic pH [108]. Together, these results demonstrated that variation of pH can be successfully incorporated into the design of drug delivery system and provide stimuli-responsive release of therapeutics.
3.3. Growth factor carriers
3.3.1. Drug/carrier interaction
The interactions between cargo proteins and their carriers are major factors to control protein release. Interactions of GFs with the ECM and ECM-derived molecules have been reviewed in previous sections, so here we focus on synthetic growth factor carriers. Growth factors are typically incorporated into carriers through two main strategies: physical adsorption and covalent immobilization. Growth factors can physically adsorb to polymeric carrier surfaces via electrostatic interactions, such as hydrogen bonds between the functional groups on the protein and the carrier [85]. One study showed that insulin stability encapsulated in PLGA microspheres was partially determined by an acylation reaction during the attachment of lactic acid/glycolic acid to the amine groups of insulin [109]. The level of deamidation, acylation and proteolysis of a protein was also influenced after insulin binding to PLGA films [110]. Bone growth factors have also been covalently immobilized onto their carriers through selective chemical coupling [105,111]. This strong interaction can slow down GF degradation or internalization as well as prolong the release from a carrier. In one study, EGF covalently tethered on a solid substrate retained its biological activity while physically adsorbed EGF on the same substrate showed no activity [112]. However, the bioactivity of growth factor incorporated through this approach might be compromised due to the structural damage to the protein caused by inappropriate coupling site selections [33].
3.3.2. Carrier degradation
Carrier degradation has been regarded as a primary mechanism dictating drug release in various bioresorbable carriers. The growth factors incorporated into synthetic polymeric carriers have been released mainly through a combined diffusion/degradation mechanism influenced by hydrolysis, which is a process of cleavage of chemical bond by the presence of water [85]. For example, the release kinetics of BMP-2 from bioresorbable scaffolds was closely associated with the degradation and erosion of the scaffolds [113,114]. Zheng et al. also reported that the amount of released BMP-2 was proportional to the loss of polymer mass during release in vitro [115]. The degradation of polymer was classified into surface (or heterogeneous) erosion and bulk (or homogeneous) erosion [116], and these two different degradation mechanisms led to distinct growth factor release kinetics [117,118]. The surface erosion rate is determined by the surface area of the carrier, which may provide a simple route to control GF release kinetics [119]. However, most bioresorbable polymers such as PLA, PLGA and PCL undergo bulk erosion with distinct degradation kinetics. In addition, a series of polymer properties such as molecular weight [120,121], crystallinity [121], and hydrophobicity [122] can affect the degradation of the polymeric carriers and in turn, affect the release rate of the growth factors. Growth factor release also heavily relies on the fabrication parameters of the carrier, because they are also closely related to the carrier degradation rate. For example, the pore size of PLG scaffolds has considerably affected the release of proteins by changing the diffusion coefficient of proteins releasing from the scaffolds [92]. A similar effect of fabrication parameters on growth factors was also observed in other formats, including microspheres [123], hydrogels [124], nanoparticles [125] and nanofibers [126]. It is noteworthy that degradation byproducts are another important factor that can influence growth factor release. In an example noted above, the degradation byproduct of poly(α-hydroxyl esters) (e.g. PLGA, PLLA and PGA) pose a threat to growth factor, due to their high acidity [93,97]. The low local pH has also catalyzed hydrolysis, which caused heterogeneous erosion inside the carrier and altered the designed growth factor release profiles [127].
Another important degradation mechanism is enzyme mediated carrier degradation which involves the use of enzyme-cleavable biopolymers. This concept has been elegantly demonstrated in studies by Hubbell et al. [128–130], in which a growth factor was fused with a factor XIIIa substrate sequence NQEQVSPL through its N-terminus and incorporated into a fibrin gel during coagulation. The release of growth factor was then controlled by the enzymatic degradation of the fibrin gel by invading cells resulting in a “cell-demanded” release mechanism coordinated with tissue growth at the desirable sites [128,130]. The technique has been successfully applied to regenerate blood vessels, bone, and neurons. A similar approach was applied to a synthetic MMP-sensitive hydrogel in which protein was liberated due to the proteolytic degradation during cell invasion into the hydrogels [131,132].
3.3.3. Minerals coatings as GF carriers
Calcium phosphate (CaP) mineral, as the major inorganic component of bone tissue, has been used for decades in orthopedics to achieve a rapid fixation and improved bone-implant bonding due to its excellent biocompatibility and osteoconductivity [133]. More recently, it has also been identified to be a versatile growth factor carrier, owing to its ability to bind to proteins via charge–charge interactions between ions and protein side chains [134–136], and dissolve at controllable rates into non-toxic, physiological mineral ions. Through the last several years, we and others have developed a series of strategies to control the growth factor release by modulating the intrinsic properties of CaP minerals, including the dissolution/reprecipitation. Here we highlight the recent progress regarding these technologies and their application in orthopedics.
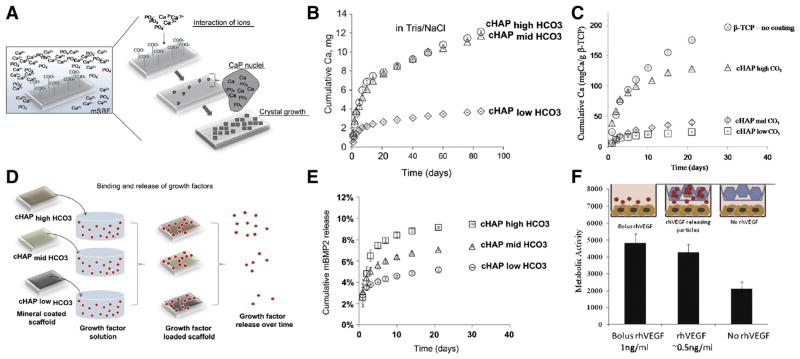
Mineral degradation (dissolution) is the primary mechanism for release of growth factors trapped/bound to mineral coated orthopedic implants [89], thus we have developed approaches to tailor the degradation of mineral coatings. Our approach involves forming various mineral coatings via a nucleation and growth process in a modified simulated body fluid (mSBF) (Fig. 3-A) [137]. Importantly, the characteristics of the mSBF can be systematically varied, and can dictate the properties of the mineral coatings, including morphology, composition, and dissolution. The systematic variation of these properties can be achieved without changing the nature of the degradation byproducts (principally Ca2+, ). In addition, mineral nucleation and growth can be achieved on virtually any underlying material. Thus, nucleation/growth of mineral coatings provides an extraordinarily adaptable approach for GF delivery on a wide variety of medical devices. For instance, by changing the carbonate concentration in mSBFs, the nanoscale morphology, composition and dissolution rate of these coatings were precisely tailored. Higher carbonate concentration led to faster dissolution of mineral coating (Fig. 3-B) which could be attributed to the substitution of by in the lattice structure of hydroxyapatite (HA) and the associated change in the crystallinity of the mineral [137–141]. A similar approach was used to modulate the degradation properties of CaP materials such as β-tricalcium phosphate (β-TCP) (Fig. 3-C) [138]. Importantly, release kinetics of growth factors in each of these studies was strongly dependent on the solubility of the mineral coatings (Fig. 3-D). Specifically, when the carbonate concentration was increased from low to high, the release of a VEGF-mimetic peptide was increased from 10% to 45% during a 30 day release study (Fig. 3-E) [140]. Similar results were also observed with other growth factors, including BMP-2, TGF-β and bFGF [138,142]. Furthermore, the released growth factors showed no significant loss of their bioactivity in multiple biological assays (Fig. 3-F) [138]. Controllable mineral coatings have been formed on various orthopedic implants including sutures, scaffolds, screws, plates, and microspheres, which combines the clinical advantages of these devices with the controllable growth factor delivery capability of the mineral coatings [137,140,143].
Fig. 3.
Controlled release of growth factors via mineral coated based delivery systems. A) Schematic of mineral coating formation process using mSBF. B) Mineral coating stability was affected by the extent of carbonate substitution in mineral coating during coating formation. C) Formation of mineral coating on β-TCP surface helped slow down the dissolution of the materials. D) Schematic of release and binding mechanism of growth factors to mineral coating. With higher carbonate concentration, the amount of growth factor released is greater over time. E) The release kinetics of VEGF mimic was controlled by the dissolution rate of mineral coating. F) VEGF bound on mineral coating fully preserved its bioactivity [138,140]. Reproduced with permission.
Another set of studies extended the control over coating degradation further by incorporation of fluoride [142]. Fluoride incorporation in the coating not only changed the nanostructure of coatings from plate-like to needle-like, but also significantly slowed down the dissolution and the release of BMP-2 (Fig. 4-A) [144]. By combining carbonate and fluoride incorporation into one coating system, we developed a multilayered microparticle platform for tunable delivery of multiple GFs. The release of BMP-2 and VEGF bound on different mineral coating layers was independently controlled by both the position and solubility of the coating. For example, one tailored microparticle formulation released up to 72% of VEGF but only 12% of BMP-2 within the same time frame (Fig. 4-B). Variation of the outer coating thickness was found to be another efficient way to modify BMP-2 release kinetics (Fig. 4-C) [142].
Fig. 4.
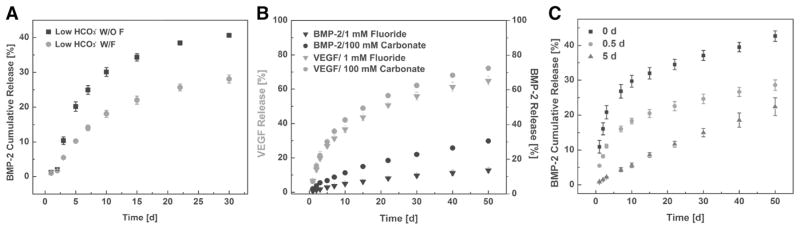
Multiple GF release via multilayer mineral coating with tuned stability: A) Incorporation of fluoride into mineral coating decelerated the dissolution of mineral coating. B) Dual release of BMP-2/VEGF was achieved by using multilayered microparicles. BMP-2 was released in a sustained manner while VEGF was liberated from mineral coating relatively faster. C) Control over BMP-2 release by changing the coating thickness on the microparticles [142]. Reproduced with permission.
3.4. Cells present in microenvironments during bone growth factor release
3.4.1. Macrophages
Macrophages are differentiated cells from the mononuclear phagocyte system that play an important role in host response to foreign bodies [145]. Almost all biomaterial implants can cause a macrophage response, regardless of their composition, dimension, and shape. Once the implants are recognized by the body’s immune system, macrophages conduct a series of actions to clear these foreign items via different cellular mechanisms, which could significantly affect the delivery of growth factor loaded on/into these implants. For example, micro/nano particles loaded with growth factors have often been cleared by macrophage via phagocytosis in a short time when these particles were injected to injury sites such as bone fractures [146]. The rapid removal of drug-releasing particles has been a significant obstacle for particulate controlled release systems [147]. To address this challenge, novel strategies such as PEGylation of the particles have been developed to mitigate phagocytosis via macrophages [148]. Recently, Zhang et al. remarkably increased the circulation lifetime of nanoparticles in vivo by disguising the particle surface with a layer of erythrocyte membrane. This biomimetic decoration of nanoparticles successfully “confused” macrophages and protected the particles from phagocytosis [149]. Macrophages are also actively involved in biodegradation of polymers, which can drastically change the release profiles of growth factors from these materials in vivo [150]. Macrophages can release reactive oxygen intermediates, enzymes and acid that serve as catalysts to accelerate implant degradation, which in turn may affect the release of growth factors loaded in these implants [151]. For instance, MMP-9 expression was found to be elevated in adherent foreign body giant cells (FBGCs) which may influence the degradation rate of natural polymer based carriers such as collagens and fibrin [152]. Clover et al. reported host mediated degradation of collagen scaffolds was mainly executed by macrophages, and that crosslinking of the collagen scaffolds increased the resistance to macrophage-mediated degradation [153].
3.4.2. Platelets
Platelets circulating in blood can be rapidly activated by external stresses such as blood vessel rupture, and quickly stop bleeding by coagulation and elaboration of a blood clot at a wound site [154]. The resulting hematoma is mainly composed of a fibrin rich ECM, which plays two major roles during wound healing: 1) it provides a template for cell migration and 2) it serves as a provisional reservoir for binding of cytokines and growth factors [68]. Platelet-triggered hematoma formation may influence growth factor delivery strategies in a series of ways. First, the presentation of delivered growth factors to target cells is realized by recruitment of the target cells into the hematoma via a series of inflammatory cells [155]. On the other hand, the released growth factor is typically able to bind to ECM proteins (e.g. fibrin) in the hematoma, which can extend GF half-life and increase bioactivity [43]. For example, released bFGF has been shown to be retained on fibrin and subsequently liberated by plasmin during fibrin degradation [48]. Further, degranulating platelets can release multiple growth factors, including EGF, bFGF, PDGF and TGF-β. These factors can either act alone or synergistically cooperate with exogenous delivered growth factors to accelerate tissue repair and regeneration [156].
3.4.3. Osteoclasts
Osteoclasts are multinucleated cells differentiated from monocyte/macrophage precursor cells surrounding the bone surface. The major function of osteoclasts is to conduct bone resorption, which is a process of bone matrix degradation during bone remodeling [157,158]. Briefly, premature osteoclasts are activated by two key signals macrophage colony-stimulating factor (M-CSF) and receptor activator of NFκB ligand (RANKL), and undergo polarization after adhering to bone surface. Mature osteoclasts generate a ruffied border that forms into a sealed compartment between the bone surface and the basal membrane of osteoclasts. Matrix degradation mediators such as hydrochloric acid and proteinases are then released into the so called “vacuole” and initiate bone resorption [158]. Osteoclasts not only play an important role in native bone remodeling, but also actively participates in implanted biomaterials degradation. The secretion of acid by osteoclasts can dissolve calcium phosphate mineral crystals, thereby influencing degradation of mineral-based biomaterials [159]. In particular, resorptive lacuna have been documented on HA, β-TCP, and biphasic calcium phosphate [160]. Importantly, osteoclast mediated resorption presents an important mechanism for growth factor liberation from mineral-based materials [89,161]. Wernike et al. showed that the presence of osteoclast-like cells sustained the long term release of protein when compared to passive diffusion, suggesting the prolonged release of protein was attributed to cell-mediated resorption [162]. Further, enzymes released by osteoclasts can degrade ECM-derived polymer, which could also influence growth factor release from polymer-based drug carriers. For example, cathepsin K, an important proteinase secreted by osteoclasts, has been shown to degrade type I and II collagens [163] as well as PEG hydrogel cross-linked by a cathepsin K degradable peptide sequence [164]. Notably, while the cell types discussed in this section do not encompass all the cell types present during GF delivery, the studies we highlighted here provide evidence of common mechanisms by which cells can influence GFs and their carrier materials.
3.5. Biomechanics
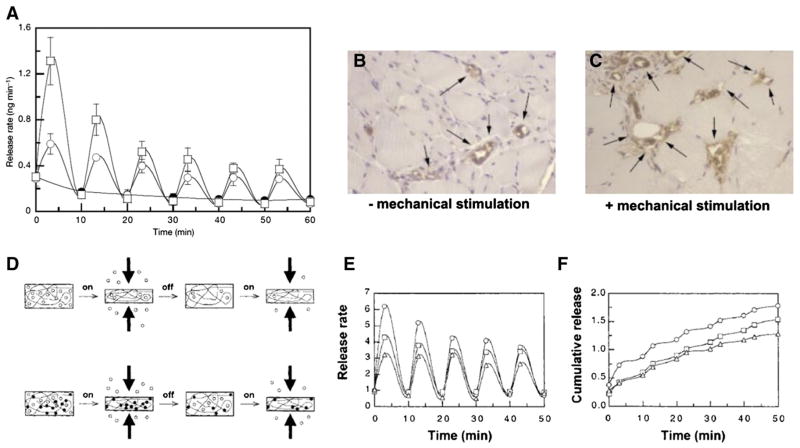
Most tissues in the human body experience a variety of mechanical stimuli, such as compression in bone and cartilage, stretching in muscle, tension in tendon and shear force in blood vessels. The existence of a mechanically dynamic environment can profoundly influence various biological processes involving both resident cells and delivered growth factors during tissue development and metabolism [165]. For example, mechanical stimuli can be converted into biochemical signals and regulate cell behaviors [166]. In one illustrative example, Little et al. reported that cyclic stretch to smooth muscle cells stimulated bFGF release and promoted the growth of the cells [167]. In other examples, mechanical stimulation to fracture hematoma has been shown to enhance secretion of VEGF and improve angiogenesis during fracture healing [168]. In view of the importance of mechanical stimuli in the body, drug delivery systems responsive to these stimuli have been designed. Mooney et al. recapitulated aspects of the mechanically dynamic environment in vivo by providing mechanical stimulation to growth factor loaded alginate hydrogels. Deformation of the hydrogel led to up to a five times greater VEGF release rate when compared to static conditions. Importantly, the increase of VEGF release promoted blood vessel formation in both severe combined immunodeficiency (SCID) and non-obese diabetic (NOD) mice (Fig. 5-A–C) [169]. They also identified that greater interactions between VEGF and the hydrogel could affect drug release under mechanical stimuli, as the release of bound VEGF was more strongly influenced by a mechanical stimulus (Fig. 5-D–F) [170]. Wang et al. reported that release of BSA encapsulated in PLGA microspheres was accelerated under mechanical loading when the microspheres were embedded in polymeric scaffolds [171]. Mechanical stimulus parameters such as frequency, duration, and amplitude have also been used to modulate growth factor release [172]. While these studies demonstrate that intentional application of mechanical stimulation can influence GF release, it is important to recognize that the surrounding mechanics are likely to be a critical parameter in virtually all GF delivery systems in orthopedics. Collectively, these studies suggest that mechanical stimulus should be taken into consideration during growth factor delivery or leveraged as an important mechanism to design new drug delivery systems.
Fig. 5.
Mechanical stimulation triggered growth factor release: A) Release rate of VEGF from alginate hydrogels under 10% (open circle) and 25% (open squares) strain amplitude, and with no compression (filled circles) as a control. In vivo response to VEGF-loaded hydrogels implanted into femoral artery ligation site: B) without mechanical stimulation, and C) with mechanical stimulation, D) Schematic of anticipated drug release from polymeric matrices under mechanical loading: open circle, free drug; filled circle, bound drug. In vitro release behaviors of trypan blue (triangle), methylene blue (square), and VEGF (circle) from alginate hydrogels under mechanical stimulus: E) in vitro release rate, and F) Cumulative release. Reproduced with permission [169,170].
4. Impact of microenvironments on orthobiologics delivery
4.1. Loading and release of biologics
Localized, sustained release of orthobiologics can potentially offer several desirable features including; lower required dose, ability to achieve long term release, and avoidance of heterotopic or even systemic exposure and associated side effects. Depending on the strategy used for protein incorporation, and the surrounding microenvironment, one can control several aspects of soluble growth factor release. This section will describe methods for loading orthobiologics and the release of growth factors from these different approaches.
4.1.1. Adsorption
Perhaps, the simplest strategy for loading orthobiologics involves adsorption to a carrier matrix. Growth factors have been adsorbed to sponges, and also to mineral surfaces. Absorbable collagen sponges are widely used as a delivery vehicle for growth factors including TGF-β, and bFGF, and they are the current clinical strategy for the release of rhBMP-2 and rhBMP-7 [173,174]. Collagen is an attractive biomaterial since it is the main non-mineral component of bone, has good biocompatibility, degradation into physiological end-products, and allows for cell infiltration. Loading of a growth factor into collagen sponges is achieved by soaking the sponge in the growth factor solution, which allows for control over the dosage by simply changing the growth factor concentration in the soaking solution. This approach can be used to load high dosages of growth factors, up to 12 mg [175]. Binding studies of rhBMP-2 to collagen sponges have demonstrated that changes in pH influence loading efficiency. At pH <4, binding of rhBMP-2 is negligible. As the pH increases from 4.5 to 6.5, more significant amounts can be loaded (up to 0.1–0.2 mg rhBMP-2 per mg collagen) and it has been reported that rhBMP2 can be loaded with over 90% efficiency [174]. However, there is typically some loss of the loaded growth factor due to mechanical manipulation of collagen sponges during implantation. BMP-2 loss might also be caused by leakage of BMP-2 due to bleeding and postoperative drains after implantation. Mok et al. studied the extravasation of rhBMP-2 after posterolateral spinal fusion and found a median 0.58% of implanted BMP-2 loss in the drains with 48 h of implantation [176], indicating the impact of these factors was in a manageable range.
In another approach, growth factors are commonly adsorbed to mineral surfaces. Metal implants, scaffolds, and polymeric devices have been coated with a mineral layer to improve the integration of the device with host tissue, and the coating can also serve as a carrier to load orthobiologics. Mineral coatings have been formed using methods such as plasma spraying, pulsed laser deposition, or electrophoretic deposition [177–179]. These methods allow for the formation of highly stable, stoichiometric hydroxyapatite. Dosages of proteins in the order of micrograms have been loaded into these coatings [180]. Another method to form mineral coatings has involved incubation of a device in a simulated body fluid solution that mimics the ionic constituents of blood plasma. The mineral formed using this method is highly porous on the nanoscale, and contains charged calcium and phosphate ions capable of efficiently binding acidic or basic growth factors via electrostatic interactions. This approach has been used to coat several devices, geometries including bone screws, sutures, microspheres, and various macroporous scaffolds [137,138,140,143,181–183]. Once the device is coated, growth factor incorporation is as simple as “dip coating” the device for several minutes in an aqueous buffer containing the growth factor of interest [183]. A broad range of growth factors and peptides have been bound to mineral coated devices using this approach including mineral-binding bone morphogenetic peptide (mBMP) [181,183,184], bFGF, rhVEGF [138,183]. This strategy offers numerous advantages, including binding of multiple proteins, control over dosage by simply changing the concentration of the growth factor solution or the dip coating time, and retention of biological activity of the bound orthobiologic [139,183]. The percentage of growth factor bound by adsorption varies since it depends on the growth factor-mineral affinity, the coating surface area, and the growth factor concentration in solution. It has been reported to range from over 90% for growth factors or peptides with high mineral binding affinity to 15% for growth factors with low mineral binding affinity [138,140,183]. This approach can be considered as a “modular design” approach, in which a controllable biologics carrier is integrated into a structural device as a thin surface coating that does not compromise the device performance.
The release kinetics of growth factors adsorbed to a carrier are highly dependent on the carrier material. On one end of the spectrum, rhBMP2 release from collagen sponges has been characterized and shown to exhibit a burst release over the first 2 days after implantation (>60% rhBMP2 is released) resulting in substantial short term release [185]. In vivo retention of rhBMP-2 has been reported to be from 2.5–4 days [174,185]. On the other end of the spectrum, highly crystalline hydroxyapatite coated onto metal implants in many cases lacks macroporosity and does not degrade. This could, in principle, lead to bone formation on the surface that would encase the growth factor and prevent its availability. Previous work has demonstrated that 15–25% of TGF-β2 loaded onto highly crystalline hydroxyapatite released in vitro within the first two days [180,186]. Release from mineral coatings is dynamic and is affected by many factors including crystallinity of the mineral [140], the affinity between the growth factor and the mineral [183], the pH, ionic composition of release medium [183], and the presence of proteins in the release medium. Mineral coatings formed after incubation in simulated body fluids can be resorbable and allow for sustained growth factor release. Suarez-Gonzalez et al. explored the differential affinity between growth factors and a coating material to achieve slower release kinetics with higher affinity, and faster release kinetics with lower affinity [183]. The release profile for rhVEGF and an engineered mBMP were characterized for periods of two months in two buffer systems; cell culture medium and simulated body fluid. Release kinetics of rhVEGF and mBMP were each slower in simulated body fluid than in cell culture medium. Simulated body fluid is supersaturated with calcium and phosphate ions, whereas cell culture medium has a lower concentration of the same ions. As the coating dissolves in SBF, the released protein may re-bind to existing or newly formed mineral or may become encapsulated within a growing mineral coating resulting in slower protein release. Re-precipitation of the mineral in cell culture medium is slower since the solution is not supersaturated with calcium and phosphate ions and therefore leads to faster release kinetics.
4.1.2. Co-precipitation
Proteins can also be loaded into materials such as mineral coatings by co-precipitation. The protein becomes incorporated into the growing mineral layer and is released gradually as the coating degrades. In a study by Liu et al., BSA was co precipitated with mineral on titanium-alloy implants [187]. BSA concentrations of 0.01–1000 μg/ml were incorporated into the supersaturated solution used to form the coating. Other groups have used similar concentrations of BSA and used longer incubation times for mineral nucleation and growth [188]. This system allows for incorporation of the protein into the growing layer, however the amount of protein required could be high because materials are typically incubated for periods of days and even weeks to achieve a continuous mineral layer. Each time that the incubating solution is renewed, the protein must be renewed, resulting in a poor effective loading efficiency. Release of proteins incorporated into the growing mineral layer by co-precipitation can also serve as vehicles for long term-sustained release. In a study by Lee et al., BSA and lysozyme were released for periods of 30 days [139]. The presence of proteins during coating precipitation can also impact the properties of mineral coatings, and therefore the subsequent release profile. In a study by Liu et al., coatings formed by incubation in simulated body fluids with and without BSA had different dissolution properties. Coatings formed in the presence of BSA degraded slower compared to those formed under the same conditions but without the protein content [187].
4.1.3. Encapsulation
Orthobiologics can also be encapsulated in various natural and synthetic polymeric matrices. The natural polymers include chitosan, alginate, dextran, and gelatin, and the synthetic polymers include PLGA, and PCL among others. Biodegradable polymer microspheres have been widely used as carriers for controlled release of growth factors. Fabrication of polymeric microspheres often relies on a water in oil emulsion or water-in-oil-in-water (W/O/W) double emulsion process with solvent extraction [189,190]. W/O/W involves dissolving the polymer in an organic solvent such as dichloromethane (DCM) and emulsifying the orthobiologic in that solution. The resulting solution is then re-emulsified in an aqueous phase. Following the double emulsion, the organic solvent is evaporated and the polymer precipitates with the orthobiologic encapsulated. Microspheres are then lyophilized for 24–48 h. Loading of proteins can be highly efficient reaching over 90% efficiency in some instances [191–193]. Milligram quantities of proteins have been loaded into polymeric microspheres [193]. Growth factors can also be incorporated in polymers used to coat metal surfaces [194]. In this case, incorporation of a growth factor is achieved by dissolving the polymer in an organic solvent followed by incorporation of the growth factor into the polymer solution [194,195]. Encapsulation of growth factors in a polymeric matrix in many instances compromises the integrity of the growth factor since it is exposed to organic solvents and polymer degradation byproducts which can result in growth factor denaturation, as discussed on previous sections [196]. Growth factor release from microspheres has drawn increasing interest and is accepted as a useful tool for controlled drug delivery due to their inherently small size, good drug loading efficiency, sustained delivery of orthobiologics, ability to release multiple growth factors, and ability to quickly respond to stimuli from the surrounding environment (e.g. temperature, pH, magnetic fields). Release kinetics from this carrier system are dependent on the properties of the polymer, as release typically involves drug diffusion through a polymer matrix that is degrading and eroding [197]. Microspheres typically exhibit burst release in the first few days (~4 days) followed by a more linear sustained release [143, 189]. A similar trend is observed when growth factors are released from polymer coatings — burst release followed by sustained release for days to weeks [143,189].
4.1.4. Affinity based binding
Natural ECM displays high affinity for many types of protein therefore is able to localize growth factor activity via the ECM binding domains on these growth factors [45,198]. This mechanism has inspired various non-covalent immobilization strategies of growth factors on orthopedic devices to achieve controllable delivery. GAGs are one of the most well-known ECM molecules used for bone growth factor immobilization owing to their high affinity to many growth factors via their negatively charged sulfate groups [199]. GAGs such as heparin and heparan sulfate have been widely exploited to load bone growth factors into different systems such as scaffolds, hydrogels and micro/nanoparticles [200]. Since their interactions with growth factors are non-covalent and reversible, this immobilization approach ensures the minimal influence on growth factor ternary structure and tends to preserve their bioactivity. A recent report by Hettiaratchi et al. demonstrated that heparin microparticles retained large amount of bioactive BMP-2 and elicited biological response comparable to soluble BMP-2 treatment [201]. Other ECM molecules such as fibronectin, fibrin and hyaluronan have also shown their capability to localize growth factor and act as sustained release platform. For example, hyaluronic acid was successful used to retain BMP-2 in its scaffold and release the BMP-2 in a sustained manner [202].
Another emerging technique for affinity based growth factor immobilization is through layer-by-layer (LBL) route, in which a thin film is formed on a charged substrate by alternately dipping in complementary charged polymer solutions [203]. One of the most attractive advantages of using LBL assembly for bone growth factor delivery is its ability to incorporate large amount of proteins with a multilayer thin film. Up to 40 wt.% of drugs can be loaded onto the LBL thin film which is about 10 times higher than most drug encapsulation systems [204]. For example, Macdonald et al. demonstrated the extremely high loading capacity of BMP-2 on LBL coatings and showed its inductivity on bone tissue formation in vivo [205]. Additionally, the binding of growth factor to LBL films are conducted in aqueous solution at mild pH and ionic strength, which can effectively preserve the activity of proteins and lower the required dosage for therapeutic applications [206]. In a study by Crouzier et al., BMP-2 loaded on TCP/HAP ceramics with a polyelectrolyte multilayer coating showed more potent inductive effect in terms of alkaline phosphatase activity, indicating the polyelectrolyte multilayer could protect protein from denaturation [207].
4.1.5. Chemical immobilization
As previously mentioned, authors Kuhl and Griffith-Cima demonstrated the feasibility of covalent growth factor tethering while preserving bioactivity. Here the authors demonstrated an increased level EGF’s mitogenic activity over adsorbed EGF when presented to rat hepatocytes. The authors argued that chemical immobilization via covalent tethering offered enhanced growth factor bioactivity via the presentation of precise concentrations and a reduction of cellular internalization and down regulation [112]. For materials used in orthopedic applications, advancements in surface chemistry have allowed for covalent tethering of growth factor orthobiologics to substrates such as titanium. Strategies for chemical immobilization on orthopedic implants often first involve functionalization of the material substrate with amines or thiols. Example functionalization techniques include the formation of a polydopamine coating on the material via dip coating, or the exposure of the material to allylic amides after plasma treatment. Attachment of the growth factor orthobiologics then proceeds through Michael Addition, imine formation, or amide formation after carbodiimide/N-hydroxysuccinimide activation of the surface amine. Variations of this strategy often employ covalent attachment of an intermediate material to the orthopedic implant surface, allowing for functionalization with a wider range of chemistries. This alternate approach often employs both natural and synthetic polymers as this intermediate, which also often confer additional surface qualities such as anti-bacterial or non-fouling properties. Examples of such polymers are the polysaccharide chitosan and PEG. Importantly, these strategies have been successful in vitro and in vivo animal models for growth factors of interest to orthopedic applications such as members of the BMP family [208,209] and VEGF [210].
Although these immobilization strategies have proved to be effective for certain specific tethering approaches, they are still dependent on the compatibility of the chemical immobilization with the growth factor variant of interest. Specifically, several of these non-selective covalent tethering strategies have resulted in reduced bioactivity of growth factors when multiple primary amines are present [33]. Chemistries that allow for tethering at selective sites on growth factors or that utilize selective electrostatic interactions are critical to improving upon chemical immobilization strategies for bioactivation of orthopedic implant surfaces. Since the publication by Kuhl and Griffith-Cima in 1996, investigators have developed several alternative strategies that avoid this limitation and have covalently tethered growth factors in ways that are more selective. Specifically, investigators have explored covalent tethering of ECM components and ECM-inspired ligands to bind growth factors to overcome this limitation [211]. Hu et al. demonstrated VEGF presented on a titanium substrate via a VEGF-binding heparin-catechol (HepC) tether. The authors demonstrated that the HepC-presented VEGF retained significantly higher activity over the direct covalent tethering of VEGF to hyaluronic acid-catechol (HAC). The authors attribute the difference in activity to the disruption of VEGF’s tertiary structure by direct covalent-tethering to HAC. Importantly, the HepC-presented VEGF enhanced in vitro tubuluogenesis and proliferation of endothelial cells and mineralization in osteoblasts/endothelial cell co-cultures over HAC-presented VEGF [211].
4.2. Impact of microenvironment on bone growth factor bioactivity during delivery
The efficacy of bone growth factors upon delivery is of paramount importance, as the success of their application in orthopedics and other fields is highly dependent on the physical integrity and biological activity of the delivered bone growth factors [198,199]. The impact of the microenvironment on bone growth factor efficacy is substantially influenced by the conformational structure of these growth factors, which is sensitive to external stress discussed in the previous sections [200]. During the course of incorporation and release, the bioactivity of growth factors is challenged by a series of microenvironmental factors, including hydrophobic interfaces, detergents used for protein encapsulation, elevated temperature and degradation byproducts of carrier materials [201]. In this section, several typical growth factor delivery systems are discussed to highlight the impact of the microenvironment on delivery efficacy.
Delivery of rhBMP-2 from collagen sponges has been successful in accelerating fracture repair, and the approach was introduced to the clinic in 2002 with the INFUSE® product. The use of INFUSE® in lumbar spine fusion has resulted in higher fusion rates, with results that are superior to lumbar fusion using autogenous bone grafts. A study by Burkus et al., compared the outcome of patients that had received the INFUSE product to patients that received iliac crest autografts. Patients treated with the INFUSE product had statistically superior outcomes with regards to operating time, blood loss, hospital stay, rate of secondary interventions, median time to return to work, and fusion rate [212]. Outcomes of the delivery of rhBMP2 from collagen sponges are summarized in [175], and serve as a demonstration that the biological activity of bone growth factors can be exploited using this carrier system. However, there is more exogenous BMP2 in a single dose of the INFUSE product than would be present in 1000 human bone defects as the physiological BMP-2 concentration is around 100–200 pg/mL in human bone tissue [213]. At concentrations so much higher than physiological range it is unclear what percentage of released rhBMP2 is biologically active.
Release of orthobiologics from mineral coated devices also results in a high level of biological activity. Previous studies by our group have demonstrated the release of bioactive growth factors in vitro and in vivo from mineral coated devices [138,181,183,184]. Suarez-Gonzalez et al. released rhVEGF from β-TCP scaffolds in an ovine intramuscular model resulting in an increase in blood vessel ingrowth [183]. Lee et al. delivered bFGF from mineral-coated sutures in a chronic rotator cuff repair model in sheep, and the local release of bFGF improved the load at failure of the repaired tendon [137]. Lu et al., delivered mBMP from mineral-coated bioresorbable interference screws in a sheep tendon-bone healing model and demonstrated improved histologic scores of early tendon-bone healing [181]. Taken together, these studies and others suggest that the mechanism of growth factor-mineral binding and subsequent release maintains growth factor biological activity and serves as a simple and perhaps broadly applicable technique [138–140,143,184].
Growth factors released after encapsulation in synthetic polymers have been reported to stimulate a biological response, therefore suggesting the release of bioactive growth factors [214–217]. For example, Richardson et al., showed that rhVEGF and platelet derived growth factor released from PLG scaffolds stimulated angiogenesis [216]. TGF-β1 released from a PLLA coating on titanium implants significantly improved biomechanical and histological aspects of fracture healing [194]. These examples show that poly(α-hydroxy ester) materials can release growth factors and influence tissue formation in vivo. This is perhaps surprising in view of studies that have reported concerns with bioactivity of growth factors released using this carrier system. Degradation of microspheres fabricated from poly(α-hydroxy-esters); the most frequently used synthetic polymers, result in acidic by products that can cause an inflammatory tissue response and denaturation/degradation of bioactive proteins [218,96]. Future studies may provide further insights into the stability of proteins encapsulated within, and released from, bioresorbable polymers.
5. Emerging directions
5.1. Relevance to other emerging molecular orthobiologics
Despite promise shown by strategies to improve bone growth factors’ stability through chemical modification and biomaterial delivery strategies, challenges with narrow therapeutic indices, short half-lives, and narrow ranges of efficacious concentrations persist. Additionally, the recombinant DNA technology that has made many growth factor-based therapies more feasible does not always produce growth factors of equivalent bioactivity when compared to their naturally derived counterparts. This is particularly true for growth factors whose bioactivity is dependent on post-translational modifications. In light of these considerations, investigators have sought strategies for delivery of other classes of orthobiologics in addition to growth factors. Here we briefly describe other classes of orthobiologics used in delivery strategies.
5.1.1. Polynucleotides for gene delivery
In the early 1990s, investigators began exploring direct polynucleotide delivery for orthopedic applications as an alternative to bone growth factor based therapies. In principle, polynucleotides in the form of plasmid DNA that encode growth factors can be delivered into target cells to induce local production of corresponding growth factor [219]. Compared to protein delivery, successful gene delivery can lead to sustained expression of physiological levels of growth factor, with authentic post-translational modification. This can potentially mitigate the need for supraphysiological dosages of recombinant growth factors, which are the major cause for the high cost and severe side effects observed in clinical studies [220]. In theory, in situ expression of bone growth factors would obviate many challenges associated with delivery of recombinant proteins. In addition, the growth factors commonly used as orthobiologics, such as BMP-2, VEGF, bFGF, and parathyroid hormone (PTH), could each be produced by cells through the delivery of their encoding genes [221–223].
Genes encoding bone growth factors must be delivered into target cells via vectors, which fall into the two broad classes of viral and non-viral vectors. Viral vectors are well known for their high efficiency of gene transfer to the cell [224]. The most commonly used viral vectors include adenovirus, retrovirus, lentivirus, and adeno-associated virus (AAV). Safety issues due to the potential for immune responses to viral gene delivery [225] and potential for insertional mutagenesis cause concerns that may limit broad clinical application [220]. However, measures can be taken to reduce safety concerns with viral vectors. Illustrating this concept, Musgrave et al. utilized a BMP-2 adenoviral vector to promote ectopic bone formation in both SCID and immunocompetent mice [226]. To extend on this concept, Okubo et al. also delivered a BMP-2 encoding gene and discovered that bone formation occurred, but immune-mediated clearance of transduced cells occurred before their beneficial effect could be realized in immunocompetent mice. They were able to circumvent this using transient immunosuppression [227]. Successful helper-virus strategies using vectors devoid of viral coding regions have shown potential for improving viral gene delivery safety [228], but viral transduction of pro-osteogenic genes in defect sites has yet to be realized in the clinic.
Investigators have also explored the use of non-viral polynucleotide vectors for gene delivery in orthopedic applications. For example, delivery of plasmid DNA encoding PTH via physically entrapment in a “gene activated matrix” resulted in sustained PTH expression for up to six weeks in a dog segmental bone defect. Remarkably, new bone on the scale of centimeters was successfully generated in the defect area [229]. Shea et al. reported that delivery of plasmid DNA encoding PDGF using a synthetic polymer matrix enhanced matrix deposition and vascularization in a rodent model [230]. Additionally, numerous non-viral vectors, such as liposomes, cationic polymers, calcium phosphates (nanoparticles and coatings) have been developed in an effort to achieve highly efficient non-viral gene delivery. However, the efficiency of non-viral vectors remains substantially lower than typical viral vectors [231]. However, substantial recent progress has been made to improve the efficiency of non-viral gene delivery by combining nanotechnology and mechanobiology, together with development of novel non-viral vectors [232–234]. With progress in both viral and non-viral gene delivery, the application of polynucleotides for gene delivery in orthopedic applications may soon become feasibly in clinical settings.
5.1.2. Peptides
Peptides are another emergent therapeutic strategy in orthobiologics. Peptide-based orthobiologics are relatively short (<50 amino acids) sequences, often derived from intact growth factors and designed to mimic their biological functions [235]. These engineered peptides can mimic naturally occurring growth factors by binding to the appropriate receptor and promoting osteogenesis during defect repair [236,237]. Importantly, their lack of a complex tertiary structure addresses some of the aforementioned stability challenges that persist in protein delivery. For example, multiple forms of BMP-2-derived short peptides have demonstrated their capability to induce osteogenic differentiation both in vitro and in vivo [238]. In addition, the synthetic adaptability of peptides relative to proteins opens up new options for controlled delivery [239]. For example, Lee et al. engineered a VEGF mimicking peptide with a unique, high affinity HA binding sequence [236]. This peptide allowed for specific activation of VEGF-dependent endothelial cell proliferation and bound tightly to HA. A similar approach has now been used with BMP mimicking peptides and others. This form of modular peptide engineering has the potential for bioactivation of a range of orthopedic devices, as well as direct activation of native bone in the form of a defect site, autograft, or allograft.
5.1.3. Cytokines
Cytokines represent another category of orthobiologics that could be utilized as therapeutics for tissue healing such as bone fracture, ligament rupture and muscle injury. Cytokines are small nonstructural proteins secreted by inflammatory cells, such as degranulating platelets, macrophages, monocytes and lymphocytes during inflammation [240]. Important cytokines involved in tissue healing include IL-1, IL-4, IL-13 tumor necrosis factor-α (TNF-α), granulocyte colony-stimulating factor (GCSF) and macrophage colony-stimulating factor (MCSF) [241]. As host regulators of inflammation at the initial stage of tissue healing, cytokines play a critical regulatory role in the healing process by initiating related cascades, recruiting precursor cells and modulating immune responses [241]. For instance, IL-4 has been shown to activate connective tissue cells and stimulate ECM deposition [242]. Chamberlain et al. reported that treatment of a ligament wound with IL-4 significantly influenced ligament healing during the first week after injury [243]. Systemic administration has been the primary mode of cytokine delivery to date. However, cytokines cause considerable systemic toxicity, thus local delivery may be preferable in emerging studies to improve therapeutic performance [244,245]. Due to the similar physiochemical properties shared by growth factors and cytokines, the considerations for growth factor delivery, including the microenvironmental factors discussed throughout this review, are also applicable to the development of drug delivery systems for cytokines.
5.2. Future directions
The vast majority of drug release studies are performed in in vitro conditions designed to mimic some limited aspects of the in vivo environment. Classical settings for drug release use a near physiologic temperature of 37 °C, near physiologic pH of 7.40, and ion concentrations similar to those in blood plasma (SBF, e.g.) [246]. While these conditions can provide a convenient framework to compare results obtained from different studies, they fail to represent critical characteristics of the in vivo environment. For example, the pH of bone defects at initial stages is usually low due to inflammation, which is likely to change the release kinetics of growth factors from various delivery systems [88]. In addition, most orthopedic implants directly contact blood and other complex biological fluids once implanted, and the release medium in vivo then becomes more complex due to the presence of serum proteins [247]. Studies from our group and others indicate that in vitro release media containing proteins can significantly influence growth factor release kinetics. For example, the presence of albumin concentrations that mimic serum amounts can significantly increase the VEGF release rate. The high complexity of growth factor release environments in vivo coupled with the influence of even simple parameters on growth factor release call for development of in vitro release environments that more closely mimic wound sites.
Another challenge for biologics delivery is maintaining bioactivity of these factors. While a substantial emphasis of growth factor release studies to date has been controlling pharmacokinetics, perhaps the more significant challenge is maintaining long term growth factor stability. Owing to their complex tertiary structure, growth factors can lose their function readily due to denaturation and associated aggregation. Denaturation can occur even more readily in response to environmental parameters such as changes in pH, temperature and solvent characteristics either during formulation or delivery [248]. Therefore, novel strategies that efficiently stabilize proteins within a carrier during these delivery systems could substantially improve the current performance of protein-based therapeutics, including bone growth factor delivery strategies. As the fundamental mechanisms causing protein denaturation have been gradually identified, corresponding protein stabilization strategies have been developed. For example, enhanced protein stability in sugar-glass nanoparticles indicates protein stabilization may be achieved by carefully controlling the microenvironment of the entrapped protein [249]. In another example, discovery of protein aggregation caused by microclimate pH changes in bioresorbable polymers have led to protein stabilization strategies that neutralize the local pH surrounding the protein during delivery [250]. These new trends in protein delivery may be translated into clinically applicable devices offering superior performance in the future.
Development of multi-component delivery systems allowing local, sustained release of multiple proteins also remains an active area of interest. While pro-osteogenic factors are of particular importance for orthopedics as they can actively promote bone formation, the presence of anti-inflammatory factors and antibiotics is also important for the success of orthopedic implants, particularly in polytrauma scenarios. For example, delivery of anti-inflammatory cytokines during the inflammatory phase of wound healing, followed by bone growth factor delivery might shorten the inflammation stage and accelerate the bone healing progress [251]. In addition, infection is still considered a primary reason for joint replacement failure, as 12% of patients receive revision replacement due to surgical or implant-related infection [252]. Thus, there is a strong need for sustained, local release of antibiotics through the process of bone healing. Delivery of multiple biologics with independent control of location, dose, and timing may be highly desirable in the context of musculoskeletal disease and trauma.
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (Grant: R01AR059916), the National Institute of General Medical Sciences’ Biotechnology Training Program (Grant: 5 T32-GM08349), and the National Science Foundation (Grant: DMR 1105591).
Abbreviations
- AAV
adeno-associated virus
- BMPs
bone morphogenetic proteins
- BSA
bovine serum albumin
- β-TCP
β-tricalcium phosphate
- CaP
calcium phosphate
- DBM
demineralized bone matrix
- DCM
dichloromethane
- ECM
extracellular matrix
- FBGCs
foreign body giant cells
- FGFs
fibroblast growth factors
- GAG
glycosaminoglycan
- GF
growth factor
- GFR
growth factor receptors
- HA
hydroxyapatite
- HAC
hyaluronic acid-catechol
- HepC
heparin-catechol
- IGF-1
insulin like growth factor 1
- IL-1
interleukin-1
- IL-4
interleukin-4
- MMPs
matrix metalloproteinases
- M-CSF
macrophage colony-stimulating factor
- mBMP
mineral binding bone morphogenetic peptide
- mSBF
modified simulated body fluid
- PDGFs
platelet-derived growth factors
- PG
proteoglycans
- PLGA
poly (lactic-co-glycolic acid)
- PEG
poly (ethylene glycol)
- PLA
poly (lactic acid)
- PCL
poly (ε-Caprolactone)
- PTH
parathyroid hormone
- RANKL
receptor activator of NFκB ligand
- RGD
Arginylglycylaspartic acid
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- W/O/W
water-in-oil-in-water
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Scaffolds, Cells, Biologics: At the Crossroads of Musculoskeletal Repair”.
References
- 1.Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H J Eng Med. 2010;224:1329–1343. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- 2.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg (Br) 1999;81:710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 3.Kirker-Head Ca, Gerhart TN, Armstrong R, Schelling SH, Carmel La. Healing bone using recombinant human bone morphogenetic protein 2 and copolymer. Clin Orthop Relat Res. 1998:205–217. doi: 10.1097/00003086-199804000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial non-unions: a prospective, randomized clinical trial comparing rhOP-1 with fresh bone autograft. J Bone Joint Surg Am. 2001;83-A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 5.Urist MR. A morphogenetic matrix for differentiation of bone tissue. Calcif Tissue Res. 1970;4:98–101. doi: 10.1007/BF02152373. [DOI] [PubMed] [Google Scholar]
- 6.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston Ra. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2002;27:2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Dimar JR, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am. 2009;91:1377–1386. doi: 10.2106/JBJS.H.00200. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 9.De Biase P, Capanna R. Clinical applications of BMPs. Injury. 2005;36(Suppl 3):S43–S46. doi: 10.1016/j.injury.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Lane JM. Bone morphogenic protein science and studies. J Orthop Trauma. 2005;19:S17–S22. doi: 10.1097/00005131-200511101-00006. [DOI] [PubMed] [Google Scholar]
- 11.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Cook SD, Rueger DC. Osteogenic protein-1: biology and applications. Clin Orthop Relat Res. 1996:29–38. [PubMed] [Google Scholar]
- 13.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC, Whitecloud TS. The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J Bone Joint Surg Am. 1994;76:827–838. doi: 10.2106/00004623-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. J Bone Joint Surg Am. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Kanczler JM, Oreffo ROC. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 16.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539–1560. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 17.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16:329–345. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lyndon JA, Boyd BJ, Birbilis N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J Control Release. 2014;179:63–75. doi: 10.1016/j.jconrel.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 20.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig Ba, Phelps Ea, Stevens HY, et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32:5241–5251. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald KM, Swanstrom MM, McCarthy JJ, Nemeth BA, Guliani TA, Noonan KJ. Exaggerated inflammatory response after use of recombinant bone morphogenetic protein in recurrent unicameral bone cysts. J Pediatr Orthop. 2010;30:199–205. doi: 10.1097/BPO.0b013e3181cec35b. [DOI] [PubMed] [Google Scholar]
- 22.Kwon B, Jenis LG. Carrier materials for spinal fusion. Spine J. 2005;5:224S–230S. doi: 10.1016/j.spinee.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Fu R, Selph S, Mcdonagh M, Peterson K, Tiwari A, Chou R. Annals of Internal Medicine Effectiveness and Harms of Recombinant Human Bone Morphogenetic. 2014. [DOI] [PubMed] [Google Scholar]
- 24.Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467:3257–3262. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill KS, Chi JH, Day A, Claus EB. Hospital Charges Associated With Use of Bone-Morphogenetic Proteins in Spinal Fusion Procedures. 2014. [DOI] [PubMed] [Google Scholar]
- 26.Shields LBE, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 27.Carragee EJ, Mitsunaga Ka, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11:511–516. doi: 10.1016/j.spinee.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Lee J-Y, Nam S-H, Im S-Y, Park Y-J, Lee Y-M, Seol Y-J, et al. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J Control Release. 2002;78:187–197. doi: 10.1016/s0168-3659(01)00498-9. [DOI] [PubMed] [Google Scholar]
- 29.Tibbitt MW, Anseth KS. Dynamic microenvironments: the fourth dimension. Sci Transl Med. 2012;4:160ps24. doi: 10.1126/scitranslmed.3004804. [DOI] [PubMed] [Google Scholar]
- 30.Lienemann PS, Lutolf MP, Ehrbar M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv Drug Deliv Rev. 2012;64:1078–1089. doi: 10.1016/j.addr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Clark RAF, Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment—implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004;58:197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, De Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36:745–757. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 36.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 37.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 38.Carey DJ. Control of growth and differentiation of vascular cells by extracellular matrix proteins. Annu Rev Physiol. 1991;53:161–177. doi: 10.1146/annurev.ph.53.030191.001113. [DOI] [PubMed] [Google Scholar]
- 39.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 40.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 41.Lauer G, Sollberg S, Cole M, Flamme I, Stürzebecher J, Mann K, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 42.Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- 43.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 44.Flaumenhaft R, Moscatelli D, Saksela O, Rifkin DB. Role of extracellular matrix in the action of basic fibroblast growth factor: matrix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J Cell Physiol. 1989;140:75–81. doi: 10.1002/jcp.1041400110. [DOI] [PubMed] [Google Scholar]
- 45.Macri L, Silverstein D, Clark RaF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell Ja. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 47.Ehrbar M, Metters A, Zammaretti P, Hubbell Ja, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release. 2005;101:93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Sahni A, Odrljin T, Francis W. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273:7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 49.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, et al. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–4520. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 50.Somasundaram R, Schuppan D. Type I, II, III, IV, V, and VI collagens serve as extracellular ligands for the isoforms of platelet-derived growth factor (AA, BB, and AB) J Biol Chem. 1996;271:26884–26891. doi: 10.1074/jbc.271.43.26884. [DOI] [PubMed] [Google Scholar]
- 51.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 52.Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-beta bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332–341. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Addison CL, Nör JE, Zhao H, Linn Sa, Polverini PJ, Delaney CE. The response of VEGF-stimulated endothelial cells to angiostatic molecules is substrate-dependent. BMC Cell Biol. 2005;6:38. doi: 10.1186/1471-2121-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiao X, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282:1080–1086. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- 55.Fthenou E, Zafiropoulos a, Katonis P, Tsatsakis a, Karamanos NK, Tzanakakis GN. Chondroitin sulfate prevents platelet derived growth factor-mediated phosphorylation of PDGF-Rbeta in normal human fibroblasts severely impairing mitogenic responses. J Cell Biochem. 2008;103:1866–1876. doi: 10.1002/jcb.21570. [DOI] [PubMed] [Google Scholar]
- 56.Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 57.Yamada KM, Even-ram S. Integrin regulation of growth factor signalling and adhesion. Nat Cell Biol. 2002;4:E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 58.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen EH, Zanotelli MR, Schwartz MP, Murphy WL. Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials. 2014;35:2149–2161. doi: 10.1016/j.biomaterials.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, et al. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, et al. alpha(v)beta(3) Integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J Biol Chem. 2004;279:37726–37733. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- 64.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 65.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 66.Maheshwari G, Wells a, Griffith LG, Lauffenburger Da. Biophysical integration of effects of epidermal growth factor and fibronectin on fibroblast migration. Biophys J. 1999;76:2814–2823. doi: 10.1016/S0006-3495(99)77435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ingber D. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 68.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16:427–434. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- 69.Everts V, Delaissé JM, Korper W, Niehof A, Vaes G, Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992;150:221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- 70.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 71.Stadelmann WK, Digenis aG, Tobin GR. Impediments to wound healing. Am J Surg. 1998;176:39S–47S. doi: 10.1016/s0002-9610(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 72.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 73.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz MA, Lechene C. Adhesion is required for protein kinase C-dependent activation of the Na+/H + antiporter by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992;89:6138–6141. doi: 10.1073/pnas.89.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stetler-stevenson WG, Aznavoorian S, Liotta A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 76.Kim J-H, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 78.Balamuralidhara V, Pramodkumar TM, Srujana N, Venkatesh MP, Vishal N, Krishna KL, et al. pH Sensitive drug delivery systems: a review. Am J Drug Discov Dev. 2011;1:24–48. [Google Scholar]
- 79.Alfaro V, Ródenas J, Palacios L, Mitjavila MT, Carbonell T. Blood acid–base changes during acute experimental inflammation in rats. Can J Physiol Pharmacol. 1996;74:313–319. [PubMed] [Google Scholar]
- 80.Steen H, Steen E, Physiologie I. A dominant role of acid pH in inflammatory excitation of nociceptors in rat skin, in vitro. J Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 82.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. (80-) [DOI] [PubMed] [Google Scholar]
- 83.Badawy SIF, Hussain MA. Microenvironmental pH modulation in solid dosage forms. 2007;96:948–959. doi: 10.1002/jps.20932. [DOI] [PubMed] [Google Scholar]
- 84.Thrimawithana TR, Rupenthal ID, Young SA, Alany RG. Environment-sensitive polymers for orthalmic drug delivery. 2014;698 [Google Scholar]
- 85.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Ghassemi AH, Hennink WE, Schwendeman SP. The microclimate pH in poly(D, L-lactide-co-hydroxymethyl glycolide) microspheres during biodegradation. Biomaterials. 2012;33:7584–7593. doi: 10.1016/j.biomaterials.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 88.Ginebra M-P, Canal C, Espanol M, Pastorino D, Montufar EB. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Wu G, de Groot K. Biomimetic coatings for bone tissue engineering of critical-sized defects. J R Soc Interface. 2010;7:S631–S647. doi: 10.1098/rsif.2010.0115.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds. J Wound Care. 2005;14:59–61. doi: 10.12968/jowc.2005.14.2.26739. [DOI] [PubMed] [Google Scholar]
- 91.Goerges AL, Nugent Ma. pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307–2315. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 92.Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21:2545–2551. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 93.Zhu G, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- 94.Kang J, Lambert O, Ausborn M, Schwendeman SP. Stability of proteins encapsulated in injectable and biodegradable poly(lactide-co-glycolide)-glucose millicylinders. Int J Pharm. 2008;357:235–243. doi: 10.1016/j.ijpharm.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang J, Schwendeman SP. Comparison of the effects of Mg(OH)2 and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D, L-lactide-co-glycolide) implants. Biomaterials. 2002;23:239–245. doi: 10.1016/s0142-9612(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 96.Van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 97.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 98.Ding AG, Schwendeman SP. Acidic microclimate pH distribution in PLGA micro-spheres monitored by confocal laser scanning microscopy. Pharm Res. 2008;25:2041–2052. doi: 10.1007/s11095-008-9594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lakshmi S, Katti D, Laurencin C. Biodegradable polyphosphazenes for drug delivery applications. Adv Drug Deliv Rev. 2003;55:467–482. doi: 10.1016/s0169-409x(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 100.Shenderova A, Burke T, Schwendeman S. The acidic microclimate in poly (lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm Res. 1999;16:241–248. doi: 10.1023/a:1018876308346. [DOI] [PubMed] [Google Scholar]
- 101.Donini C, Robinson D, Colombo P, Giordano F, Peppas N. Preparation of poly(methacrylic acid-g-poly(ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int J Pharm. 2002;245:83–91. doi: 10.1016/s0378-5173(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 102.Patel VR, Amiji MM. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res. 1996;13:588–593. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 103.Podual K. Preparation and dynamic response of cationic copolymer hydrogels containing glucose oxidase. Polymer (Guildf) 2000;41:3975–3983. [Google Scholar]
- 104.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 105.Lee K, Silva Ea, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsusaki M, Akashi M. Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly(gamma-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules. 2005;6:3351–3356. doi: 10.1021/bm050369m. [DOI] [PubMed] [Google Scholar]
- 107.Banerjee I, Mishra D, Das T, Maiti TK. Wound pH-responsive sustained release of therapeutics from a poly(NIPAAm-co-AAc) hydrogel. J Biomater Sci Polym Ed. 2012;23:111–132. doi: 10.1163/092050610X545049. [DOI] [PubMed] [Google Scholar]
- 108.Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibrahim MA, Ismail A, Fetouh MI, Göpferich A. Stability of insulin during the erosion of poly(lactic acid) and poly(lactic-co-glycolic acid) microspheres. J Control Release. 2005;106:241–252. doi: 10.1016/j.jconrel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 110.Houchin ML, Heppert K, Topp EM. Deamidation, acylation and proteolysis of a model peptide in PLGA films. J Control Release. 2006;112:111–119. doi: 10.1016/j.jconrel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 111.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 112.Kuhl P, Griffith-Cima L. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 113.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–117. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Lo KW-H, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64:1277–1291. doi: 10.1016/j.addr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niu X, Feng Q, Wang M, Guo X, Zheng Q. In vitro degradation and release behavior of porous poly(lactic acid) scaffolds containing chitosan microspheres as a carrier for BMP-2-derived synthetic peptide. Polym Degrad Stab. 2009;94:176–182. [Google Scholar]
- 116.Gijpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 117.Park TG, Lu W, Crotts G. controlled release Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly (D, L-lactic acid-co-glycolic acid) microspheres. J Control Release. 1995;33:211–222. [Google Scholar]
- 118.Bittner B, Witt C, Mäder K, Kissel T. Degradation and protein release properties of microspheres prepared from biodegradable poly(lactide-co-glycolide) and ABA tri-block copolymers: influence of buffer media on polymer erosion and bovine serum albumin release. J Control Release. 1999;60:297–309. doi: 10.1016/s0168-3659(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 119.Von Burkersroda F, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 120.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 121.Kamei S, Inoue Y, Okada H, Yamada M, Ogawa Y, Toguchi H. New method for analysis of biodegradable polyesters by high-performance liquid chromatography after alkali hydrolysis. Biomaterials. 1992;13:953–958. doi: 10.1016/0142-9612(92)90120-d. [DOI] [PubMed] [Google Scholar]
- 122.Iii P, Pistner H, Bendix DR, Mtiling J, Reuther JF. Poly (L-lactide): a long-term degradation study in vivo. Biomaterials. 1993;14:291–298. doi: 10.1016/0142-9612(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 123.Fan D, De Rosa E, Murphy MB, Peng Y, Smid CA, Chiappini C, et al. Mesoporous Silicon-PLGA Composite Microspheres for the Double Controlled Release of Biomolecules for Orthopedic Tissue Engineering. Adv Funct Mater. 2012;22:282–293. [Google Scholar]
- 124.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 125.Choi SW, Kim JH. Design of surface-modified poly(D, L-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J Control Release. 2007;122:24–30. doi: 10.1016/j.jconrel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Sill TJ, von Recum HA. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 127.Li S, Mccarthy S. Further investigations on the hydrolytic degradation of poly (DL-lactide) Biomaterials. 1999;20:35–44. doi: 10.1016/s0142-9612(97)00226-3. [DOI] [PubMed] [Google Scholar]
- 128.Schense JC, Hubbell Ja. Cross-linking exogenous bifunctional peptides into fibrin gels with factor xiiia. Bioconjug Chem. 1999;10:75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 129.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF–fibrin matrices for endothelialization. J Control Release. 2001;72:101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 130.Sakiyama S, Schense J, Hubbell J. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension : an example of designer matrices in tissue engineering. FASEB J. 1999;13:2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 131.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 132.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888-+. [Google Scholar]
- 133.LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev. 2008;108:4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 134.Dowd TL, Rosen JF, Li L, Gundberg CM. The three-dimensional structure of bovine calcium ion-bound osteocalcin using 1H NMR spectroscopy. Biochemistry. 2003;42:7769–7779. doi: 10.1021/bi034470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luong LN, Hong SI, Patel RJ, Outslay ME, Kohn DH. Spatial control of protein within biomimetically nucleated mineral. Biomaterials. 2006;27:1175–1186. doi: 10.1016/j.biomaterials.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 136.Yu X, Wang L, Jiang X, Rowe D, Wei M. Biomimetic CaP coating incorporated with parathyroid hormone improves the osseointegration of titanium implant. J Mater Sci Mater Med. 2012;23:2177–2186. doi: 10.1007/s10856-012-4682-7. [DOI] [PubMed] [Google Scholar]
- 137.Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. Controllable protein delivery from coated surgical sutures. J Mater Chem. 2010;20:8894–8903. [Google Scholar]
- 138.Suárez-González D, Lee JS, Lan Levengood SK, Vanderby R, Murphy WL. Mineral coatings modulate β-TCP stability and enable growth factor binding and release. Acta Biomater. 2012;8:1117–1124. doi: 10.1016/j.actbio.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater. 2011;23:4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suárez-González D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials. 2012;33:713–721. doi: 10.1016/j.biomaterials.2011.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan H, Darvell BW. Effect of carbonate on hydroxyapatite solubility. Cryst Growth Des. 2010;10:845–850. [Google Scholar]
- 142.Yu X, Khalil A, Dang PN, Alsberg E, Murphy WL. Multilayered Inorganic Microparticles for Tunable Dual Growth Factor Delivery. Adv Funct Mater. 2014;24:3082–3093. doi: 10.1002/adfm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jongpaiboonkit L, Franklin-Ford T, Murphy WL. Mineral-Coated Polymer Micro-spheres for Controlled Protein Binding and Release. Adv Mater. 2009;21:1960–1963. [Google Scholar]
- 144.Budz JA, Nancollas GH. The mechanism of dissolution of hydroxyapatite and carbonated apatite in acidic solutions. J Cryst Growth. 1988;91:490–496. [Google Scholar]
- 145.Anderson JM, Miller KM. Biomaterial biocompatibility and the macrophage. Biomaterials. 1984;5:5–10. doi: 10.1016/0142-9612(84)90060-7. [DOI] [PubMed] [Google Scholar]
- 146.Kwiatkowska K, Sobota a. Signaling pathways in phagocytosis. Bioessays. 1999;21:422–431. doi: 10.1002/(SICI)1521-1878(199905)21:5<422::AID-BIES9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 147.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 149.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–R9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 151.Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000;7:40–47. doi: 10.1097/00062752-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 152.Jones JA, Mcnally AK, Chang DT, Qin LA, Meyerson H, Colton E, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A. 2007;84:158–166. doi: 10.1002/jbm.a.31220. [DOI] [PubMed] [Google Scholar]
- 153.Yahyouche a, Zhidao X, Czernuszka JT, Clover aJP. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater. 2011;7:278–286. doi: 10.1016/j.actbio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 154.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 155.Grundnes O, Reikerås O. The importance of the hematoma for fracture healing in rats. Acta Orthop Scand. 1993;64:340–342. doi: 10.3109/17453679308993640. [DOI] [PubMed] [Google Scholar]
- 156.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 157.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 158.Väänänen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–138. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 159.Taylor JC, Cuff SE, Leger JPL, Morra A, Anderson GI. In vitro osteoclast resorption of bone substitute biomaterials used for implant site augmentation: a pilot study. Int J Oral Maxillofac Implants. n.d;17:321–30. [PubMed] [Google Scholar]
- 160.Duplat D, Chabadel A, Gallet M, Berland S, Bédouet L, Rousseau M, et al. The in vitro osteoclastic degradation of nacre. Biomaterials. 2007;28:2155–2162. doi: 10.1016/j.biomaterials.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 161.Roldán JC, Detsch R, Schaefer S, Chang E, Kelantan M, Waiss W, et al. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J Craniomaxillofac Surg. 2010;38:423–430. doi: 10.1016/j.jcms.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 162.Wernike E, Hofstetter W, Liu Y, Wu G, Sebald HJ, Wismeijer D, et al. Long-term cell-mediated protein release from calcium phosphate ceramics. J Biomed Mater Res A. 2009;92:463–474. doi: 10.1002/jbm.a.32411. [DOI] [PubMed] [Google Scholar]
- 163.Hou WS, Li Z, Gordon RE, Chan K, Klein MJ, Levy R, et al. Cathepsin k is a critical protease in synovial fibroblast-mediated collagen degradation. Am J Pathol. 2001;159:2167–2177. doi: 10.1016/S0002-9440(10)63068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hsu C-W, Olabisi RM, Olmsted-Davis Ea, Davis AR, West JL. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J Biomed Mater Res A. 2011;98:53–62. doi: 10.1002/jbm.a.33076. [DOI] [PubMed] [Google Scholar]
- 165.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 166.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sudhir K, Hashimura K, Bobik a, Dilley RJ, Jennings GL, Little PJ. Mechanical strain stimulates a mitogenic response in coronary vascular smooth muscle cells via release of basic fibroblast growth factor. Am J Hypertens. 2001;14:1128–1134. doi: 10.1016/s0895-7061(01)02189-6. [DOI] [PubMed] [Google Scholar]
- 168.Groothuis A, Duda GN, Wilson CJ, Thompson MS, Hunter MR, Simon P, et al. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: involvement of VEGF mechano-regulation. Bone. 2010;47:438–444. doi: 10.1016/j.bone.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 169.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 170.Lee KY, Peters MC, Mooney DJ. Controlled Drug Delivery from Polymers by Mechanical Signals. Adv Mater. 2001;13:837–839. [Google Scholar]
- 171.Yang Y, Tang G, Zhang H, Zhao Y, Yuan X, Fan Y, et al. Controlled release of BSA by microsphere-incorporated PLGA scaffolds under cyclic loading. Mater Sci Eng C. 2011;31:350–356. [Google Scholar]
- 172.Urciuolo F, Imparato G, Netti PA. Effect of Dynamic Loading on Solute Transport in Soft Gels Implication for Drug Delivery. Bioeng Food Nat Prod Eff. 2008;54:824–834. [Google Scholar]
- 173.McPherson JM. The utility of collagen-based vehicles in delivery of growth factors for hard and soft tissue wound repair. Clin Mater. 1992;9:225–234. doi: 10.1016/0267-6605(92)90103-z. [DOI] [PubMed] [Google Scholar]
- 174.Geiger M. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 175.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mok JM, Durrani SK, Piper SL, Hu SS, Deviren V, Berven SH, et al. Extravasation of rhBMP-2 with use of postoperative drains after posterolateral spinal fusion. Spine (Phila Pa 1976) 2008;33:1668–1674. doi: 10.1097/BRS.0b013e31817b6229. [DOI] [PubMed] [Google Scholar]
- 177.Tsui YC, Doyle C, Clyne TW. Plasma sprayed hydroxyapatite coatings on titanium substrates Part 1: Mechanical properties and residual stress levels. Biomaterials. 1998;19:2015–2029. doi: 10.1016/s0142-9612(98)00103-3. [DOI] [PubMed] [Google Scholar]
- 178.Bao Q, Chen C, Wang D, Ji Q, Lei T. Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl Surf Sci. 2005;252:1538–1544. [Google Scholar]
- 179.Zhitomirsky I, Gal-or L. Electrophoretic deposition of hydroxyapatite. J Mater Sci Mater Med. 1997;8:213–219. doi: 10.1023/a:1018587623231. [DOI] [PubMed] [Google Scholar]
- 180.Sumner DR, Turner TM, Urban RM, Virdi AS, Inoue N. Additive enhancement of implant fixation following combined treatment with rhTGF-beta2 and rhBMP-2 in a canine model. J Bone Joint Surg Am. 2006;88:806–817. doi: 10.2106/JBJS.E.00846. [DOI] [PubMed] [Google Scholar]
- 181.Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, Murphy WL. Influence of hydroxyapatite-coated and growth factor-releasing interference screws on tendon-bone healing in an ovine model. Arthroscopy. 2009;25:1427–1434.e1. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suárez-González D, Barnhart K, Saito E, Vanderby R, Hollister SJ, Murphy WL. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J Biomed Mater Res A. 2010;95:222–234. doi: 10.1002/jbm.a.32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Suárez-González D, Lee JS, Diggs A, Lu Y, Nemke B, Markel M, et al. Controlled Multiple Growth Factor Delivery from Bone Tissue Engineering Scaffolds Via Designed Affinity. Tissue Eng Part A. 2013;20:2077–2087. doi: 10.1089/ten.tea.2013.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lu Y, Lee JS, Nemke B, Graf BK, Royalty K, Illgen R, et al. Coating with a modular bone morphogenetic peptide promotes healing of a bone-implant gap in an ovine model. PLoS One. 2012;7:e50378. doi: 10.1371/journal.pone.0050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Friess W. Characterization of absorbable collagen sponges as rhBMP-2 carriers. Int J Pharm. 1999;187:91–99. doi: 10.1016/s0378-5173(99)00174-x. [DOI] [PubMed] [Google Scholar]
- 186.Matsumoto T, Okazaki M, Inoue M, Yamaguchi S, Kusunose T, Toyonaga T, et al. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials. 2004;25:3807–3812. doi: 10.1016/j.biomaterials.2003.10.081. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y, Hunziker E, Randall N, de Groot K, Layrolle P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials. 2003;24:65–70. doi: 10.1016/s0142-9612(02)00252-1. [DOI] [PubMed] [Google Scholar]
- 188.Azevedo HS, Leonor IB, Alves CM, Reis RL. Incorporation of proteins and enzymes at different stages of the preparation of calcium phosphate coatings on a degradable substrate by a biomimetic methodology. Mater Sci Eng C. 2005;25:169–179. [Google Scholar]
- 189.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buket Basmanav F, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 191.Meng FT, Ma GH, Qiu W, Su ZG. W/O/W double emulsion technique using ethyl acetate as organic solvent: effects of its diffusion rate on the characteristics of microparticles. J Control Release. 2003;91:407–416. doi: 10.1016/s0168-3659(03)00273-6. [DOI] [PubMed] [Google Scholar]
- 192.Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, Scurr D, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77:26–35. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 193.Bilati U, Allémann E, Doelker E. Poly(D, L-lactide-co-glycolide) protein-loaded nanoparticles prepared by the double emulsion method—processing and formulation issues for enhanced entrapment efficiency. 2008. [DOI] [PubMed] [Google Scholar]
- 194.Schmidmaier G, Wildemann B, Bail H, Lucke M, Fuchs T, Stemberger A, et al. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-β1) from a biodegradable poly(d, l-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone. 2001;28:341–350. doi: 10.1016/s8756-3282(00)00456-7. [DOI] [PubMed] [Google Scholar]
- 195.Strobel C, Bormann N, Kadow-Romacker A, Schmidmaier G, Wildemann B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J Control Release. 2011;156:37–45. doi: 10.1016/j.jconrel.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 196.Cleland JL, Jones AJ. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm Res. 1996;13:1464–1475. doi: 10.1023/a:1016063109373. [DOI] [PubMed] [Google Scholar]
- 197.Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 198.Belair DG, Le NNT, Murphy WL. Design of Growth Factor Sequestering Biomaterials. Chem Commun. 2014 doi: 10.1039/c4cc04317k. http://dx.doi.org/10.1039/C4CC04317K. [DOI] [PMC free article] [PubMed]
- 199.Lindahl U, Hook M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- 200.Hudalla GA, Murphy WL. Biomaterials that Regulate Growth Factor Activity via Bioinspired Interactions. Adv Funct Mater. 2011;21:1754–1768. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.05.011. http://dx.doi.org/10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed]
- 202.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 203.Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science. 1997;277:1232–1237. (80-) [Google Scholar]
- 204.Hammond PT. Building biomedical materials layer-by-layer. Mater Today. 2012;15:196–206. [Google Scholar]
- 205.Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32:1446–1453. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Layer-By-layer films as a biomimetic reservoir for rhBMP-2 Delivery: Controlled Differentiation of Myoblasts to Osteoblasts. Small. 2009;5:598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- 207.Crouzier T, Sailhan F, Becquart P, Guillot R, Logeart-Avramoglou D, Picart C. The performance of BMP-2 loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer film coating. Biomaterials. 2011;32:7543–7554. doi: 10.1016/j.biomaterials.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 208.Puleo Da, Kissling Ra, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23:2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 209.Shi Z, Neoh KG, Kang ET, Poh CK, Wang W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules. 2009;10:1603–1611. doi: 10.1021/bm900203w. [DOI] [PubMed] [Google Scholar]
- 210.Hu X, Neoh K-G, Shi Z, Kang E-T, Poh C, Wang W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials. 2010;31:8854–8863. doi: 10.1016/j.biomaterials.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 211.Hu X, Neoh KG, Zhang J, Kang E-T, Wang W. Immobilization strategy for optimizing VEGF’s concurrent bioactivity towards endothelial cells and osteoblasts on implant surfaces. Biomaterials. 2012;33:8082–8093. doi: 10.1016/j.biomaterials.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 212.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 213.Park Y, Kim JW, Kim DS, Kim EB, Park SJ, Park JY, et al. The Bone Morphogenesis Protein-2 (BMP-2) is associated with progression to metastatic disease in gastric cancer. Cancer Res Treat. 2008;40:127–132. doi: 10.4143/crt.2008.40.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci U S A. 2008;105:11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–1241. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 217.Chen RR, Mooney DJ. Polymeric Growth Factor Delivery Strategies for Tissue Engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 218.Wang H, Leeuwenburgh SCG, Li Y, Jansen JA. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng Part B Rev. 2012;18:24–39. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv Drug Deliv Rev. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 220.Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012;64:1331–1340. doi: 10.1016/j.addr.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010;12 doi: 10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: Transgene expression and cellular Transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rose LC, Kucharski C, Uludağ H. Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model. Biomaterials. 2012;33:3363–3374. doi: 10.1016/j.biomaterials.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 224.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Trentin D, Hubbell J, Hall H. Non-viral gene delivery for local and controlled DNA release. J Control Release. 2005;102:263–275. doi: 10.1016/j.jconrel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 226.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 227.Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake SI. Osteoinduction by bone morphogenetic protein-2 via adenoviral vector under transient immunosuppression. Biochem Biophys Res Commun. 2000;267:382–387. doi: 10.1006/bbrc.1999.1975. [DOI] [PubMed] [Google Scholar]
- 228.Abe N, Lee Y-P, Sato M, Zhang X, Wu J, Mitani K, et al. Enhancement of bone repair with a helper-dependent adenoviral transfer of bone morphogenetic protein-2. Biochem Biophys Res Commun. 2002;297:523–527. doi: 10.1016/s0006-291x(02)02193-9. [DOI] [PubMed] [Google Scholar]
- 229.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 230.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 231.He CX, Tabata Y, Gao JQ. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386:232–242. doi: 10.1016/j.ijpharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 232.Choi S, Yu X, Jongpaiboonkit L, Hollister SJ, Murphy WL. Inorganic coatings for optimized non-viral transfection of stem cells. Sci Rep. 2013;3:1567. doi: 10.1038/srep01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kong HJ, Hsiong S, Mooney DJ. Nanoscale cell adhesion ligand presentation regulates nonviral gene delivery and expression. Nano Lett. 2007;7:161–166. doi: 10.1021/nl062485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bab I, Chorev M. Osteogenic growth peptide: from concept to drug design. Biopolymers. 2002;66:33–48. doi: 10.1002/bip.10202. [DOI] [PubMed] [Google Scholar]
- 236.Lee JS, Wagoner Johnson AJ, Murphy WL. A modular hydroxyapatite-binding version of vascular endothelial growth factor. Adv Mater. 2010;22:5494–5498. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 237.Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Saito A, Suzuki Y, Ogata S-I, Ohtsuki C, Tanihara M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res A. 2005;72:77–82. doi: 10.1002/jbm.a.30208. [DOI] [PubMed] [Google Scholar]
- 239.Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, et al. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275:16213–16218. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- 240.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 241.Feghali Ca, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 242.Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Lab Invest. 2000;80:1337–1343. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- 243.Chamberlain CS, Leiferman EM, Frisch KE, Wang S, Yang X, Brickson SL, et al. The influence of interleukin-4 on ligament healing. Wound Repair Regen. 2011;19:426–435. doi: 10.1111/j.1524-475X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.He H, Grignol V, Karpa V, Yen C, LaPerle K, Zhang X, et al. Use of a nanoporous biodegradable miniature device to regulate cytokine release for cancer treatment. J Control Release. 2011;151:239–245. doi: 10.1016/j.jconrel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mejías R, Costo R, Roca AG, Arias CF, Veintemillas-Verdaguer S, González-Carreño T, et al. Cytokine adsorption/release on uniform magnetic nanoparticles for localized drug delivery. J Control Release. 2008;130:168–174. doi: 10.1016/j.jconrel.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 246.Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm. 2006;314:189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 247.Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. J Control Release. 2012;161:429–445. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 248.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 249.Giri J, Li W-J, Tuan RS, Cicerone MT. Stabilization of proteins by nanoencapsulation in sugar-glass for tissue engineering and drug delivery applications. Adv Mater. 2011;23:4861–4867. doi: 10.1002/adma.201102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fu K, Klibanov K, Langer R. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18:24–25. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- 251.Ji W, Wang H, van den Beucken JJJP, Yang F, Walboomers XF, Leeuwenburgh S, et al. Local delivery of small and large biomolecules in craniomaxillofacial bone. Adv Drug Deliv Rev. 2012;64:1152–1164. doi: 10.1016/j.addr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 252.Labek G, Thaler M, Janda W, Agreiter M, Stöckl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. J Bone Joint Surg (Br) 2011;93:293–297. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]