Abstract
Hypocalcemia and hyperphosphatemia because of resistance towards parathyroid hormone (PTH) in the proximal renal tubules are the most prominent abnormalities in patients affected by pseudohypoparathyroidism type Ib (PHP-Ib). In this rare disorder that is caused by GNAS methylation changes, resistance can occur towards other hormones, such as thyroid-stimulating hormone (TSH), that mediate their actions through G protein-coupled receptors. However, these additional laboratory abnormalities are usually not recognized until PTH-resistant hypocalcemia becomes clinically apparent. We now describe four pediatric patients, first diagnosed with subclinical or overt hypothyroidism between the ages of 0.2 and 15 years, who developed overt PTH-resistance 3-20 years later. Although anti-TPO antibodies provided a plausible explanation for hypothyroidism in one of these patients, this and two other patients revealed broad epigenetic GNAS abnormalities, which included loss of methylation (LOM) at exons AS, XL and A/B, and gain of methylation at exon NESP55, i.e. findings consistent with PHP-Ib. LOM at GNAS exon A/B alone led in the fourth patient to the identification of a maternally inherited 3-kb STX16 deletion, a well-established cause of autosomal dominant PHP-Ib. Although GNAS methylation changes were not detected in additional pediatric and adult patients with subclinical hypothyroidism (23 pediatric and 39 adult cases), hypothyroidism can obviously be the initial finding in PHP-Ib patients. One should therefore consider measuring PTH, along with calcium and phosphate, in patients with unexplained hypothyroidism for extended periods of time to avoid hypocalcemia and associated clinical complications.
Keywords: Epigenetics, pseudohypoparathyroidism, hypothyroidism, PTH, GNAS
Introduction
Pseudohypoparathyroidism (PHP) is characterized by resistance to parathyroid hormone (PTH) in the proximal renal tubules, which leads to hypocalcemia, hyperphosphatemia, and impaired 1,25(OH)2 vitamin D production. PHP type Ia (PHP-Ia) is caused by maternally inherited, heterozygous inactivating mutations affecting those GNAS exons encoding the alpha-subunit of the stimulatory G protein (Gsα) 1-3. In addition to PTH-resistance, PHP-Ia patients present with features of Albrights Hereditary Osteodystrophy (AHO) and frequently with resistance to thyroid-stimulating hormone (TSH), growth hormone-releasing hormone (GHRH), calcitonin (CT), or other hormones that mediate their actions through Gsα-coupled receptors.
In contrast to PHP-Ia, patients affected by PHP type Ib (PHP-Ib) typically present only with PTH-resistant hypocalcemia and hyperphosphatemia, and these individuals usually show no evidence for AHO. Several forms of PHP-Ib can be distinguished. The most common form of autosomal dominant PHP-Ib (AD-PHP-Ib) is caused by maternally inherited, heterozygous deletions in the STX16 gene, which is located approximately 220 kb up-stream of GNAS exon A/B 4-6, or by deletions within GNAS 7-9. These deletions are associated either with a loss of methylation at GNAS exon A/B alone 4-6,9 or a loss of all maternal methylation imprints 7,8. Patients affected by the sporadic form of PHP-Ib present with laboratory abnormalities and epigenetic GNAS changes that are largely indistinguishable from those observed in AD-PHP-Ib due to deletions within GNAS 10-15. The lack of methylation at exon A/B (also referred to as exon 1A) results in biallelic expression of this presumably non-coding transcript, which leads through unknown mechanisms to reduced expression of Gsα. The Gsα promoter does not undergo parent-specific methylation thus allowing biallelic expression in most cells. However, in certain tissues, such as proximal renal tubules, thyroid, paraventricular nucleus of the hypothalamus, brown fat, ovaries and pituitary, Gsα is derived predominantly from the maternal allele, leading to deficiency of this ubiquitously expressed signaling protein and thus hormonal resistance, if this parental allele carries a mutation 16-20. The mechanism(s) that silences Gsα expression from the non-methylated paternal allele is unknown, but seems to involve a protein(s) that is particularly abundantly expressed in those tissues where Gsα is derived predominantly from the maternal allele. Furthermore, production of this unknown factor appears to vary, since PTH-resistance in the proximal renal tubules develops only later in life in humans and mice 5,11,21 and it is known to vary considerably even among individuals with the same maternally inherited STX16 deletion 22,23.
PHP-Ib patients can present with resistance towards hormones other than PTH that mediate their actions through G protein-coupled receptors. For example, TSH elevations have been observed in some PHP-Ib patients, but these elevations are usually only mild and are typically detected after symptomatic hypocalcemia has been observed 3,11,22,24-26. We now describe four patients, who had been diagnosed first with hypothyroidism, yet PTH-resistance became clinically apparent only with a significant delay, in one case not until twenty years later. Silencing of paternal Gsα expression thus can be similarly efficient in the thyroid as in the proximal renal tubules.
Materials and Methods
Genetic and epigenetic studies
To assess copy number and methylation status, respectively, for the GNAS-STX16 region, multiplex ligation-dependent probe amplification assay (MLPA) and methylation-specific multiplex ligation-dependent probe amplification assay (MS-MLPA) were performed following the instructions of the kit's manufacturer (MRC-Holland B.V. Willem Schoutenstraat 6, 1057 DN Amsterdam, the Netherlands). Amplicons were submitted for capillary electrophoresis to the Massachusetts General Hospital DNA Core Facility. Copy number was assessed by comparing for each amplicon patient data to the mean of at least three healthy subjects. Information about the GNAS methylation status was obtained by comparing the peaks obtained from reactions using a methylation-sensitive endonuclease with those obtained without enzymatic digestion.
The PCR to search for the 3-kb STX16 deletion was performed with QIAGEN Taq DNA polymerase and the other reagents supplied with the same kit following the manufacturer's protocols; the following PCR primers were used: “a”, 5′-TTGGCAGATAACTGCTGTGG-3′; “b”, 5′- GGAAGAGCTAAGAGAACAAG-3′; “c”, 5′-GGTGGAGCAGAACACACTGA-3′; “d”, 5′-CCACCTGTGGCATCATGTTA-3′. Cycler program: denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min, followed by an additional elongation step at 72°C for 10 min.
Analysis of microsatellites markers D20S86, 907-rep2, 261P9-CA, 806-CA, 543J19-TTA, and D20S171 was performed by the Center for Human Genetic Research of the Massachusetts General Hospital.
All biochemical results were obtained in the clinical laboratory of the referring hospitals.
Pediatric PHP-Ib patients presenting first with an elevated TSH level
The male patient 130/II-1 was conceived through in vitro fertilization and was born by Cesarean section after 26 weeks of gestation because of extreme intrauterine growth retardation and severe premature contractions. Neonatal screening for hypothyroidism on the 3rd day of life was normal. On the 70th day of life his TSH levels were measured again, as is routinely done before discharge from the neonatal intensive care unit, and it was found to be elevated to 10.6 μU/ml (normal range: 0.5-6.0) with an FT4 of 0.67 ng/dl (normal range: 0.95-2.10) consistent with overt hypothyroidism. He was therefore referred to D.C., who initiated treatment with L-thyroxine. Ultrasonography revealed a normal thyroid gland; anti-thyroglobulin and anti-thyroperoxidase antibodies were negative. At the age of 17 months his total serum calcium level was normal (10.4 mg/dl; normal range for this age: 8.9-10.1), while his serum phosphate level was at the upper end of the age-appropriate normal range (5.9 mg/dl; normal range for this age: 4.6-5.6). At the age of 3.5 years, he was hospitalized because of a urinary tract infection and the laboratory work-up at that time revealed significant, albeit asymptomatic hypocalcemia (7.0 mg/dl; normal range at this age: 8.9-10.1) and hyperphosphatemia (6.4 mg/dl; normal range at this age: 3.9-5.3), with an elevated PTH (291 pg/ml) and a normal 25OH vitamin D level (31 ng/ml). He had no dysmorphic features and his development was age-appropriate. Oral calcium (1,000 mg/day) and calcitriol (0.25 μg/day) were started; dosages of both medications were adjusted over the time. His linear growth remained appropriate for his target height.
The female patient 148/II-1 was born at term; her neonatal TSH screening was normal. When investigated at the age of 6 months for obesity and developmental delay by D.T., she was found to have elevated TSH of 13.2 μU/ml (normal range: 0.4-5.5) with an FT4 of 1.25 ng/dl (normal range: 0.85-1.46), and treatment with L-thyroxine was started; anti-thyroglobulin and anti-thyroperoxidase antibodies were absent and ultrasonography revealed a normal thyroid gland. At that time she had a normal serum calcium level (10.5 mg/dl; normal range at this age: 8.0-11.0) with an elevated serum phosphate (7.5 mg/dl; normal range at this age: 4.9-6.1); no PTH level was measured. At the age of 3 years, she showed no clinical evidence for hypocalcemia, but was found to have an elevated PTH level (668 pg/ml) with low calcium (7.9 mg/dl; normal range at this age: 8.9-10.1) and elevated phosphate (8.5 mg/dl; normal range at this age: 3.9-5.3); treatment with 1-alpha vitamin D (1 μg/day) and calcium carbonate (2,400 mg/day) was therefore initiated.
The female patient 149/II-1 was born at term. Her growth and development had been normal, but menarche did not occur until 15 years. At that age, she presented with an enlarged thyroid gland and was found to have an elevated TSH (21.9 μU/ml; normal range: 0.5-5.0) and low FT4 (0.28 ng/dl; normal range: 0.7-1.48). A thyroid ultrasonography revealed no abnormalty and she showed no evidence for an abnormal regulation of calcium homeostasis. Her thyroglobulin antibodies (titer: 1:25,600; normal: <1:25) and thyroid peroxidase antibodies (76 IU/ml; normal: <50) were elevated. L-thyroxine treatment was therefore started with 100 μg per day. Her older sister had a goiter, but normal thyroid function tests; her maternal grandmother and several relatives of her maternal grandfather had hyperthyroidism. At the age of 28, she was investigated further because of cramping pains in the upper gastrointestinal and esophageal region; an endoscopic evaluation revealed no macroscopic or microscopic abnormalities. Although showing no clinical evidence for hypocalcemia, she was found to have a low total serum calcium (6.7 mg/dl) and an elevated alkaline phosphatase (134 U/L; normal range: 35-105). Treatment with cholecalciferol (20 μg) and 1000 mg calcium carbonate was started, which resulted in resolution of the abdominal symptoms. Ten days later, serum PTH was found to be elevated (1186 pg/ml) and serum phosphate was at the upper end of normal (4.3 mg/dl); total serum calcium was again significantly below the normal range and the ionized calcium was only 0.76 mmol/L. Her 1.25(OH)2 vitamin D level was normal (166 pmol/L). She was referred to an endocrine unit, where gastroscopy, neck ultrasound, abdominal ultrasound, and radiographic examination of long bones revealed no abnormalities. On treatment with cholecalciferol (50 μg/day) and calcium carbonate (3000 mg/day), the patient remained asymptomatic. When referred to P.A. and O.M., her PTH levels had improved (501 pg/ml), but total serum calcium had not reached the normal range (8.44 mg/dl). Alfacalcidol 0.25 μg twice per day was therefore started and 3,000 mg/day of calcium carbonate was continued. Two months after starting alfacalcidol serum total calcium was normal (8.92 mg/dl).
The male patient 147/II-1 was jaundiced during the perinatal period, but had a normal TSH on the neonatal screen. At 3 years of age, he showed increasing lethargy compared to his non-identical twin brother. Upon referral to W.R., he was found to have a normal total T4 (8.3 μg/dl; normal range: 4.6-12.0) and a mildly elevated TSH level (5.9 μU/mL; normal range: 0.2-4.0 μU/ml) and treatment with L-thyroxine (25 μg/day) was therefore started. Anti-thyroglobulin and anti-thyroperoxidase antibodies as well as islet cell antibodies and anti-nuclear antibodies (ANA) were negative. The family history was positive for multiple autoimmune disorders. His mother, maternal grandmother, and a maternal great aunt were diagnosed with hypothyroidism. The maternal great-grandmother had scleroderma and lupus, and the mother was positive for ANA. The father's first cousin had type 1 diabetes. Clinical examination revealed a normally developed child without AHO features; his thyroid gland was normal to palpation. Over the subsequent years, the dosage of L-thyroxine was adjusted in order to maintain TSH values in the normal range. At the age of 22 years, concurrent with mononucleosis, he presented with muscle cramps, tetany, and tingling of the face and hands, and was found to be hypocalcemic (calcium 5.6 mg/dL). Further investigations revealed an elevated PTH (795 pg/mL), reduced 25OH vitamin D (17 ng/mL), and low magnesium (1.2 mg/dL) and potassium levels (3 mmol/L). Serum phosphorus was always within normal limits. After treating 147/II-1 with cholecalciferol his serum 25OH vitamin D level normalized, but his PTH remained elevated and he was therefore started on calcitriol (0.5 μg/day) and calcium carbonate (3,000 mg/day). When last investigated at the age of 25.5 years, while on treatment with L-thyroxine his FT4 and TSH were normal (1.4 ng/dl and 4.0 μU/ml, respectively); he had no detectable anti-thyroperoxidase antibodies, and his thyroid was normal in size and echogenicity as determined by ultrasonography.
Pediatric and adult patients with sporadic subclinical hypothyroidism
Because of the delayed onset of PTH-resistance in the above patients, we also studied 62 patients (23 pediatric and 39 adult cases) with sporadic subclinical hypothyroidism, who were referred from different regions of Italy to M.T. and A.M.
Pediatric patients
15 males and 8 females with subclinical hypothyroidism (defined as an elevation in TSH without a reduction in FT4; data are presented as mean±SD) had mildly elevated TSH (5.78±1.74 μU/ml; normal range: 0.5-3.5) and normal FT4 levels (1.13±0.25 ng/dl; normal range: 0.7-1.7). Two of these patients were identified at the neonatal screening for congenital hypothyroidism, and both had thyroid glands that were normal in size and location. All other individuals had been negative in the neonatal screening test, but TSH levels were found to be elevated at 6.3±4.2 years when measured for non-specific complaints.
Adult patients
11 males and 28 females were diagnosed with subclinical hypothyroidism (levels of TSH: 8.08±2.21 μU/ml; FT4: 0.96±0.2 ng/dl) at 30.7±9.9 years of age.
Additional biochemical and clinical investigations allowed in all the above pediatric and adult patients exclusion of autoimmune thyroiditis, since testing for anti-thyroperoxidase, anti-thyroglobulin, and anti-TSH receptor antibodies was negative. For all cases, serial serum dilutions excluded assay interference with heterophilic antibodies and all TSH measurements run in parallel to the standard curve (data not shown). Imaging by ultrasound showed properly located thyroid glands with normal echogenicity. Nucleotide sequence analysis of all exons encoding the TSH receptor revealed no pathogenetic mutation except for a well-known polymorphisms, a C to A transversion in nucleotide 154 (CCC/ACC) affecting the proline residue at position 52 which is replaced by threonine. The elevated TSH levels therefore remained unexplained in these patients, who were followed for 4.4±3.4 years (range: 0.5-13) and did not develop alterations in PTH, calcium, and phosphate concentration during that time.
The study was approved by the local ethical committees and informed consent was obtained from all subjects or their guardians.
Results
We studied four individuals, who were diagnosed initially with subclinical or overt hypothyroidism, yet developed PTH-resistant hypocalcemia and hyperphosphatemia 3-20 years later (see Table 1). These changes in the regulation of mineral ion homeostasis were associated with abnormal GNAS methylation in genomic DNA from blood leukocytes, thus establishing PHP-Ib as the underlying disorder. Only patient 149/II-1 had elevated thyroglobulin and thyroid peroxidase antibodies. All four patients revealed a complete loss of methylation when tested by MS-MLPA with two probes specific for GNAS exon A/B. Patients 130/II-1, 148/II-1, and 149/II-1 furthermore showed a loss of methylation at exons XL and AS (4.9±1.9% and 3.1±1.4% of control, respectively; mean±SD; testing with four different probes), and a gain of methylation at exon NESP55 (102.1±13.8% of control; testing with three different probes), but no evidence for a variation in copy number for STX16 exons 5 and 6 (Suppl. Fig. 1a-c). In contrast, DNA from patient 147/II-1 revealed a complete loss of methylation at GNAS exon A/B alone and only half the copy number of STX16 exons 5 and 6 (data not shown). Consistent with these findings, analysis of DNA from this patient showed the previously identified 3-kb deletion within STX16 that comprises exons 4-6 4,27 and is the most frequent cause of AD-PHP-Ib 28. As shown in Fig. 2, the 663-bp PCR product was present in 147/II-1 (lane #1) and his mother 147/I-2 (lane #2), who is clinically unaffected and had normal serum levels of TSH (2.26 μU/ml), PTH (27 pg/ml), and calcium (9.6 mg/dl); the 663-bp band was absent in DNA from a healthy control (lane #3). The wild-type allele, which would give rise to a PCR product of about 3.8 kb (3812 nt), could not be amplified with the reagents that were used for PCR. These studies showed that the affected individual 147/II-1 had inherited the deletion from his mother, who is an unaffected carrier of the genetic defect.
Table 1.
Patient characteristics, laboratory, and epigenetic findings in four patients initially diagnosed with hypothyroidism before establishing the diagnosis of PHP-Ib; note that patient 149/II-1 has autoimmune thyroid disease and that PHP-Ib most likely developed independently. The normal ranges for PTH, calcium, and phosphate (adult) were similar for the laboratories at the different institutions; for the other parameters, institution-specific references ranges are provided; * = only total T4 was measured at the time (normal range: 4.6-12.0 μg/dl). To convert FT4 from ng/dl to pmol/L multiply by 12.87. Analysis of GNAS methylation at exons A/B, XL, AS, and NESP55: -non-methylated; + methylated. 3-kb STX16 deletion: yes, present; no, absent.
| 130/II-1 | 148/II-1 | 149/II-1 | 147/II-1 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age (yrs) | 0,2 | 3,5 | 0,5 | 3,0 | 15,0 | 28,0 | 3,0 | 23,0 |
|
| ||||||||
| TSH (μU/ml) | 10,6 (0.5‐6.0) | 1,9 (0.5‐5.0) | 13,2 (0.4‐5.5) | 8,0 (0.4‐4.2) | 21,9 (0.5‐5.0) | 0,1 (0.5‐5.0) | 5,9 (0.2‐4.0) | 4,0 (0.5‐4.5) |
| FT4 (ng/dl) | 0,67 | 1,90 (0.95‐2.10) | 1,25 | 1,49 (0.85‐1.46) | 0,28 | 1,19 (0.70‐1.48) | 8.3* | 1,39 (0.9‐1.80) |
|
| ||||||||
| PTH (pg/ml) (10‐65) | n.d. | 291 | n.d. | 668 | n.d. | 1186 | n.d | 795 |
| Calcium (mg/dl) (8.5‐10.5) | 10,0 | 7,0 | 10,5 | 7,9 | n.d. | 6,7 | n.d | 5,6 |
| Phosphate (mg/dl) (adult: 3.0‐4.5) | 5.4 | 6,4 | 7,5 | 8,5 | n.d. | 4,3 | n.d | 3,5 |
| 25OHD (ng/ml) | n.d. | 31 (>30) | n.d. | 19 (30‐100) | n.d. | 34 (>30) | n.d | 17 (30‐100) |
|
| ||||||||
| GNAS methylation | maternal | paternal | maternal | paternal | maternal | paternal | maternal | paternal |
|
| ||||||||
| A/B | - | - | - | - | - | - | - | - |
| XL | - | - | - | - | - | - | + | - |
| AS | - | - | - | - | - | - | + | - |
| NESP55 | + | + | + | + | + | + | + | - |
|
| ||||||||
| 3‐kb STX16 deletion | no | no | no | Yes | ||||
Figure 2.
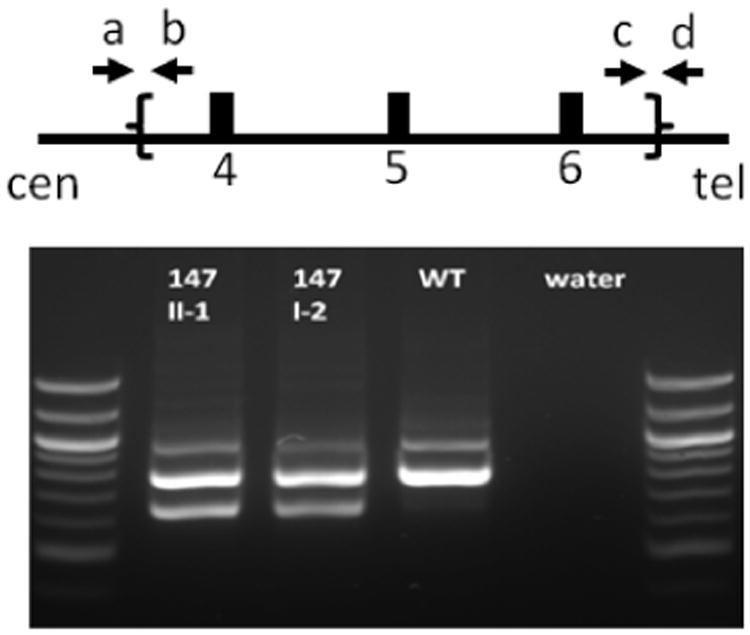
Schematic presentation of STX16 exons 4-6 (open boxes) and location of the primers (indicated by arrows a-d) that were used for multiplex PCR to assess the presence of the previously described 3-kb STX16 deletion 27. Lane #1, the patient 147/II-1; lane #2, the patient's healthy carrier mother; lane #3, healthy individual; lane #4, negative control. The 663 bp band is only observed when using primers a and d that are outside of the deletion borders and visible only in subjects with the 3-kb STX16 deletion.
Expected bands: Wild-type DNA: primers a and b, 966 bp; primers c and d, 793 bp; primers a and d, 3641 bp (too long to be amplified with the PCR system used). DNA from patient with 3-kb STX16 deletion: additional band of 663 bp with primers a and d. The 100 bp ladders are shown on both sides of the gel.
The findings in patients 130/II-1, 148/II-1, and 149/II-1, namely broad GNAS methylation changes are consistent with sporadic PHP-Ib. Some of these sporadic cases were previously shown to be affected by paternal uniparental isodisomy/heterodisomy involving chromosome 20q (patUPD20q), a rare cause of PHP-Ib 25,26,29,30. However, analysis of several microsatellite markers (i.e. D20S86, 907-rep2, 261P9-CA, 806-CA, 543J19-TTA, D20S171) located around the GNAS locus revealed no evidence for homozygosity in either of these patients, thus making patUPD comprising a large region of chromosome 20q unlikely.
Since our four pediatric patients presented first with an elevated TSH level, we next determined whether pediatric and adult patients (n=62) with subclinical hypothyroidism could also have GNAS methylation changes. Parameters of calcium homeostasis were normal in these individuals and MS-MLPA revealed no changes in GNAS methylation status.
Discussion
Modifications at the differentially methylated regions within GNAS, namely loss of one or all maternal methylation imprints, are typically observed in familial and most sporadic cases of PHP-Ib 3. These epigenetic changes in affected individuals impair Gsα expression from the maternal allele in all tissues, but the alterations have in most cells little or no functional consequences since the quantities of Gsα made from the paternal allele are sufficient to maintain cAMP-dependent signalling. However, in the proximal renal tubules, paternal Gsα expression appears to be silenced through as-of-yet unknown mechanisms. In the presence of inactivating mutations in GNAS exons 1-13 that are located on the maternal allele, as in PHP-Ia, or in the presence of maternal deletions within or up-stream of GNAS, as in PHP-Ib, little or no Gsα protein is made in this portion of the kidney thus leading to hormonal resistance 1-3. The responsible mechanisms that reduce Gsα expression from the paternal allele appear to have variable efficacy, since PTH-resistance does not become apparent in PHP-Ib patients until the ages of 2-3 years, and clinically symptomatic hypocalcemia usually does not develop until the beginning of the second decade of life 5,11. Furthermore, even in families in which the frequently encountered 3-kb deletion in STX16 has been documented on the maternal allele, laboratory evidence for PTH-resistance can be highly variable with some patients showing little or no elevation of PTH levels 5,11,22,23,31 although GNAS methylation changes are established shortly after fertilization and thus affect all tissues. Consistent with these findings in patients, mice show biallelic Gsα expression in the proximal renal tubules during early post-natal development. Subsequently, however, a major reduction of Gsα expression occurs at the paternal Gnas locus, thus leading, at least, in rodents with maternal ablation of Gnas exon 1 to PTH-resistance by the third week of life 21.
The silencing mechanisms that reduce the amount of Gsα derived from the paternal allele appear to be present not only in the proximal renal tubules 21, but also in other tissues, including pituitary, brown fat, and thyroid 21,32,33. In the latter tissue, substantially reduced paternal Gsα signalling is thus expected to lead in the presence of maternal GNAS mutations to transient or persistent TSH-resistance, possibly influenced further by genetic factors, environmental influences (i.e. iodine intake), or physiological changes during puberty and pregnancy. It is therefore conceivable that silencing of paternal Gsα transcription, combined with maternal GNAS methylation changes can cause TSH-resistance and consequently elevated TSH levels and even reduced FT4 levels. In three of our patients, these abnormalities occurred in the absence of anti-thyroid antibodies and a thyroid gland that appeared normal in size and texture as determined by ultrasonography.
However, patient 149/II-1 presented with autoimmune thyroiditis as well as TSH-resistance due to GNAS methylation changes. Her hypothyroidism could thus be explained by two distinct, possibly additive mechanisms, just like in a previously described patient with a homozygous TSH-receptor mutation, who later developed autoimmune thyroiditis 34. Since autoimmune thyroid disease occurs relatively frequently in the general population, our findings in patient 149/II-1 emphasize that GNAS methylation changes need to be considered even if anti-TPO antibodies have been detected.
By using MS-MLPA, we have now demonstrated broad GNAS methylation changes in three of our patients and an isolated loss of exon A/B methylation in one patient. The initial laboratory and clinical findings in one patient (149/II-1) were consistent with autoimmune hypothyroidism, but the epigenetic GNAS changes were undoubtedly present before and may have contributed to the TSH elevation and the reduced FT4 levels. The remaining three individuals revealed no evidence for anti-thyroid antibodies, thus their subclinical or overt hypothyroidism had developed well before PTH-resistant hypocalcemia. These findings suggest that silencing of Gsα transcription from the paternal allele and thus predominantly maternal expression of this ubiquitously expressed signaling protein can be as efficient in the thyroid as in the proximal renal tubules. The protein(s) contributing to the silencing of the Gsα promoter thus appear to be expressed at variable levels in different tissues with predominantly monoallelic Gsα expression. In fact, its expression levels may vary throughout life thus explaining the lack of PTH-resistance in infants, who later develop PHP-Ib, and the major delay in disease onset in some adult patients 5,11, including those presented in this report.
We failed to demonstrate GNAS methylation changes in a cohort of pediatric and adult patients with idiopathic subclinical hypothyroidism. However, more such patients need to be investigated to exclude epigenetic GNAS changes and thus diminished Gsα expression as a cause of this disorder. The observation that one of our PHP-Ib patients, namely patient 147/II-1, did not develop symptomatic hypocalcemia until 20 years after establishing the diagnosis of hypothyroidism, raises the question whether hypothyroid patients need to be screened for extended periods of time for changes in serum calcium and phosphate. While the genetic and/or epigenetic analysis of the GNAS locus is unlikely to be cost-effective, the periodic measurement of serum PTH, calcium, and phosphate levels should be considered. Because PTH levels are highly variable in PHP-Ib patients, even when caused by the same genetic mutation 4,11, it would be reasonable to search for GNAS methylation changes in all those hypothyroid patients, who present with any PTH elevation, unless 25OH vitamin D levels are reduced as evidence for vitamin D deficiency. Measuring only calcium, without PTH and phosphate, is probably insufficient for establishing the diagnosis of PHP-Ib in patients with idiopathic hypothyroidism since normal calcium blood levels can be maintained for extended periods of time because of PTH-stimulated bone resorption.
In conclusion, TSH elevation as evidence for subclinical or overt hypothyroidism can occur in some PHP-Ib patients with methylation change at the GNAS locus before PTH-resistant hypocalcemia becomes apparent. Our current data and previously reported findings indicate that significant silencing of Gsα expression from the paternal GNAS allele may occur in tissues other than the proximal renal tubules, including the thyroid. In fact, it is conceivable that other disorders, such as isolated obesity, intrauterine growth retardation, or short stature can be caused in some patients by GNAS methylation changes without obvious changes in PTH and/or TSH levels.
Supplementary Material
Figure 1.
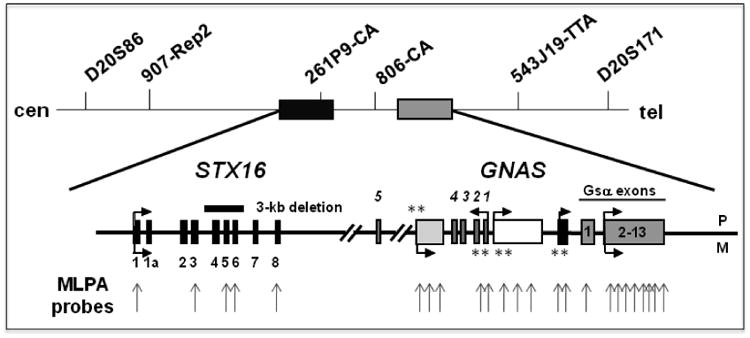
Schematic presentation of the GNAS-STX16 region on chromosome 20q13.3. The approximate locations of different microsatellites markers used in this study are shown, as are the multiple probes used MLPA and MS-MLPA (grey vertical arrows). Horizontal arrows depict direction of transcription; **, indicate the locations of differentially methylated regions and whether methylation occurs on either the paternal (P) or the maternal (M) allele. The location of the 3-kb deletion involving STX16 exons 4-6 is indicated by a horizontal black bar.
Acknowledgments
This work was supported by the National Institutes of Health (RO1 DK 46718-20 to H.J.)
Abbreviations
- PHP
Pseudohypoparathyroidism
- LOM
loss of methylation
- AHO
Albrights Hereditary Osteodystrophy
- PTH
parathyroid hormone
- TSH
thyroid-stimulating hormone
- GHRH
growth hormone-releasing hormone
- CT
calcitonin
- ANA
anti-nuclear antibodies
- SD
standard deviation
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.2408]
Disclosure Statement: The authors have nothing to disclose
Additional Supporting Information may be found in the online version of this article.
References
- 1.Weinstein L, Yu S, Warner D, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 2.Levine M. Hypoparathyroidism and pseudohypoparathyroidism. In: DeGroot L, Jameson J, editors. Endocrinology. Philadelphia, PA: W.B. Saunders Company; 2005. pp. 1611–36. [Google Scholar]
- 3.Bastepe M, Jüppner H. Pseudohypoparathyroidism, Albright's Hereditary Osteodystrophy, and Progressive Osseous Heteroplasia: disorders caused by inactivating GNAS mutations. In: DeGroot LJ, Jameson JL, editors. Endocrinology. 6th. Philadelphia, PA: W.B. Saunders Company; 2010. pp. 1223–35. [Google Scholar]
- 4.Bastepe M, Fröhlich LF, Hendy GN, et al. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest. 2003;112:1255–63. doi: 10.1172/JCI19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M. A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet. 2005;76:804–14. doi: 10.1086/429932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elli FM, de Sanctis L, Peverelli E, et al. Autosomal Dominant Pseudohypoparathyroidism type Ib: a novel inherited deletion ablating STX16 causes Loss of Imprinting at the A/B DMR. The Journal of clinical endocrinology and metabolism. 2014:jc20133704. doi: 10.1210/jc.2013-3704. [DOI] [PubMed] [Google Scholar]
- 7.Bastepe M, Fröhlich LF, Linglart A, et al. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet. 2005;37:25–7. doi: 10.1038/ng1487. [DOI] [PubMed] [Google Scholar]
- 8.Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. The Journal of clinical endocrinology and metabolism. 2010;95:3993–4002. doi: 10.1210/jc.2009-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard N, Abeguile G, Coudray N, et al. A new deletion ablating NESP55 causes loss of maternal imprint of A/B GNAS and autosomal dominant pseudohypoparathyroidism type Ib. The Journal of clinical endocrinology and metabolism. 2012;97:E863–7. doi: 10.1210/jc.2011-2804. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Nealon J, Weinstein L. Distinct patterns of abnormal GNAS imprinting in familial and sporadic pseudohypoparathyroidism type IB. Hum Mol Genet. 2005;14:95–102. doi: 10.1093/hmg/ddi009. [DOI] [PubMed] [Google Scholar]
- 11.Linglart A, Bastepe M, Jüppner H. Similar clinical and laboratory findings in patients with symptomatic autosomal dominant and sporadic pseudohypoparathyroidism type Ib despite different epigenetic changes at the GNAS locus. Clin Endocrinol (Oxf) 2007;67:822–31. doi: 10.1111/j.1365-2265.2007.02969.x. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani G, de Sanctis L, Barbieri AM, et al. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of Albright hereditary osteodystrophy and molecular analysis in 40 patients. The Journal of clinical endocrinology and metabolism. 2010;95:651–8. doi: 10.1210/jc.2009-0176. [DOI] [PubMed] [Google Scholar]
- 13.Zazo C, Thiele S, Martin C, et al. Gsα activity is reduced in erythrocyte membranes of patients with pseudohypoparathyroidism due to epigenetic alterations at the GNAS locus. J Bone Miner Res. 2011;26:1864–70. doi: 10.1002/jbmr.369. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Rebollo E, Pérez de Nanclares G, Lecumberri B, et al. Exclusion of the GNAS locus in PHP-Ib patients with broad GNAS methylation changes: evidence for an autosomal recessive form of PHP-Ib? J Bone Miner Res. 2011;26:1854–63. doi: 10.1002/jbmr.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Nanclares G, Romanelli V, Mayo S, et al. Detection of hypomethylation syndrome among patients with epigenetic alterations at the GNAS locus. The Journal of clinical endocrinology and metabolism. 2012;97:E1060–7. doi: 10.1210/jc.2012-1081. [DOI] [PubMed] [Google Scholar]
- 16.Wroe SF, Kelsey G, Skinner JA, et al. An imprinted transcript, antisense to Nesp, adds complexity to the cluster of imprinted genes at the mouse Gnas locus. Proc Natl Acad Sci U S A. 2000;97:3342–6. doi: 10.1073/pnas.050015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters J, Wroe SF, Wells CA, et al. A cluster of oppositely imprinted transcripts at the Gnas locus in the distal imprinting region of mouse chromosome 2. Proc Natl Acad Sci USA. 1999;96:3830–5. doi: 10.1073/pnas.96.7.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward BE, Moran V, Strain L, Bonthron DT. Bidirectional imprinting of a single gene: GNAS1 encodes maternally, paternally, and biallelically derived proteins. Proc Natl Acad Sci USA. 1998;95:15475–80. doi: 10.1073/pnas.95.26.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson CM, Ball ST, Nottingham WT, et al. A cis-acting control region is required exclusively for the tissue-specific imprinting of Gnas. Nat Genet. 2004;36:894–9. doi: 10.1038/ng1398. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Yu D, Lee E, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc Natl Acad Sci U S A. 1998;95:8715–20. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turan S, Fernandez-Rebollo E, Aydin C, et al. Postnatal establishment of allelic galphas silencing as a plausible explanation for delayed onset of parathyroid hormone resistance owing to heterozygous galphas disruption. J Bone Miner Res. 2014;29:749–60. doi: 10.1002/jbmr.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jüppner H, Schipani E, Bastepe M, et al. The gene responsible for pseudohypoparathyroidism type Ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. Proc Natl Acad Sci USA. 1998;95:11798–803. doi: 10.1073/pnas.95.20.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastepe M, Jüppner H. The GNAS locus and pseudohypoparathyroidism. Hormone Research. 2005;63:65–74. doi: 10.1159/000083895. [DOI] [PubMed] [Google Scholar]
- 24.Bastepe M, Pincus JE, Sugimoto T, et al. Positional dissociation between the genetic mutation responsible for pseudohypoparathyroidism type Ib and the associated methylation defect at exon A/B: evidence for a long-range regulatory element within the imprinted GNAS1 locus. Hum Mol Genet. 2001;10:1231–41. doi: 10.1093/hmg/10.12.1231. [DOI] [PubMed] [Google Scholar]
- 25.Bastepe M, Lane AH, Jüppner H. Paternal uniparental isodisomy of chromosome 20q (patUPD20q) - and the resulting changes in GNAS1 methylation - as a plausible cause of pseudohypoparathyroidism. Am J Hum Genet. 2001;68:1283–9. doi: 10.1086/320117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastepe M, Altug-Teber O, Agarwal C, Oberfield SE, Bonin M, Jüppner H. Paternal uniparental isodisomy of the entire chromosome 20 as a molecular cause of pseudohypoparathyroidism type Ib (PHP-Ib) Bone. 2011;48:659–62. doi: 10.1016/j.bone.2010.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turan S, Akin L, Akcay T, et al. Recessive versus imprinted disorder: consanguinity can impede establishing the diagnosis of autosomal dominant pseudohypoparathyroidism type Ib. Eur J Endocrinol. 2010;163:489–93. doi: 10.1530/EJE-10-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turan S, Ignatius J, Moilanen J, et al. De novo STX16 deletions: an infrequent cause of pseudohypoparathyroidism type Ib that should be excluded in sporadic cases. The Journal of clinical endocrinology and metabolism. 2012 doi: 10.1210/jc.2012-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Rebollo E, Lecumberri B, Garin I, et al. New mechanisms involved in paternal 20q disomy associated with pseudohypoparathyroidism. Eur J Endocrinol. 2011;163:953–62. doi: 10.1530/EJE-10-0435. [DOI] [PubMed] [Google Scholar]
- 30.Dixit A, Chandler KE, Lever M, et al. Pseudohypoparathyroidism type 1b due to paternal uniparental disomy of chromosome 20q. The Journal of clinical endocrinology and metabolism. 2013;98:E103–8. doi: 10.1210/jc.2012-2639. [DOI] [PubMed] [Google Scholar]
- 31.Bastepe M, Jüppner H. Pseudohypoparathyroidism: new insights into an old disease. In: Strewler GJ, editor. Endocrinology and Metabolism Clinics of North America: Hormones and Disorders of Mineral Metabolism. Philadelphia: W. B. Saunders; 2000. pp. 569–89. [DOI] [PubMed] [Google Scholar]
- 32.Hayward B, Barlier A, Korbonits M, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107:R31–6. doi: 10.1172/JCI11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The gsalpha gene: predominant maternal origin of transcription in human thyroid gland and gonads. The Journal of clinical endocrinology and metabolism. 2002;87:4736–40. doi: 10.1210/jc.2002-020183. [DOI] [PubMed] [Google Scholar]
- 34.Tonacchera M, Agretti P, De Marco G, et al. Thyroid resistance to TSH complicated by autoimmune thyroiditis. The Journal of clinical endocrinology and metabolism. 2001;86:4543–6. doi: 10.1210/jcem.86.9.7791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


