Abstract
Summary
We performed a systematic review and meta-analysis of the performance of clinical risk assessment instruments for screening for DXA-determined osteoporosis or low bone density. Commonly evaluated risk instruments showed high sensitivity approaching or exceeding 90 % at particular thresholds within various populations but low specificity at thresholds required for high sensitivity. Simpler instruments, such as OST, generally performed as well as or better than more complex instruments.
Introduction
The purpose of the study is to systematically review the performance of clinical risk assessment instruments for screening for dual-energy X-ray absorptiometry (DXA)-determined osteoporosis or low bone density.
Methods
Systematic review and meta-analysis were performed. Multiple literature sources were searched, and data extracted and analyzed from included references.
Results
One hundred eight references met inclusion criteria. Studies assessed many instruments in 34 countries, most commonly the Osteoporosis Self-Assessment Tool (OST), the Simple Calculated Osteoporosis Risk Estimation (SCORE) instrument, the Osteoporosis Self-Assessment Tool for Asians (OSTA), the Osteoporosis Risk Assessment Instrument (ORAI), and body weight criteria. Meta-analyses of studies evaluating OST using a cutoff threshold of <1 to identify US postmenopausal women with osteoporosis at the femoral neck provided summary sensitivity and specificity estimates of 89 % (95%CI 82–96 %) and 41 % (95%CI 23–59 %), respectively. Meta-analyses of studies evaluating OST using a cutoff threshold of 3 to identify US men with osteoporosis at the femoral neck, total hip, or lumbar spine provided summary sensitivity and specificity estimates of 88 % (95%CI 79–97 %) and 55 % (95%CI 42–68 %), respectively. Frequently evaluated instruments each had thresholds and populations for which sensitivity for osteoporosis or low bone mass detection approached or exceeded 90 % but always with a trade-off of relatively low specificity.
Conclusions
Commonly evaluated clinical risk assessment instruments each showed high sensitivity approaching or exceeding 90 % for identifying individuals with DXA-determined osteoporosis or low BMD at certain thresholds in different populations but low specificity at thresholds required for high sensitivity. Simpler instruments, such as OST, generally performed as well as or better than more complex instruments.
Keywords: Low bone density, Meta-analysis, Osteoporosis, Risk assessment, Screening, Systematic review
Introduction
Osteoporosis affects over 200 million people worldwide and is associated with significant costs, morbidity, and mortality secondary to fractures [1–4]. Osteoporosis is underdiagnosed and undertreated, despite availability of effective treatments [5–7]. Osteoporosis screening is recommended by many national clinical practice guidelines [8].
The gold standard test for diagnosing osteoporosis is dual-energy X-ray absorptiometry (DXA), which measures bone mineral density (BMD). Evidence of osteoporosis treatment efficacy to reduce fracture risk has been shown in clinical trials for individuals with osteoporosis by DXA criteria or prior osteoporotic fracture. For this reason, DXA testing is typically done to identify individuals who have not previously experienced an osteoporotic fracture who are most likely to benefit from treatment. In recent years, there has been interest in the use of osteoporosis clinical risk assessment instruments as an initial prescreening tool prior to DXA. These risk instruments/tools assess individuals’ clinical risk factors for osteoporosis to help gauge whether risk is sufficient for further evaluation with DXA. There are several potential advantages to use of clinical risk assessment tools as an initial osteoporosis screening test, including greater accessibility—these instruments can be used anywhere, including outpatient offices, nursing homes, etc., compared with DXA which is typically done at referral centers—and lower costs—the cost associated with risk instruments is primarily the time it takes to administer them, typically several minutes, in comparison to DXA which costs approximately $50 in the USA in 2014 [9].
The purpose of this study was to perform a systematic review and meta-analysis of the performance of clinical risk assessment instruments for identifying individuals with osteoporosis or low BMD by DXA criteria.
Methods
Data sources and search strategies
Literature search strategies were developed and performed in collaboration with a professional research librarian (AAS) to locate studies reporting the performance of clinical risk assessment instruments for identifying individuals with osteoporosis or low bone density (osteopenia) by DXA criteria in addition to studies that assessed instruments for predicting absolute fracture risk. This study reports findings for the systematic review and meta-analysis of clinical risk assessment instruments for identifying individuals with osteoporosis or low bone density; findings for the performance of risk instruments for predicting absolute fracture risk were reported in a prior study [10].
Databases searched included the following: Embase.com Embase (1974–2011), Wiley Cochrane Library (1898–2011), OvidSP MEDLINE (1948–June 2011), OvidSP MEDLINE Daily Update (June 2011), OvidSP In Process & Other Non-Indexed Citations (June 2011), ISI Web of Science limited to Proceeding Papers and Meeting Abstracts (1945–2011), ISI BIOSIS Previews limited to Meetings (1969–2011), Scopus (1960–2011), ClinicalTrials.gov (1999–2011), Health Services Research Projects in Progress (1995–2011), VHL LILACS (1982–2011), VHL IBECS (1999–2009), ProQuest Dissertations & Theses (1861–2011), NRR Archives (2000–2007), and OpenGrey (1980–2005). All initial literature searches were completed in June and July 2011. The MEDLINE search was updated in August 2014. The detailed MEDLINE search strategy used has been reported in the study of Nayak et al. [10]. Other database search strategies are available upon request.
Supplementary literature search methods included reviewing reference lists of included studies and topical reviews to locate additional studies. We also handsearched Osteoporosis International, Endocrine Reviews, and the Journal of Bone and Mineral Research for relevant articles from 1990 to 2011.
Study selection
Inclusion and exclusion criteria were applied to yield relevant studies. We included studies that evaluated the performance of a clinical risk assessment instrument to identify individuals with central DXA-determined osteoporosis or low BMD, provided sensitivity and specificity values (or sufficient data to calculate these values) for at least one specified cutoff threshold of the evaluated instrument used to identify individuals with BMD T-scores below a specified DXA threshold, reported original data, and had adult participants. We included studies of clinical risk tools combined with quantitative ultrasound, X-ray, or other non-DXA test results in the risk tool algorithm, provided that the instrument contained a clinical risk factor component. We included studies published in any format (e.g., journal article, government report, abstract) and any language of publication and had no restrictions on study participant characteristics (other than age ≥18 years) or co-morbidities. We excluded studies that did not evaluate clinical risk assessment instrument performance in populations independent of the instrument development cohort.
We reviewed studies for inclusion in two stages, title/ abstract followed by full text. We used Google Translate translation system to translate foreign language studies. One reviewer assessed all studies for inclusion or exclusion at the title/abstract stage (DLE); a second reviewer (SN) assessed all studies for which there were questions about potential eligibility at that stage. Two reviewers (DLE and SN) assessed all studies retrieved for full-text review for eligibility.
Data extraction
Information extracted from eligible studies included participant numbers, participant characteristics, study location, clinical risk assessment instrument(s) evaluated, DXA reference sites assessed, risk instrument thresholds (cutoff values used to separate positive from negative results) assessed, DXA low BMD or osteoporosis thresholds used, sensitivity and specificity associated with each threshold, area under the receiver operating characteristic (ROC) curve (AUC) if reported, and potential sources of bias.
Data analysis
We performed random-effects meta-analysis using the DerSimonian and Laird method to calculate summary estimates of sensitivity and specificity for each separate risk assessment instrument for which there were at least three studies evaluating performance in a similar population within the same country and reporting sensitivity and specificity estimates for the same combination of risk tool cutoff threshold and DXA reference sites and threshold. All analyses were performed using Stata version 11.0 (StataCorp, College Station, TX). Furthermore, we qualitatively described findings for instruments and thresholds for which data were insufficient for meta-analysis and evaluated potential sources of bias.
Results
Literature search and study selection
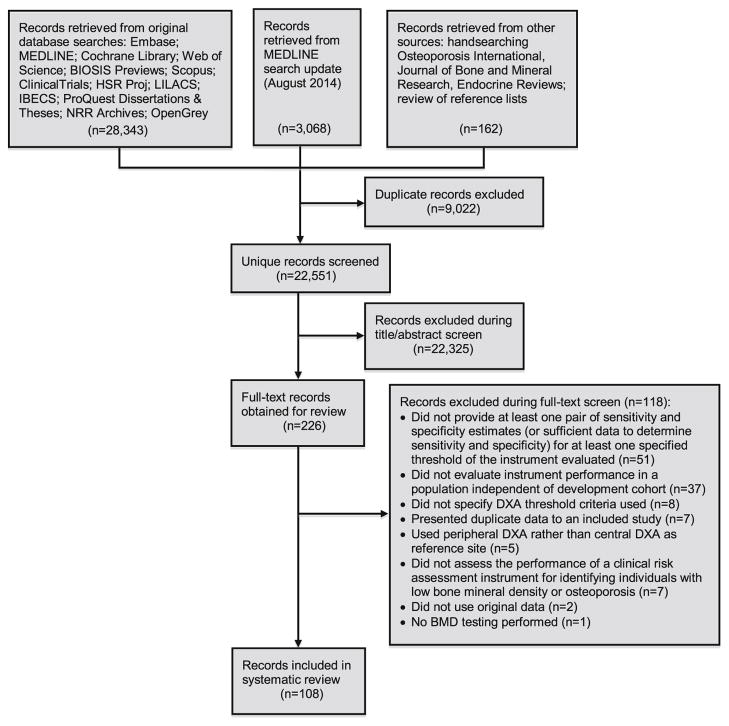
The literature search yielded 22,551 separate records for review, of which 108 met inclusion criteria [11–118]. A flow diagram of the literature search and study selection process is shown in Fig. 1.
Fig. 1.
Flow diagram of literature search and study selection
Study characteristics
Included study characteristics are described in the Electronic Supplementary Material (Appendix Table 1). The most commonly assessed instruments were the Osteoporosis Self-Assessment Tool (OST) (55 studies), the Simple Calculated Osteoporosis Risk Estimation (SCORE) instrument (32 studies), the Osteoporosis Self-Assessment Tool for Asians (OSTA) (27 studies), the Osteoporosis Risk Assessment Instrument (ORAI) (26 studies), and body weight criteria (15 studies). Many studies assessed multiple clinical risk assessment instruments. Only five studies evaluated a combination of a peripheral bone density assessment test with a clinical risk assessment tool [33, 59, 86, 88, 117]. Eighty-five studies were published as full-text articles, while 23 were abstracts only. Included studies were performed in 34 different countries. The number of study participants ranged from 60 to 21,063, and a mean participant age in the 60s was most common. Most studies (78) included female participants only, 24 studies had only male participants, and 6 studies included both sexes. The majority of studies did not report participants’ medical comorbidities; 5 studies included only individuals with rheumatoid arthritis [16, 43, 44, 47, 92]. Reported osteoporosis prevalence in study populations ranged from 4.1 to 44.8 %. Studies assessed the sensitivity and specificity of a variety of risk assessment instrument and DXA threshold combinations; most studies evaluated risk instruments for identifying participants with DXA-determined osteoporosis (T-score≤2.5) and assessed DXA sites of the femoral neck, total hip, and/or lumbar spine. Included studies were published between 1998 and 2014, and a large majority (101) was published in English.
Meta-analysis of performance of clinical risk assessment instruments for identifying individuals with osteoporosis by DXA criteria
Sufficient data was available for meta-analyses of the sensitivity and specificity of the OST risk assessment instrument for women and men in the USA, and the OSTA risk assessment instrument for women in Thailand. The total number of participants in all studies included in each of these meta-analyses was 31, 779 for OST for women in the USA [45, 51, 80], 760 for OST with a threshold (risk instrument cutoff value used to separate positive from negative results) of 3 for men in the USA [11, 60, 107], 5260 for OST with thresholds of 2 and 1 for men in the USA [11, 60, 71], 3079 and 2780 for OSTA with a threshold of ≤−1 for women in Thailand with DXA reference sites of the femoral neck [26, 39, 56, 84, 87, 88, 98, 109] and lumbar spine [26, 39, 56, 84, 87, 98, 109], respectively, and 1201 for OSTA with a threshold of ≤0 for women in Thailand [39, 84, 109]. Meta-analysis results are shown in Table 1. Meta-analysis of studies evaluating OST in US postmenopausal women with a threshold of <1 to identify individuals with osteoporosis at the femoral neck provided summary sensitivity and specificity estimates of 89 % (95%CI 82–96 %) and 41 % (95%CI 23–59 %), respectively. Meta-analysis of studies evaluating OST in predominantly older men in the USAwith a threshold of 3 to identify individuals with osteoporosis at the femoral neck, total hip, or lumbar spine provided summary sensitivity and specificity estimates of 88 % (95%CI 79–97 %) and 55 % (95%CI 42–68 %), respectively. Meta-analysis of studies evaluating OSTA in postmenopausal women in Thailand using a threshold of ≤0 to identify individuals with osteoporosis at the femoral neck provided summary sensitivity and specificity estimates of 90 % (95%CI 84–95 %) and 47 % (95%CI 30–64 %), respectively. Summary sensitivity estimates were lower (and specificity estimates typically higher) for the meta-analyses performed using other OST and OSTA thresholds and DXA references site combinations for US older men and postmenopausal women in Thailand (Table 1). There was significant between-study heterogeneity in all but one of the performed meta-analyses, as demonstrated by high I-squared values.
Table 1.
Meta-analysis results
| Risk instrument | Country | Population | Risk instrument threshold | DXA reference site and T-score threshold | Summary sensitivity estimate from meta-analysis (%c (95%CI; I2 valued)) | Summary specificity estimate from meta-analysis (%c (95%CI; I2 valued)) |
|---|---|---|---|---|---|---|
| OSTa | USA | Postmenopausal women [40, 45, 68] | <1 | Femoral neck, ≤−2.5 | 89 (82–96; I2=98.8 %) | 41 (23–59; I2=99.9 %) |
| OSTa | USA | Men [11, 52, 90] | 3 | Femoral neck, total hip, or lumbar spine, ≤−2.5 | 88 (79–97; I2=68.5 %) | 55 (42–68; I2=89.6 %) |
| OSTa | USA | Men [11, 52, 59] | 2 | Femoral neck, total hip, or lumbar spine, ≤−2.5 | 81 (70–92; I2=83.2 %) | 54 (32–76; I2=98.5 %) |
| OSTa | USA | Men [11, 52, 59] | 1 | Femoral neck, total hip, or lumbar spine, ≤−2.5 | 73 (62–84; I2=81.0 %) | 64 (45–83; I2=98.2 %) |
| OSTAb | Thailand | Postmenopausal women [26, 39, 56, 84, 87, 88, 98, 109] | ≤−1 | Femoral neck, ≤−2.5 | 84 (76–92; I2=76.8 %) | 61 (50–72; I2=97.4 %) |
| OSTAb | Thailand | Postmenopausal women [26, 39, 56, 84, 87, 98, 109] | ≤−1 | Lumbar spine, ≤−2.5 | 71 (60–82; I2=89.7 %) | 62 (52–73; I2=96.6 %) |
| OSTAb | Thailand | Postmenopausal women [39, 84, 109] | ≤0 | Femoral neck, ≤−2.5 | 90 (84–95; I2=10.4 %) | 47 (30–64; I2=97.1 %) |
| OSTAb | Thailand | Postmenopausal women [39, 84, 109] | ≤0 | Lumbar spine, ≤−2.5 | 83 (67–99; I2=91.1 %) | 48 (36–60; I2=93.4 %) |
Osteoporosis Self-Assessment Tool
Osteoporosis Self-Assessment Tool for Asians
Random-effects meta-analysis using DerSimonian and Laird method
Percentage of variation across studies attributable to heterogeneity
There were insufficient numbers of studies evaluating other risk assessment instruments within similar populations within the same country and using the same risk tool and DXA thresholds to perform meta-analysis for other instruments or for meta-analysis of the performance of OST and OSTA in other countries or for identifying individuals with low BMD rather than osteoporosis.
Qualitative review of the performance of clinical risk assessment instruments for identifying individuals with osteoporosis or low BMD by DXA criteria
We qualitatively reviewed the performance of frequently evaluated risk assessment instruments (OST, SCORE, ORAI, OSTA, and body weight criteria) for identifying individuals with DXA-determined osteoporosis (T-score≤−2.5) or low BMD with T-scores≤−2.0 at the femoral neck, total hip, or lumbar spine—commonly accepted reference sites for the diagnosis of osteoporosis [119]. Studies that evaluated OST for women reported performance estimates ranging from a high sensitivity of 99 % and corresponding specificity of 15 % with use of a threshold of <6 to identify perimenopausal and early postmenopausal women in Denmark with osteoporosis [96] to a low sensitivity of 17.7 % (95%CI 16.0–19.5 %) and corresponding specificity of 95.7 % (95%CI 94.8–96.4 %) with use of a threshold of −3 to identify postmenopausal women in Argentina with osteoporosis [100]; AUCs ranged from 0.652 (95%CI 0.604–0.699) [72] to 0.77 [95]. Studies that evaluated OST to identify men with osteoporosis or low BMD at the femoral neck, total hip, or lumbar spine reported performance estimates ranging from a high sensitivity of 93 % and corresponding specificity of 66 % with use of a threshold of 3 to identify US male veterans with osteoporosis [11] to a low sensitivity of 6 % and corresponding specificity of 94 % with use of a threshold of −2 to identify US men with rheumatoid arthritis with osteoporosis [92]; AUCs ranged from 0.590 (95%CI 0.492–0.688) when evaluating OST with a threshold of <4 in Portuguese men aged ≥50 [73] to 0.993 in a subgroup analysis of US male veterans aged ≥80 [11].
For the SCORE instrument, performance estimates for identifying women with osteoporosis or low BMD at the femoral neck, total hip, or lumbar spine ranged from a high sensitivity of 98.9 % and corresponding specificity of 5.7 % with use of a threshold of 6 to identify postmenopausal Belgian women aged >65 with osteoporosis [15] to a low sensitivity of 44 % and corresponding specificity of 77 % with use of a threshold of >7 to identify perimenopausal and early postmenopausal women in Denmark with osteoporosis [96]; AUCs ranged from 0.64 to 0.76 [34]. For ORAI, reported sensitivity for identifying women with osteoporosis ranged from a high value of 100 % (95%CI 94.9–100 %) with corresponding specificity reported as not applicable (no individuals tested negative) with use of a threshold of ≥9 for Portuguese post-menopausal women aged ≥65 years [72] to a low sensitivity of 3 % with corresponding specificity of 98 % with use of a threshold of >11 for perimenopausal and early postmenopausal women in Denmark [96]; AUCs ranged from 0.64 (95%CI 0.58–0.70) [96] to 0.703 [32].
For the OSTA instrument for identifying women with osteoporosis at the femoral neck, total hip, or lumbar spine, performance estimates ranged from a high sensitivity of 93.0 % (95%CI 84.3–97.7 %) and corresponding specificity of 20.2 % (95%CI 12.5–30.1 %) with use of a threshold of <2 for Portuguese postmenopausal women aged ≥65 years [72] to a low sensitivity of 29.9 % (95%CI 19.3–42.3 %) and corresponding specificity of 91.1 % (95%CI 88.2–93.5 %) with use of a threshold of ≤−1 for postmenopausal Chinese women aged 45–59 [114]; AUCs ranged from 0.62 (95%CI 0.56–0.68) [84] to 0.668 (95%CI 0.619–0.716) [72]. Reported sensitivities and specificities for OSTA for identifying men with osteoporosis at the femoral neck, total hip, or lumbar spine ranged from a high sensitivity of 87.33 % and corresponding specificity of 56.20 % with use of a threshold of ≤−1 for Chinese men aged ≥50 years [69] to a low sensitivity of 38.2 % and corresponding specificity of 82.1 % with use of a threshold of <1 for Portuguese men aged ≥50 years [73]; AUCs ranged from 0.597 (95%CI 0.497–0.697) [73] to 0.676 (95%CI 0.612–0.732) [117].
Two studies evaluated body weight criteria for identifying women with osteoporosis at the femoral neck, total hip, or lumbar spine [72, 78]. The highest reported sensitivity was 88.0 % (95%CI 68.8–97.5 %) with a corresponding specificity of 43.6 % (95%CI 36.2–51.2 %) when using a threshold of <70 kg for 40–54-year-old Portuguese postmenopausal women [72], and the lowest reported sensitivity was 39.6 % (95%CI 38.5–40.6 %) with a corresponding specificity of 82.8 % (95%CI 82.0–83.6 %) when using a threshold of ≤57 kg for Canadian women aged 40–59 years [78]. AUCs ranged from 0.611 (95%CI 0.562–0.661) for Portuguese post-menopausal women [72] to 0.71 (95%CI 0.68–0.75) for a subgroup of 40–49-year-old Canadian women [78]. One study evaluated body weight criteria for identifying men with osteoporosis at the femoral neck, total hip, or lumbar spine [73]. This study, which included Portuguese men aged ≥50 years, reported a high sensitivity of 82.4 % and corresponding specificity of 35.7 % when evaluating a threshold of <80 kg (AUC 0.590 (95%CI 0.492–0.689)) and conversely a low sensitivity of 26.5 % and specificity of 89.3 % when evaluating a threshold of <65 kg (AUC 0.579 (95%CI 0.467–0.691)) [73].
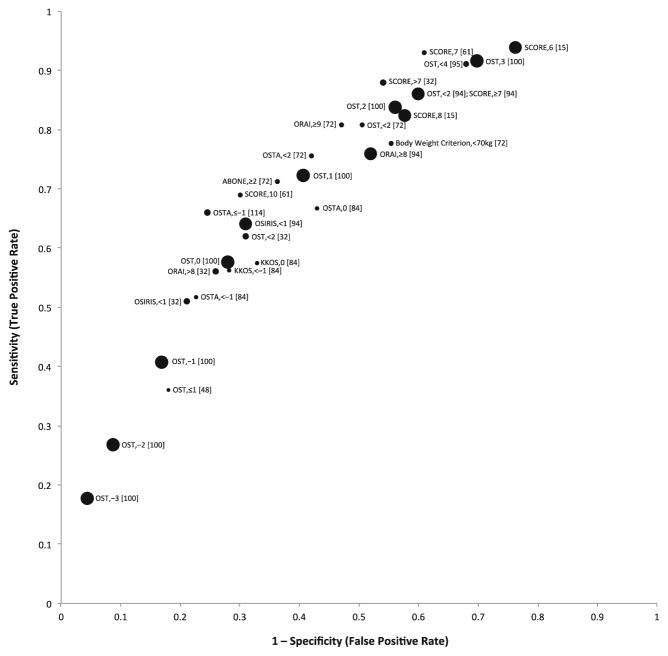
Figure 2 is a plot of overall analysis results for sensitivity (true positive rate) versus 1 minus specificity (false positive rate) from studies that evaluated the performance of clinical risk assessment instruments in postmenopausal female study populations for identifying DXA-determined osteoporosis as defined by T-score≤−2.5 at the femoral neck, total hip, or lumbar spine. Risk assessment instrument performance estimates for postmenopausal women varied by study, risk instrument assessed, and risk instrument threshold assessed. The OST and SCORE instruments at several different thresholds (3 or 4 for OST and 6 or 7 for SCORE) demonstrated the highest sensitivities for identifying postmenopausal women with osteoporosis in these studies.
Fig. 2.
Scatter plot of sensitivity (true positive rate) versus 1–specificity (false positive rate) for studies evaluating clinical risk assessment instrument performance for identifying postmenopausal women with DXA-determined osteoporosis (T-score≤−2.5) at the femoral neck, total hip, or lumbar spine. Each point is labeled with the corresponding risk instrument, risk instrument cutoff threshold (cutoff value used to separate positive from negative results), and associated reference. Points are proportional to the number of study participants; however, sizes are not to scale
Performance of clinical risk assessment instruments by age
A number of studies assessed performance of SCORE, OST, ORAI, OSTA, or body weight criteria in different age subgroups when using the same risk instrument thresholds for identifying individuals with osteoporosis or low BMD at various DXA references sites. Six studies evaluated SCORE performance in younger and older perimenopausal or postmenopausal women [15, 34, 57, 75, 81, 97]; four found lower sensitivity of SCORE for younger women in their studies than older women when using the same thresholds, and all found higher specificity for younger women than older women. Three studies assessed OST in older and younger subgroups of postmenopausal women [72, 74, 80]; all found that that OST had lower sensitivity but higher specificity for younger postmenopausal women when using the same thresholds. Four studies assessed OST performance for age subgroups of men over the age of 50; three found lower sensitivity for the younger men in their analyses, and all four studies found higher specificity for the younger men subgroup [11, 60, 91, 93]. Four studies evaluated ORAI performance in younger compared to older postmenopausal women; three found lower sensitivity for younger women, and all found higher specificity for younger women [72, 74, 75, 81]. Three studies evaluated OSTA performance in younger and older perimenopausal or postmenopausal women, and all found lower OSTA sensitivity but higher OSTA specificity in younger women [72, 87, 114]. Two studies evaluated body weight criteria (<70 kg) performance in younger and older postmenopausal women; one study found higher sensitivity for women 40–55 years of age and similar specificity for younger and older women [72], and the other found similar sensitivity and specificity for younger and older postmenopausal women [74].
Study quality and potential sources of bias
We evaluated studies on several quality criteria to assess potential for bias, including sample size, recruitment of a cohort unclassified by disease (osteoporosis or low BMD) state, time between risk tool administration and DXA testing, independence of interpretation of risk tool and DXA results, and source of funding. A large majority of studies had at least 30 participants with and 30 participants without osteoporosis or low bone density, including all studies included in the meta-analyses for OST for US women. However, several studies included in meta-analyses of OSTA for women in Thailand did not have at least 30 participants with osteoporosis at either the femoral neck or lumbar spine [26, 56, 84], and two studies included in meta-analyses of OST for men in the USA [11, 107] did not have 30 participants with osteoporosis. Nearly all studies implied recruitment of participants as a cohort unclassified by disease state and did not report independence of interpretation of risk tool and DXA results. A large majority of studies did not report the time between risk instrument and DXA testing for participants. Most studies did not report their funding source; 28 studies reported pharmaceutical company funding or author association with a pharmaceutical company [11, 16–18, 20, 23, 36, 38, 40, 41, 49–51, 58, 59, 64, 71–73, 76, 78, 85, 91–93, 102, 103, 118], including three that were included in meta-analyses of OST for US men [11, 71, 93].
Discussion
Our systematic review and meta-analysis of the performance of clinical risk assessment instruments for screening for osteoporosis or low bone density found that many studies have been performed to assess performance of these tools; however, evidence for each tool’s performance when using the same risk instrument threshold and DXA threshold and reference sites within a particular country’s population is limited, with only OST (for US women and men) and OSTA (for women in Thailand) having a sufficient number of similar studies for meta-analysis of sensitivity and specificity estimates. Our meta-analysis findings showed reasonably high (88–90 %) summary sensitivity estimates for OST for postmenopausal women in the USA and OSTA for postmenopausal women in Thailand when using thresholds of <1 and ≤0, respectively, to identify women with osteoporosis at the femoral neck and for OST for older US men when using a threshold of 3 to identify men with osteoporosis at the femoral neck, total hip, or lumbar spine. However, the corresponding specificity estimates at these thresholds were low, in the range of 40–55 %. Our qualitative review of studies that evaluated risk instruments of OST, SCORE, ORAI, OSTA, and body weight criteria revealed that each showed sensitivity near or exceeding 90 % for identifying individuals with DXA-determined osteoporosis or low BMD with a T-score≤−2.0 at the femoral neck, total hip, or lumbar spine when using various risk instrument thresholds within different populations but low specificity, frequently below 40 %, at thresholds required for high sensitivity. Thus, at clinical risk tool thresholds required to identify nearly or more than 90 % of individuals with osteoporosis or low BMD with a T-score≤−2.0, many false positives can also be expected, with approximately half to most individuals without osteoporosis or DXA T-score≤−2.0 also testing positive. AUCs for commonly evaluated risk instruments to identify individuals with osteoporosis or T-score≤−2.0 at the femoral neck, total hip, or lumbar spine varied in different study populations, with values most commonly in the 0.6 to 0.8 range; an AUC of 1 indicates a perfectly accurate test for distinguishing individuals with and without the condition of interest, whereas an AUC of 0.5 indicates a useless test. In general, AUCs of 0.80–0.90 are considered good, 0.70–0.80 are considered fair, and 0.60–0.70 are considered poor [120].
Among studies that evaluated the performance of clinical risk assessment instruments in postmenopausal female study populations for identifying DXA-determined osteoporosis as defined by T-score≤−2.5 at the femoral neck, total hip, or lumbar spine, OST and SCORE evaluated at several different thresholds (3 or 4 for OST and 6 or 7 for SCORE) demonstrated the highest sensitivities. OSTA, body weight criterion, and ORAI at various thresholds demonstrated somewhat lower sensitivity among these studies. Studies that evaluated the Age, Body Size, No Estrogen (ABONE) and OSIRIS risk tools for postmenopausal women at cut points of ≥2 or <1, respectively, for diagnosis of osteoporosis at various DXA reference sites generally found insufficient sensitivity compared to other options; thus, we recommend against use of ABONE or OSIRIS at these cut points given better alternatives. In general, simpler instruments, such as OST, OSTA, or even body weight criteria alone performed as well as (and in several studies, most frequently for OST, better than) more complex instruments, and thus, we recommend use of a simple instrument, such as OST, over more complex instruments. Even body weight criteria, with its simplicity (no calculation required), performed comparably in several studies to some tools with more risk factors (e.g., ORAI), although generally not as well as OST or SCORE. However, given its simplicity and that primary care patients are weighed at almost every appointment, body weight criteria have potential for easy clinical use.
Even within our meta-analyses limited to studies done within similar populations within the same country using identical risk instrument and DXA thresholds, there was significant between-study heterogeneity. One possible source of heterogeneity is variation in participant age; for instance, of the studies included in the meta-analyses of OST for postmenopausal US women, the study by Gourlay et al. that included only older postmenopausal women aged 67 years and older [45] reported higher sensitivity and lower specificity than the other two included studies, one which included participants aged 45–81 [51] and the other which had a majority of participants younger than age 65 [80]. Our qualitative review of studies that performed age subgroup analyses for the OST, SCORE, OSTA, or ORAI risk instruments revealed that for each of these instruments when using the same threshold for different age subgroups, sensitivity for identifying individuals with osteoporosis or low BMD was lower at a majority of the time (and specificity consistently higher) for younger compared to older postmenopausal women or older men. Our findings suggest that for many instruments, different thresholds may be needed for screening individuals in different age ranges to ensure adequate sensitivity, particularly for younger (such as early postmenopausal) individuals. Another potential source of heterogeneity in our analyses is slight variation in how risk instrument scores were calculated between studies. For example, of the studies that were included in the meta-analyses of OST for US men, three calculated OST values using the formula [0.2×(weight in kilograms-age in years), truncated to give an integer] [11, 71, 107], whereas one study calculated OST using the formula [0.2×(weight in kilograms -age in years), rounding to the nearest integer] (personal communication) [60].
Our findings indicate a general pattern of osteoporosis clinical risk assessment instruments having a significant trade-off between sensitivity and specificity, and fair at best overall ability to distinguish individuals with and without osteoporosis or T-scores≤−2.0 by DXA criteria at the femoral neck, total hip, or lumbar spine. Our findings generally agree with prior more limited reviews evaluating the performance of these instruments. A systematic review by Rubin et al. that included 31 studies of risk instruments for predicting low BMD also found no consistently best performing tool, and that simple tools performed equal to or better than more complex instruments [121]. A systematic review by Nelson et al. in 2010 compared AUC performance estimates for 23 studies of risk assessment instruments to predict BMD T-score≤−2.5 and also found that most AUCs were in the range of 0.6 to 0.8, indicating modest prediction of DXA-determined osteoporosis [122]. This study also found that instruments with fewer risk factors often performed equally to or better than more complicated instruments and did not identify a clearly best performing instrument [122]. A systematic review by Rud et al. that included studies comparing OST to other tests to select women for BMD testing found that the diagnostic odds ratio did not differ significantly among OST, SCORE, and ORAI instruments [123].
Osteoporosis clinical risk assessment instruments are not currently widely used for screening by primary care physicians, unlike some risk instruments for other medical conditions—for example, the Framingham Risk Score for 10-year cardiovascular event risk. This is despite the fact that many osteoporosis risk instruments are simpler than the Framingham Risk Score, and discrimination performance of the Framingham Risk Score does not appear to be better than osteoporosis clinical risk assessment instruments, with c statistic values (analogous to AUCs) ranging from 0.63 to 0.83 in different populations [124]. Several factors may contribute to greater use of the Framingham Risk Score. First, heart disease is the leading cause of death of women and men, with mortality rates substantially higher than that associated with osteoporosis; thus, given that physicians have competing preventive care demands, it is not surprising that they may prioritize heart disease prevention. Another factor that may contribute to lower use for osteoporosis clinical risk instruments is lack of evidence for whether their standardized use would reduce fracture rates. An additional barrier is the existence of different osteoporosis clinical risk instrument cutoff thresholds to define a positive test result when screening among different populations, such as women versus men, or individuals of different ages. Such “moving-target” thresholds are an impediment for busy clinicians who have limited time in a brief patient visit to identify the appropriate threshold. This problem could be addressed by providing an easy-to-use online osteoporosis risk instrument calculator for physicians to enter key data about their patient (e.g., age and sex) and have this data automatically processed to report whether a patient’s risk instrument score is sufficient to warrant further evaluation.
Our systematic review and meta-analysis results by themselves are insufficient to answer the question of whether osteoporosis clinical risk assessment tools should be used routinely in clinical practice. This question would be best addressed with a comprehensive comparative effectiveness analysis that compares different screening tests and thresholds to identify the best strategies for patients with different key characteristics such as age and sex. It is likely that the best screening strategies would vary according to patient characteristics. Although specificity is generally poor for osteoporosis clinical risk assessment instruments at the thresholds required to identify approximately 90 % of individuals with osteoporosis or low BMD, it is possible that it may still be worthwhile to prescreen individuals with a clinical risk assessment instrument and reduce the number of people without osteoporosis or low BMD referred for DXA testing by 50 % or so. Several previous studies have found that osteoporosis risk assessment instruments can be cost-effective screening tools, despite their low specificity [125, 126]. Our findings can be applied to future comparative effectiveness analyses to evaluate whether prescreening with clinical risk assessment instruments may be a good option for patients with different characteristics. In the absence of an up-to-date comparative effectiveness analysis of all available osteoporosis screening options for women and men of different ages, evidence is currently lacking to recommended routine use of osteoporosis clinical risk assessment instruments as an initial screening test over DXA. However, these tools are a viable screening option for individuals who are not able to easily access DXA testing or who would prefer a non-DXA initial screening test. If an osteoporosis clinical risk assessment instrument is chosen for initial screening, we recommend use of a simple instrument (e.g., OST).
Our study has several limitations. First, the heterogeneity of included studies limited our ability to perform meta-analysis of all the data. Even within the meta-analyses performed, there was significant statistical heterogeneity. Another limitation was the total number of study participants included in the studies in our meta-analysis of OST for screening US men with a threshold of 3 was relatively small (760). Furthermore, we found mixed quality of the studies included in this systematic review. Moreover, publication bias is a possibility, with studies showing favorable performance results being preferentially published; however, we included abstracts in addition to full-text articles to mitigate this potential bias. Our study had several notable strengths. This study is the most comprehensive review of the performance of clinical risk assessment instruments for identifying individuals with osteoporosis or low BMD to date; we included greater than 70 more studies than any prior review on this topic that we are aware of, after performing an exhaustive literature search. Additionally, we performed meta-analyses of performance estimates of OST for women and men in the USA and OSTA for women in Thailand, including data from nearly 32,000 participants in several different studies for the meta-analyses of OST for US postmenopausal women.
In conclusion, our findings show that commonly evaluated risk instruments of OST, SCORE, ORAI, OSTA, and body weight criteria each demonstrate high sensitivity approaching or exceeding 90 % for identifying individuals with DXA-determined osteoporosis or low BMD with a T-score≤−2.0 at particular thresholds within various populations but with a trade-off of low specificity at thresholds required for high sensitivity. Simpler instruments, such as OST, generally perform as well as or better than more complex instruments. Currently, the lack of standardized cutoff thresholds for these instruments limits their potential for clinical use; thus, cut point standardization is an important area for future research. Additional studies are also needed to evaluate the comparative effectiveness and cost-effectiveness of use of clinical risk assessment instruments for initial prescreening of individuals for osteoporosis or low BMD compared with other screening strategies.
Supplementary Material
Acknowledgments
Sources of funding Smita Nayak was supported by grant 7R01AR060809-04 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; Susan L. Greenspan was supported by NIH grant P30AG024827 from the National Institute on Aging.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00198-015-3025-1) contains supplementary material, which is available to authorized users.
Conflicts of interest None.
Contributor Information
S. Nayak, Email: smita.nayak@swedish.org, Swedish Center for Research and Innovation, Swedish Health Services, Swedish Medical Center, 747 Broadway, Seattle, WA 98122-4307, USA
D. L. Edwards, Swedish Center for Research and Innovation, Swedish Health Services, Swedish Medical Center, 747 Broadway, Seattle, WA 98122-4307, USA
A. A. Saleh, Arizona Health Sciences Library, University of Arizona, Tucson, AZ, USA
S. L. Greenspan, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1.Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004:126–134. [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord. 2003;4:24. doi: 10.1186/1471-2474-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 5.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment, 1988–2003. Arch Intern Med. 2004;164:1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TV, Center JR, Eisman JA. Osteoporosis: underrated, underdiagnosed and undertreated. Med J Aust. 2004;180:S18–S22. doi: 10.5694/j.1326-5377.2004.tb05908.x. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P, Rejnmark L, Mosekilde L. Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int. 2005;16:134–141. doi: 10.1007/s00198-004-1680-8. [DOI] [PubMed] [Google Scholar]
- 8.Leslie WD, Schousboe JT. A review of osteoporosis diagnosis and treatment options in new and recently updated guidelines on case finding around the world. Curr Osteoporos Rep. 2011;9:129–140. doi: 10.1007/s11914-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 9.CMS Medicare Physician Fee Schedule. http://www.cms.gov/apps/physician-fee-schedule/
- 10.Nayak S, Edwards DL, Saleh AA, Greenspan SL. Performance of risk assessment instruments for predicting osteoporotic fracture risk: a systematic review. Osteoporos Int. 2014;25:23–49. doi: 10.1007/s00198-013-2504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723–727. doi: 10.4065/78.6.723. [DOI] [PubMed] [Google Scholar]
- 12.Anders ME. In: Evaluation of clinical decision rules for bone mineral density testing in a nationally representative sample. Turner L, editor. University of Arkansas; United States: 2006. [Google Scholar]
- 13.Anders ME, Turner LW, Wallace LS, Spencer HJ, Simpson DD. Predictive accuracy of two cut scores for the Osteoporosis Self-Assessment Tool in a nationally representative sample of men. Osteoporos Int. 2006;17:960. [Google Scholar]
- 14.Ben Sedrine W, Ethgen O, Devogelaer J-P, Depresseux G, Kaufman J-M, Goemaere S, Reginster J-Y. Evaluation of proposals of Belgian Social Security Institute for reimbursement of bone densitometry tests. Toward a cost-effective strategy for osteoporosis screening? Aging Clin. 2004;16:413–419. doi: 10.1007/BF03324573. [DOI] [PubMed] [Google Scholar]
- 15.Ben Sedrine W, Devogelaer JP, Kaufman JM, Goemaere S, Depresseux G, Zegels B, Deroisy R, Reginster JY. Evaluation of the simple calculated osteoporosis risk estimation (SCORE) in a sample of white women from Belgium. Bone. 2001;29:374–380. doi: 10.1016/s8756-3282(01)00583-x. [DOI] [PubMed] [Google Scholar]
- 16.Brand C, Lowe A, Hall S. The utility of clinical decision tools for diagnosing osteoporosis in postmenopausal women with rheumatoid arthritis. BMC Musculoskelet Disord. 2008;9:13. doi: 10.1186/1471-2474-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenneman SK, Lacroix AZ, Buist DSM, Chen YT, Abbott TA., III Evaluation of decision rules to identify postmenopausal women for intervention related to osteoporosis. Dis Manage. 2003;6:159–168. doi: 10.1089/109350703322425509. [DOI] [PubMed] [Google Scholar]
- 18.Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP Canadian Multicentre Osteoporosis S. Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA. 2001;286:57–63. doi: 10.1001/jama.286.1.57. [DOI] [PubMed] [Google Scholar]
- 19.Cadarette SM, McIsaac WJ, Hawker GA, Jaakkimainen L, Culbert A, Zarifa G, Ola E, Jaglal SB. The validity of decision rules for selecting women with primary osteoporosis for bone mineral density testing. Osteoporos Int. 2004;15:361–366. doi: 10.1007/s00198-003-1552-7. [DOI] [PubMed] [Google Scholar]
- 20.Cadarette SM, Jaglal SB, Murray TM. Validation of the Simple Calculated Osteoporosis Risk Estimation (SCORE) for patient selection for bone densitometry. Osteoporos Int. 1999;10:85–90. doi: 10.1007/s001980050199. [DOI] [PubMed] [Google Scholar]
- 21.Cass AR, Shepherd AJ. Validation of the Male Osteoporosis Risk Estimation Score (MORES) in a primary care setting. J Am Board Fam Med JABFM. 2013;26:436–444. doi: 10.3122/jabfm.2013.04.120182. [DOI] [PubMed] [Google Scholar]
- 22.Cass AR, Shepherd AJ, Carlson CA. Osteoporosis risk assessment and ethnicity: validation and comparison of 2 clinical risk stratification instruments. J Gen Intern Med. 2006;21:630–635. doi: 10.1111/j.1525-1497.2006.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan SP, Yeap SS, Hew FL, Chee SS, Zaitun Y, Daurio C, Ross PD. The osteoporosis self-assessment tool for Asians (OSTA): validation in Malaysia. Bone. 2003;32:S176. [Google Scholar]
- 24.Chan SP, Teo CC, Ng SA, Goh N, Tan C, Deurenberg-Yap M. Validation of various osteoporosis risk indices in elderly Chinese females in Singapore. Osteoporos Int. 2006;17:1182–1188. doi: 10.1007/s00198-005-0051-4. [DOI] [PubMed] [Google Scholar]
- 25.Chang SFY, RS Determining the cut-off point of osteoporosis based on the osteoporosis self-assessment tool, body mass index and weight in Taiwanese young adult women. J Clin Nurs. 2013;23:2628–2636. doi: 10.1111/jocn.12483. [DOI] [PubMed] [Google Scholar]
- 26.Chaovisitsaree S, Namwongprom SAN, Morakote N, Suntornlimsiri N, Piyamongkol W. Comparison of osteoporosis self assessment tool for Asian (OSTA) and standard assessment in menopause clinic, Chiang Mai. J Med Assoc Thai. 2007;90:420–425. [PubMed] [Google Scholar]
- 27.Cons Molina F, Moroyoqui Navarro LA, García Agramont M, Searcy B. Validación en español de un instrumento de pre-escrutinio para la detección de masa ósea baja en mujeres posmenopáusicas: índice SCORE / SCORE Index ia pre-screening questionary used in post-menopausal women in risk of low bone mass. Rev Mex Reumatol. 1998;13:135–143. [Google Scholar]
- 28.Cook RB, Collins D, Tucker J, Zioupos P. Comparison of questionnaire and quantitative ultrasound techniques as screening tools for DXA. Osteoporos Int. 2005;16:1565–1575. doi: 10.1007/s00198-005-1864-x. [DOI] [PubMed] [Google Scholar]
- 29.Crandall CJ, Larson J, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, Wactawski-Wende J, Gass ML, Robbins JA, Watts NB, Ensrud KE. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US Preventive Services Task Force strategy and two traditional strategies in the Women’s Health Initiative. J Bone Min Res. 2014;29:1661–1666. doi: 10.1002/jbmr.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ceulaer F, Hanssens L, Tancredi A, Bruyere O, Richy F, Pire G, Maassen P, Reginster JY. Evaluation of the osteoporosis self-assessment tool (OST) as a screening test for osteoporosis and osteopenia in men. Osteoporos Int. 2003;14:S93. [Google Scholar]
- 31.Delialioglu SU, Kaya K, Ozisler Z, Ozel S. Performance of risk assessment indices for the prediction of Postmenopausal Osteoporosis. Osteoporoz Dunyasindan. 2009;15:21–25. [Google Scholar]
- 32.El Maghraoui A, Habbassi A, Ghazi M, Achemlal L, Mounach A, Nouijai A, Bezza A. Validation and comparative evaluation of four osteoporosis risk indexes in Moroccan menopausal women. Arch Osteoporos. 2006;1:1–6. [Google Scholar]
- 33.Espinosa V, Riedemann P, Bustos L, Alvarado M, Fulgeri V, Pena N, Sanhueza D. Evaluation of screening tests for detection of risk of osteoporosis and its combination with heel ultrasound in Chilean women over 50 years. JCR-J Clin Rheumatol. 2010;16:S8. [Google Scholar]
- 34.Falasca GF, Dunston C, Banglawala YA. Further validation of a questionnaire to identify women likely to have low bone density. J Clin Densitom. 2003;6:231–236. doi: 10.1385/jcd:6:3:231. [DOI] [PubMed] [Google Scholar]
- 35.Fransiska Y, Tiksnadi B, Chaidir R, Ismiarto YD. The male osteoporosis risk estimation score and the osteoporosis self-assessment screening tool for Indonesian men. J Orthop Surg. 2012;20:205–208. doi: 10.1177/230949901202000214. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara S, Masunari N, Suzuki G, Ross PD. Performance of osteoporosis risk indices in a Japanese population. Curr Ther Res Clin Exp. 2001;62:586–594. [Google Scholar]
- 37.Gambacciani M, Genazzani AR. Comparison of osteoporosis screening tools the heel ultrasound measurement versus the calculated risk assessment tool. Calcif Tissue Int. 2004;74:S98. [Google Scholar]
- 38.Gaugris S, Tenenhouse A, Desjardin B, et al. Validating the Osteoporosis Self-Assessment Tool (OST) in Canada [Abstract] Osteoporos Int. 2005;16(Suppl 3):S18. [Google Scholar]
- 39.Geater S, Leelawattana R, Geater A. Validation of the OSTA index for discriminating between high and low probability of femoral neck and lumbar spine osteoporosis among Thai postmenopausal women. J Med Assoc Thai. 2004;87:1286–1292. [PubMed] [Google Scholar]
- 40.Geusens P, Dumitrescu B, van Geel T, van Helden S, Vanhoof J, Dinant GJ. Impact of systematic implementation of a clinical case finding strategy on diagnosis and therapy of postmenopausal osteoporosis. J Bone Miner Res. 2008;23:812–818. doi: 10.1359/jbmr.080212. [DOI] [PubMed] [Google Scholar]
- 41.Geusens P, Hochberg MC, van der Voort DJM, Pols H, van der Klift M, Siris E, Melton ME, Turpin J, Byrnes C, Ross P. Performance of risk indices for identifying low bone density in postmenopausal women. Mayo Clin Proc. 2002;77:629–637. doi: 10.4065/77.7.629. [DOI] [PubMed] [Google Scholar]
- 42.Ghazi M, Mounach A, Nouijai A, Ghozlani I, Bennani L, Achemlal L, Bezza A, El Maghraoui A. Performance of the osteoporosis risk assessment tool in Moroccan men. Clin Rheumatol. 2007;26:2037–2041. doi: 10.1007/s10067-007-0611-4. [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Vaquero C, Martinez Aguila D, Rozadilla A, Romera M, Narvaez J, Nolla JM. Evaluation of two proposals based on clinical factors for selecting what male patients with rheumatoid arthritis should undergo a bone densitometry. Reumatol Clin. 2007;3:63–66. doi: 10.1016/S1699-258X(07)73603-3. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Lopez L, Gamez-Nava JI, Vega-Lopez A, Rodriguez-Jimenez NA, Gonzalez-Montoya N, Aguilar-Chavez E, Alcaraz-Lopez MF, Rocha-Munoz AD, Castro-Lizano N, Morales-Romero J, Salazar-Paramo M, Suarez-Almazor ME. Performance of risk indices for identifying low bone mineral density and osteoporosis in Mexican Mestizo women with rheumatoid arthritis. J Rheumatol. 2012;39:247–253. doi: 10.3899/jrheum.110467. [DOI] [PubMed] [Google Scholar]
- 45.Gourlay ML, Powers JM, Lui LY, Ensrud KE. Clinical performance of osteoporosis risk assessment tools in women aged 67 years and older. Osteoporos Int. 2008;19:1175–1183. doi: 10.1007/s00198-007-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gourlay ML, Miller WC, Richy F, Garrett JM, Hanson LC, Reginster J-Y. Performance of osteoporosis risk assessment tools in postmenopausal women aged 45–64 years. Osteoporos Int. 2005;16:921–927. doi: 10.1007/s00198-004-1775-2. [DOI] [PubMed] [Google Scholar]
- 47.Haugeberg G, Orstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK. Clinical decision rules in rheumatoid arthritis: do they identify patients at high risk for osteoporosis? Testing clinical criteria in a population based cohort of patients with rheumatoid arthritis recruited from the Oslo Rheumatoid Arthritis Register. Ann Rheum Dis. 2002;61:1085–1089. doi: 10.1136/ard.61.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawker G, Mendel A, Lam MA, Akhavan PS, Cancino-Romero J, Waugh E, Jamal S, Mian S, Jaglal S. A clinical decision rule to enhance targeted bone mineral density testing in healthy mid-life women. Osteoporos Int. 2012;23:1931–1938. doi: 10.1007/s00198-011-1862-0. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg MC, Tracy JK, van der Klift M, Pols H. Validation of a risk index to identify men with an increased likelihood of osteoporosis. J Bone Miner Res. 2002;17:SA95. [Google Scholar]
- 50.Hochberg MC, Thompson DE, Ross PD. Validation of a simple clinical risk index to identify postmenopausal Asian women with osteoporosis: the osteoporosis self-assessment tool for Asians (OSTA) Osteoporos Int. 2002;13:S115. [Google Scholar]
- 51.Hochberg MC, Thompson DE, Ross PD. Validation of risk indices to identify postmenopausal women with an increased likelihood of osteoporosis. Osteoporos Int. 2002;13:S111. [Google Scholar]
- 52.Horner K, Devlin H, Harvey L. Detecting patients with low skeletal bone mass. J Dent. 2002;30:171–175. doi: 10.1016/s0300-5712(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 53.Horner K, Karayianni K, Mitsea A, Berkas L, Mastoris M, Jacobs R, Lindh C, van der Stelt P, Marjanovic E, Adams J, Pavitt S, Devlin H. The Mandibular Cortex on Radiographs as a Tool for Osteoporosis Risk Assessment: the OSTEODENT Project. J Clin Densitom. 2007;10:138–146. doi: 10.1016/j.jocd.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez-Nunez FG, Manrique-Arija S, Urena-Garnica I, Romero-Barco CM, Panero-Lamothe B, Descalzo MA, Carmona L, Rodriguez-Perez M, Fernandez-Nebro A. Reducing the need for central dual-energy X-ray absorptiometry in postmenopausal women: efficacy of a clinical algorithm including peripheral densitometry. Calcif Tissue Int. 2013;93:62–68. doi: 10.1007/s00223-013-9728-4. [DOI] [PubMed] [Google Scholar]
- 55.Junior PDP, Jr, Chahade WH. Risk factors associated to osteoporosis in Brazilian Women from sao Jose do Rio Pardo, Sao Paulo. Rev Bras Reumatol. 2007;47:16–24. [Google Scholar]
- 56.Kamondetdecha R, Panyakhamlerd K, Chaikittisilpa S, Chaiwatanarat T, Tepmongkol S, Taechakraichana N. Value of Osteoporosis Self-assessment Tools for Asians (OSTA) with or without Brown’s clinical risk factors in detection of post-menopausal osteoporosis. Climacteric. 2013;16:127–132. doi: 10.3109/13697137.2012.678913. [DOI] [PubMed] [Google Scholar]
- 57.Karkucak M, Capkin E, Kerimoglu S, Serdaroglu M, Topbas M, Yildiz H, Guler M. Performance of simple calculated osteoporosis risk estimation in a sample of women with suspected osteoporosis in the Turkish population. Rheumatol Int. 2008;28:825–830. doi: 10.1007/s00296-008-0546-3. [DOI] [PubMed] [Google Scholar]
- 58.Koh LKH, Ben Sedrine W, Torralba TP, Kung A, Fujiwara S, Chan SP, Huang QR, Rajatanavin R, Tsai KS, Park HM, Reginster JY. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 59.Kung AWC, Ho AYY, Sedrine WB, Reginster JY, Ross PD. Comparison of a simple clinical risk index and quantitative bone ultrasound for identifying women at increased risk of osteoporosis. Osteoporos Int. 2003;14:716–721. doi: 10.1007/s00198-003-1428-x. [DOI] [PubMed] [Google Scholar]
- 60.Kyaw T, Naing S, Mallios R, McFarland S, Cohen A, Huang J. Application of osteoporosis screening tools in males among VA patients. J Gen Intern Med. 2010;25:S223. [Google Scholar]
- 61.Lauwerier D, Hoet H, Roman E, De Boever E, Zmierczak H, Goemaere S. Prescreening of postmenopausal osteoporosis with ultrasound measurements and a risk questionnaire in primary care: a critical evaluation. Tijdschrift voor Geneeskunde. 2002;58:1107–1113. [Google Scholar]
- 62.Leeangkoonsathian E, Boonyanuruk P, Pongchaiyakul C, Panichkul S. Validate of clinical risk index for osteoporosis in Thai women at Phramongkutklao Hospital. J Med Assoc Thail. 2012;95:487–492. [PubMed] [Google Scholar]
- 63.Lerttrakul S, Soontrapa S. Modified OSTA index for referring women for DEXA measurement. J Med Assoc Thai. 2005;88(Suppl 5):S80–S83. [PubMed] [Google Scholar]
- 64.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, Program MBD. Selection of women aged 50–64 yr for bone density measurement. J Clin Densitom. 2013;16:570–578. doi: 10.1016/j.jocd.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Lesnyak Y, Ershova O, Menshikova L, Lesnyak O. Performance of the osteoporosis risk assessment tool (OST) in Russian women. Osteoporos Int. 2003;14:S53. [Google Scholar]
- 66.Li-Yu JT, Llamado LJQ, Torralba TP. Validation of OSTA among Filipinos. Osteoporos Int. 2005;16:1789–1793. doi: 10.1007/s00198-005-1929-x. [DOI] [PubMed] [Google Scholar]
- 67.Li YM. Concordance of a self assessment tool and measurement of bone mineral density in identifying the risk of osteoporosis in elderly Taiwanese women. Tzu Chi Med J. 2008;20:206–212. [Google Scholar]
- 68.Liu MY, Li CL, Pei Y, Xiao YJ, Zhang Y, Cheng XL. Diagnostic values of self-assessment tool for Asians for osteoporosis in aged men. Chung-Hua i Hsueh Tsa Chih [Chin Med J] 2011;91:2112–2115. [PubMed] [Google Scholar]
- 69.Liu M, Zhang Y, Cheng X, Lu Y, Li N, Gong Y, Pei Y, Li C. The effect of age on the changes in bone mineral density and osteoporosis detection rates in Han Chinese men over the age of 50. Aging Male. 2014;17:166–173. doi: 10.3109/13685538.2014.940308. [DOI] [PubMed] [Google Scholar]
- 70.Lu C, Chen D, Cai Y, Wei S. Concordane of OSTA and lumbar spine BMD by DXA in identifying risk of osteoporosis. J Orthop Surg. 2006;1:14. doi: 10.1186/1749-799X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynn HS, Woo J, Leung PC, Barrett-Connor EL, Nevitt MC, Cauley JA, Adler RA, Orwoll ES. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087–1092. doi: 10.1007/s00198-007-0553-3. [DOI] [PubMed] [Google Scholar]
- 72.Machado P, da Silva JAP. Performance of decision algorithms for the identification of low bone mineral density in Portuguese postmenopausal women. Acta Reumatol. 2008;33:314–328. [PubMed] [Google Scholar]
- 73.Machado P, Coutinho M, da Silva JAP. Selecting men for bone densitometry: performance of osteoporosis risk assessment tools in Portuguese men. Osteoporos Int. 2010;21:977–983. doi: 10.1007/s00198-009-1036-5. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Aguila D, Gomez-Vaquero C, Rozadilla A, Romera M, Narvaez J, Nolla JM. Decision rules for selecting women for bone mineral density testing: application in postmenopausal women referred to a bone densitometry unit. J Rheumatol. 2007;34:1307–1312. [PubMed] [Google Scholar]
- 75.Mauck KF, Cuddihy MT, Atkinson EJ, Melton LJ., III Use of clinical prediction rules in detecting osteoporosis in a population-based sample of postmenopausal women. Arch Intern Med. 2005;165:530–536. doi: 10.1001/archinte.165.5.530. [DOI] [PubMed] [Google Scholar]
- 76.Mellstrom D, Rubinsky S, Waern E, Sen SS. Validating the osteoporosis self-assessment tool (OST) in Sweden. Osteoporos Int. 2003;14:S80. [Google Scholar]
- 77.Mittal N, Ridella M, Felton C, Xu T. Performance of screening tools in diagnosing osteoporosis in postmenopausal Mexican American women. J Invest Med. 2011;59:606. [Google Scholar]
- 78.Morin S, Tsang JF, Leslie WD. Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int. 2009;20:363–370. doi: 10.1007/s00198-008-0688-x. [DOI] [PubMed] [Google Scholar]
- 79.Mossman EA, Grinnell NC, Cole L, Osborn KL, McClung MR. Screening methods for DXA referral: the Osteoporosis Self-Assessment Tool for Asians and quantitative ultrasound sonography. Osteoporos Int. 2002;13:P60. [Google Scholar]
- 80.Mossman EA, Luckey M, McClung MR. The osteoporosis self-assessment tool (OST) performs differently in younger versus older postmenopausal women. J Bone Miner Res. 2003;18:S358. [Google Scholar]
- 81.Mottaghi P, Karimifar M, Salesi M, Mehrabi A. Osteoporosis screening tools in Iranian postmenopausal women. Iran Red Crescent Med J. 2010;12:122–126. [Google Scholar]
- 82.Oh SM, Nam BH, Rhee Y, Moon SH, Kim DY, Kang DR, Kim HC. Development and validation of osteoporosis risk-assessment model for Korean postmenopausal women. J Bone Min Metab. 2013;31:423–432. doi: 10.1007/s00774-013-0426-0. [DOI] [PubMed] [Google Scholar]
- 83.Pang WY, Inderjeeth CA. FRAX without bone mineral density versus osteoporosis self-assessment screening tool as predictors of osteoporosis in primary screening of individuals aged 70 and older. J Am Geriatr Soc. 2014;62:442–446. doi: 10.1111/jgs.12696. [DOI] [PubMed] [Google Scholar]
- 84.Panichyawat N, Tanmahasamut P. Comparison of OSTA index and KKOS scoring system for prediction of osteoporosis in postmenopausal women who attended Siriraj Menopause Clinic. J Med Assoc Thail. 2012;95:1365–1371. [PubMed] [Google Scholar]
- 85.Park HM, Ben Sedrine W, Reginster JY, Ross PD. Korean experience with the OSTA risk index for osteoporosis: a validation study. J Clin Densitom. 2003;6:247–250. doi: 10.1385/jcd:6:3:247. [DOI] [PubMed] [Google Scholar]
- 86.Perez-Castrillon JL, Sagredo MG, Conde R, del Pino-Montes J, de Luis D. OST risk index and calcaneus bone densitometry in osteoporosis diagnosis. J Clin Densitom. 2007;10:404–407. doi: 10.1016/j.jocd.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Pongchaiyakul C, Nguyen ND, Eisman JA, Nguyen TV. Clinical risk indices, prediction of osteoporosis, and prevention of fractures: diagnostic consequences and costs. Osteoporos Int. 2005;16:1444–1450. doi: 10.1007/s00198-005-1996-z. [DOI] [PubMed] [Google Scholar]
- 88.Pongchaiyakul C, Panichkul S, Songpatanasilp T. Combined clinical risk indices with quantitative ultrasound calcaneus measurement for identifying osteoporosis in Thai postmenopausal women. J Med Assoc Thai. 2007;90:2016–2023. [PubMed] [Google Scholar]
- 89.Pongchaiyakul C, Wanothayaroj E. Performance of the Khon Kaen Osteoporosis Study (KKOS) score for identifying osteoporosis in men. J Med Assoc Thai. 2007;90:1518–1523. [PubMed] [Google Scholar]
- 90.Reginster JY, Ben Sedrine W, Viethel P, Micheletti MC, Chevallier T, Audran M. Validation of OSIRIS, a prescreening tool for the identification of women with an increased risk of osteoporosis. Gynecol Endocrinol. 2004;18:3–8. doi: 10.1080/09513590310001651713. [DOI] [PubMed] [Google Scholar]
- 91.Richards J, Lazzari AA, Qualler DA, Desale S, Howard R, Kerr GS. Validation of the osteoporosis self-assessment tool in US male veterans. J Clin Densitom. 2014;17:32–37. doi: 10.1016/j.jocd.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Richards JS, Peng J, Amdur RL, Mikuls TR, Hooker RS, Michaud K, Reimold AM, Cannon GW, Caplan L, Johnson D, Hines AE, Kerr GS. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12:434–440. doi: 10.1016/j.jocd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Richards JS, Lazzari AA, Amdur RL, Teves DA, Howard R, Davis SA, Dussault P, Stukes DL, Coote-Johnson S, Howard H, Liu FM, Kerr GS. The osteoporosis self assessment tool in US male veterans. Arthritis Rheum. 2008;58:S745. [Google Scholar]
- 94.Richy F, Gourlay M, Ross PD, Sen SS, Radican L, De Ceulaer F, Ben Sedrine W, Ethgen O, Bruyere O, Reginster JY. Validation and comparative evaluation of the osteoporosis self-assessment tool (OST) in a Caucasian population from Belgium. QJM. 2004;97:39–46. doi: 10.1093/qjmed/hch002. [DOI] [PubMed] [Google Scholar]
- 95.Rud B, Abrahamsen B, Rejnmark L, Jensen JB. How does estrogen use in early postmenopausal women affect the diagnostic performance of the osteoporosis self-assessment tool and quantitative ultrasonography? Bone. 2005;36:S345. [Google Scholar]
- 96.Rud B, Jensen JEB, Mosekilde L, Nielsen SP, Hilden J, Abrahamsen B. Performance of four clinical screening tools to select peri- and early postmenopausal women for dual X-ray absorptiometry. Osteoporos Int. 2005;16:764–772. doi: 10.1007/s00198-004-1748-5. [DOI] [PubMed] [Google Scholar]
- 97.Russell AS, Morrison RT. An assessment of the new “SCORE” index as a predictor of osteoporosis in women. Scand J Rheumatol. 2001;30:35–39. doi: 10.1080/030097401750065300. [DOI] [PubMed] [Google Scholar]
- 98.Saetung S, Ongphiphadhanakul B, Rajatanavin R. The relationship of an Asian-specific screening tool for osteoporosis to vertebral deformity and osteoporosis. J Bone Miner Metab. 2008;26:47–52. doi: 10.1007/s00774-007-0796-2. [DOI] [PubMed] [Google Scholar]
- 99.Saravi FD, Riveros OP, Andrada MA, Ortiz PF. Osteoporosis self-assessment tool performance in postmenopausal women of Mendoza, Argentina. Bone. 2007;41:S9. doi: 10.1155/2013/150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saravi FD. Osteoporosis self-assessment tool performance in a large sample of postmenopausal women of Mendoza, Argentina. J Osteoporos. 2013;2013:150–154. doi: 10.1155/2013/150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scholtissen S, Guillemin F, Bruyere O, Collette J, Dousset B, Kemmer C, Culot S, Cremer D, Dejardin H, Hubermont G, Lefebvre D, Pascal-Vigneron V, Weryha G, Reginster JY. Assessment of determinants for osteoporosis in elderly men. Osteoporos Int. 2009;20:1157–1166. doi: 10.1007/s00198-008-0789-6. [DOI] [PubMed] [Google Scholar]
- 102.Sen SS, Rives VP, Pharm D, Frisoli A, Neto JFM. Validating the OsteoRisk, an osteoporosis risk assessment tool for Latin America, in Brazil. Osteoporos Int. 2003;14:S60. [Google Scholar]
- 103.Sen SS, Geling O, Ross PD, Reid I. Validating the osteoporosis self-assessment tool in New Zealand. Osteoporos Int. 2002;13:P77. [Google Scholar]
- 104.Shan LP, Bee OF, Suniza SS, Adeeb N. Developing a Malaysian Osteoporosis Screening Tool (MOST) for early osteoporosis detection in Malaysian women. Sex Reprod Healthc. 2011;2:77–82. doi: 10.1016/j.srhc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Shepherd AJ, Cass AR, Ray L. Determining risk of vertebral osteoporosis in men: validation of the male osteoporosis risk estimation score. J Am Board Fam Med. 2010;23:186–194. doi: 10.3122/jabfm.2010.02.090027. [DOI] [PubMed] [Google Scholar]
- 106.Sinnott B, Kukreja S, Barengolts E. Utility of screening tools for the prediction of low bone mass in African American men. Osteoporos Int. 2006;17:684–692. doi: 10.1007/s00198-005-0034-5. [DOI] [PubMed] [Google Scholar]
- 107.Sybrowsky CL, Skedros JG. Comparison of the OST Versus a Typical Risk-Factor Questionnaire Evaluating Osteoporosis in Men. J Bone Miner Res. 2004;19:S164. [Google Scholar]
- 108.Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, Ohama K, Tanimoto K. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol Am J Roentgenol. 2004;183:1755–1760. doi: 10.2214/ajr.183.6.01831755. [DOI] [PubMed] [Google Scholar]
- 109.Tanprasertkul C, Wattanaruangkowit P, Panyakhamlerd K. The combination of body mass index and age as a new index for identifying osteoporosis in Thai postmenopausal women. J Med Assoc Thai. 2010;93(Suppl 7):S76–S82. [PubMed] [Google Scholar]
- 110.Vilaseca RD, Garcia VC, Boncompte Vilanova PM, Lopez MJ, Garcia CC, Baures RM on behalf of the densitometry referral study group. Sensitivity, specificity, positive and negative predictive values of the criteria for indicating a bone densitometry in the evaluation of medical techniques and research in Cataluna. Reumatol Clin. 2011;7:161–166. doi: 10.1016/j.reuma.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Wallace LS, Ballard JE, Holiday D, Turner LW, Keenum AJ, Pearman CM. Evaluation of decision rules for identifying low bone density in postmenopausal African-American women. J Natl Med Assoc. 2004;96:290–296. [PMC free article] [PubMed] [Google Scholar]
- 112.Whelan BR, Falvey E, O’Dwyer D, Daly M, Drummond F, Shanahan F, Molloy MG. Selection of men for osteoporosis screening using DXA - A comparison of four assessment tools. Arthritis Rheum. 2005;52:1982. [Google Scholar]
- 113.Whelan BR, Falvey E, Daly M, Crowley M, Shanahan F, Harney S, Molloy MG. Use of the Osteoporosis Self Assessment Tool (OST) in pre- and post-menopausal women - Setting cutoffs for risk of osteoporosis. Rheumatology (Oxford) 2006;45:I24–I25. [Google Scholar]
- 114.Yang Y, Wang B, Fei Q, Meng Q, Li D, Tang H, Li J, Su N. Validation of an osteoporosis self-assessment tool to identify primary osteoporosis and new osteoporotic vertebral fractures in postmenopausal Chinese women in Beijing. BMC Musculoskelet Disord. 2013;14:271. doi: 10.1186/1471-2474-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yaraman N, Celik C, Karaoglan B. Using of “score” index for osteoporosis in postmenopausal women. J Rheumatol Med Rehabil. 2003;14:2–8. [Google Scholar]
- 116.Yeoum SG, Lee JH. Usefulness of estimated height loss for detection of osteoporosis in women. J Korean Acad Nurs. 2011;41:758–767. doi: 10.4040/jkan.2011.41.6.758. [DOI] [PubMed] [Google Scholar]
- 117.Zha XY, Hu Y, Pang XN, Chang GL, Li L. Diagnostic value of Osteoporosis Self-Assessment Tool for Asians (OSTA) and quantitative bone ultrasound (QUS) in detecting high-risk populations for osteoporosis among elderly Chinese men. J Bone Miner Metab. 2014 doi: 10.1007/s00774-014-0587-5. [DOI] [PubMed] [Google Scholar]
- 118.Zimering MB, Shin JJ, Shah J, Wininger E, Engelhart C. Validation of a novel risk estimation tool for predicting low bone density in Caucasian and African American men veterans. J Clin Densitom. 2007;10:289–297. doi: 10.1016/j.jocd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 119.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 120.Duncan IG. Healthcare Risk Adjustment and Predictive Modeling. ACTEX Publications; 2011. [Google Scholar]
- 121.Rubin KH, Friis-Holmberg T, Hermann AP, Abrahamsen B, Brixen K. Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res. 2013;28:1701–1717. doi: 10.1002/jbmr.1956. [DOI] [PubMed] [Google Scholar]
- 122.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:99–111. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- 123.Rud B, Hilden J, Hyldstrup L, Hrobjartsson A. The Osteoporosis Self-Assessment Tool versus alternative tests for selecting postmenopausal women for bone mineral density assessment: a comparative systematic review of accuracy. Osteoporos Int. 2009;20:599–607. doi: 10.1007/s00198-008-0713-0. [DOI] [PubMed] [Google Scholar]
- 124.D’Agostino RB, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 125.Nayak S, Roberts MS, Greenspan SL. Cost-effectiveness of different screening strategies for osteoporosis in postmenopausal women. Ann Intern Med. 2011;155:751–761. doi: 10.1059/0003-4819-155-11-201112060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ito K, Hollenberg JP, Charlson ME. Using the osteoporosis self-assessment tool for referring older men for bone densitometry: a decision analysis. J Am Geriatr Soc. 2009;57:218–224. doi: 10.1111/j.1532-5415.2008.02110.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.