Abstract
Summary
Anti-osteoporosis medication (AOM) use in patients exposed to glucocorticoids is thought to reduce fractures. We found post-menopausal women using glucocorticoids for at least 90 days who also used an AOM within 90 days had 48 % fewer fractures by 1 year and 32 % fewer fractures by 3 years compared to non-AOM users.
Introduction
The purpose of this study is to explore the effectiveness of adherence to quality measures by estimating the effect of anti-osteoporosis medication (AOM) initiation within 90 days after chronic (≥90 days) glucocorticoid (GC) therapy on osteoporotic fracture.
Methods
A new-user cohort was assembled using the MarketScan databases between 2000 and 2012. Included patients were female, age ≥50 at GC initiation, had a first GC fill daily dose ≥10 mg and persisted for at least 90 days. During a 365-day baseline period, patients were excluded for prior GC or AOM (bisphosphonate, denosumab, teriparatide) use, fracture, or cancer diagnosis. Initiators of an AOM in the 14 days pre- or 90 days post-GC fill were characterized as AOM users; those without, AOM non-users. Follow-up began 91 days after GC fill with patients followed until fracture, loss of continuous enrollment, initiation of AOM by AOM non-users, or end of study period. A propensity score was estimated for AOM receipt using all measured covariates and converted to a stabilized inverse probability of treatment weights (IPTW). Weighted hazard ratios (HR) and associated 95 % confidence intervals (95 % CI) were estimated using weighted Cox proportional hazard models.
Results
Of the 7885 women eligible for the study, 12.1 % were AOM users. AOM use was associated with lower fracture incidence: weighted HR of 0.52 (95 % CI 0.29, 0.94) at 1 year and weighted HR of 0.68 (95 % CI 0.47, 0.99) at 3 years.
Conclusions
AOM initiation within 90 days of chronic GC use was associated with a fracture reduction of 48 % at 1 year and 32 % at 3 years.
Keywords: Anti-osteoporosis medication, Glucocorticoid-induced osteoporosis, Osteoporotic fracture, Quality measures
Introduction
Glucocorticoid-induced osteoporosis (GIO) is the most common secondary cause of osteoporosis [1, 2]. In the USA glucocorticoids are estimated to be used by 1.2 % (~1.5 million) of persons aged ≥20, while worldwide estimates have ranged between 0.5 and 0.9 % [3-7]. In the USA, between 1.4 and 2.7 % of women aged ≥50 were current oral glucocorticoid users with most having chronic (≥90 days) use [3]. Daily glucocorticoids doses as low as 2.5 mg prednisone equivalence are associated with an increased fracture risk, with the most rapid loss of bone density occurring in the first 3 to 6 months after initiation [8-10]. Post-menopausal women have higher fracture rates than men and are particularly susceptible to fractures after menopause [11]. Based on criteria from the 2010 American College of Rheumatology (ACR) guidelines for the prevention and treatment of GIO, 1.7 % of US post-menopausal women were at risk for GIO between 2005 and 2010 [12]. Fractures due to GIO, particularly hip fractures, increase mortality risk, health-care costs, risk of future fractures, and comorbid conditions while decreasing patient’s quality of life [13, 14].
US clinical guidelines for the treatment and prevention of GIO recommend anti-osteoporosis medications (AOM) in patients initiating or expecting to be on glucocorticoid therapy for at least 90 days [2, 15-17]. The 2010 ACR GIO guidelines include recommendations for treatment with AOMs—bisphosphonates (alendronate, risedronate, and zoledronic acid), or teriparatide—based on clinical risk factors [15]. These four AOMs have been approved by the United States Food and Drug Administration (FDA) for GIO and have demonstrated efficacy in randomized controlled trials (RCT) for prevention of bone loss [10, 13, 18]. Clinical guidelines have recommended the use of denosumab, although it has not been FDA approved for GIO [2, 17]. AOM treatment is expected to reduce and prevent fractures in GIO based on RCTs of alendronate and risedronate which demonstrated attenuated bone mineral density (BMD) loss (primary endpoint) and a reduction in vertebral fractures (secondary endpoint) [10, 19, 20]. To date, no GIO RCTs have used fracture as the primary endpoint.
Two GIO-related quality measures have been endorsed by the National Quality Forum based on expert opinion and results of the aforementioned RCTs; within 180 days of initiation of a glucocorticoid, patients should receive the following: (1) treatment with an AOM or (2) a diagnostic dual energy X-ray absorptiometry (DXA) scan [21, 22]. To our knowledge, no clinical studies have attempted to estimate the effect of AOM initiation on fracture risk based on receipt of quality measure care in new glucocorticoid users.
Two 2013 observational studies produced conflicting results related to use of AOM in GIO [23, 24]. A population-based registry conducted in Manitoba, Canada, reported that AOM or DXA use within 180 days of chronic glucocorticoid initiation increased the odds of fracture by 30% within 3 years [23]. The second study was a retrospective cohort study of bisphosphonate users, reporting a decrease in fracture risk at 12 months [24]. Both studies’ estimates may be subject to selection bias by including long-term users of therapy, or confounding by disease severity and/or underlying fracture risk according to gender and age. These conflicting findings have resulted in persistent questions regarding the effectiveness of preventive AOM treatment in GIO despite evidence of beneficial effects on BMD [25].
With reports of 1.7 % of the US post-menopausal population at risk for GIO, it is important to determine if AOM treatment concordant with currently endorsed quality measures reduces fracture risk in GIO. To investigate the association between AOM use and fracture risk among those at risk of GIO, we conducted a retrospective cohort study of women aged 50 years and older newly initiating chronic glucocorticoids based on administrative claims data. Specifically, we aimed to estimate the effect of AOM initiation within 90 days after chronic glucocorticoid initiation on osteoporotic fracture.
Materials and methods
Data source
We used MarketScan (Truven Health Analytics, Ann Arbor, MI) Commercial Claims and Encounters (CCAE) and Medicare Supplemental Coordination of Benefits (COB) databases (January 1, 2000–December 31, 2012) for this study. These combined databases are de-identified and contain information from a large population of patients with employer-provided insurance residing in all 50 states and the District of Columbia. The MarketScan databases capture individual-level clinical utilization, expenditures, and enrollment across inpatient, outpatient, prescription drug, and carve-out services from a wide selection of employer-sponsored health plans. In 2011 the databases contained data on approximately 60 million covered lives [26]. The Institutional Review Board at the University of North Carolina at Chapel Hill determined that this study did not constitute human subjects research and so did not require IRB approval.
Study design and population
We conducted an administrative claims-based retrospective cohort study of the impact of AOM initiation on GIO-fracture risk in women aged 50 and older on ≥10 mg prednisone equivalent glucocorticoids. We define AOMs as alendronate, ibandronate, risedronate, zoledronic acid, teriparatide, or denosumab. We have included denosumab in our AOMs based on an ongoing phase III trial of its use for prevention of bone loss in glucocorticoid treatment patients, while ibandronate has been shown to significantly increase lumbar spine and total hip BMD in glucocorticoid patients [27, 28]. Our study employs a new user design, focusing on incident rather than prevalent users of glucocorticoids and AOM, as prevalent users may have exceeded the quality measure period and likely have differing baseline characteristics and fracture risks than new users [29]. The date of initial glucocorticoid fill is considered the index date, the 365 days pre-index are considered the baseline period, with 14 days pre- and 90 days post-index considered the quality measure period (see Fig. 1).
Fig. 1.
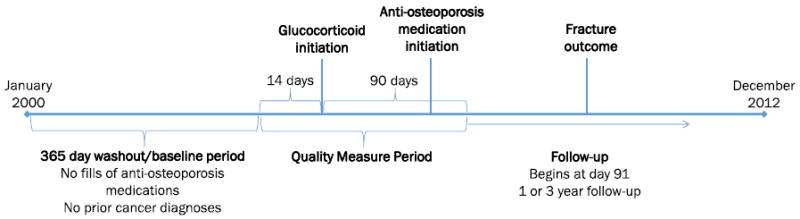
Study schematic
We identified women age ≥50 with a new outpatient pharmacy dispensing of a ≥10 mg prednisone equivalent oral glucocorticoid that did not fill either a glucocorticoid or AOM during the baseline period or at any other point prior to the index date [30, 31]. Patients were required to have continuous health plan enrollment during both the baseline and quality measure periods. We required patients’ first period of glucocorticoid use to be ≥90 days including the index date; we allowed a gap of up to 30 days between the last supplied day and first date of new fill to still be considered continuous use. We used International Classification of Diseases, Ninth Revision, ClinicalModification (ICD-9), Common Procedure Terminology (CPT), or Healthcare Common Procedure Coding System (HCPCS) codes to identify patient characteristics, inclusion, and exclusion criteria during the baseline window. Patients with a diagnosis claim for cancer (ICD-9 140-208) at any time during their enrollment prior to the index date were excluded due to cancer-related fractures being pathophysiologically different from osteoporotic fractures [31, 32]. If no medication dispensing of any kind was observed during baseline, the patient was excluded due to the possibility of missing pharmacy claims during the baseline which could cause misclassification. As prior fractures are associated with future fracture risk, we excluded patients with any fractures (ICD-9 733.1–733.19 or 800–829) during baseline and the quality measure period. Follow-up began at the end of the quality measure period (day 91) and fractures during the quality measure period represented outcomes that were unlikely to have been affected by AOM use and may have prompted AOM initiation. Further details of inclusion/exclusion are presented as Fig. 2.
Fig. 2.
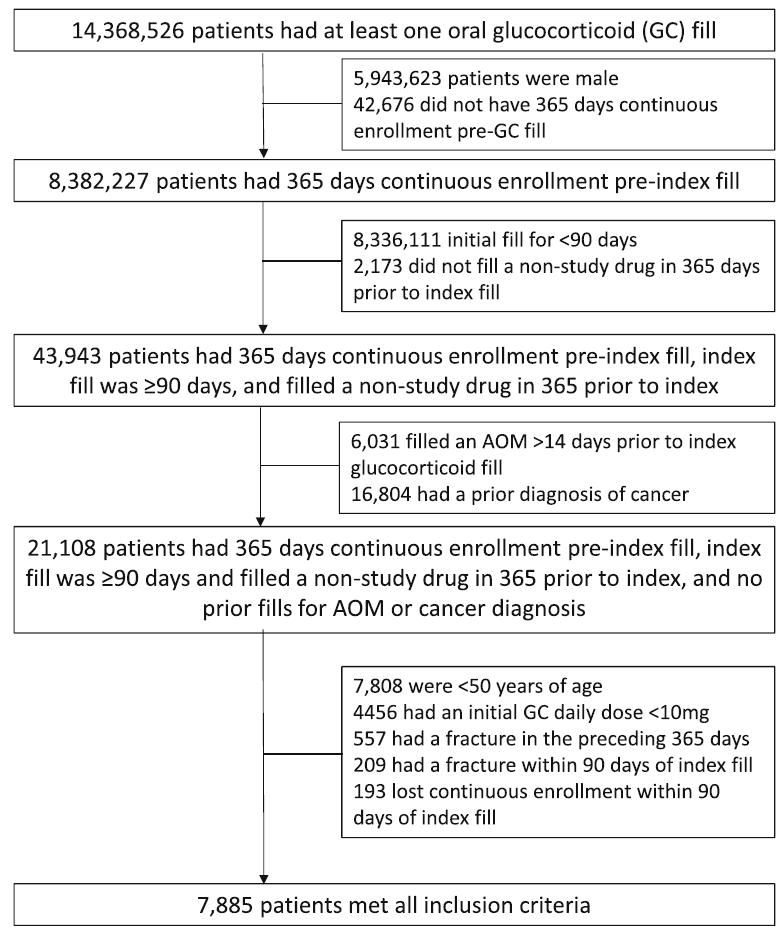
Inclusion and exclusion diagram. GC oral glucocorticoid, Index fill date of first oral GC fill, AOM anti-osteoporosis medication (bisphosphonate, teriparatide, denosumab). Cancer diagnosis: ICD-9 (140-208.xx); fracture in the preceding 365 days or within 90 days: ICD-9 733.1-733.19 or 800-829)
Exposure assessment
We assessed AOM initiation during the quality measure period, which extended from 14 days prior to the index date to 90 days after. Although clinical guidelines recommend initiation of an AOM within 180 days of GC initiation, our main analysis uses a 90-day exposure period to reduce attrition due to loss of continuous enrollment or early fracture. To address the current clinical quality measure, we conducted a sensitivity analysis using 180 days as the quality measure period (see “Sensitivity Analyses” below). Patients who filled an AOM during the quality measure period are referred to as AOM users, and those without any use during the quality measure period are referred to as AOM non-users. The 14 days prior to index glucocorticoid fill are considered part of the quality measure period due to the assumption that these patients were being treated with AOM in the anticipation of glucocorticoid use.
Covariate assessment
Baseline characteristics were assessed during baseline based on ICD-9 and CPT codes including a diagnosis of osteoporosis, continuous age, region of health plan, type of insurance, year of index fill, conditions associated with secondary osteoporosis per the 2004 Surgeon General’s Report, Charlson comorbidity index, conditions associated with falling, DXA scan use, healthcare utilization (number of outpatient visits and number of inpatient admissions), calcitonin, raloxifene, and hormone therapy use [33-36]. Codes used to define each of the covariates are presented in Supplementary Table 1 with specific conditions associated with secondary osteoporosis and falling listed. Selected covariates are presented in Table 1, with a full listing presented in Supplementary Table 2.
Table 1.
Selected demographics of patients who filled (AOM users) or did not fill (AOM non-users) an anti-osteoporosis medication within 90 days of starting a glucocorticoid (2000–2012)
| Characteristic, N (%) unless otherwise specified | Crude population
|
Weighted population
|
||
|---|---|---|---|---|
| AOM users | AOM non-users | AOM users | AOM non-users | |
| N | 952 | 6933 | 937 | 6937 |
| Age, mean (SD) | 67.5 (10.6) | 66.6 (11.4) | 67.0 (11.4) | 66.7 (11.3) |
| 50–59a | 260 (27.3) | 2421 (34.9) | 296 (31.6) | 2360 (34.0) |
| 60–69a | 276 (29.0) | 1839 (26.5) | 256 (27.4) | 1867 (26.9) |
| 70–79a | 270 (28.4) | 1535 (22.1) | 221 (23.6) | 1576 (22.7) |
| 80+a | 146 (15.3) | 1138 (16.4) | 163 (17.4) | 1135 (16.4) |
| In 365 days prior to index | ||||
| Osteoporosis | 80 (8.4) | 222 (3.2) | 41 (4.3) | 272 (3.9) |
| Diabetes mellitus (types 1 and 2) | 117 (12.3) | 1182 (17.0) | 138 (14.8) | 1143 (16.5) |
| Central adiposity | 16 (1.7) | 244 (3.5) | 27 (2.9) | 229 (3.3) |
| Inflammatory bowel disease | 12 (1.3) | 271 (3.9) | 49 (5.3) | 249 (3.6) |
| Rheumatoid arthritis | 135 (14.2) | 1078 (15.5) | 128 (13.7) | 1066 (15.4) |
| Stroke | 90 (9.5) | 621 (9.0) | 83 (8.9) | 626 (9.0) |
| Congestive heart failure | 76 (8.0) | 697 (10.1) | 84 (9.0) | 677 (9.8) |
| Asthma/COPD | 158 (16.6) | 1352 (19.5) | 175 (18.7) | 1328 (19.1) |
| Proton pump inhibitors | 257 (27.0) | 1951 (28.1) | 280 (29.9) | 1943 (28.0) |
| Anticonvulsants | 93 (9.8) | 868 (12.5) | 133 (14.2) | 847 (12.2) |
| Calcitonin | 11 (1.2) | 86 (1.2) | 10 (1.1) | 86 (1.2) |
| Hormone replacement therapy | 198 (20.8) | 1347 (19.4) | 186 (19.9) | 1364 (19.7) |
| Raloxifene | 23 (2.4) | 124 (1.8) | 21 (2.2) | 131 (1.9) |
| DXA | 177 (18.6) | 708 (10.2) | 111 (11.8) | 788 (11.4) |
| Charlson Comorbidity Score | ||||
| 0 | 811 (85.2) | 5682 (82.0) | 777 (83.0) | 5716 (82.4) |
| 1 | 27 (2.8) | 212 (3.1) | 28 (3.0) | 210 (3.0) |
| 2 | 55 (5.8) | 498 (7.2) | 71 (7.6) | 487 (7.0) |
| 3–5 | 52 (5.5) | 431 (6.2) | 51 (5.4) | 422 (6.1) |
| ≥6 | 7 (0.7) | 110 (1.6) | 10 (1.0) | 103 (1.5) |
| In 90 days post-index | ||||
| Osteoporosis | 100 (10.5) | 151 (2.2) | 34 (3.6) | 225 (3.2) |
| DXA | 264 (27.7) | 626 (9.0) | 115 (12.3) | 787 (11.3) |
All demographic variables are listed in Supplementary Table 3
COPD chronic obstructive pulmonary disease, DXA dual energy X-ray absorptiometry
Age by decade has been presented here for descriptive purposes but is not included in the propensity score
Outcome assessment
The primary fracture outcome is defined as a fracture at the hip, pelvis, humerus, wrist, or spine (vertebral, cervical, lumbar, and/or thoracic fractures). The specific codes are available in Supplementary Table 3 [37, 38]. This fracture definition is based on two separate published fracture algorithms, with ICD-9 diagnoses of hip, humerus, and wrist fractures which required having accompanying CPT codes for treatment within 30 days [37]. Our use of all four spinal sites and associated codes were based on fractures at these sites likely being associated with osteoporosis [38].
Follow-up began the day after the end of the quality measure period (91 days after index-fill). Patients were followed until fracture, loss of health plan enrollment, end of study period (December 31, 2012), or fill of an AOM by a participant who was designated an AOM non-user at baseline. Analyses were administratively censored at 1 and 3 years in separate analyses, if no events were present. We present event rates per 1000 person-years for fractures in both the AOM users and AOM nonusers as well as AOM use by non-AOM users as well as mean (standard deviation [SD]) days follow-up at a maximum of 1 and 3 years for both groups.
Statistical methods
To control confounding by measured characteristics, we estimated a propensity score for receipt of an AOM within the quality measure period using logistic regression models including all measured covariates [39, 40]. The resulting propensity scores were the probability of receipt of an AOM based on all the measured covariates. We then converted the propensity score into stabilized inverse probability of treatment weights (IPTW). When IPTW weights are applied, a pseudo-population is created where the measured covariates are not associated with the outcome, and both the treated and untreated groups are standardized to the overall population [39]. Advantages of IPTW weighting includes retention all study patients and estimate of an average treatment effect in the whole population of eligible patients.
Statistical analysis
Baseline differences between the treatment groups were assessed using Student’s t tests for continuous and chi-square tests for categorical variables to determine if differences in covariate distributions between AOM users and non-users were present (presented in Supplementary Table 3). We conducted a modified intent-to-treat (ITT) by censoring at AOM initiation in baseline non-users. The modified ITT was used as subsequent initiation was thought to lower fracture risk from that point forward. To address the use of the modified ITT, a true ITT was also undertaken (see “Sensitivity Analyses”). Hazard ratios (HR) and 95% confidence intervals (95 % CI) were estimated using Cox proportional hazard models. HRs are reported for unadjusted, adjusted for all measured covariates, and IPTW-weighted analyses. All analyses were performed with SAS 9.3 (SAS Institute, Cary, NC).
Sensitivity analyses
We undertook three types of sensitivity analyses associated with this study. First, as our primary analysis uses a quality measure period of 90 days rather than the quality measure-recommended 180 days, we varied the length of the quality measure period to determine its effect on treatment estimates. Analyses using 30-, 60-, 120-, 150-, and 180-day quality measure periods were conducted using the same fracture definition and exclusion criteria as the main analysis. Second, to determine the sensitivity of our results to the fracture definition, we employed varying fracture definitions, including (1) composite of hip, pelvis, humerus, or wrist fractures with associated CPTs (non-spine); (2) spine fractures; (3) vertebral fractures alone; and (4) spine fractures excluding cervical fractures. Third, as we employed a modified ITT for the primary analysis, we conducted a true ITT analysis by only censoring non- AOM users for loss of enrollment or the end of the study period. To better visualize the effect of these multiple estimates, sensitivity analysis results are presented as forest plots.
Results
There were 7885 women who met all inclusion criteria (see Fig. 2). Among those, 952 patients (12.1 %) were classified as AOM users. Bisphosphonates accounted for 95.5 % of all AOMs initiated during this quality measure period (alendronate 67.5 %, ibandronate 4.1 %, risedronate 26.9 %, zoledronic acid 0.9 %) with teriparatide accounting for the remaining 0.5 %. Selected baseline characteristics of AOM users and non-users before and after weighting are presented in Table 1 with all baseline characteristics presented in Supplementary Table 3. Before weighting both during baseline and quality measure period, AOM users had osteoporosis diagnoses more often (baseline 8.4 vs. 3.2 %, quality measure period 10.5 % vs. 2.2 %) and received DXAs more often (baseline 18.6 vs. 10.2 %, quality measure period 27.7 vs. 9.0 %). AOM non-users were more likely to have a diagnosis for asthma/chronic obstructive pulmonary disease (COPD) (16.6 vs. 19.5 %) and based on region, women in the southern region of the US used AOMs less often (23.9 vs. 28.2 %). Although the mean ages were similar (AOM users [67.5] vs. AOM non-users [66.6]) a greater proportion of non-users were aged 50–59 (27.3 vs. 34.9 %), while a greater proportion of those aged 70–79 were AOM users (28.4 vs. 22.1 %). After weighting, all measured covariates were balanced between the treatment groups, except for barbiturate use (0.8 vs. 0.2 %), inflammatory bowel disease (5.2 vs. 3.6 %), and end-stage renal disease (4.5 vs. 3.2 %).
With a maximum of 1-year follow-up, fractures occurred at a rate of 20.1 per 1000 person years in AOM users, 29.7 per 1000 person years in AOM non-users, and 129.7 per 1000 person years for AOM use in non-AOM users. Mean follow-up time with a maximum at 1 year was 300.5 days (SD 109.1) for AOM users and 274.6 days (SD 123.8) for non-users. AOM use was associated with a decreased fracture risk: unadjusted HR=0.68 (95 % CI 0.41, 1.14), adjusted HR=0.67 (95 % CI 0.39, 1.15), and weighted HR=0.52 (95 % CI 0.29, 0.94) at 1 year (Table 2).
Table 2.
Fracture rates associated with AOM (anti-osteoporosis medication) use within 90 days of chronic glucocorticoid initiation
| Study group | Unweighted
|
Weighted
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fracture rate | AOM censor ratea | Mean follow-up | HR | aHR | Fracture rate | AOM censor ratea | Mean follow-up | wHR | ||
| AOM users | 1 year | 20.1 | N/A | 306.3 (106.1) | 0.68 (0.41, 1.14) | 0.67 (0.39, 1.15) | 15.3 | N/A | 300.5 (109.1) | 0.52 (0.29, 0.94) |
| AOM non-users | 1 year | 29.7 | 129.7 | 274.8 (123.4) | REF | REF | 29.6 | 136.3 | 274.6 (123.8) | REF |
| AOM users | 3 years | 21.4 | N/A | 680.8 (398.5) | 0.79 (0.56, 1.11) | 0.78 (0.54, 1.12) | 18.4 | N/A | 639.8 (392.0) | 0.68 (0.47, 0.99) |
| AOM non-users | 3 years | 27.5 | 91.2 | 539.7 (395.6) | REF | REF | 27.3 | 94.8 | 541.2 (397.1) | REF |
Rate is event per 1000 person years
HR unadjusted hazard ratio, aHR hazard ratio adjusted for all available covariates, wHR stabilized inverse probability of treatment weighted (IPTW) hazard ratio
AOM non-users were censored at use of AOM after the end of the QM period
With a maximum of 3 years follow-up, fractures occurred at a rate of 21.4 per 1000 person years in AOM users, 27.5 per 1000 person years in AOM non-users, and 91.2 per 1000 person years for AOM use in non-AOM users. Mean follow-up time with a maximum of 3 years was 639.8 days (SD 392.0) for AOM users and 541.2 days (SD 397.1) for AOM non-users. AOM use was associated with a slightly more attenuated reduction in fracture risk: unadjusted HR=0.79 (95 % CI 0.56, 1.11), adjusted HR=0.78 (95 % CI 0.54, 1.12), and weighted HR=0.68 (95 % CI 0.47, 0.99) (Table 2).
Our sensitivity analyses varying the length of the quality measure period found that in all time period lengths, AOM use was associated with lower fracture rates at both 1 and 3 years (see Supplementary Figs. 1 and 2). Specifically, weighted HRs for 60 and 120 day quality measure periods indicated AOM use within these time periods reduced fracture rates at 3 years. Of note, after accounting for exclusions at 180 days, AOMs were filled by 16.6 % (1174/7093) of patients without a fracture by 180 days. Separating the fracture definition in non-spine, spine, vertebral alone, and without cervical yielded protective weighted HRs at both 1 and 3 years for all three fracture definitions (see Supplementary Figs. 3 and 4). The third sensitivity analysis using a true ITT design is presented as Supplementary Figs. 5 and 6. We found that when patients were only censored at loss of continuous enrollment or end of the study period, weighted estimates were more protective than the main analysis at both 1 and 3 years.
Excluded patients
Patients who were excluded from the analysis due to fractures in the quality measure period included 174 women, equivalent to 2.2 % of the total study population from our analysis (refer to Fig. 1). Vertebral fractures were the exclusion for 10.3 % of this population, with an additional 7.5 % having hip fractures, and the remaining 82.2 % with a fracture at another site. The mean age was 70.1 (11.3) years with 4.6 % having a diagnosis of osteoporosis and 9.8 % having a DXA prior to GC initiation. A total of 34.8 % of the excluded population initiated an AOM within 90 days, with 41.7 % of these patients filling an AOM prior to fracture.
Discussion
In this study, women ≥50 who initiated glucocorticoid therapy at ≥10 mg daily for 90 days or more and filled an AOM within a quality measure period 14 days prior or up to 90 days after initiation showed a fracture risk reduction of 48 % at 1 year and 32 % at 3 years compared to patients who did not fill an AOM. Our results suggest initiation of AOM therapy within 90 days can effectively reduce fracture risk for GIO patients. These results represent an estimate of the longer-term efficacy of AOM initiation in older US women without known previous glucocorticoid or AOM use initiating high-dose glucocorticoids. With only an additional 5 % of patients without fracture filling an AOM at 180 days compared to 90 days, our results suggest that adherence to the 180-day AOM prescribing goal in current clinical quality measures can help reduce fracture in the high-risk population of chronic oral GC users.
We report a reduction in fracture risk similar to estimates by Thomas et al., but in the opposite direction of Majumdar et al. [23, 24]. Majumdar et al. found that compared to long-term oral GC users without documented AOM or DXA, those with documentation of receipt of an AOM or DXA scan within 180 days of initiation of ≥10 mg prednisone equivalent dose was associated with an increased fracture HR at 1 and 3 years [23]. However, Majumdar et al. did not account for differing baseline fracture risks as evidenced by including all ages and genders in the study population, nor did they account for future fracture risk due to previous fracture, likely causing confounding by indication. Also, Majumdar et al. defined high-quality GIOP care by a composite endpoint of a BMD test or a dispensing of prescription osteoporosis medications within 6 months of a new long-term systemic GC initiation. If most individuals in the GIO care group received a BMD test but not osteoporosis medication, similar fracture rates would be expected in the test vs. comparison group. Our study focused only on treatment to estimate outcomes associated with therapeutic interventions, not diagnostic testing.
Our study used the same database as Thomas et al., though their study only included patients who were concurrently using glucocorticoids and bisphosphonates [24]. Thomas et al. required bisphosphonate use prior or concurrent to glucocorticoid use which may not be representative of common clinical use and likely measures the effect of bisphosphonate continuation with glucocorticoid use, rather than the effect of AOM use after glucocorticoid initiation. Similarly, the comparison of fractures at 3 months to fractures at 12 months does not address if bisphosphonates use is more effective than nonuse, only that long-term use of bisphosphonates are effective in reducing fractures.
Previous estimates of the prevalence of AOM use with chronic glucocorticoids have ranged from 14.5 to 50 %, suggesting our study population received AOM at a lower rate (12.1 %) compared to other study populations [6, 12, 41, 42]. This likely is due to our exclusion of prevalent fragility fractures and prevalent glucocorticoid users, which, if included, may have increased the treatment percentage due to higher baseline fracture risks. AOM users received DXAs more often in the year prior as well as the 90 days post glucocorticoid initiation compared to AOM non-users which is similar to DXA utilization in the Global Longitudinal Study of Osteoporosis in Women (GLOW) study [43]. All patients in this analysis based on a prednisone equivalent dose >7.5 mg would be recommended an AOM by the 2010 ACR guidelines, though only 24.8 % of this at-risk population has been reported as treated [12, 15]. In our current study, we observed a similarly low initiation rate (12.1 %) in women who were new glucocorticoid initiators previously unexposed to AOMs. Although current US quality measures measure receipt of an AOM within 180 days of glucocorticoid initiation, we found that after exclusion of prevalent fractures, only an additional 5 % of patients filled an AOM at 180 days compared to 90 days. Additionally, the effect measure estimate was attenuated as the length of the period increased, likely due to the exclusion of early events in the longer windows. For example, when using a 180-day window, an additional 1113 patients would have been excluded from the analysis for either fracture or loss of continuous enrollment, further reducing the sample size. This finding suggests that most clinicians who adhere to GIO quality measures do so shortly before or after the patient initiates GC use. Therefore, protocols to improve GIO care may be most successful if they are implemented at the clinic visit when the first GC prescription is issued.
Our main analysis defined osteoporotic fracture as those at the hip, pelvis, humerus, wrist, and spine showing a significant reduction in fracture risk at 1 and 3 years. However, there is no definitive, validated definition for osteoporotic fracture in administrative claims data. The algorithms used in this analysis have previously been published and, in the case of the non-spine codes, have been validated [37, 44].We included the spine sites in the analysis primarily due to the secondary findings of reduction in vertebral fractures in RCTs. The sites and codes are based on fractures that are regarded as likely due to osteoporosis [38]. All four fracture definitions in the sensitivity analysis produced similar protective estimates at both 1 and 3 years, though less precise due to the small number of events. When the outcomes were combined, the increased number of events allowed increased precision. The directionality of our sensitivity analysis strengthens our conclusions of a protective effect for AOM use.
Our study possessed several limitations that should be noted. The MarketScan databases include both commercially insured patients and patients with Medicare supplemental insurance who may not be representative of the Medicare, uninsured, or publically insured populations. Though, our use of IPTW weights reduced confounding which would have been associated with patient location. Our estimates likely have some unmeasured confounding present as the MarketScan database did not have clinical variables such as bone mineral density, frailty, or functional impairment available which could have contributed to the prescription of AOMs and future fracture risk. Our study methodology does not account for any additional variability due to persistence or adherence to AOM, or cumulative dose or duration of glucocorticoid therapy. However, we viewed a modified ITT analysis as the most conservative estimate of AOM effectiveness as subsequent AOM was thought to reduce future fracture risk. Additionally, our sensitivity analyses using a true ITT produced more protective HRs than the main analysis, suggesting validity of our main analysis results. Exposure is based on AOM fill within 90 days, which may introduce selection bias due to all patients having to live and be without fracture for the entire quality measure period to be included in the study. However, we view this exclusion as essential for comparable fracture risks at the end of the quality measure period and describe the women who had fractures in the 90 days post-index in the results. Due to small sample sizes, stratified analyses of individual AOM agents were not conducted. Because we used administrative claims, patient’s prior exposure to glucocorticoids or AOM may not be accurate. To minimize misclassification, we excluded all patients who had a fill of either of these therapies recorded in the database prior to index fill regardless if it fell within the baseline period [31]. Exposure was based on first fill daily dose, which may not be indicative of daily dose for the duration of use. Patients who had fractures greater than 1 year prior to glucocorticoid initiation were not identified and excluded in this analysis, therefore there may be patients with prior fractures in this analysis. Although the primary aim of our paper was to examine fracture outcomes in glucocorticoid users receiving AOM versus patients not receiving AOM, future studies may also want to consider the effect of AOM initiation on health-care resource utilization and patient characteristics associated with AOM initiation. A composite fracture outcome was used for the main analysis and was based on two previously defined algorithms which may not capture all osteoporotic fractures and which might have included traumatic fractures. We could not verify menopausal status in these databases; and providers may have been less willing to prescribe AOM for women in their 50s who were premenopausal. Our study results are generalizable to women ≥50 years of age who are new initiators of glucocorticoids and AOMs. Patients excluded from our analysis due to fractures within 90 days of glucocorticoid initiation represent a population who would benefit from AOM treatment but were not analyzed; to account for this at-risk population, we describe the population characteristics in the “Results” section. Lastly, our study period spanned 12 years wherein AOM and guidelines for AOM treatment have changed; this may result in differential treatment based on when the glucocorticoid was initiated.
Based on our results, AOM fill within 90 days of initiating ≥10 mg daily glucocorticoids was associated with a 48 % reduction at 1 year and 32 % at 3 years for osteoporotic fracture, suggesting that treatment for GIO based on quality measures can be effective in preventing fractures. Less than 12 % of glucocorticoid initiators without prior AOM treatment filled an AOM during the 90 days after glucocorticoid initiation, continuing to demonstrate a care gap in glucocorticoid-induced osteoporosis. Our findings suggest that to reduce fractures, quality measures for GIO may be more effective if AOM treatment is recommended within 90 days rather than 180 days. Continued screening, assessment of fracture risk, and appropriate treatment are essential to prevent fractures in GIO. To further close the care gap in GIO, clinicians should consider initiating AOM therapy if they believe their patients may use a glucocorticoid for 90 or more days.
Supplementary Material
Acknowledgments
The database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (1 ULI RR025747); and the UNC School of Medicine.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00198-014-3022-9) contains supplementary material, which is available to authorized users.
Conflicts of interest Robert A. Overman, Margret L. Gourlay, Chad L. Deal, and J. Bradley Layton declare that they have no conflicts of interest. Joel F. Farley has received prior consulting support from Daiichi-Sankyo Pharmaceuticals for unrelated research. M. Alan Brook hart has received research support Amgen and has sat on advisory boards for Amgen, Merck, and Pfizer (honoraria received by institution). He has received consulting fees from RxAnte, Inc. and World Health Information Science Consultants, LLC.
Contributor Information
R. A. Overman, Email: overmar@unc.edu, Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Campus Box 7573, Chapel Hill, NC 27599-7573, USA; Department of Rheumatology, Orthopedic and Rheumatologic Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
M. L. Gourlay, Department of Family Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
C. L. Deal, Department of Rheumatology, Orthopedic and Rheumatologic Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
J. F. Farley, Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Campus Box 7573, Chapel Hill, NC 27599-7573, USA
M. A. Brookhart, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
J. B. Layton, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
References
- 1.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 2.Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, Cooper C, Diez Perez A, Eastell R, Hofbauer LC, Kanis JA, Langdahl BL, Lesnyak O, Lorenc R, McCloskey E, Messina OD, Napoli N, Obermayer-Pietsch B, Ralston SH, Sambrook PN, Silverman S, Sosa M, Stepan J, Suppan G, Wahl DA, Compston JE Joint IOF-ECTS GIO Guidelines Working Group. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23:2257–2276. doi: 10.1007/s00198-012-1958-1. [DOI] [PubMed] [Google Scholar]
- 3.Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthr Care Res (Hoboken) 2013;65:294–298. doi: 10.1002/acr.21796. [DOI] [PubMed] [Google Scholar]
- 4.Fardet L, Petersen I, Nazareth I. Description of oral glucocorticoid prescriptions in general population. Rev Med Interne. 2011;32:594–599. doi: 10.1016/j.revmed.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 5.van Staa TP, Leufkens HGM, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AC, Elmer PJ, Nichols GA, Herson M. Practice patterns in patients at risk for glucocorticoid-induced osteoporosis. Osteoporos Int. 2005;16:2168–2174. doi: 10.1007/s00198-005-2016-z. [DOI] [PubMed] [Google Scholar]
- 7.Walsh LJ, Wong CA, Pringle M, Tattersfield AE. Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross sectional study. Br Med J. 1996;313:344–346. doi: 10.1136/bmj.313.7053.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, III, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–899. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 10.Compston J. Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol. 2010;6:82–88. doi: 10.1038/nrrheum.2009.259. [DOI] [PubMed] [Google Scholar]
- 11.Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469:1900–1905. doi: 10.1007/s11999-011-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overman RA, Toliver JC, Yeh JY, Gourlay ML, Deal CL. U.S. Adults meeting 2010 American College of Rheumatology criteria for treatment and prevention of glucocorticoid-induced osteoporosis. Arthr Care Res (Hoboken) 2014;66:1644–1652. doi: 10.1002/acr.22346. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess. 2007;11:iii,iv, ix–xi. 1–231. doi: 10.3310/hta11070. [DOI] [PubMed] [Google Scholar]
- 14.Chrischilles E, Shireman T, Wallace R. Costs and health effects of osteoporotic fractures. Bone. 1994;15:377–386. doi: 10.1016/8756-3282(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 15.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM, Volkmann E, Saag KG. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthr Care Res (Hoboken) 2010;62:1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 16.Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013;75:392–396. doi: 10.1016/j.maturitas.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, Caeyers N, Cutolo M, Halliday S, Da Silva JA, Kirwan JR, Ray D, Rovensky J, Severijns G, Westhovens R, Bijlsma JW. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2013;72:1905–1913. doi: 10.1136/annrheumdis-2013-203249. [DOI] [PubMed] [Google Scholar]
- 18.Homik JE, Cranney A, Shea B, Tugwell P, Wells G, Adachi R, Suarez-Almazor M. Bisphosphonates for steroid induced osteoporosis. Cochrane Database of Systematic Reviews. 2000 doi: 10.1002/14651858.CD001347. CD001347. [DOI] [PubMed] [Google Scholar]
- 19.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J, Melo-Gomes JA, Gruber BL, Yanover MJ, Leite MO, Siminoski KG, Nevitt MC, Sharp JT, Malice MP, Dumortier T, Czachur M, Carofano W, Daifotis A. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, Doherty SM, Maricic M, Rosen C, Brown J, Barton I, Chines AA. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–285. doi: 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]
- 21.National Quality Forum. 0614 Steroid use—osteoporosis screening. [01 September 2014];2012 http://www.qualityforum.org/QPS/0614.
- 22.National Quality Forum. [01 September 2014];0633 Osteopenia and chronic steroid use—treatment to prevent osteoporosis. 2012 http://www.qualityforum.org/QPS/0633.
- 23.Majumdar SR, Lix LM, Morin SN, Yogendran M, Metge CJ, Leslie WD. The disconnect between better quality of glucocorticoid-induced osteoporosis preventive care and better outcomes: a population-based cohort study. J Rheumatol. 2013;40:1736–1741. doi: 10.3899/jrheum.130041. [DOI] [PubMed] [Google Scholar]
- 24.Thomas T, Horlait S, Ringe JD, Abelson A, Gold DT, Atlan P, Lange JL. Oral bisphosphonates reduce the risk of clinical fractures in glucocorticoid-induced osteoporosis in clinical practice. Osteoporos Int. 2013;24:263–269. doi: 10.1007/s00198-012-2060-4. [DOI] [PubMed] [Google Scholar]
- 25.Saag KG, Curtis J, Warriner A. Challenges in defining quality of care for glucocorticoid-induced osteoporosis: defending good against perfect. J Rheumatol. 2013;40:1640–1642. doi: 10.3899/jrheum.130980. [DOI] [PubMed] [Google Scholar]
- 26.Hansen L, Chang S. White paper: health research data for the real world: the MarketScan(R) databases 2013 [Google Scholar]
- 27.ClinicalTrials.gov. Amgen. [01 September 2014];Study to show treatment with d’mab as good as treatment with risedronate in subjects start to take or taking GCs (GIOP) 2014 http://clinicaltrials.gov/show/NCT01575873 NLM Identifier: NCT01575873.
- 28.Hakala M, Kroger H, Valleala H, Hienonen-Kempas T, Lehtonen-Veromaa M, Heikkinen J, Tuomiranta T, Hannonen P, Paimela L ONCE trial group. Once-monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: a 12-month, randomized, double-blind, placebo-controlled trial. Scand J Rheumatol. 2012;41:260–266. doi: 10.3109/03009742.2012.664647. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 30.Webb R, Singer M. Oxford handbook of critical care. Oxford University Press; New York: 2005. [Google Scholar]
- 31.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, Seeger JD. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22:542–550. doi: 10.1002/pds.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis JR, Taylor AJ, Matthews RS, Ray MN, Becker DJ, Gary LC, Kilgore ML, Morrisey MA, Saag KG, Warriner A, Delzell E. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int. 2009;20:1969–1972. doi: 10.1007/s00198-009-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 34.Office of the Surgeon General (US) Bone health and osteoporosis: a report of the surgeon general. Office of the Surgeon General (US); Rockville: 2004. [PubMed] [Google Scholar]
- 35.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014 doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38:1103–1120. doi: 10.1111/1475-6773.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Schneeweiss S, Liu J, Daniel GW, Chang CL, Garneau K, Solomon DH. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, Saag K. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64:46–5339. doi: 10.1016/j.jclinepi.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 41.Duyvendak M, Naunton M, Atthobari J, van den Berg PB, Brouwers JR. Corticosteroid-induced osteoporosis prevention: longitudinal practice patterns in The Netherlands 2001-2005. Osteoporos Int. 2007;18:1429–1433. doi: 10.1007/s00198-007-0345-9. [DOI] [PubMed] [Google Scholar]
- 42.Saag KG, Gehlbach SH, Curtis JR, Youket TE, Worley K, Lange JL. Trends in prevention of glucocorticoid-induced osteoporosis. J Rheumatol. 2006;33:1651–1657. [PubMed] [Google Scholar]
- 43.Silverman S, Curtis J, Saag K, Flahive J, Adachi J, Anderson F, Chapurlat R, Cooper C, Diez-Perez A, Greenspan S, Hooven F, Le Croix A, March L, Netelenbos JC, Nieves J, Pfeilschifter J, Rossini M, Roux C, Siris E, Watts N, Compston J. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2014 doi: 10.1007/s00198-014-2883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


