Abstract
Purpose
Fanconi anemia (FA) is an inherited disorder associated with a constitutional defect in the FA DNA repair machinery that is essential for resolution of DNA interstrand crosslinks. Individuals with FA are predisposed to formation of head and neck squamous cell carcinomas (HNSCCs) at a young age. Prognosis is poor, partly due to patient intolerance of chemotherapy and radiation requiring dose reduction, which may lead to early recurrence of disease.
Experimental Design
Using HNSCC cell lines derived from the tumors of FA patients, and murine HNSCC cell lines derived from the tumors of wild type and Fancc−/− mice, we sought to define FA-dependent chemosensitivity and DNA repair characteristics. We utilized DNA repair reporter assays to explore the preference of FA HNSCC cells for non-homologous end joining (NHEJ).
Results
Surprisingly, interstrand crosslinker (ICL) sensitivity was not necessarily FA-dependent in human or murine cell systems. Our results suggest that the increased Ku-dependent NHEJ that is expected in FA cells did not mediate relative ICL resistance. ICL exposure resulted in increased DNA damage sensing and repair by poly(ADP-ribose) polymerase (PARP) in FA-deficient cells. Moreover, human and murine FA HNSCC cells were sensitive to PARP inhibition, and sensitivity of human cells was attenuated by FA gene complementation.
Conclusions
The observed reliance upon PARP-mediated mechanisms reveals a means by which FA HNSCCs can acquire relative resistance to the ICL-based chemotherapy that is a foundation of HNSCC treatment, as well as a potential target for overcoming chemoresistance in the chemosensitive individual.
Keywords: Fanconi anemia, head and neck/oral cancers, interstrand crosslinker resistance, genotype-phenotype correlations, PARP inhibitors
INTRODUCTION
Fanconi anemia (FA) is a genetic disorder characterized by congenital abnormalities, progressive bone marrow failure, and cancer predisposition (1, 2). FA results from germ-line mutations in one of sixteen genes that participate in a common DNA repair pathway, thus deregulating DNA damage responses and leading to the disorder’s clinical phenotypes (3-5). It has been demonstrated that FA-deficient cells exhibit reduced capacity for homologous recombination (HR), while non-homologous end joining (NHEJ) is elevated and even functionally contributes to FA phenotypes under certain circumstances (6, 7). While acute myelogenous leukemia (AML) is the most frequently-occurring malignancy in FA, individuals with the disease also possess a strong predisposition to the development of solid tumors, particularly squamous cell carcinomas of the head and neck (HNSCCs), as well as of the anogenital region (8-11). FA HNSCCs occur primarily in the oral cavity and in the absence of traditional risk factors for HNSCC such as tobacco and alcohol use (11, 12).
Data from the International Fanconi Anemia Registry indicates that the cumulative incidence of non-hematologic malignancies in FA patients may be as high as 28% by 40 years of age (11). This dramatic risk of HNSCC is, for unclear reasons, increased further by the allogeneic hematopoietic stem cell transplantation that is the treatment of choice to correct the disorder’s progressive bone marrow failure (13, 14). The hypothetical cumulative incidence of HNSCC, defined as the cumulative incidence of HNSCC development if the competing risks of death due to other causes are removed, has been estimated to approach 100% in transplanted patients versus 50% in non-transplanted patients that reach their maximal life expectancy (14). Thus, as improved transplantation and supportive care measures prolong survival, the risk of HNSCC will become an increasingly prominent issue for FA patients.
Once HNSCCs are clinically manifest, patients with FA fare exceedingly poorly with two-year overall and relapse-free survival rates of less than 50% (15). Patients tolerate surgery well, but experience significant morbidity and also mortality with the radiation and/or interstrand crosslinker (ICL)-based chemotherapy that, depending upon tumor stage at presentation, may be necessary components of treatment (12, 15, 16). While the poor prognosis of FA HNSCC patients has been attributed to intolerance of conventional clastogenic therapy due to their constitutional sensitivity to DNA damaging agents, a high rate of early locoregional recurrence (15) may suggest that the tumors are not adequately controlled by the degree of genotoxic therapy that they can tolerate.
The desire to avoid severe toxicity and the hope that FA HNSCCs will share in the individual’s DNA damage sensitivity makes the use of low-dose clastogenic treatments a possible option for therapy. However, the increased genomic instability caused by an underlying defect in error-free DNA repair by homologous recombination may facilitate FA tumor evolution by inducing genomic adaptations that could mitigate any inherent sensitivity to DNA damage, particularly in light of the ability of FA-deficient oral keratinocytes to proliferate more rapidly compared to controls, despite exhibiting increased DNA damage both in vitro and in vivo (17, 18). Thus far, the extent to which FA HNSCC phenotypes remain dependent on a dysfunctional FA pathway remains unclear, and direct and systematic examination of FA-dependent biological and molecular properties of FA HNSCCs has been limited (19, 20), predominantly due to the paucity of available isogenic human and murine HNSCC model systems.
The poly(ADP-ribose) polymerase, or PARP, family of proteins contains 18 distinct proteins that catalyze the covalent attachment of ADP-ribose units from donor NAD+ molecules onto target proteins, resulting in the attachment of monomers or linear or branched poly(ADP-ribose) (PAR) polymers that modify the receiving protein’s function (21, 22). Two of these, PARP1 and PARP2, bind to sites of DNA damage and recruit and activate effector proteins that participate in numerous DNA damage repair mechanisms. PARP1 has also been shown to PARylate itself as a means of enhancing its own activity (21, 22). Although PARP proteins have been implicated in chemoresistance of several solid tumor types including non-small cell lung cancer and sporadic head and neck cancers (23, 24), and their inhibition has been associated with synthetic lethality in tumor cells defective in BRCA1 or BRCA2 (25), they have not yet been studied in FA HNSCC.
In order to characterize the pathway-dependent cellular and molecular phenotypes of FA HNSCC cells, we generated isogenic cellular models of FA-deficient and proficient HNSCC cells, and characterize here their comparative biological and molecular properties and DNA repair capabilities. Human patient-derived FANCA−/− and FANCC−/− HNSCC cells were transduced with either control or FA-complementing retroviral vectors prior to analysis. Surprisingly, ICL sensitivity of FA-deficient tumor cells was not increased compared to their FA–complemented cellular counterparts or to sporadic HNSCC cells. Additionally, a murine HNSCC model was generated by exposing wild type (WT) and Fancc−/− mice to the carcinogen 4-nitroquinolone 1-oxide (4-NQO). Although non-neoplastic Fancc−/− epithelial cells were hypersensitive to crosslinking agents, some Fancc−/− tumor cells lost their characteristic sensitivity, similar to the human model. To investigate potential compensatory mechanisms in DNA repair pathways of FA HNSCCs, we tested the degree to which PARP proteins are engaged in the repair process in both FA-proficient and FA-deficient cells. The results show that PARP activity is specifically upregulated in FA-deficient HNSCCs, and this increased activity is associated with a selective sensitivity to PARP inhibitors in both human and murine FA HNSCC cells. Taken together, the data question the expectation that FA HNSCCs share the individual’s global DNA damage hypersensitivity, thus perhaps contributing to the high rate of early locoregional recurrence in patients treated with reduced-intensity genotoxic therapies. Importantly, we also demonstrate that this increased resistance to ICLs is caused, at least in part, through PARP activation. PARP inhibitors may thus provide new avenues for treatment of HNSCC in FA.
MATERIALS AND METHODS
Human cell cultures and vectors
Three FA patient-derived HNSCC cell lines used in this study were kind gifts from other institutions. VU-1131 (FANCC−/−) and VU-1365 (FANCA−/−) lines were obtained from Drs. Johan de Winter and Ruud Brakenhoff at VU University, Amsterdam, Netherlands, and OHSU-974 (FANCA−/−) cells were obtained from Dr. Grover Bagby at the Oregon Health and Science University (OHSU). These have been described previously as HPV-negative head and neck cancer cells, and the respective patients were not treated with cisplatin or other ICL-causing agents prior to creation of the cell lines (19). Human sporadic HNSCC cell lines CAL-27, FADU, and SCC-4 were obtained from American Type Culture Collection. Cell culture conditions are detailed in the Supplemental Materials and Methods. All cell lines were authenticated regularly by their morphologic characteristics and analysis of FA status and corresponding genetic and molecular markers.
The cDNAs for human FANCA and FANCC were cloned into the multicloning site of the oncoretroviral vector S91IN, which co-expresses an IRES-neomycin phosphotransferase cassette, thus conferring resistance to G418 (Invitrogen, Grand Rapids, NY). S91IN and the two FA vectors, S91FAIN and S91FCIN, were transfected into ecoPhoenix cells and then supernatant generated to stable transduce PG13 cells, as previously described (26, 27). Supernatant from G418 resistant PG13 cells were collected, filtered through 0.45 μm, stored at −80°C, thawed, and then tested functionally for correction of FANCA- and FANCC-deficient reference cells with known bi-allelic mutations (data not shown). Subsequently, supernatants were utilized to transduce human HNSCC cell lines. Cultures with 0.8 mg/mL medium G418 were used for selection of transduced polyclonal HNSCC cell populations.
Murine HNSCC tumor induction
Fancc−/− mice were described previously (28) and were maintained and treated according to Institutional Animal Care and Use Committee guidelines at the Portland VA Medical Center. To generate murine oral HNSCCs, two to four month-old mice (22 WT and 18 Fancc−/−) were treated with 20 μg/mL 4-nitroquinoline-1-oxide (4-NQO; Sigma, St. Louis, MO) in water for up to 45 weeks. Mice were monitored weekly for tumor development and euthanized at the first signs of morbidity. Following euthanasia, tumor masses were preserved in formalin for histologic analyses and/or prepared for cell isolation and culture. Tumor grade and type were determined by hematoxylin and eosin (H&E) staining and analysis by a cancer pathologist at OHSU blinded to the genotype of the specimens.
Murine cell culture
Cell isolation and culture of primary tongue epithelial cells and HNSCC cells from WT and Fancc−/− mice are described in the Supplemental Materials and Methods.
Western blot analysis
Trypsinized cells were washed with PBS and collected by centrifugation. For FANCA, FANCC, FANCD2, and actin immunoblots, whole-cell protein extracts were lysed using the Laemmli method (29). For DNA-PKcs and pDNA-PKcsS2056 immunoblots, whole-cell protein extracts were lysed using RIPA buffer (1% Triton X-100, 1% DOC, 0.1% SDS, 0.16 M NaCl, 10 mM Tris pH 7.4, and 5 mM EDTA) supplemented with a protease inhibitor cocktail (BD Biosciences, San Jose, CA), 10 mM NaF, and 5 mM NaVO3. Protein concentrations were determined using a Pierce™ BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). Lysates were resolved by SDS-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane (BioRad, Hercules, CA). Membranes were probed with the appropriate primary antibody overnight. Primary antibodies used were: FANCA (Cascade, Grand Island, NY), FANCC (a kind gift from the Fanconi Anemia Research Fund through OHSU), FANCD2 (Novus, Littleton, CO), actin (Seven Hills Bioresearch, Cincinnati, OH), DNA-PKcs (Abcam, Cambridge, England), and pDNA-PKcsS2056 (Abcam, Cambridge, England). Membranes were washed with TNET (10 mm Tris, 2.5 mm EDTA, 50 mm NaCl, and 0.1% Tween 20) and secondary anti-mouse (GE, Pittsburgh, PA) or anti-rabbit (Jackson Immunoresearch, West Grove, PA) antibodies conjugated to horseradish peroxidase were added for 30 minutes. Membranes were then exposed to chemiluminescence reagents (Thermo Scientific, Waltham, MA) for protein detection. For detection of monoubiquitinated FANCD2, cells were plated for 24 hours and subsequently left untreated or treated with 2 mM hydroxyurea for 24 hours prior to collection. For detection of DNA-PKcs and pDNA-PKcsS2056, cells were pre-treated with DNA-PKcs inhibitors DNA-PK inhibitors NU-7026 (Tocris, Bristol, United Kindgom) or NU-7771 (Tocris, Bristol, United Kindgom) for 24 hours and subsequently with 2 μg/mL bleomycin for 20 minutes prior to collection.
Organotypic epithelial raft culture
Three-dimensional organotypic rafts were generated as described previously and as detailed in the Supplemental Materials and Methods (18). H&E staining was performed for morphologic examination by a cancer pathologist at Cincinnati Children’s Hospital Medical Center blinded to the gene complementation status of the specimens. Photographs were obtained on a Leica DM2500 microscope using Leica Application Suite software. Immunofluorescence for BrdU was performed as described below. The percent BrdU-positive cell population was quantified as the ratio of total BrdU-positive nuclei to total nuclei per 200x field. Such ratios were determined for three fields of each raft and averaged.
Immunofluorescence microscopy
Preparation of coverslips and epithelial raft sections for immunofluorescence and performance of immunofluorescence microscopy is described in the Supplemental Materials and Methods.
Cell cycle analysis by flow cytometry
Assays were performed as previously described (30). Briefly, FA-deficient and complemented HNSCC cells were either left untreated or treated with 0.25 μg/mL melphalan (Sigma, St. Louis, MO) for 48 hours. Cells were trypsinized, washed in PBS, and fixed in 100 μL BD Cytofix/Cytoperm (BD Biosciences, San Jose, CA). Cells were prepared using the protocol for the APC BrdU Flow Kit (BD Biosciences, San Jose, CA). Cell cycle profiles were detected using 7AAD on a BD FACSCanto instrument (BD Biosciences, San Jose, CA), and the data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Cellular proliferation assays
Cellular growth was measured by MTS assays as described (31) and by viable cell counts over time using dye exclusion and counted live cell assays as described in the Supplemental Materials and Methods.
DNA repair assays
Flow cytometry-based DNA repair assays were performed as described (32) using constructs designed to measure the proportion of cells engaged in NHEJ. Briefly, equal numbers of FA-deficient and complemented VU-1131 cells were plated in 6-well plates. Following 24 hours of growth, transfections were performed utilizing FuGENE HD transfection reagent (Promega, Madison, WI) and Opti-MEM reduced serum media (Invitrogen, Grand Rapids, NY). Following 24 hours, GFP expression was measured using a BD FACSCanto instrument (BD Biosciences, San Jose, CA). The data was analyzed using FlowJo software (Tree Star, Ashland, OR). At least four independent experiments were performed with each construct.
Statistical analysis
Graphs were created and statistical analyses performed using GraphPad Prism software (GraphPad, La Jolla, CA). Data points and error bars indicate mean and standard deviation, respectively, of the raw data.
RESULTS
FA complementation of patient-derived HNSCC cells reverses characteristic cellular FA phenotypes
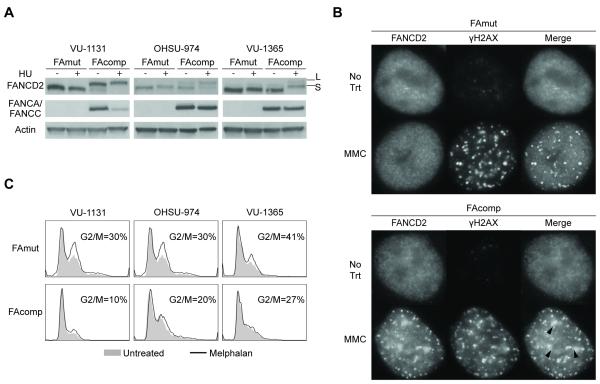
The goal of this study was to determine FA-dependent growth and chemosensitivity properties of patient-derived HNSCC cells, with the expectation that substantial ICL sensitivity was to be observed in FA-deficient cells. One FANCC-deficient cell line (VU-1131) and two FANCA-deficient cell lines (OHSU-974 and VU-1365), all originally cultured from the HNSCCs of FA patients, were utilized for gene correction. The cells were transduced with either control retroviral vector, FANCC retroviral vector for VU1131, or FANCA vector for OHSU-974 and VU-1365. FANCA and FANCC expression was confirmed in each case at the protein level by immunoblotting. Complementation restored pathway activation as demonstrated by FANCD2 monoubiquitination following HU treatment; thus the mutant FA gene was corrected in each case (Fig 1A). Additionally, immunofluorescence experiments demonstrated that monoubiquitinated FANCD2 in complemented, but not control cells, was capable of localizing to sites of double-stranded DNA breaks following MMC treatment as shown by colocalization of FANCD2 and γH2AX foci (Fig 1B). To verify FA pathway functionality, isogenic cell populations were treated with melphalan and subjected to cell cycle analysis. As predicted, FA complementation rescued cells from accumulation in the G2/M phase of the cell cycle, a hallmark of FA pathway deficiency, following melphalan treatment (Fig 1C) (30).
Figure 1. Gene correction of FA patient-derived HNSCC cell lines.
A) Immunoblot of isogenic FA patient-derived HNSCC cell lines. Complementation of the relevant FA gene restores FANCD2 activity upon treatment with hydroxyurea (HU). FAmut: FA-deficient; FAcomp: FA-complemented; S: FANCD2; L: Monoubiquitinated FANCD2. B) Immunofluorescence for FANCD2 and γH2AX shows localization of FANCD2 to sites of DNA damage in FAcomp cells following MMC treatment. Images shown are representative of three independent experiments, each with similar results. C) FAcomp cells are rescued from cell cycle arrest in the G2/M phase caused by melphalan treatment.
FA complementation does not affect HNSCC proliferation in three dimensions
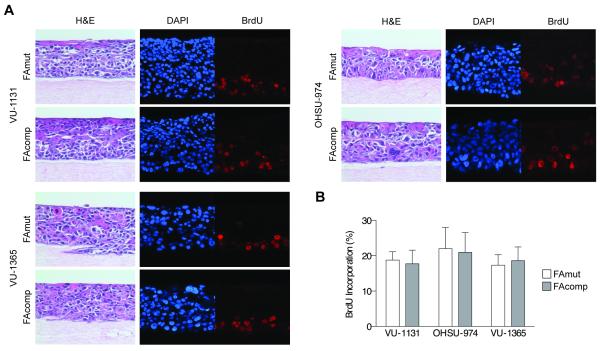
Our previous work utilizing HPV E6/E7-immortalized FA patient-derived and FA knockdown keratinocyte models had shown that FA loss confers a proliferative advantage specifically in the environment of three-dimensional organotypic epithelial rafts, despite characteristic FA phenotypes and increased DNA damage (18). In order to examine the growth of FA HNSCC in the context of the epithelial milieu wherein they arise, we generated rafts utilizing the above FA-deficient and complemented HNSCC cells. H&E staining revealed comparable raft thickness, as well as similar morphological features of the constituent cells (Fig 2A). Immunofluorescence detection of BrdU incorporation revealed no significant differences, indicating that FA correction in malignant HNSCC cells does not affect proliferation (Fig 2B). From this, we concluded that although differentiation-associated cell cycle exit of non-malignant, HPV-positive keratinocytes is FA-dependent and reversible upon complementation, the FA pathway is unable to exert any such anti-proliferative influence following tumorigenesis.
Figure 2. Three-dimensional organotypic epithelial rafts generated from human FA HNSCC cells.
A) H&E and immunofluorescence staining of rafts created from isogenic HNSCC cell lines. Images are representative of three independent experiments, each with similar results. H&E sections of FAmut and FAcomp rafts are equivalent in mitotic index, cellular differentiation, and stromal content. Immunofluorescence for BrdU incorporation indicates similar proliferative rates. B) Quantification of BrdU incorporation of FAmut and FAcomp cells; a t-test indicated no significant difference.
FA HNSCC cells acquire relative resistance to interstrand crosslinkers
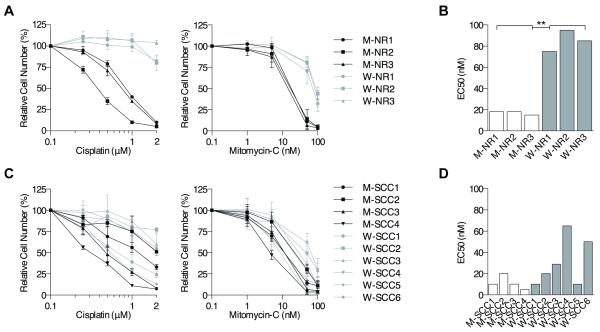
The expectation that FA-deficient HNSCC cells possess the same hypersensitivity to ICLs as non-malignant cells from FA patients has not previously been tested in murine or human systems. We therefore sought to develop a murine model of non-malignant oral keratinocytes and HNSCCs using Fancc−/− and WT mice. Oral keratinocytes were harvested from either WT (W-NR) or Fancc−/− (M-NR) mice, SV40-transduced for immortalization, and analyzed in survival assays to test for relative sensitivities to MMC and cisplatin. As expected, SV40-immortalized Fancc−/− oral keratinocytes exhibited significantly increased sensitivity to MMC and cisplatin when compared to their WT counterparts (Fig 3A). Specifically, Fancc−/− cells displayed an approximately five-fold average decrease in half maximal effective concentration (EC50) compared to WT cells (Fig 3B, Table S1).
Figure 3. Interstrand crosslinker sensitivity of murine FA HNSCC.
A) Cisplatin (left) and MMC (right) cellular growth assays of immortalized, non-malignant oral epithelial cells of Fancc−/− (M-NR) and WT (W-NR) mice shows significantly increased sensitivity of Fancc−/− cell lines. B) MMC half-maximal effective concentrations (EC50s) of immortalized, non-malignant murine Fancc−/− and WT oral epithelial cells. ** p < 0.01 (t-test). C) Cisplatin (left) and MMC (right) cellular growth assays of murine Fancc−/− (M-SCC) and WT (WSCC) mice indicate overlap of sensitivities. D) MMC EC50s of Fancc−/− and WT HNSCC cell lines following 5 days of exposure; a t-test revealed no significant difference.
For HNSCC induction, we utilized a well-known carcinogen, 4-NQO, which has been shown to cause the development of murine HNSCCs that closely mimic human tumors histopathologically (33, 34). WT and Fancc−/− mice were treated with 4-NQO in water for up to 45 weeks. Mice were monitored weekly for visible tumor development and euthanized at the first signs of morbidity. Survival (time to morbidity that necessitated sacrifice) and tumor incidence were similar for WT and Fancc−/− mice (Figs S1A, B). Median survival for both cohorts of mice was 40 weeks. Greater than 80% of mice of both genotypes developed tumors that were located mainly on the tongue, with a subset developing on or in the lip, buccal mucosa, and esophagus (Fig S1C). All tumors were well-differentiated HNSCCs ranging from low to high-grade (Fig S1D, Table S1). We did not detect metastases in either genotype, analogous to previous studies (33, 34), perhaps due to the necessity of early euthanasia after tumor development. Tumors were harvested for generation of WT (W-SCC) or Fancc−/− (M-SCC) cell lines. These were subsequently tested in survival assays for relative sensitivities to MMC and cisplatin. Interestingly, Fancc−/− mutant compared to WT HNSCC cells did not differ significantly in their sensitivity to MMC or cisplatin (Fig 3C). In fact, three of six WT lines displayed an MMC EC50 of 10-20 nM, similar to an EC50 of 5-20 mM in Fancc−/− lines, while one other WT line displayed an only slightly higher EC50 of 29 nM (Figs 3D, Table S1). The lack of uniform ICL sensitivity in Fancc−/− versus WT cell lines does not appear to be due to increased chromosomal instability in WT cells during malignant transformation, as Fancc−/− cell lines showed more complex karyotypes and had greater levels of MMC-induced chromosomal breakage (Table S1).
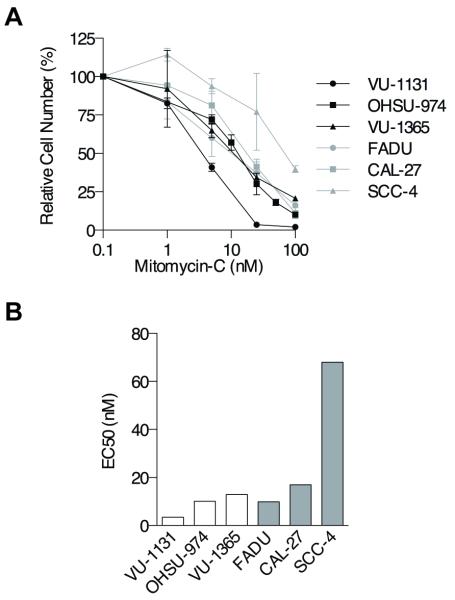
To compare the murine with human FA HNSCC cell models, we also subjected uncorrected FA patient-derived cell lines and cell lines derived from sporadically-occurring HNSCCs to MMC treatment and performed viable cell counts after 5 days of exposure. These experiments revealed results similar to those obtained with murine HNSCC cells; overlap of survival curves of FA and sporadic cell lines was observed, and two of three FA and two of three sporadic lines had an EC50 of 9-17 nM (Figs 4A, B). Taken together, we concluded that FA-deficient HNSCC cells can largely overcome FA-dependent sensitivity to chemical crosslinkers.
Figure 4. Interstrand crosslinker sensitivity of human FA HNSCC.
A) MMC cellular growth assays of FA patient-derived (black) and sporadic (gray) HNSCC cell lines indicate overlap of sensitivities following 5 days of treatment. B) MMC EC50s of FA patient-derived and sporadic HNSCC cell lines following 5 days of exposure; a t-test revealed no significant difference.
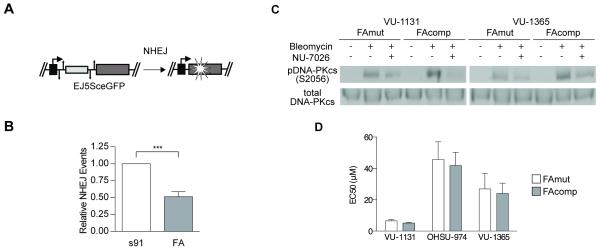
FA HNSCC cells engage in increased NHEJ at baseline, but do not require Ku-dependent NHEJ for repair of cisplatin-induced DNA damage
Given the reported stimulation of NHEJ that is regulated by FA in other cellular models (6, 7), we next sought to define FA-dependent NHEJ DNA repair properties of FA HNSCC using established reporter constructs (Fig 5A). Isogenic VU-1131 cell lines were co-transfected with I-SceI endonuclease plus NHEJ-GFP reporter plasmids as described in mammary epithelial cell lines (32). Flow cytometry was then used to detect the percentage of cells with the corresponding repair events. As expected, FA HNSCC cells had significantly increased occurrences of NHEJ in comparison with their complemented counterparts (Fig 5B).
Figure 5. Preference for NHEJ of mutant and complemented FA HNSCC cells.
A) Schematic of DNA repair reporter assay constructs for NHEJ. B) Reporter assays performed on isogenic VU-1131 cells indicate that FA gene correction decreases baseline preference for NHEJ. ** p < 0.01 (t-test). C) Immunoblot of total DNA-PKcs and pDNA-PKcsS2056 in FAmut and FAcomp cell lines treated with 2 μg/mL bleomycin for 20 minutes in the presence and absence of 24 hours pre-treatment with the DNA-PKcs inhibitor NU-7026 (2 μM). Pretreatment with NU-7026 decreases phosphorylation of DNA-PKcs caused by bleomycin treatment. D) Chemical inhibition of DNA-PKcs does not decrease the cisplatin EC50 of FAmut HNSCC cells relative to FAcomp cells following 2 days of exposure.
NHEJ has been identified as encompassing two distinct and competing pathways (35). Classical NHEJ is dependent upon recruitment of the Ku70/80 heterodimer to DNA double-strand breaks (DSBs) and subsequent activation by phosphorylation of DNA-PKcs (36); alternative NHEJ is suppressed by the binding of Ku70/80 to DSBs and is initiated by binding of PARP1 to DSB ends (37, 38). The performance of Ku-dependent NHEJ has been implicated in the increased defective DNA repair that occurs in FA-deficient cells (6, 7). To determine whether FA HNSCC cells relied upon increased performance of Ku-dependent NHEJ in response to ICLs, we next investigated the effect of its inhibition using the DNA-PKcs inhibitors NU-7026 and NU-7441 on cisplatin sensitivity of human FA-deficient and complemented cell populations. We hypothesized that, if Ku-dependent NHEJ were the necessary DNA repair pathway used by FA HNSCC cells following ICL exposure, then inhibition would produce an early decrease in survival in deficient versus complemented cells. To understand baseline behavior, isogenic cell lines were first treated with cisplatin alone for two days, following which growth was quantified by MTS assays. FA deficient and corrected cells for each donor possessed similar sensitivities (Fig S2A). Viable cell counts following two days of MMC treatment of VU-1131 and OHSU-974 cell lines also revealed comparable survival (Fig S2B). Sensitivity to other chemotherapeutic agents that are used clinically for the treatment of head and neck cancer was also evaluated, including paclitaxel, 5-fluorouracil, and rapamycin. No differences in the response to these drugs were observed between FA-deficient versus proficient cells (Figs S2C, D). Reduced DNA-PKcs phosphorylation in the presence of NU-7026 or NU-7441 was confirmed via immunoblotting (Figs 5C, S3A). Next, the cells were exposed to cisplatin, and treated versus untreated cells were subjected to cellular growth assays. Interestingly, DNA-PKcs inhibition did not differentially affect the cisplatin sensitivity of FA-deficient and complemented human HNSCC cells (Figs 5D, S3B), suggesting that Ku-dependent NHEJ was not specifically upregulated by FA HNSCC cells following ICL exposure.
PARP activity is required by FA HNSCC, and tumor cells are sensitive to PARP inhibitors
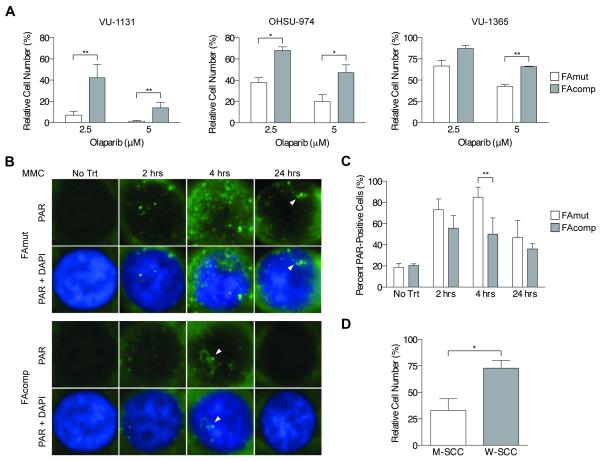
PARP inhibitors were initially developed as chemotherapeutic agents for BRCA-deficient cancers following the identification of synthetic lethality of PARP inhibition in BRCA1-mutated cells (39). In light of the intrinsic relationship between the FA and BRCA pathways, we sought to determine the effect of PARP inhibition on the growth of FA HNSCC cells. Viable cell counts were taken over time in the presence of the combined PARP1/PARP2 inhibitor olaparib and the PARP1 inhibitor PJ-34. The results indicated profound sensitivity of human FA HNSCC cells to olaparib that was significantly decreased by complementation (Fig 6A). A similar result was observed in VU-1131 cells treated with PJ-34 (Fig S4A). Intranuclear PAR foci, but not cytoplasmic signal, are an indicator of PARP-mediated DNA damage sensing and repair activity (40). Thus we next quantified PAR polymer foci following MMC treatment, and detected increased formation of intranuclear PAR foci in FA-deficient cells (Figs 6B, C). In order to test olaparib sensitivity in the above malignant murine tumor cell system, we quantified viable cell counts using WT and Fancc−/− cell lines. PARP inhibitor sensitivity was present uniformly in the Fancc−/− cell lines (Figs 6D, S4B). Taken together, activation of PARP-mediated DNA damage responses provides a mechanism upon which FA HNSCC cells can rely for response to both endogenous and exogenous DNA damage.
Figure 6. PARP inhibitor sensitivity of human and murine FA HNSCC cells.
A) Cellular growth assays on isogenic human HNSCC cells exposed to the PARP1/PARP2 inhibitor olaparib shows uniform sensitivity of FAmut cell lines. * p < 0.05; ** p < 0.01 (t-test). B) Immunofluorescence for PAR foci in VU-1131 cells shows increased PAR foci formation in FAmut cells over a 24-hour course of MMC treatment. Results shown are representative of two (no treatment) or three (2, 4, and 24 hours) independent experiments of each time point, each with similar results. C) Quantification of intranuclear PAR foci in VU-1131 cells over a 24-hour course of MMC treatment reveals a significantly increased number of PAR foci in FAmut cells at 4 hours of exposure. ** p < 0.01 (t-test). D) Cellular growth assays performed on murine Fancc−/− (M-SCC) and WT (W-SCC) HNSCC cell lines treated with olaparib. shows significantly increased sensitivity of Fancc−/− cell lines. * p < 0.05.
DISCUSSION
The lack of knowledge about the natural behavior and response to therapy of HNSCCs arising in FA patients is a major hindrance to their successful treatment. Therapy for HNSCC includes surgery and possibly radiation therapy or chemotherapy, depending upon disease stage. In light of the established sensitivity of FA patients to genotoxic agents their poor survival has traditionally been attributed to intolerance of therapy. However, long-term follow-up of patients who survive initial therapy and obtain a complete response indicates a very high rate of recurrence of 50% by age 40 (15). Most of these recurrences are at the original site of disease, suggesting incomplete disease control rather than origination of a metachronous tumor. While the rate of second or multiple primary tumor formation in FA HNSCC patients had been reported to be over 60% (15), the majority of these are in the anogenital regions, further underscoring that tumors arising in the head and neck area after a first occurrence of HNSCC are likely to be recurrent tumor. Given that current therapy provided for these tumors may be insufficient to provide lasting progression-free survival, and that treatment of FA patients with HNSCC could benefit from an in-depth understanding of tumor biology and response to therapy, we considered whether patients’ poor prognosis extends beyond their constitutional susceptibility to DNA damage. In order to address this lack of understanding in human and murine models, we utilized a panel of HNSCC cell lines derived from the tumors of FA patients or mice and their FA-proficient counterparts.
A significant body of research has provided insight into the behavior of FA hematopoietic cells. Bone marrow transplantation for FA patients with severe bone marrow failure, acute myelogenous leukemia, or myelodysplastic syndrome can be successfully performed with low rates of toxicity-related morbidity using T cell-depleted grafts and reduced-intensity preparative regimens (41). Unfortunately, the hope that FA HNSCC could also be treated both effectively and safely with low-dose clastogenic therapies may be incorrect. Published research suggests that the extra-hematopoietic compartments of these patients possess a distinct set of characteristics; for instance, in both in vitro and in vivo models of the epidermal compartment, FA deficiency leads to unique and unexpected gains in keratinocyte proliferation despite increased DNA damage (17, 18).
Thorough understanding of FA HNSCC has been impaired by the need of a comprehensive model. We used an isogenic human FA HNSCC model that allowed for observations of tumor cell characteristics that were strictly FA-dependent. Three-dimensional organotypic tumor rafts utilized here provide a view of FA HNSCC as a carcinoma in situ, and allow for quantifiable examination of tumor cell proliferation in a physiologic but controlled environment. However while available human FA HNSCC cell lines are well-characterized (19), they are few in number. The difficulty in faithfully recapitulating the FA epithelial compartment is underscored by the fact that FA mice do not spontaneously form HNSCCs (42). We thus used 4-NQO to induce HNSCCs in WT and Fancc−/− mice. The cell lines isolated from these and non-malignant oral keratinocytes of WT and Fancc−/− mice reveal data similar to that obtained in the human FA HNSCC cell system.
We find that the growth characteristics between FA-deficient and FA-complemented HNSCC cells are similar. In contrast, FA complementation of patient-derived non-malignant keratinocytes decreases hyperplasia (17, 18). In light of the chromosomal instability induced by FA deficiency, loss of the suppressive effect of the FA pathway on proliferation of the pre-malignant epithelium could conceivably contribute to the increased risk of HNSCC in FA patients. However the loss of growth suppression seen in FA-complemented HNSCC cells suggests that, following malignant transformation, cellular machineries become less dependent upon FA deficiency.
Previous work utilized colony assays to explore the chemosensitivity of FA compared to sporadic HNSCC cells, and found a lack of MMC sensitivity in the FANCA-deficient OHSU-974 cell line (20). Importantly the present study confirms this result. In contrast, ICL sensitivity has been observed in FA fibroblasts (5, 20, 43, 44). We postulated that, in the background of FA deficiency, tumorigenesis and the resulting genomically unstable environment, as illustrated by the complex karyotypes of Fancc−/− HNSCCs (Table S1), could lead to adaptations in cellular processes that may confer relative chemoresistance. Such adaptation is in line with comparisons between murine keratinocytes versus HNSCC-derived cell lines; early passage immortalized oral keratinocytes are consistently hypersensitive to ICLs, whereas HNSCC cell populations are not (Figs 3A-D). Alterations in DNA repair mechanisms are one of a variety of means for tumor cells to become chemoresistant, and would be especially advantageous to a cancer arising in a patient with intrinsic DNA damage sensitivity. It thus stands to reason that FA HNSCCs would, in the process of tumor generation and development, and in response to the increased cellular stress during transformation, be preferentially selected for cells that have enhanced DNA repair mechanisms.
Increased performance of NHEJ at the expense of HR is an expected result of FA pathway loss and so is a natural first choice for examination of the impact of DNA repair on chemosensitivity of FA HNSCCs. However the extent to which NHEJ participates in the survival of FA HNSCC has not previously been explored, nor has DNA repair by NHEJ been directly measured in FA HNSCC cells. Using DNA repair reporter assays we show that, as expected, FA-deficient VU-1131 cells exhibit increased NHEJ (Fig 5B). We found that DNA-PKcs inhibition does not decrease the cisplatin EC50 of the human FA-deficient HNSCC cell lines (Fig 5D), while all are uniformly sensitive to PARP inhibition. The lack of enhanced cisplatin sensitivity of FA-deficient HNSCC cells following DNA-PKcs inhibition suggests that Ku-dependent NHEJ is not the DNA repair mechanism required by FA HNSCC cells for repair of damage caused by ICLs.
In contrast to the NHEJ machinery, PARP appears to be a more promising target in FA HNSCCs. We show increased activation of PARP in FA-deficient HNSCC cells by greater formation of intranuclear PAR foci following MMC treatment (Figs 6B, C). In addition, rescue of PARP inhibitor sensitivity of human FA HNSCC cells occurred by gene complementation (Figs 6A, S4A) and uniform PARP inhibitor sensitivity was additionally observed in murine FA HNSCC cells (Figs 6D, S4B). PARP inhibitor sensitivity has previously been examined in MMC-sensitive fibroblasts derived from FA mice as well as FA patients, with conflicting results (5, 44); the present work adds to this not only by showing PARP sensitivity in FA HNSCC cells but by linking PARP activity to cellular response to ICLs and subsequent relative resistance. We thus postulate that PARP hyperactivation is a mechanism frequently acquired during malignant transformation whereby FA HNSCC overcome constitutional DNA damage sensitivity.
PARP activation could conceivably overcome FA pathway deficiency by multiple mechanisms. PARP1, which comprises approximately 90% of intranuclear PARP, engages numerous modes of DNA repair, including single-strand break repair (45), base excision repair (45), nucleotide excision repair (46), Ku-independent NHEJ (37), and HR (47). PARP1 has also been implicated in Chk1 signaling at stalled replication forks (40), plays a role in control of transcription by maintaining chromatin in a transcriptionally active state (48), and may promote survival by functioning as a cofactor for NF-κB-dependent transcription (49). PARP2 has been associated with the later steps of single-strand break repair and base excision repair (50). It remains to be seen what aspects of PARP protein function are most critical for FA HNSCC cell adaptation.
The relative ICL resistance of FA HNSCC cells highlights the delicate balance between providing effective therapy and avoiding excessive toxicity in cancer treatment. The difficulty in achieving this balance becomes especially profound in FA HNSCC patients, as the therapy de-escalation that may be necessary in order to avoid overwhelming toxicity-related morbidity may simultaneously undertreat their malignancy. In this light, it is essential to identify new therapies that will enhance survival of this fragile patient population. Identification of PARP-mediated DNA repair as a key survival mechanism employed by FA HNSCCs provides a promising new potential avenue of treatment. PARP inhibitor therapy could enhance efficacy of low-dose clastogenic treatments via synergistic effects. PARP inhibition could greatly benefit patients that have undergone bone marrow transplantation that are at the highest risk for HNSCC development, as the presence of a hematopoietic compartment unaffected by FA could prevent excessive myelotoxicity in a patient group with an otherwise grim prognosis. Further studies targeting PARP will hopefully allow for forward progress in improvement of outcomes of FA patients with HNSCC.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Due to the sensitivity of Fanconi anemia (FA) patients to DNA damage caused by interstrand crosslinks, current therapy for head and neck squamous cell carcinomas (HNSCCs) developing in FA patients requires either dose reduction or omission of the radiation and chemotherapy that are mainstays of treatment for sporadically-occurring HNSCCs. However, frequent early locoregional recurrence suggests a discontinuity between constitutional DNA damage sensitivity and tumor cell chemotherapy sensitivity. The surprising degree of interstrand crosslinker (ICL) resistance of FA HNSCC cells questions the efficacy of low-dose conventional therapies. By identifying sensitivity to PARP inhibitors, this study demonstrates that systematic testing of alternative agents will be necessary using our established murine and human FA HNSCC models, and that results obtained from these studies may be directly translatable into phase I/II clinical trials for treatment of FA HNSCC using PARP inhibition via either systemic or directed means.
ACKNOWLEDGMENTS
We are grateful to Dr. James Lessard of Cincinnati Children’s Hospital Medical Center (CCHMC) and Seven Hills Bioresearch (Cincinnati, OH) for his gift of the C4 pan-actin monoclonal antibody used in this work. We thank Dr. Jeremy Stark, Department of Cancer Biology, Division of Radiation Biology, Beckmann Research Institute of the City of Hope (Duarte, CA), for the NHEJ reporter EJ5SceGFP. We are grateful to Dr. Adam Lane, also of CCHMC, for assistance with statistical analysis. We thank Drs. Parinda Mehta, Stella Davies, and Kasiani Myers of CCHMC and the Cincinnati Children’s Fanconi Anemia Comprehensive Care Center for thoughtful experimental guidance and discussion. This work was supported in part by NIH award RO1 CA102357 (S.I.W.), NHLBI grant PO1HL048546 (S.B.O.), and a grant from the Fanconi Anemia Research Fund (L.E.H).
Footnotes
Conflicts of interest: The authors claim no potential conflicts of interest
REFERENCES CITED
- 1.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–63. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005:2925–40. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- 4.Kee Y, D’Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. 2012;122:3799–806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Spitz GS, Veturi U, Lach FP, Auerbach AD, Smogorzewska A. Regulation of multiple DNA repair pathways by the Fanconi anemia protein SLX4. Blood. 2013;121:54–63. doi: 10.1182/blood-2012-07-441212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol Cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Pace P, Mosedale G, Hodskinson MR, Rosado IV, Sivasubramaniam M, Patel KJ. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329:219–23. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP. Fanconi’s anemia and malignancies. Am J Hematol. 1996;53:99–110. doi: 10.1002/(SICI)1096-8652(199610)53:2<99::AID-AJH7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511–7. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–6. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 11.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 12.Birkeland AC, Auerbach AD, Sanborn E, Parashar B, Kuhel WI, Chandrasekharappa SC, et al. Postoperative clinical radiosensitivity in patients with fanconi anemia and head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:930–4. doi: 10.1001/archoto.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masserot C, Peffault de Latour R, Rocha V, Leblanc T, Rigolet A, Pascal F, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113:3315–22. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 15.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Marcou Y, D’Andrea A, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiother Oncol. 2001;60:75–9. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoskins EE, Morris TA, Higginbotham JM, Spardy N, Cha E, Kelly P, et al. Fanconi anemia deficiency stimulates HPV-associated hyperplastic growth in organotypic epithelial raft culture. Oncogene. 2009;28:674–85. doi: 10.1038/onc.2008.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zeeburg HJ, Snijders PJ, Pals G, Hermsen MA, Rooimans MA, Bagby G, et al. Generation and molecular characterization of head and neck squamous cell lines of fanconi anemia patients. Cancer Res. 2005;65:1271–6. doi: 10.1158/0008-5472.CAN-04-3665. [DOI] [PubMed] [Google Scholar]
- 20.Kachnic LA, Li L, Fournier L, Willers H. Fanconi anemia pathway heterogeneity revealed by cisplatin and oxaliplatin treatments. Cancer Lett. 2010;292:73–9. doi: 10.1016/j.canlet.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 22.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–67. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 23.Michels J, Vitale I, Galluzzi L, Adam J, Olaussen KA, Kepp O, et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 2013;73:2271–80. doi: 10.1158/0008-5472.CAN-12-3000. [DOI] [PubMed] [Google Scholar]
- 24.Forster M, Mendes R, Fedele S. Synthetic lethality and PARP-inhibitors in oral and head & neck cancer. Curr Pharm Des. 2012;18:5431–41. doi: 10.2174/138161212803307608. [DOI] [PubMed] [Google Scholar]
- 25.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–93. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanenberg H, Batish SD, Pollok KE, Vieten L, Verlander PC, Leurs C, et al. Phenotypic correction of primary Fanconi anemia T cells with retroviral vectors as a diagnostic tool. Exp Hematol. 2002;30:410–20. doi: 10.1016/s0301-472x(02)00782-8. [DOI] [PubMed] [Google Scholar]
- 27.Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–4. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Tomkins DJ, Auerbach W, McKerlie C, Youssoufian H, Liu L, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet. 1996;12:448–51. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Chandra S, Levran O, Jurickova I, Maas C, Kapur R, Schindler D, et al. A rapid method for retrovirus-mediated identification of complementation groups in Fanconi anemia patients. Mol Ther. 2005;12:976–84. doi: 10.1016/j.ymthe.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Keimling M, Wiesmuller L. DNA double-strand break repair activities in mammary epithelial cells--influence of endogenous p53 variants. Carcinogenesis. 2009;30:1260–8. doi: 10.1093/carcin/bgp117. [DOI] [PubMed] [Google Scholar]
- 33.Steidler NE, Reade PC. Experimental induction of oral squamous cell carcinomas in mice with 4-nitroquinolone-1-oxide. Oral Surg Oral Med Oral Pathol. 1984;57:524–31. doi: 10.1016/0030-4220(84)90312-8. [DOI] [PubMed] [Google Scholar]
- 34.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42:655–67. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;4:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H, et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–82. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Q, Barboule N, Frit P, Gomez D, Bombarde O, Couderc B, et al. Ku counteracts mobilization of PARP1 and MRN in chromatin damaged with DNA double-strand breaks. Nucleic Acids Res. 2011;39:9605–19. doi: 10.1093/nar/gkr656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 40.Min W, Bruhn C, Grigaravicius P, Zhou ZW, Li F, Kruger A, et al. Poly(ADP-ribose) binding to Chk1 at stalled replication forks is required for S-phase checkpoint activation. Nat Commun. 2013;4:2993. doi: 10.1038/ncomms3993. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhury S, Auerbach AD, Kernan NA, Small TN, Prockop SE, Scaradavou A, et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. Br J Haematol. 2008;140:644–55. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 42.Parmar K, D’Andrea A, Niedernhofer LJ. Mouse models of Fanconi anemia. Mutat Res. 2009;668:133–40. doi: 10.1016/j.mrfmmm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobs PM, Sahaayaruban P, Saito H, Reifsteck C, Olson S, Joenje H, et al. Immortalization of four new Fanconi anemia fibroblast cell lines by an improved procedure. Somat Cell Mol Genet. 1996;22:151–7. doi: 10.1007/BF02369905. [DOI] [PubMed] [Google Scholar]
- 44.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 45.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robu M, Shah RG, Petitclerc N, Brind’Amour J, Kandan-Kulangara F, Shah GM. Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair. Proc Natl Acad Sci U S A. 2013;110:1658–63. doi: 10.1073/pnas.1209507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, Yu X. Function of BRCA1 in the DNA damage response is mediated by ADP-ribosylation. Cancer cell. 2013;23:693–704. doi: 10.1016/j.ccr.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–14. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–64. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 50.Yelamos J, Farres J, Llacuna L, Ampurdanes C, Martin-Caballero J. PARP-1 and PARP-2: New players in tumour development. Am J Cancer Res. 2011;1:328–46. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.