Abstract
Cumulatively, B cell research in multiple sclerosis (MS) is an example for a translational medicine effort that resulted in a promising therapeutic approach for one of the most debilitating chronic neurological diseases of young adults. Experimental autoimmune/allergic encephalomyelitis, the animal model for MS, was first described in 1935, and has since provided the scientific basis for important developments in targeted therapeutics. In 2008, the first CD20-targeting B cell-depleting therapeutic trials using rituximab in MS were published. Owing to the now repeatedly shown significant amelioration of MS disease activity using anti-CD20 B cell depleting strategies, scientific interest in the immunopathological relevance of B cells gained further traction and has since undergone a renaissance of innovative investigations. While additional B cell therapies for MS are presently being developed by the biopharma industry, much remains to be understood about the role B cells in MS. The goal of this review article is to summarize how B cells may contribute to MS pathogenesis as basis to understanding why B cell-depletion is effective in MS.
Keywords: Multiple sclerosis, B cells, B cell-depleting therapy
Multiple Sclerosis – A brief overview
Multiple sclerosis (MS) is the most common chronic neurological disease of young adults, affecting about 2.5 million people worldwide. In countries populated by Northern Europeans and their descendants the incidence is about 7/100,000, and prevalence is about 120/100,000[1]. The incidence of MS seems to have increased over the last century, particularly in women, leading to a sex-ratio of 3:1 (female to male)[2]. The peak age of onset is between 20 and 40 years of age. At disease onset, ∼80% of patients are diagnosed with relapsing-remitting MS (RRMS); over time about 60% of RRMS patients will develop secondary progressive MS; about 25% never experience sustained neurological disability, whereas a smaller percentage become severely disabled within short time after the MS diagnosis. Pathologically, MS is characterized by chronic CNS inflammation accompanied by demyelination, gliosis, and axonal loss. Axonal pathology is believed to be ultimately responsible for progressive neurological disability. The most accepted view of MS pathogenesis includes autoimmune-mediated myelin injury in a susceptible host. MS behaves as a complex genetic trait[3], and exposure to infectious, climatic and other environmental variables likely have a considerable effect on an individual's risk to develop MS. Disease-specific, immune modulatory therapies became available in the mid-to-late 1990's; currently, seven substances are approved for the treatment of MS (interferon-β1, glatiramer acetate, mitoxantrone, natalizumab, fingolimod, dimethyl fumarate, teriflunomide). These compounds have been extensively studied and discussed elsewhere. In this review article, we will focus on B cells, their immunological properties relevant to MS and how B cell depleting therapeutic strategies currently in development affect B cell functions.
B cells – MS disease drivers
B cells can exert effector functions as antigen-presenting cells, by cytokine and antibody production, and they participate in the formation of ectopic lymphoid tissues (Figure 1). The strongest evidence to date for B cells playing a crucial role in MS immune pathology stems from studies evaluating the effect and efficacy of anti-CD20 B cell depleting therapy such as rituximab, ocrelizumab, and ofatumumab[4-7]. Interestingly, the initial impetus for B cell depleting therapy was to remove autoantibody-producing plasma cells after multiple experimental autoimmune encephalitis (EAE) studies had demonstrated critical roles of antibody responses in the development of CNS demyelination[8-11]. However, since the late 1990's it has become increasingly appreciated, that antigen-presentation by B cells is necessary to trigger autoimmunity against the CNS myelin oligodendrocyte glycoprotein[12-14]. B cells can provide activation/effector mechanisms, and can assume pro-inflammatory, anti-inflammatory and/or regulatory roles. To date, the exact target antigens of pathogenic B cell responses in MS remain unknown, despite our knowledge that disease-associated B cells result from antigen-driven affinity maturation. Needless to say, not all B cells in MS patients support detrimental autoimmunity. Therefore, being able to clearly differentiate pathologically relevant from irrelevant B cells in the future will set the stage for treatments with enhanced and possibly personalized therapeutic precision and further improved safety profiles.
Figure 1. B cell functions.
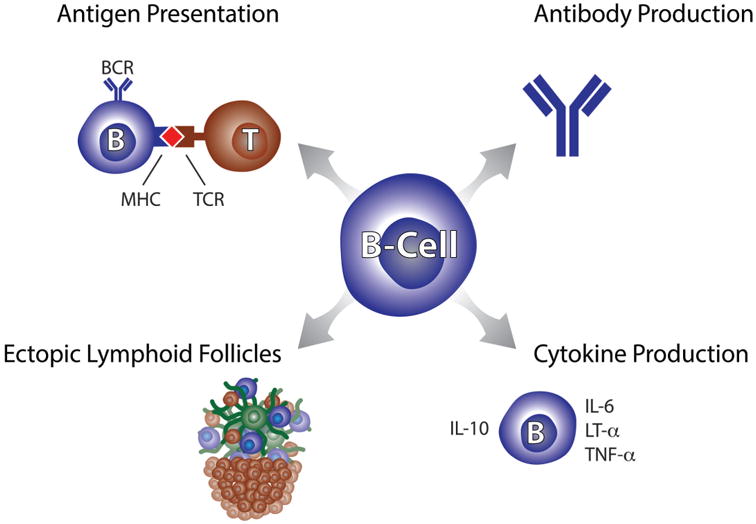
Depicted are basic immunological functions provided by B cells as relevant to MS immune pathology. Autoantigen presentation was shown to be the key B cell function for experimental CNS autoimmunity in myelin-oligodendrocyte induced EAE. Antibodies have long been hypothesized to play a role in MS; for example, clonal antibodies can be found as OCB in CSF. Ectopic lymphoid follicles are tertiary lymphoid tissues that become established at sites of inflammation. In MS, lymphoid follicle-like structures have been found associated with meningeal tissues. Cytokine production by B cells can support regulatory (IL-10) and pro-inflammatory T cell functions (IL-6, LT-α, TNF-α); cytokine production by B cells can occur following recognition of specific antigens or in an antigen-independent fashion. See text for detailed explanations.
In the following paragraphs, we will discuss B cell functions that have either been demonstrated or are likely to be involved in MS immune pathology. We will focus mainly on human data but will include experimental animal data where appropriate.
The peripheral B cell compartment in MS
There is ample evidence for peripheral B cell responses to be tightly involved in the immune pathology of MS through pro-inflammatory mechanisms, bystander activation, or through regulatory functions. B cell tolerance is necessary to control autoimmunity that can randomly develop during early B cell development[15] or even during targeted immunity against foreign pathogens[16]. Central B cell tolerance, which reduces the autoimmune potential of B cells developing in the bone marrow appears to be intact in the majority of MS patients; conversely, B cell tolerance mechanisms controlling autoimmunity of B cells circulating between peripheral lymphoid tissues seems to be defective [17]. Cervical lymph nodes have been described as possible sites supporting CNS directed autoimmunity in both humans [18] and mice [19].
Memory B cells serve as highly efficient antigen-specific antigen-presenting cells (APC)[20,21]. In this regard, myelin-reactive memory B cells can be found in the peripheral blood of MS patients[22]. Memory B cells express high levels of CD20; they are effectively depleted and repopulate slowly following treatment with anti-CD20 targeting monoclonal antibodies[23] concurrent with sustained suppression of MS disease activity[24]. In MS, B cells were shown to secrete increased levels of IL-6 when compared to healthy controls; this pro-inflammatory skewing was not seen in returning B cells 12 months after B cell depleting therapy[25]. Given that the repopulating B cell compartment is mainly composed of mature naïve and immature B cells[26], it is likely that increased IL-6 production in MS patients is a function associated with antigen-experienced memory B cells. Memory B cells are also effective supporters of T cell immune mechanisms; their depletion reduces IL-17 production by peripheral blood lymphocytes further supporting a role of B cells in supporting pro-inflammatory T helper 17 cell (Th17) responses[13,25]. Furthermore, B cells from MS patients were shown to respond to unspecific activating stimuli such as CpG or IFN-γ with an exaggerated pro-inflammatory cytokine profile[27]. Accordingly, pro-inflammatory functions of B cells in MS can also occur in an antigen-independent fashion, by way of bystander activation of T cells, a mechanism that explains the association of MS relapses with systemic infections[28,29].
In humans and mice, IL-10 secreting B cells (B10 cells) exert regulatory roles by suppressing T helper 1 (Th1) differentiation[30,31] and by down-regulating TNF-α production by monocytes[32]. Conflicting data exists regarding the B cell subpopulation responsible for IL-10 secretion. One study found IL-10 expressing B cells amongst activated memory B cells suggesting that B10 cells provide antigen-specific regulatory functions[32]; increased numbers of IL-10 producing B cells were reported in patients with autoimmune disease although most MS patients in this study were treated with immune-suppressive or immune-modulatory therapies[32]. Conversely, another study reported that IL-10 secretion is mainly a function of naïve B cells[21] and that a switch from regulatory B10 cells to pro-inflammatory B cells may occur as B cells transition from naïve to memory phenotypes[21]. In EAE, B10 cells inhibit autoimmune T cell responses, an effector function that is dependent on IL-21 and CD40-mediated interaction with T cells[33]. Interestingly, under certain circumstances therapeutic B cell depletion in EAE may also eliminate regulatory B cells and will result in exacerbation of disease activity[14]. To date, disease worsening in direct response to CD20-targeting B cell depleting therapy has not been observed in humans; however, an increased pro-inflammatory monocytic phenotype has been described in some MS patients after treatment with rituximab[34].
We recently demonstrated that in MS clonally related B cells provide an antigen-specific and immunologically active link between the periphery and cerebrospinal fluid[35,36] and that OCB-producing B cells are not only present in the CNS but also in peripheral blood[37]. Antigen-stimulated B cells provide an active immune axis between CNS and periphery and may undergo immune stimulation in both compartments[35-37], further supporting the important pathological role of peripheral B cell immunity in MS (Figure 2).
Figure 2. B cells provide an immunologically active axis between the periphery and CNS.
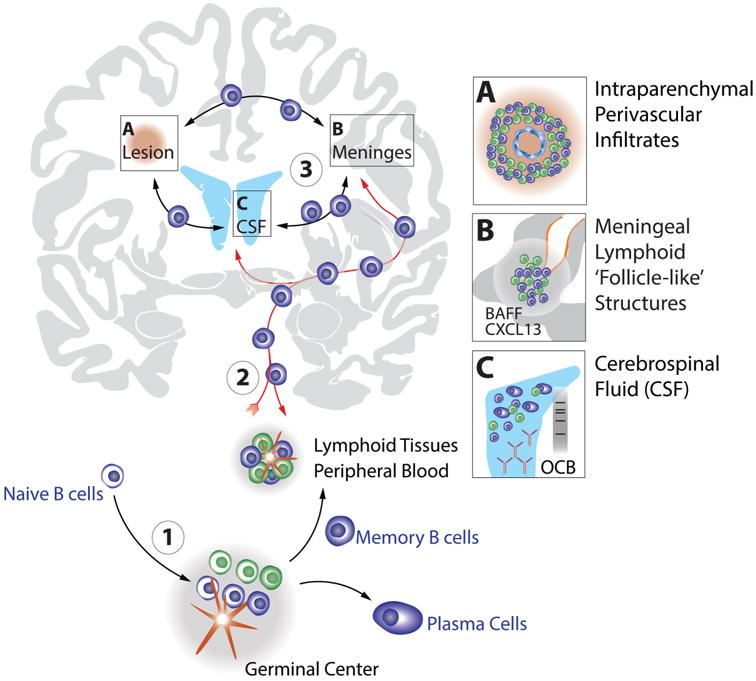
Naïve B cells emerge from the bone marrow (1) and undergo initial antigen-training and affinity maturation in peripheral germinal centers (GC). Memory B cells arising from GCs can be further stimulated in peripheral lymphoid tissues and/or migrate to the CNS compartment (2) where they participate in, and establish immunologically active sites in MS lesions (A) and pial meningeal tissues (B). An immunological continuum and “circulation” (3) of antigen-experienced B cells also involves the CSF compartment represented by schematic lateral ventricles in blue (C). Clonal and clonally related B cell receptors suggesting ongoing antigen-stimulation can be detected in all three CNS-sites (i.e. lesions, meninges, CSF) and in the periphery, suggesting MS disease-driving immunity to be active on both sides of the blood-brain barrier. The CSF (C) also contains OCB in the majority of MS patients, another sign of antigen-driven stimulation of B cells to differentiate into antibody producing plasmablasts or plasma cells. B cells are in blue shades, T cells in green shades. See text for details.
B cells in the CNS
A growing body of evidence has established the CNS not only as target tissue of autoimmunity in MS, but has found sub-compartments (i.e. brain parenchyma, cerebrospinal fluid, meningeal tissue) to reflect immunological activity, supporting B cell affinity-maturation, proliferation, and terminal differentiation to antibody-producing plasma cells. B cells are commonly found in MS lesions, albeit predominantly in active lesions and at significantly lower numbers compared to T cells[38]. Lymphoid B cell follicle-like structures featuring characteristics of germinal centers were observed in the cerebral meninges of SPMS patients [39] and are associated with cortical neuronal loss and demyelination[40,41]. While the pathological importance of such ectopic B cell follicles as drivers of CNS targeted autoimmunity remains to be fully understood, the presence of CD35+ follicular dendritic cells and proliferating B cells, together with expression of the B cell-attracting chemokine CXCL13 and of the B cell-activation factor (BAFF) suggest active immune responses to occur in meningeal tertiary lymphoid tissues in SPMS patients[39,42]. Further, CXCL13 and BAFF were also described in MS lesions [43,44] where these factors could mediate local B cell recruitment and maturation at sites of active demyelination. B cells and B cells present in MS CNS and CSF are clonally expanded[45-47] and IgG class-switched[48,49], and their immunoglobulin genes are somatically hypermutated and appear to be subjected to intrathecal affinity-maturation[36,47,50-52], additional evidence for active B cell immune mechanisms. The fact that overlapping B cell repertoires expressing related Ig-VH sequences were found in MS brain parenchyma, meningeal lymphoid follicles, CSF, and the periphery [35,36,53,54], suggests an intrathecal immunological continuum that may be exposed to immune-stimulation on both sides of the blood-brain barrier (Figure 2).
Memory B cells in the CSF display up-regulation of co-stimulatory molecules[55] suggesting active B-T cell interactions. Different stages of B cell development are present in CSF[55] and CSF plasma cells are producers of soluble clonal IgG[56,57], further supporting antigen-driven B cell immune responses to be active intrathecally. It has been repeatedly shown that antigen-experienced B cell subsets predominate in the CSF and CNS. Accordingly, >90% of B cells in the CSF carry the memory B cell marker CD27 and a fraction of CSF B cells express CD138 and/or CD38, supporting presence of mechanisms stimulating the maturation of clonally activated memory B cells into antibody producing plasmablasts and plasma cells[58]; CD27-IgD+ naïve B cells are significantly lower in the CSF compared to blood[59].
To date, the pathological relevance of antibodies in MS remains unclear despite intrathecal presence of clonal IgG (oligoclonal bands, OCB) and IgM [60-62] and significant IgG deposits in some demyelinating MS lesions [63]. The rapid response to B cell depleting therapy, leaving antibody levels nearly unchanged[5,6], has led some to speculate that antibodies play a less important pathogenic role. However, CNS-directed autoantibodies require a permissible inflammatory environment[8] or at the very least a functional complement system to exert their pathogenic function[64]. Eliminating the T cell activating, antigen-presenting functions of B cells by way of B cell depletion, likely reduces intraparenchymal inflammatory effectors to a degree that will render autoantibodies ineffective promoters of tissue damage. Overall, memory B cells and plasmablasts/plasma cells are the most abundant B cell subsets in MS CNS and CSF. However, these B cells do not represent a static immune response but rather engage in active affinity maturation with help from other immune cell types, cytokines, and survival factors.
B cell-depleting therapy in MS
CD20-targeting lymphocyte-depleting therapy was shown to effectively suppress MS disease-activity measures including new enhancing lesions and relapse-rates[4-7]. Beginning with the first studies describing successful treatment of MS with the anti-CD20 antibody rituximab [4,5], further efforts ensued to develop B cell depletion as therapeutic paradigm in MS. In the following paragraphs we will briefly discuss emerging therapies that were developed to directly target B cells: anti-CD19, anti-CD20, and anti-BAFF-R (Table 1). All are IgG1 antibodies and can mediate complement-dependent (CDC), and antibody-dependent cell-mediated (ADCC) cytotoxic effects on their target cells.
Table 1.
Biologics targeting B cells or B cell activating factors.
| Biologic | Molecular Characteristics | Targets | Effects in MS |
|---|---|---|---|
| Rituximab | murine/human chimeric monoclonal IgG1 | - CD20 | - reduced MRI measures of disease activity[4, 5] |
| - B cells: See Figure 3. | - reduced relapse rate[4, 5] | ||
| - CD20+ T cells | - reduced intrathecal B cells | ||
|
| |||
| Ocrelizumab | humanized (90%) monoclonal IgG1 | - CD20 | - reduced MRI measures of disease activity[6] |
| - B cells: See Figure 3. | - reduced relapse rate[6] | ||
| - Effect on CD20+ T cells not yet known | |||
|
| |||
| Ofatumumab | fully human monoclonal IgG1 | - CD20 | - reduced MRI measures of disease activity[7] |
| - B cells: See Figure 3. | |||
| - Effect on CD20+ T cells not yet known | |||
|
| |||
| MEDI-551 | humanized monoclonal IgG1 | - CD19 | unknown |
| - B cells: see Figure 3 | |||
|
| |||
| VAY736 | fully human monoclonal IgG1 | - BAFF-R | unknown |
| - B cells: see Figure 3 | |||
| - BAFF-R+ T cells? | |||
|
| |||
| Atacicept | recombinant fusion protein with extracellular domain of TACI receptor and Fc domain of human Ig | - BAFF | unexpected inflammatory effects[81] |
| - APRIL | |||
Anti-CD20 therapy
Three monoclonal anti-CD20 antibodies have been or are currently studied for the treatment of MS: rituximab (chimeric human/mouse IgG1), ocrelizumab (humanized IgG1), and ofatumumab (fully human IgG1); they differ in their recognition of CD20 epitopes and in intensity of CDC or ADCC elicited, but all mediate near-complete depletion of CD19+ B cells in peripheral blood (reviewed in [65]). Very low numbers of B cells remain in the circulation following CD20-targeted depletion[66], and certain B cell populations resident in lymphoid tissues may also display resistance to B cell depleting therapies[67]. CD20 is expressed on a wide-range of B cell subsets starting at the Pre-B cell stage and extending through memory B cells (Figure 3). Accordingly, Pro-B cells and antibody-producing plasmablasts/plasma cells are not primarily affected by anti-CD20 therapy; levels of soluble immunoglobulins in serum[5,6] remain mostly unchanged, at least in the short-term. Rituximab was shown to reduce CSF B cell counts, but at 6 months OCB and CSF IgG-Index were unchanged[68]. Anti-CD20 mediated B cell depletion using rituximab was the first therapy to directly target B cells in MS [4,5]. In the 48 week phase II trial[5], patients who received rituximab had a highly significant >90% reduction in total gadolinium-enhancing lesions on brain MRI over the course of the study, beginning at week 12; clinically, a significant relapse-rate reduction of about 50% was observed at 24 and 48 weeks[5]. At 48 weeks, 24.1% of patients tested for the presence of anti-human chimeric antibodies (HACA, i.e. antibodies against rituximab) had developed HACA without apparent association with efficacy measures. The phase II trials with ocrelizumab and ofatumumab cite similar highly statistically significant reduction of new and total numbers of gadolinium-enhancing lesions[6,7]; ocrelizumab resulted in relapse-rate reductions between 73% and 80%[6]. Ocrelizumab is currently in phase III clinical development for relapsing-remitting MS and primary progressive MS (ClinicalTrials.gov Identifiers: NCT01247324, NCT01412333, and NCT01194570). As detailed earlier in this article, multiple functions of B cells can be affected by anti-CD20 B cell depletion. The long-term effects of anti-CD20 therapy in MS have yet to be fully understood, both, with respect to efficacy, and safety. Ocrelizumab was reported to result in sustained suppression of clinical MRI disease-activity 72 weeks after the last of 4 dosages applied at 24-week intervals[24]. In the short-term, anti-CD20 therapy can result in mostly mildly reduced serum immunoglobulin levels; however, prolonged exposure to anti-CD20 antibodies may induce delayed depletion of the plasma cell population and reduction of soluble immunoglobulins due to reduced memory B cell formation and terminal differentiation into antibody-producing subsets[69]. Overall, by now CD20-targeting therapy was repeatedly shown to be highly effective in reducing MRI disease-activity outcome measures and phase III clinical trials are expected to fully reveal the therapeutic potential of this approach.
Figure 3. B cell development and expression of surface markers targeted by emerging MS therapeutics.
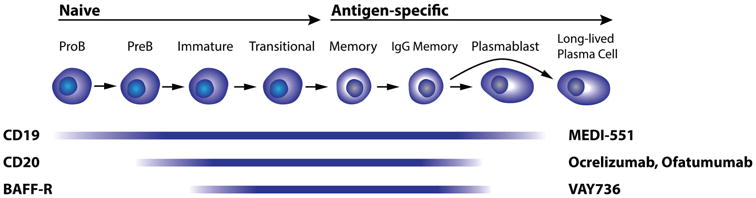
CD19 is a pan-B cell marker expressed on nearly the entire B cell lineage starting from early Pro-B cells and disappearing on long-lived plasma cells; MEDI-551 (anti-CD19 antibody) currently in clinical development for MS therapy cuts deeply into the B cell compartment. CD20 is expressed on the majority of B cells; it appears on Pre B cells and becomes downregulated during terminal differentiation to plasmablasts/plasma cells. Interestingly, CD20 is also expressed by a small subset of T cells, which become depleted by rituximab. BAFF-R has a similar expression pattern as CD20; VAY736 is now also being studied as MS therapy.
Interestingly, CD20 was also reported to be expressed at low levels on a small subset of T cells [66,70] in healthy donors and patients with rheumatoid arthritis (RA); rituximab effectively depletes these T cells from peripheral blood RA patients [71]. CD20-expressing T cells are likely functionally diverse but harbor pro-inflammatory Th17 properties in RA [72]. Our own work confirms depletion of CD3+CD20dim T cells from peripheral blood of MS patients treated with rituximab [66]. Clearly, further work is necessary to determine whether CD20-expressing T cells contribute to MS immune pathology.
Anti-CD19 therapy
More recently, a humanized anti-CD19 IgG1 antibody[73], MEDI-551 entered phase II clinical development for treatment of MS (ClinicalTrials.gov Identifier: NCT01585766). At this point, no data of MEDI-551 in MS have been reported. CD19 is expressed on B cells starting with Pro-B cells through the antibody-producing plasmablast stage (Figure 3), and is gradually lost during terminal differentiation to plasma cells[74]. In an animal model with humanized CD19 and CD20, MEDI-551 induced longer-lasting B cell depletion compared to rituximab due to significant effects on early B cells (Pro-B cells) in bone marrow[73]. Also in animals, it was shown that anti-CD19 therapy reduces levels of serum immunoglobulins including autoantibodies due to plasmablast/plasma cell depletion[75]. It is unclear whether autoantibodies play a significant role in MS immune pathology and whether the enhanced antibody-reducing functionality of MEDI-551 will provide increased efficacy and/or risk of infection when compared to anti-CD20 therapy. Aside from the effect on plasmablasts and plasma cells and slower B cell repopulation after treatment, MEDI-551 is expected to affect the same B cell functions as CD20-targeting strategies. Anti-CD19 therapy will not deplete CD3+CD20dim T cells; however, a small number of reports describe CD19-expressing dendritic cells [74,76], but their existence in human has not been diligently studied and an effect of anti-CD19 therapy on dendritic cells is merely speculative at this point.
Anti-BAFF-R therapy
The most recent addition to the armamentarium of biologics directly targeting B cells is VAY736, a fully human IgG1 antibody against the B cell activating factor-receptor (BAFF-R)[77], currently in phase II clinical development for MS (ClinicalTrials.gov Identifier: NCT02038049). In healthy humans BAFF-R is expressed on naïve B cells and can be found through post-germinal center memory B cell and plasma blast stages (Figure 3)[78]. Low levels of BAFF-R have also been described on human central and effector T cells[79]. BAFF-R is the receptor for BAFF (also BLyS, B lymphocyte stimulator); BAFF promotes B cell survival at numerous stages throughout B cell development. In MS, BAFF was found to be elevated in demyelinating lesions[80], and has also been suggested to be involved in the formation of lymphoid follicle-like meningeal structures[42]. Interestingly, therapeutic targeting and neutralization of soluble BAFF and APRIL (A Proliferation-Inducing Ligand), another B cell stimulator, by atacicept leads to increased inflammatory activity in MS[81]. No data has been reported regarding the biological effects of VAY736 in humans. However, given its IgG1 isotype which can induce CDC and ADCC, and that BAFF-R is expressed on a wide range of B cell subsets, VAY736 will likely show similar effects on the B cell compartment as anti-CD20 antibodies but may also lead to reduced serum immunoglobulin levels. Like anti-CD20 antibodies, VAY736 may induce depletion of a small portion of T cells, which may, or may not be of therapeutic relevance. Theoretically, VAY736 could exert additional effects by blocking BAFF-binding to BAFF-R on B cells that escaped depletion in peripheral blood and lymphoid tissues, and may therefore interfere with a similar immunological pathway as atacicept, i.e. BAFF/BAFF-R interaction. However, atacicept was designed to target and neutralize soluble B cell activation factors, while VAY736 appears to have been developed to primarily target B cells for depletion, making for an overall different mode of action. In that context it is interesting to note that BAFF-R-deficient mice have fewer mature B cells [82] and develop increased EAE severity[83], a scenario that is probably reflective of the effects of atacicept in humans.
Summary
B cells play important roles in the initiation and perpetuation of CNS-targeting inflammation in MS. B cell repertoires on both sides of the blood-brain barrier overlap, suggesting disease-driving immunological stimuli to be active not only in the CNS but also the periphery. Multiple B cell mediated mechanisms are likely involved in MS immune pathology, with antigen-presentation by B cells occupying a central role. B cell depletion is a highly effective and promising therapeutic approach to MS. Four different biologics that directly target B cells for depletion are currently in clinical development, two targeting CD20, one targeting CD19, and one targeting the BAFF-R. Each therapeutic approach has or, in the case of anti-BAFF-R is expected to have, in common significant depletion of the B cell compartment. However, each strategy also has its unique features, which may, or may not, contribute to differences in therapeutic efficacy and/or safety profiles. Additional work is required to further pinpoint the features of pathologically relevant B cells and their target antigens in human MS.
Key messages.
- B cells are key players in MS immune pathogenesis
- B cell depletion ameliorates MS disease activity
- Further research is necessary to fully understand pathologically relevant B cell subsets
Acknowledgments
Funding and Disclosures: Dr. von Büdingen is supported by grants from the NMSS (RG-4868), and the NIH (K02NS072288), and by an endowment from the Rachleff Family Foundation. He has received Research Funding from Pfizer and Roche, and consulting fees from Novartis and Roche. Dr. Zamvil is supported by research grants from the NIH (R01AI073737 and R01NS063008), the NMSS (RG-4124), The Guthy Jackson Charitable Foundation, The Maisin Foundation, Biogen Idec, Inc., Teva Pharmaceuticals, Inc., Five Prime, Inc. and Boehringer-Ingelheim, Inc. He has served as a consultant and received honoraria from Biogen-Idec, EMD-Serono, Genzyme, Novartis, Questcor, Roche, and Teva Pharmaceuticals, Inc., and has served or serves on Data Safety Monitoring Boards for Lilly, BioMS, Teva and Opexa Therapeutics. Dr. Palanichamy is an employee of Biogen.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in canada: A longitudinal study. Lancet neurology. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: Genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: A 72-week, open-label, phase i trial. Annals of neurology. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: A phase 2 study. Neurology. 2014 doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 8.Genain CP, Nguyen MH, Letvin NL, et al. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. The Journal of clinical investigation. 1995;96:2966–2974. doi: 10.1172/JCI118368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linington C, Engelhardt B, Kapocs G, Lassman H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic t cells and demyelinating antibody. J Neuroimmunol. 1992;40:219–224. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 10.Lyons JA, Ramsbottom MJ, Cross AH. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol. 2002;32:1905–1913. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Schluesener HJ, Sobel RA, Linington C, Weiner HL. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987;139:4016–4021. [PubMed] [Google Scholar]
- 12.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. Mhc class ii-dependent b cell apc function is required for induction of cns autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber MS, Prod'homme T, Patarroyo JC, et al. B-cell activation influences t-cell polarization and outcome of anti-cd20 b-cell depletion in central nervous system autoimmunity. Annals of neurology. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human b cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 16.Mouquet H, Scheid JF, Zoller MJ, et al. Polyreactivity increases the apparent affinity of anti-hiv antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinnunen T, Chamberlain N, Morbach H, et al. Specific peripheral b cell tolerance defects in patients with multiple sclerosis. The Journal of clinical investigation. 2013;123:2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern JN, Yaari G, Vander Heiden JA, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6:248ra107. doi: 10.1126/scitranslmed.3008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Or A, Oliveira EM, Anderson DE, et al. Immunological memory: Contribution of memory b cells expressing costimulatory molecules in the resting state. J Immunol. 2001;167:5669–5677. doi: 10.4049/jimmunol.167.10.5669. [DOI] [PubMed] [Google Scholar]
- 21.Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human b cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 22.Harp CT, Ireland S, Davis LS, et al. Memory b cells from a subset of treatment-naive relapsing-remitting multiple sclerosis patients elicit cd4(+) t-cell proliferation and ifn-gamma production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. European journal of immunology. 2010;40:2942–2956. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of b cell subsets after transient b cell depletion using anti-cd20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 24.Hauser SL, Li D, Calabresi P, et al., editors. Week 144 results of a phase ii, randomized, multicenter trial assessing the safety and efficacy of ocrelizumab in patients with relapsing–remitting multiple sclerosis (rrms) San Diego, CA: 2013. p. S31.004. [Google Scholar]
- 25.Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of il-6-producing b cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood b cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 27.Bar-Or A, Fawaz L, Fan B, et al. Abnormal b-cell cytokine responses a trigger of t-cell-mediated disease in ms? Annals of neurology. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 28.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- 29.Buljevac D, Flach HZ, Hop WC, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- 30.Blair PA, Norena LY, Flores-Borja F, et al. Cd19(+)cd24(hi)cd38(hi) b cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory b cells inhibit eae initiation in mice while other b cells promote disease progression. The Journal of clinical investigation. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare il-10-competent b-cell subset in humans that parallels mouse regulatory b10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory b cells control t-cell autoimmunity through il-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann-Horn K, Schleich E, Hertzenberg D, et al. Anti-cd20 b-cell depletion enhances monocyte reactivity in neuroimmunological disorders. Journal of neuroinflammation. 2011;8:146. doi: 10.1186/1742-2094-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palanichamy A, Apeltsin L, Kuo TC, et al. Immunoglobulin class-switched b cells form an active immune axis between cns and periphery in multiple sclerosis. Sci Transl Med. 2014;6:248ra106. doi: 10.1126/scitranslmed.3008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Büdingen HC, Kuo TC, Sirota M, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. The Journal of clinical investigation. 2012;122:4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bankoti J, Apeltsin L, Hauser SL, et al. In multiple sclerosis, oligoclonal bands connect to peripheral b-cell responses. Annals of neurology. 2014;75:266–276. doi: 10.1002/ana.24088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic b-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134:2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 41.Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Annals of neurology. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 42.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of cxcl13 and baff is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 43.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: Cxcl12 and cxcl13 up-regulation is differentially linked to cns immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 44.Krumbholz M, Theil D, Derfuss T, et al. Baff is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, Oksenberg JR. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol. 1999;163:5133–5144. [PubMed] [Google Scholar]
- 46.Owens GP, Ritchie AM, Burgoon MP, Williamson RA, Corboy JR, Gilden DH. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the b cell response in multiple sclerosis cerebrospinal fluid. J Immunol. 2003;171:2725–2733. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- 47.Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hypermutation of v(h) genes of b cells from cerebrospinal fluid in multiple sclerosis. The Journal of clinical investigation. 1998;102:1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandberg-Wollheim M, Turesson I. Lymphocyte subpopulations in the cerebrospinal fluid and peripheral blood in patients with multiple sclerosis. Scand J Immunol. 1975;4:831–836. doi: 10.1111/j.1365-3083.1975.tb03724.x. [DOI] [PubMed] [Google Scholar]
- 49.Ritchie AM, Gilden DH, Williamson RA, et al. Comparative analysis of the cd19+ and cd138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- 50.Colombo M, Dono M, Gazzola P, et al. Accumulation of clonally related b lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 51.Smith-Jensen T, Burgoon MP, Anthony J, Kraus H, Gilden DH, Owens GP. Comparison of immunoglobulin g heavy-chain sequences in ms and sspe brains reveals an antigen-driven response. Neurology. 2000;54:1227–1232. doi: 10.1212/wnl.54.6.1227. [DOI] [PubMed] [Google Scholar]
- 52.Beltran E, Obermeier B, Moser M, et al. Intrathecal somatic hypermutation of igm in multiple sclerosis and neuroinflammation. Brain. 2014 doi: 10.1093/brain/awu205. [DOI] [PubMed] [Google Scholar]
- 53.Lovato L, Willis SN, Rodig SJ, et al. Related b cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain: a journal of neurology. 2011;134:534–541. doi: 10.1093/brain/awq350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obermeier B, Lovato L, Mentele R, et al. Related b cell clones that populate the csf and cns of patients with multiple sclerosis produce csf immunoglobulin. Journal of neuroimmunology. 2011;233:245–248. doi: 10.1016/j.jneuroim.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corcione A, Casazza S, Ferretti E, et al. Recapitulation of b cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obermeier B, Mentele R, Malotka J, et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14:688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 57.von Büdingen HC, Gulati M, Kuenzle S, Fischer K, Rupprecht TA, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of patients with central nervous system autoimmune demyelination produce “oligoclonal bands”. J Neuroimmunol. 2010;218:134–139. doi: 10.1016/j.jneuroim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main b cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 59.Haas J, Bekeredjian-Ding I, Milkova M, et al. B cells undergo unique compartmentalized redistribution in multiple sclerosis. J Autoimmun. 2011;37:289–299. doi: 10.1016/j.jaut.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Kostulas VK, Link H, Lefvert AK. Oligoclonal igg bands in cerebrospinal fluid. Principles for demonstration and interpretation based on findings in 1114 neurological patients. Archives of neurology. 1987;44:1041–1044. doi: 10.1001/archneur.1987.00520220043014. [DOI] [PubMed] [Google Scholar]
- 61.Villar LM, Masjuan J, Gonzalez-Porque P, et al. Intrathecal igm synthesis predicts the onset of new relapses and a worse disease course in ms. Neurology. 2002;59:555–559. doi: 10.1212/wnl.59.4.555. [DOI] [PubMed] [Google Scholar]
- 62.Sindic CJ, Monteyne P, Laterre EC. Occurrence of oligoclonal igm bands in the cerebrospinal fluid of neurological patients: An immunoaffinity-mediated capillary blot study. Journal of the neurological sciences. 1994;124:215–219. doi: 10.1016/0022-510x(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 63.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Annals of neurology. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 64.Saadoun S, Waters P, Anthony Bell B, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobin g and human complement produces neuromyelitis optica lesions in mice. Brain. 2010 doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robak T, Robak E. New anti-cd20 monoclonal antibodies for the treatment of b-cell lymphoid malignancies. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2011;25:13–25. doi: 10.2165/11539590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased cd20-expressing t cells in multiple sclerosis patients. J Immunol. 2014 doi: 10.4049/jimmunol.1400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong Q, Ou Q, Ye S, et al. Importance of cellular microenvironment and circulatory dynamics in b cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 68.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces b cells and t cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brinkman IH, van de Laar MA, Jansen TL, van Roon EN. The potential risk of infections during (prolonged) rituximab therapy in rheumatoid arthritis. Expert opinion on drug safety. 2011;10:715–726. doi: 10.1517/14740338.2011.562188. [DOI] [PubMed] [Google Scholar]
- 70.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. Cd20 (pan-b cell) antigen is expressed at a low level on a subpopulation of human t lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 71.Wilk E, Witte T, Marquardt N, et al. Depletion of functionally active cd20+ t cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–3571. doi: 10.1002/art.24998. [DOI] [PubMed] [Google Scholar]
- 72.Eggleton P, Bremer E, Tarr JM, et al. Frequency of th17 cd20+ cells in the peripheral blood of rheumatoid arthritis patients is higher compared to healthy subjects. Arthritis research & therapy. 2011;13:R208. doi: 10.1186/ar3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herbst R, Wang Y, Gallagher S, et al. B-cell depletion in vitro and in vivo with an afucosylated anti-cd19 antibody. The Journal of pharmacology and experimental therapeutics. 2010;335:213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 74.Tedder TF. Cd19: A promising b cell target for rheumatoid arthritis. Nature reviews Rheumatology. 2009;5:572–577. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 75.Yazawa N, Hamaguchi Y, Poe JC, Tedder TF. Immunotherapy using unconjugated cd19 monoclonal antibodies in animal models for b lymphocyte malignancies and autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:15178–15183. doi: 10.1073/pnas.0505539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of clinical investigation. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morphosys: Vay736.
- 78.Carter RH, Zhao H, Liu X, et al. Expression and occupancy of baff-r on b cells in systemic lupus erythematosus. Arthritis Rheum. 2005;52:3943–3954. doi: 10.1002/art.21489. [DOI] [PubMed] [Google Scholar]
- 79.Ng LG, Sutherland AP, Newton R, et al. B cell-activating factor belonging to the tnf family (baff)-r is the principal baff receptor facilitating baff costimulation of circulating t and b cells. J Immunol. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 80.Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E. Baff is elevated in serum of patients with wegener's granulomatosis. J Autoimmun. 2005;25:298–302. doi: 10.1016/j.jaut.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Hartung HP, Kieseier BC. Atacicept: Targeting b cells in multiple sclerosis. Therapeutic advances in neurological disorders. 2010;3:205–216. doi: 10.1177/1756285610371146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. Tnf family member b cell-activating factor (baff) receptor-dependent and -independent roles for baff in b cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 83.Kim SS, Richman DP, Zamvil SS, Agius MA. Accelerated central nervous system autoimmunity in baff-receptor-deficient mice. Journal of the neurological sciences. 2011;306:9–15. doi: 10.1016/j.jns.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


