Abstract
Glioma stem-like cells (GSCs) are a subpopulation of cells in tumors that are believed to mediate self-renewal and relapse in glioblastoma (GBM), the most deadly form of primary brain cancer. In radiation oncology, hyperthermia is known to radiosensitize cells and it is re-emerging as a treatment option for patients with GBM. In this study, we investigated the mechanisms of hyperthermic radiosensitization in GSCs by a phosphokinase array that revealed the survival kinase AKT as a critical sensitization determinant. GSCs treated with radiation alone exhibited increased AKT activation, but the addition of hyperthermia before radiotherapy reduced AKT activation and impaired GSC proliferation. Introduction of constitutively active AKT in GSCs compromised hyperthermic radiosensitization. Pharmacologic inhibition of PI3K further enhanced the radiosensitizing effects of hyperthermia. In a preclinical orthotopic transplant model of human GBM, thermoradiotherapy reduced pS6 levels, delayed tumor growth and extended animal survival. Together, our results offer a preclinical proof-of-concept for further evaluation of combined hyperthermia and radiation for GBM treatment.
Keywords: hyperthermia, radiation, glioma stem cell, glioblastoma, glioma, AKT, cancer stem cell, PI3K
INTRODUCTION
Glioblastoma (GBM) is the most common malignant primary brain tumor (1). The median survival for patients with newly diagnosed GBM is about one year despite aggressive therapy with surgery, radiation and chemotherapy (2). Radiation is the most efficacious, non-surgical treatment but control rates remain poor (3). Glioma stem cells (GSCs) are increasingly recognized as playing important roles in tumor progression and therapeutic resistance (4–10). Therefore, treatments that improve targeting of GSCs are needed to provide durable tumor control.
Hyperthermia is one of the oldest and most potent radiosensitizers (11, 12) and has been shown to improve cancer control in numerous phase III clinical trials (13–17). When used in conjunction with brachytherapy, hyperthermia significantly improves the survival of patients with GBM (15). However, widespread adoption of hyperthermia has been limited due to technical challenges in its administration. In addition, brachytherapy has failed to demonstrate a clinical benefit over standard external beam irradiation (18, 19). Laser interstitial hyperthermia is emerging as an innovative, minimally invasive surgery that permits real-time three-dimensional thermometry within the operating theater. Initial studies of interstitial hyperthermia for patients with GBM have been promising, and this technique is gaining momentum as a new therapeutic option (20–22). In light of this developing treatment modality, we sought to determine the efficacy of hyperthermia and external beam radiation in treating GBM and to characterize the molecular mechanisms of radiosensitization by hyperthermia to uncover potential targets for improving GSC radiosensitivity.
MATERIALS AND METHODS
Cell culture and treatment conditions
Glioma stem cells (specimens 3691 and 387) were isolated and functionally validated as previously described (23, 24) and cultured as neurospheres in neural basal medium (NBM) enriched with B27 supplement (Gibco), 10 ng/ml basic fibroblast growth factor (bFGF) and 10 ng/ml epidermal growth factor (EGF) (R&D Systems) (24) . Only low-passage GSCs were used (< 5 passages). Non-stem tumor cells (NSTCs) were cultured in DMEM containing 10% fetal bovine serum (FBS). GSCs were sham-treated or treated with hyperthermia (HT, incubated in a humidified incubator, 5% CO2temperature set at 42.5°C for 1 hour), radiation (RT, 2 Gy), or combined hyperthermia and radiation treatment (HT+RT, incubated in a 42.5°C incubator for 1 hour followed by 2 Gy irradiation within 30 minutes of completion of HT). We chose to use a clinically relevant dose of 2 Gy, the standard fraction size used for radiotherapy for patients with GBM (2) to investigate the effects of hyperthermia on GSCs. Cells were treated with a Cesium-137 irradiator. The pCDH-T2A-Puro-MSCV-GFP and pCDH-T2A-Puro-MSCV-MYR-AKT1 plasmids were introduced into GSCs as previously described (24). GSCs were treated with 0µM or 5µM of the small molecular inhibitor of PI3K, LY294002 (Cell Signaling) for 2 hours before HT and/or RT.
Cell proliferation and colony formation
Cells were plated in 6-well plates at 1x104 cells/well and incubated overnight in a humidified 37°C incubator. Treatment occurred 24 hours post-plating and live cells were assessed via a hemocytometer and trypan blue exclusion. Cells were harvested and counted for each treatment group on days 0, 1, 3, 5 and 7. For GSC colony formation, cells were plated in 12-well plates at a density of 5×103 cells/well and incubated in a 37°C incubator. At day 7 post-treatment, cells were induced to attach using 5% FBS (v/v) for 24 hours (5, 23). The media was removed and the attached colonies were then washed with PBS, fixed with 100% ice-cold methanol for 20 minutes and stained with crystal violet.
Radiation dose-response analysis
Cells were plated in 10 cm plates at a density of 1.0×104 cells/plate and incubated overnight in a 37°C incubator. Treatment occurred 24 hours post-plating with increasing doses of radiation (0 Gy, 2 Gy, 4 Gy or 6 Gy) and hyperthermia and radiation treatment (42.5°C for 1 hr followed by irradiation). At 7 days post-treatment, a fraction of cells, 7.5% of cells (v/v), were transferred to a 96-well plate and incubated for 1 hour in a 37°C incubator to allow tumorspheres to settle in the wells. All tumorspheres in each well (n=3) were assessed via bright-field microscopy.
Cell cycle analysis
Cells were collected and washed once with ice cold PBS and dissociated with StemPro Accutase Cell Dissociation Reagent (Gibco). Cells were washed again with 1x PBS and stored at −20°C in 70% ethanol until used for analysis. Samples were washed and then suspended in 1x PBS. DNA extraction buffer was added to facilitate extraction of low molecular weight DNA. Cell pellets were incubated at room temperature in DNA staining solution (0.2% Propidium Iodide and 2% RNAase A) for 30 minutes. Cells were subsequently analyzed by flow cytometry.
Western blot analysis
Cells were washed with ice-cold PBS and lysed in M2 buffer (20 mM Tris pH 7.6, 0.5% NP40, 250 mM NaCl, 2.5 mM EDTA, 3 mM EGTA, and protease and phosphatase inhibitors). Protein concentration in the supernatants was measured using the Bio-Rad protein assay dye reagent (Bio-Rad). Protein lysates were resolved on 8–12% SDS-polyacrylamide gels and transferred to PVDF membranes (Millipore). The following primary antibodies were used: AKT, phospho-AKT (Ser473), pS6K (Thr421/Ser424), GAPDH, phospho-ERK1/2 (Thr202/Tyr204), phospho-RSK2 (Ser227) (Cell Signaling).
Immunofluorescence and immunohistochemistry
GSCs derived from patient specimens 3691 and 387 were plated on Matrigel-coated plate (BD) and then treated as indicated. Cells were fixed with 4% PFA for 10 min at room temperature. Cells or sections were blocked with PBS containing 10% normal goat serum (Sigma) and 0.1% Triton X-100 (Sigma). Cells or sections were incubated overnight with the appropriate primary antibody at 4°C and then washed with PBS three times prior to incubation with the appropriate secondary antibody for 1 hour at room temperature. Nuclei were counterstained with DAPI. TUNEL assay was performed using ApopTag Red in Situ Apoptosis Detection Kit (Millipore) in accordance with the manufacturer’s protocol. For immunohistochemistry, tissue sections were deparaffinized and processed for immunoperoxidase staining with the primary antibody incubated at 4°C overnight, followed by the appropriate biotinylated secondary antibody and detected using the VECTASTAIN Elite kit (Vector Laboratories). Sections were counter-stained with either Fast Red or hematoxylin. The following primary antibodies were used: 53BP1 (Bethyl Laboratories), pS6 (Ser240/Ser244) (Cell Signaling), Ki67 (Vector Laboratories). Cells with any detectable Ki67 staining were scored as being positive.
Human phospho-kinase array
Human phospho-kinase array kit was purchased from R&D systems (ARY003B) and protocol was followed as recommended in the vendor’s handbook.
Animal procedures and treatments
All animal experiments were performed in accordance with protocols approved by the Cleveland Clinic Institutional Animal Care and Use committee (IACUC) and in agreement to the National Institute of Health animal welfare guidelines. 20,000 GSCs derived from human glioblastoma and passaged as xenografts were intracranially injected into the right cerebrum of four to six week old athymic nude mice as previously described (24). Hyperthermia was administered using the FDA-approved BSD 500 commercial hyperthermia unit (Salt Lake City, UT). Heating was provided by 915 MHz microwaves emanating from two antennae on the left and right aspects of the tumor-bearing area. A thermistor was placed between the antennae that controlled the microwave power to monitor real-time temperature. A circulating water bolus set at 42°C was placed on top of the mice, antennae and thermistors. Tumors received an average T90 of 42.4°C for 1 hour, where T90 represented 90% of all measured intratumoral temperatures exceeding 42.4°C. Radiation therapy of 2 Gy in 1 fraction was delivered to the mouse head using a Pantak XRAY cabinet irradiator (300 kVp, 10 mA). Lead shielding was used to ensure that only the head was irradiated. For mice receiving hyperthermia and radiation, radiation was delivered within 30 minutes of completion of hyperthermia. The IVIS-100 bioluminescence imaging system was used to assess tumor growth. Five to seven mice were included in each treatment group, total n = 26. Animal experiments were confirmed using independent groups of mice, total n = 38.
Statistical analyses
All graphs, statistical significances and Kaplan-Meier survival curves were generated using GraphPad Prism software. One-way ANOVA or t-test assuming unequal variances was used for all continuous measures and chi square or Fisher’s exact testing for proportion comparisons. Mouse survival comparisons were determined by log-rank analysis.
RESULTS
Hyperthermia improves the radiosensitivity of GSCs
GSCs contribute to tumor progression by resisting radiation therapy (5, 7, 25). We first investigated the ability of hyperthermia to improve the radiosensitivity of GSCs by assessing tumorsphere formation and colony formation, two indicators of the self-renewal capacity of GSCs in vitro (5, 26). Two different GSC specimens were treated with escalating doses of radiation (0–6 Gy) or combined hyperthermia and radiation (42.5°C for 1 hour followed by radiation). With increasing doses of radiation, thermoradiotherapy decreased the surviving fraction of GSCs significantly more than radiation alone (Figure 1A-B). The dose-modifying factor (DMF, ratio of radiation dose with hyperthermia to radiation alone to achieve 90% cell kill) was 0.66 and 0.62 for GSC 387 and GSC 3691, respectively, showing a considerable enhancement in cell killing with hyperthermia. There were no appreciable differences in GSC death treated with hyperthermia alone (42.5°C for 1 hour) or the control, sham-treated cells (data not shown).
Figure 1. Hyperthermia sensitizes GSCs to radiation and impairs self-renewal.
(A-B) GSC specimens 3691 (A) and 387 (B) were treated with increasing doses of radiation (RT) or combined hyperthermia and radiation (HT+RT, 42.5°C for 1 hr followed by the indicated RT dose) and spheres were counted on day 7. (C-D) Representative images (left) of colony formation in GSC specimens 3691 (C) and 387 (D) treated with hyperthermia (HT, 42.5°C for 1 hr), radiation (RT, 2 Gy) or combined hyperthermia and radiation (HT+RT, 42.5°C for 1 hr followed by 2 Gy) and quantification of colonies (right). (E-F) Representative images of tumorspheres (left) of GSCs 3691 (E) and 387 (F) treated as indicated with quantification of tumorspheres (right). Graphed data are means ± SD (n=3). * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001.
We next determined the effect of hyperthermia on GSC colony formation. Hyperthermia alone had little effect on GSC colony formation (Figure 1C-D) or tumorsphere formation (Figure 1E-F). Radiation alone attenuated GSC colony formation and tumorsphere growth by about 50%, but a significant number of GSCs remained and were able to form tumorspheres. The most effective treatment was thermoradiotherapy, which reduced GSC self-renewal by an additional 50% compared to radiation alone (Figure 1C-F). Together, these studies demonstrate that thermoradiotherapy is more effective than radiation alone in reducing the clonogenic capacity of GSCs.
The addition of hyperthermia to radiation reduces GSC proliferation
We next assessed changes in cell survival and proliferation. Hyperthermia alone had little impact on cell number or proliferation compared to control cells (Figure 2 A-B). Seven days after treatment, radiation reduced cell counts by about 50%, consistent with colony formation and tumorsphere formation assays. Treatment with combined hyperthermia and radiotherapy significantly reduced cell number compared to radiation therapy alone (Figure 2A-B).
Figure 2. Thermoradiotherapy reduces GSC viability and proliferation.
(A-B) GSCs were treated as described in Figure 1 and absolute cell counts are shown. Cell numbers were counted every 2 days. Data are means ± SD (n=3). (C-D) Quantification of relative fraction of Ki67+ cells on day 0 and day 3 post-treatment are shown. The fractions of Ki67+ cells were normalized to the control group. (E-F) Representative staining of Ki67 in GSCs is shown. Nuclei were counterstained with DAPI (blue). HT: hyperthermia, RT: radiation, HT+RT: hyperthermia and radiation. * denotes p < 0.05.
To determine whether the reduction in cell number reflected changes in the rate of proliferation, we assessed immunofluorescence staining of Ki67, a marker of proliferation, after the indicated treatments. Ki67 staining revealed that hyperthermia or radiation alone had little effect on GSC proliferation compared to control-treated cells. Compared to radiation alone, thermoradiotherapy efficiently reduced by about 5-fold GSC proliferation (Figure 2C-F).
Thermoradiotherapy reduces DNA repair and promotes cell death
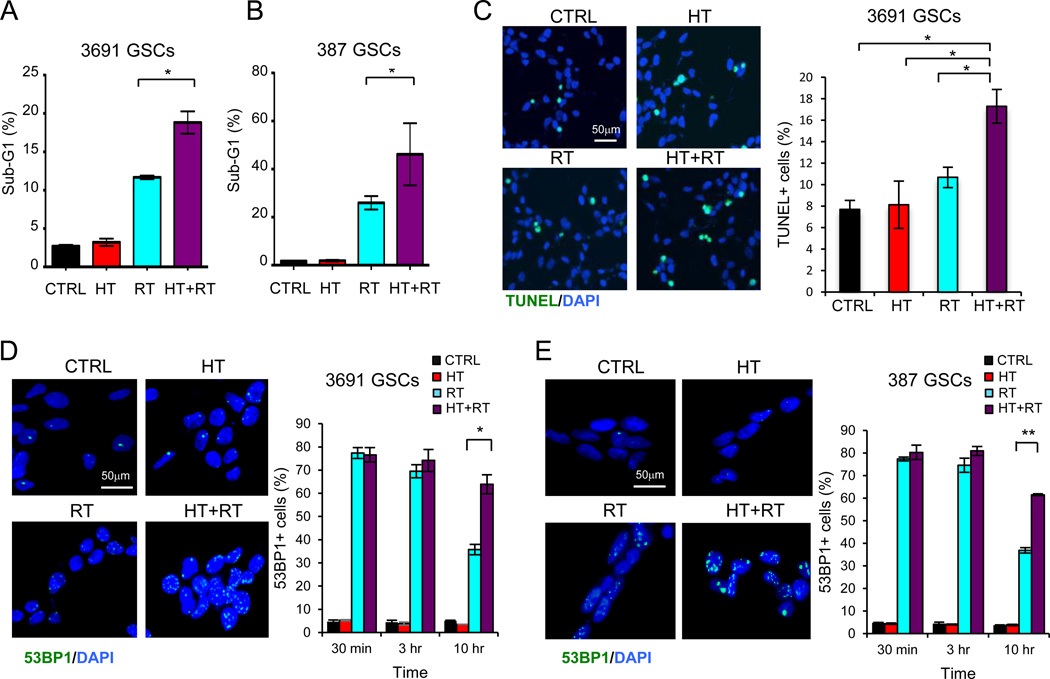
GSCs preferentially activate their DNA repair machinery after radiation and resist apoptosis compared to non-stem tumor cells (NSTCs) (6). Inhibition of DNA repair improves the radiosensitivity of GSCs (27). Hyperthermia is thought to act as a radiosensitizer primarily by interference of DNA damage repair (28). We examined whether combined thermoradiotherapy could reduce the efficiency of double strand break repair in GSCs and induce cell death. Cell cycle analysis by flow cytometry showed an increase in the fraction of dying cells, as indicated by the sub-G1 population, in the radiation group and an even greater increase in the sub-G1 population in the thermoradiotherapy group (Figure 3A-B). Furthermore, GSCs treated with thermoradiotherapy exhibited higher levels of apoptosis as assessed by TUNEL staining (Figure 3C).
Figure 3. Thermoradiotherapy impairs DNA damage repair and induces cell death.
(A-B) GSCs were treated as described in Figure 1. Quantification of the sub-G1 population of GSCs as assessed by flow cytometry. (C) Representative TUNEL (green) staining on 3691 GSCs 48 hours after indicated therapy (left) and quantification of fraction of TUNEL+ cells are shown (right). (D-E) Representative 53BP1 staining (green) in GSCs 10 hours after the indicated treatments is shown (left). Nuclei were counterstained with DAPI (blue). Quantification of the fraction of 53BP1+ cells at indicated times after treatment is shown (left). Nuclei with more than five 53BP1 foci staining were scored as positive. Graphs represent the fraction of 53BP1+ cells over total cell number. HT: hyperthermia, RT: radiation, HT+RT: hyperthermia and radiation. Graphed data are means ± SD (n=3). * denotes p < 0.05, ** denotes p < 0.01.
We next assessed the efficiency of DNA strand break repair by 53BP1 staining. Cells that underwent thermoradiotherapy showed similar levels of 53BP1 staining 30 minutes and 3 hours after treatment compared to those that were treated only with radiation (Figure 3D-E). At 10 hours post-treatment, while 53BP1 levels decreased for GSCs treated only with radiation, it remained elevated in GSCs treated with thermoradiotherapy, suggesting that hyperthermia impairs DNA strand break repair (Figure 3D-E). Hyperthermia itself had minimal effect on 53BP1 foci staining or apoptosis compared to control sham-treated cells. Together, these data suggest that inhibition of DNA repair and enhanced cell death contribute to hyperthermic radiosensitization.
Hyperthermia reduces AKT activation after irradiation
To study the molecular events underlying thermoradiotherapy, we performed a human phospho-kinase array (Figure 4A). There was no significant difference in phospho-kinase profile in GSCs treated with hyperthermia alone compared to the control GSCs. Radiation treatment increased p53 and CHK2 phosphorylation as expected (Figure 4A). Radiation alone also induced the phosphorylation of multiple kinases including ERK, AKT, RSK1/2 and p70 S6K. However, thermoradiotherapy reduced phosphorylation of AKT and its downstream kinases RSK1/2 and p70 S6K (Figure 4A). pERK levels did not appear to differ between radiation and thermoradiotherapy-treated cells. We did not detect significant changes in the phosphorylation of multiple other proteins on the array including p38 MAPK, AMPK, Src, JNK or STAT (refer to Supplemental Figure 1 for map of array components).
Figure 4. Hyperthermia inhibits radiation-induced AKT activation in GSCs.
(A) GSCs were treated as described in Figure 1 and human phospho-kinase array was performed with GSCs lysates as indicated. Select phosphorylated proteins on the kinase array membrane are indicated. (B) Cells were treated as described in Figure 1. Western blot analysis on components of AKT signaling and phosphor-ERK in GSCs and non-stem tumor cells (NSTCs) are shown. Quantification of Western blot analysis was done by ImageJ, and the relative density is provided at the bottom of each Western blot, respectively. (C) Western blot of phosphorylated AKT in GSCs stably transduced with constitutively activated MYR-AKT or GFP is shown. (D) Representative images of colony formation (left) and quantification of colony number (right) is shown in GSCs expressing MYR-AKT or GFP. (E) Representative images of GSC colony formation after treatment with LY294002 or vehicle control as indicated. Graphed data are means ± SD (n=3). HT: hyperthermia, RT: radiation, HT+RT: hyperthermia and radiation. * denotes p < 0.05.
We next confirmed these results by immunoblot in paired GSCs and NSTCs derived from the same patient specimen. Hyperthermia alone had no obvious effect on the phosphorylation of AKT, RSK1/2 or p70 S6K (Figure 4B and supplemental Figure 2). However, radiotherapy significantly increased levels of phosphorylated AKT, RSK1/2, p70 S6K and ERK in GSCs. Radiation-induced activation of AKT appeared significant in GSCs but not in NSTCs. These results are consistent with increased AKT signaling mediating radiation resistance (29–31). Combined thermoradiotherapy reduced radiation-induced phosphorylation of AKT, RSK1/2 and p70 S6K in GSCs but had no demonstrable effect on ERK phosphorylation (Figure 4B).
We next tested the ability of constitutively active AKT to rescue hyperthermic radiosensitization of GSCs using cells that stably expressed myristoylated AKT (MYR-AKT) or control GFP (Figure 4C). In GFP-expressing cells, thermoradiotherapy reduced colony formation compared to radiation alone. However, in MYR-AKT expressing cells, radiation and thermoradiotherapy groups exhibited similar numbers of colonies (Figure 4D). Treatment of GSCs with the PI3K inhibitor LY294002 further reduced GSC colony formation (Figure 4E). Together, these data suggest that a major mechanism by which thermoradiotherapy mediates GSC radiosensitization is through down-regulation of AKT signaling.
Thermoradiotherapy extends survival in a mouse model of GBM
To further investigate the potential therapeutic benefit of thermoradiotherapy in glioblastoma, we examined the effects of thermoradiotherapy on tumor growth of GSC-derived glioblastoma xenografts. GSCs stably expressing luciferase were transplanted into the forebrains of immunocompromised mice. When tumors reached similar levels of luminescence, mice were randomized to receive sham treatment, hyperthermia, radiation or thermoradiotherapy. Mice bearing tumors treated with thermoradiotherapy consistently displayed reduced tumor size and significantly increased animal survival relative to tumors treated with hyperthermia or radiation alone (HT+RT vs. RT alone, p=0.0231) (Figure 5A, F). This survival advantage was confirmed with an independent cohort of 38 mice (data not shown). Consistent with our in vitro data, thermoradiotherapy reduced levels of phosphorylated S6, an effector of AKT (Figure 5B, D), and proliferation (Figure 5C, E) in tumors. These data suggest that the combination of hyperthermia and radiation reduces tumor growth and improves survival potentially by abrogation of radiation-induced AKT signaling.
Figure 5. Thermoradiotherapy suppressed GBM growth and increased survival.
(A) Representative images of cross-sections (hematoxylin and eosin [H&E] stained) of mouse brains harvested on day 5 post-treatment. (B-C) Immunohistochemical staining for phospho-S6 (B) and Ki67 (C) in GBM xenografts treated as indicated are shown. (D-E) Quantification of fraction of phospho-S6+ and Ki67+ tumor cells to total tumor cells in the indicated group is shown. (F) Kaplan-Meier analysis of neurologic-sign-free survival of mice (n=26) treated as indicated revealing improved survival in mice treated with thermoradiotherapy vs. radiation alone, p=0.0231. Mouse survival data was confirmed in an independent cohort of 38 mice. Graphed data are means ± SD (n=3). HT: hyperthermia, RT: radiation, HT+RT: hyperthermia and radiation. * denotes p < 0.05, ** denotes p< 0.01, *** denotes p< 0.001.
DISCUSSION
The PI3K/AKT pathway is aberrantly regulated in over 40% of GBM and is associated with poor patient prognosis (32–34). This pathway is frequently over-activated in brain tumor stem cells to mediate radiation resistance (29, 31, 35). The proposed stem cell marker CD133/Prominin directly interacts with the p85 subunit of PI3K to facilitate AKT signaling in GSCs (36), and GSCs are particularly sensitive to AKT pathway inhibition (31, 37, 38). Therefore, targeting this pathway may improve therapy for patients with GBM.
Our study reveals that hyperthermia can abrogate radiation-induced activation of AKT in GSCs, and this translated into reduced tumor growth and improved animal survival in a preclinical model of GBM. We found that thermoradiotherapy reduced levels of phosphorylated AKT and its downstream kinases, p70 S6K and RSK1/2, but had minimal effect on other pathways, including ERK, p38 MAPK, Src, JNK or STAT. In tumors, combined hyperthermia and radiation diminished phospho-S6 levels and decreased proliferation to baseline levels. Introduction of constitutively activated AKT rescued GSCs from cell death induced by thermoradiotherapy. In addition, inhibition of AKT signaling by a PI3K inhibitor further sensitized GSCs to radiotherapy and thermoradiotherapy. Together, these data suggest that hyperthermia may improve the radiosensitivity of GSCs primarily by inhibition of AKT proliferative and pro-survival signaling. These results are consistent with results of gold nanoshell-mediated hyperthermia in improving the radiosensitivity of breast cancer stem cells (39) and suggest that maximizing PI3K-AKT inhibition with hyperthermia and pharmacologic inhibition may further improve radiosensitization of cancer stem cells.
In our preclinical model, tumors exhibited suppression of AKT signaling that persisted for over 5–7 days after a single hyperthermia treatment and low-dose radiation. If hyperthermia were followed immediately by fractionated radiation, then we anticipate that GSCs would remain radiosensitive for at least the first few fractions of radiotherapy. Currently in clinical practice, a delay of over 3–4 weeks occurs between surgery, including interstitial hyperthermia, and the start of radiation and chemotherapy. This delay in treatment allows for healing after surgery and devising a custom radiation plan. Because interstitial hyperthermia is minimally invasive and delivered through small burr holes, less time is needed for healing. Furthermore, radiotherapy may be pre-planned as there is less tissue distortion with interstitial hyperthermia compared to traditional craniotomy and tumor resection. Our studies suggest that decreasing the time interval between these treatment modalities would maximize hyperthermic radiosensitization of GSCs. Additional studies are needed to determine the optimal timing between interstitial hyperthermia and radiotherapy in patients.
Impairment of DNA damage repair is one of the major mechanisms attributed to hyperthermic radiosensitization (11, 40, 41). The PI3K-AKT pathway is increasingly recognized as a modulator of DNA double strand break repair (42–45). Radiation facilitates activation of AKT via ATM or DNA-dependent protein kinase (DNA-PK) (46, 47). PTEN, a negative regulator of the PI3K/AKT signaling pathway, regulates DNA damage response by regulating CHK1 localization (48) and nuclear PTEN regulates sensitivity to radiation damage in an ATM-dependent manner (49). We found that hyperthermia reduced the efficiency of DNA damage repair in GSCs after clinically relevant doses of radiation.
Historically, monotherapy against one signaling pathway in cancer is often ineffective in the clinic due to redundant pathways. Our data suggest that maximizing AKT inhibition with pharmacologic inhibitors and hyperthermia may enhance cancer control and potentially overcome resistance mechanisms. In summary, our studies reveal that hyperthermia improves the radiosensitivity of GSCs by suppressing radiation-induced AKT activation and proliferation. Our preclinical mouse models further support that combined thermoradiotherapy impairs tumor growth and extends animal survival. These studies support clinical translation of combined hyperthermia and radiation for patients with GBM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chaomei Xiang for critical review of the manuscript, and Erin Jesse and Stephen Bao for their technical support.
Financial support: American Cancer Society, B*CURED Foundation, Cleveland Clinic Foundation, and Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and the NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Authors report no conflict of interest.
REFERENCE
- 1.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet oncology. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. International journal of radiation oncology, biology, physics. 1979;5(10):1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer cell. 2013;24(3):331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura K, Aoyagi M, Ando N, Ogishima T, Wakimoto H, Yamamoto M, et al. Expansion of CD133-positive glioma cells in recurrent de novo glioblastomas after radiotherapy and chemotherapy. Journal of neurosurgery. 2013;119(5):1145–1155. doi: 10.3171/2013.7.JNS122417. [DOI] [PubMed] [Google Scholar]
- 8.Lim YC, Roberts TL, Day BW, Harding A, Kozlov S, Kijas AW, et al. A role for homologous recombination and abnormal cell-cycle progression in radioresistance of glioma-initiating cells. Molecular cancer therapeutics. 2012;11(9):1863–1872. doi: 10.1158/1535-7163.MCT-11-1044. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Molecular cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2005;21(8):779–790. doi: 10.1080/02656730500271668. [DOI] [PubMed] [Google Scholar]
- 12.Overgaard J. The heat is (still) on--the past and future of hyperthermic radiation oncology. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;109(2):185–187. doi: 10.1016/j.radonc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(13):3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 14.Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345(8949):540–543. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 15.Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/− hyperthermia for glioblastoma multiforme. International journal of radiation oncology, biology, physics. 1998;40(2):287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- 16.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355(9210):1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 17.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. International journal of radiation oncology, biology, physics. 1996;35(4):731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 18.Selker RG, Shapiro WR, Burger P, Blackwood MS, Arena VC, Gilder JC, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51(2):343–355. discussion 55-7. [PubMed] [Google Scholar]
- 19.Laperriere NJ, Leung PM, McKenzie S, Milosevic M, Wong S, Glen J, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. International journal of radiation oncology, biology, physics. 1998;41(5):1005–1011. doi: 10.1016/s0360-3016(98)00159-x. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi AM, Hawasli AH, Rodriguez A, Schroeder JL, Laxton AW, Elson P, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer medicine. 2014;3(4):971–979. doi: 10.1002/cam4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpentier A, Chauvet D, Reina V, Beccaria K, Leclerq D, McNichols RJ, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers in surgery and medicine. 2012;44(5):361–368. doi: 10.1002/lsm.22025. [DOI] [PubMed] [Google Scholar]
- 22.Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. Journal of neurosurgery. 2013;118(6):1202–1219. doi: 10.3171/2013.1.JNS1291. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man J, Shoemake J, Zhou W, Fang X, Wu Q, Rizzo A, et al. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell reports. 2014;9:1–15. doi: 10.1016/j.celrep.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rycaj K, Tang DG. Cancer stem cells and radioresistance. International journal of radiation biology. 2014;90(8):615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 27.Lim YC, Roberts TL, Day BW, Stringer BW, Kozlov S, Fazry S, et al. Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells. Molecular oncology. 2014 doi: 10.1016/j.molonc.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007;19(6):418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes & development. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlow B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PloS one. 2014;9(4):e94621. doi: 10.1371/journal.pone.0094621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn J, Hayman TJ, Jamal M, Rath BH, Kramp T, Camphausen K, et al. The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro-oncology. 2014;16(1):29–37. doi: 10.1093/neuonc/not139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer research. 1997;57(19):4183–4186. [PubMed] [Google Scholar]
- 34.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 35.Osuka S, Sampetrean O, Shimizu T, Saga I, Onishi N, Sugihara E, et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells. 2013;31(4):627–640. doi: 10.1002/stem.1328. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Jiang Y, Zou F, Liu Y, Wang S, Xu N, et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(17):6829–6834. doi: 10.1073/pnas.1217002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26(12):3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becher OJ, Hambardzumyan D, Walker TR, Helmy K, Nazarian J, Albrecht S, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer research. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Science translational medicine. 2010;2(55):55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(24):9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genet SC, Fujii Y, Maeda J, Kaneko M, Genet MD, Miyagawa K, et al. Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time- and temperature-dependent manner. Journal of cellular physiology. 2013;228(7):1473–1481. doi: 10.1002/jcp.24302. [DOI] [PubMed] [Google Scholar]
- 42.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer biology & therapy. 2009;8(8):730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98(3):834–841. doi: 10.1182/blood.v98.3.834. [DOI] [PubMed] [Google Scholar]
- 44.Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, et al. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Molecular and cellular biology. 2002;22(22):7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. The Journal of biological chemistry. 2007;282(29):21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Molecular cell. 2008;30(2):203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Viniegra JG, Martinez N, Modirassari P, Hernandez Losa J, Parada Cobo C, Sanchez-Arevalo Lobo VJ, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. The Journal of biological chemistry. 2005;280(6):4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 48.Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer cell. 2005;7(2):193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341(6144):395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.