Abstract
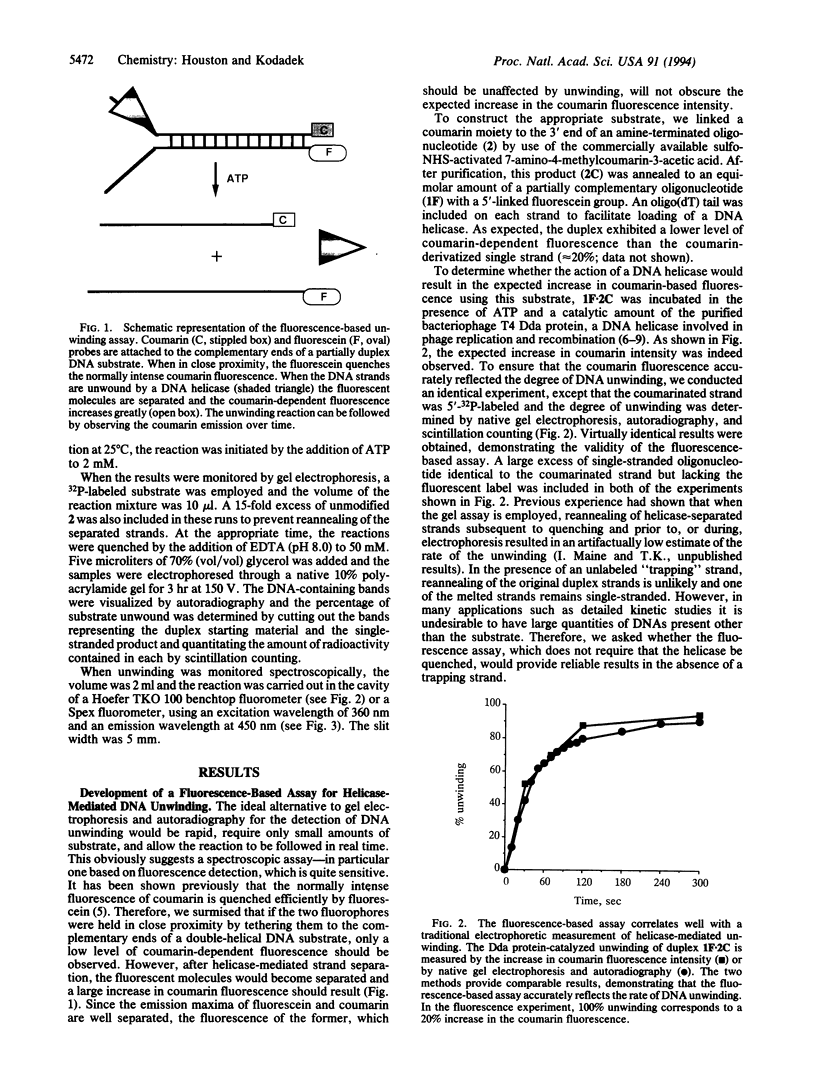
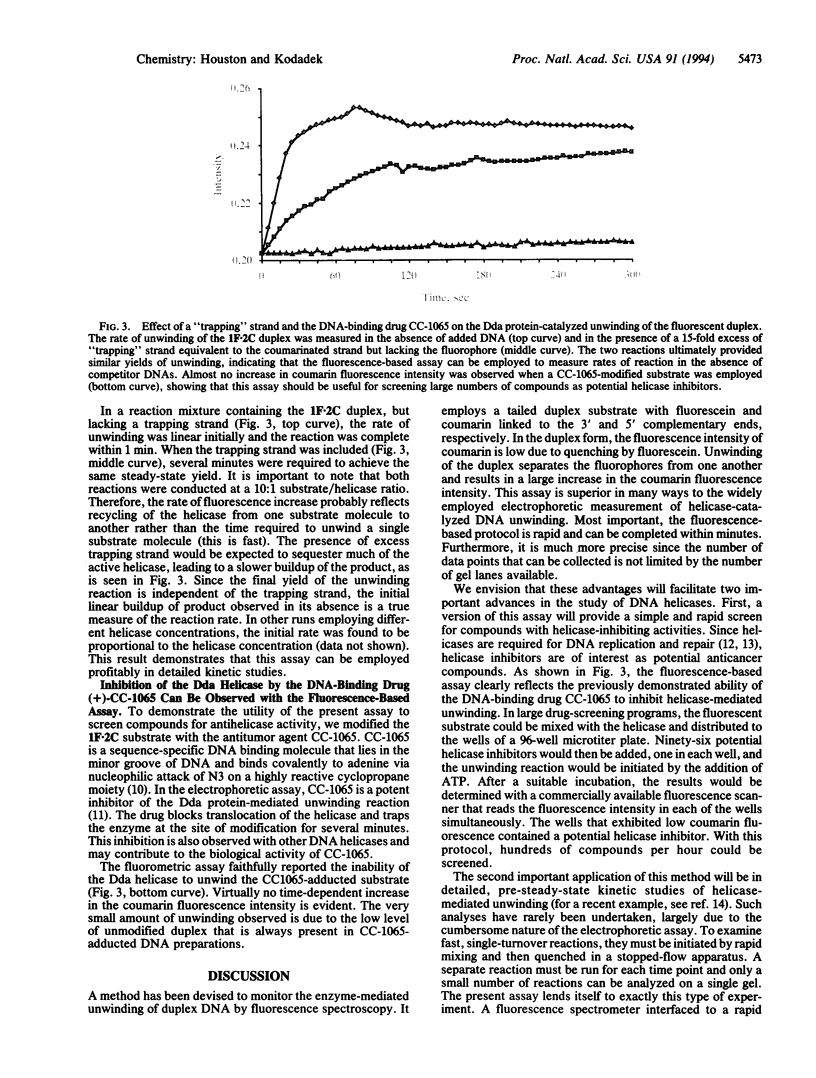
A method is described for monitoring the enzyme-mediated unwinding of duplex DNA spectrophotometrically. The assay employs a partially duplex oligonucleotide substrate modified at the complementary end with coumarin and fluorescein moieties. When in close proximity the fluorescein quenches the fluorescence of coumarin. However, when the strands are separated by the action of a DNA helicase, the coumarin fluorescence increases greatly. Therefore, the progress of enzyme-mediated DNA unwinding can be measured in real time by fluorescence spectroscopy. This assay provides a simple method to screen for helicase inhibitors, which are of growing interest as potential anticancer agents. The application of this technique to kinetic analyses of the mechanism of action of DNA helicases is also discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaratunga M., Lohman T. M. Escherichia coli rep helicase unwinds DNA by an active mechanism. Biochemistry. 1993 Jul 13;32(27):6815–6820. doi: 10.1021/bi00078a003. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Wong M. L., Alberts B. M. In vitro replication through nucleosomes without histone displacement. Nature. 1990 Feb 22;343(6260):719–726. doi: 10.1038/343719a0. [DOI] [PubMed] [Google Scholar]
- Cardullo R. A., Agrawal S., Flores C., Zamecnik P. C., Wolf D. E. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8790–8794. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czworkowski J., Odom O. W., Hardesty B. Fluorescence study of the topology of messenger RNA bound to the 30S ribosomal subunit of Escherichia coli. Biochemistry. 1991 May 14;30(19):4821–4830. doi: 10.1021/bi00233a026. [DOI] [PubMed] [Google Scholar]
- Eis P. S., Millar D. P. Conformational distributions of a four-way DNA junction revealed by time-resolved fluorescence resonance energy transfer. Biochemistry. 1993 Dec 21;32(50):13852–13860. doi: 10.1021/bi00213a014. [DOI] [PubMed] [Google Scholar]
- Hacker K. J., Alberts B. M. Overexpression, purification, sequence analysis, and characterization of the T4 bacteriophage dda DNA helicase. J Biol Chem. 1992 Oct 15;267(29):20674–20681. [PubMed] [Google Scholar]
- Jongeneel C. V., Bedinger P., Alberts B. M. Effects of the bacteriophage T4 dda protein on DNA synthesis catalyzed by purified T4 replication proteins. J Biol Chem. 1984 Oct 25;259(20):12933–12938. [PubMed] [Google Scholar]
- Jongeneel C. V., Formosa T., Alberts B. M. Purification and characterization of the bacteriophage T4 dda protein. A DNA helicase that associates with the viral helix-destabilizing protein. J Biol Chem. 1984 Oct 25;259(20):12925–12932. [PubMed] [Google Scholar]
- Kodadek T., Alberts B. M. Stimulation of protein-directed strand exchange by a DNA helicase. Nature. 1987 Mar 19;326(6110):312–314. doi: 10.1038/326312a0. [DOI] [PubMed] [Google Scholar]
- Lohman T. M. Helicase-catalyzed DNA unwinding. J Biol Chem. 1993 Feb 5;268(4):2269–2272. [PubMed] [Google Scholar]
- Maine I. P., Sun D., Hurley L. H., Kodadek T. The antitumor agent CC-1065 inhibits helicase-catalyzed unwinding of duplex DNA. Biochemistry. 1992 Apr 28;31(16):3968–3975. doi: 10.1021/bi00131a012. [DOI] [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Naegeli H., Modrich P., Friedberg E. C. The DNA helicase activities of Rad3 protein of Saccharomyces cerevisiae and helicase II of Escherichia coli are differentially inhibited by covalent and noncovalent DNA modifications. J Biol Chem. 1993 May 15;268(14):10386–10392. [PubMed] [Google Scholar]
- Reynolds V. L., Molineux I. J., Kaplan D. J., Swenson D. H., Hurley L. H. Reaction of the antitumor antibiotic CC-1065 with DNA. Location of the site of thermally induced strand breakage and analysis of DNA sequence specificity. Biochemistry. 1985 Oct 22;24(22):6228–6237. doi: 10.1021/bi00343a029. [DOI] [PubMed] [Google Scholar]
- Roman L. J., Kowalczykowski S. C. Characterization of the helicase activity of the Escherichia coli RecBCD enzyme using a novel helicase assay. Biochemistry. 1989 Apr 4;28(7):2863–2873. doi: 10.1021/bi00433a018. [DOI] [PubMed] [Google Scholar]
- Sung P., Bailly V., Weber C., Thompson L. H., Prakash L., Prakash S. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993 Oct 28;365(6449):852–855. doi: 10.1038/365852a0. [DOI] [PubMed] [Google Scholar]
- Taylor A., Smith G. R. Unwinding and rewinding of DNA by the RecBC enzyme. Cell. 1980 Nov;22(2 Pt 2):447–457. doi: 10.1016/0092-8674(80)90355-4. [DOI] [PubMed] [Google Scholar]
- Warpehoski M. A., Hurley L. H. Sequence selectivity of DNA covalent modification. Chem Res Toxicol. 1988 Nov-Dec;1(6):315–333. doi: 10.1021/tx00006a001. [DOI] [PubMed] [Google Scholar]