Abstract
Cre-recombinase mediated conditional deletion of Lox-P site flanked ("floxed") genes is widely used for functional gene annotation in mice. Many different Cre-transgenic mouse lines have been developed for cell-type specific gene disruption. But often, the precise tissue-patterns of Cre activity remain incompletely characterized. Two widely used transgenes for conditional gene recombination in hematopoietic cells are Vav-iCre driven from the murine Vav1 promotor, and hCD2-iCre driven from the human CD2 promotor. Vav-iCre expresses active Cre in fetal and adult hematopoietic stem cells and all descendants, hCD2-iCre in immature and mature B and T lymphocytes. To better characterize which hematopoietic cells contain hCD2-iCre activity, we compared EYFP fluorescence in hCD2-iCre+/- R26-stop-EYFP+/- and Vav-iCre+/- R26-stop-EYFP+/-mice. R26-stop-EYFP ubiquitously encodes EYFP preceded by a floxed stop cassette. By removing it, Cre activity induces measurable EYFP expression. Our results confirm the known activity patterns for both Cre transgenes and unveil additional hCD2-iCre mediated reporter gene recombination in common lymphoid progenitors, in natural killer cells and their progenitors, and in plasmacytoid and conventional dendritic cells. This supports previously proposed common lymphoid origins for natural killer cells and subsets of dendritic cells, and indicates the need to consider pleiotropic effects when studying hCD2-iCre mediated conditional knockout mice. Vav-iCre+/- R26-stop-EYFP+/-mice did not show the non-hematopoietic recombination in vascular endothelial cells seen in other Vav-Cre mouse lines, but displayed an unexpected Vav-iCre mediated recombination in a bone cell subset lacking hematopoietic markers. This pinpoints the need to consider stromal cell contributions to phenotypes of Vav-iCre mediated conditional knockout mice. Altogether, our data provide the first detailed assessment of hCD2-iCre and Vav-iCre mediated deletion of floxed genes during lymphocyte development from hematopoietic stem cells and open up novel applications for either Cre-transgenic mouse line.
Introduction
Cre-recombinase mediated conditional deletion of Lox-P site flanked ("floxed") genes is widely used for functional gene annotation in mice. Many different Cre-transgenes are available for cell-type specific or drug-induced disruption of a targeted gene [1,2]. Transgenes encoding improved Cre (iCre) optimize Cre expression through optimized codon usage, removed putative cryptic splice sites and reduced CpG content to limit epigenetic silencing [3]. However, transgenic Cre activity in untargeted cell types can compromise the desired cell type specificity of Cre mediated gene recombination [4]. For example, Vav1 promoter driven Cre (Vav-Cre) or improved Cre (Vav-iCre) disrupt floxed genes in fetal and adult hematopoietic stem cells (HSC) and their descendants, which ultimately form all blood cells. Thus, Vav-Cre is commonly used for pan-hematopoietic gene disruption [2,3,5–9]. But certain Vav-Cre lines also delete in vascular endothelial cells (EC) or in precursors which do not express Vav, possibly due to Cre-transgene or LacZ reporter insertion effects [6]. This might indirectly affect hematopoiesis, in particular HSC quiescence in bone marrow (BM) vascular endothelial niches [10]. Vav-iCre transgene expression also occurs in the testis [3]. Thus, for many Cre-lines a better characterization of the precise tissue-patterns of Cre activity is required before observed phenotypes can be unambiguously linked to the specific disruption of the targeted gene in the targeted cell type.
Cell type-specific Cre activity can be visualized in R26-stop-EYFP mice where a EYFP gene preceded by a floxed stop cassette was knocked into the ubiquitously expressed ROSA26 locus. Stop removal by Cre recombination induces measurable EYFP expression in those cells harboring active Cre without significant leaky EYFP expression [11]. Flow cytometry can quantify both the proportion of EYFP expressing cells, and the extent of Cre activity in these which correlates with the EYFP mean fluorescence intensity (MFI). Once the Stop cassette has been removed, EYFP expression continues even in the absence of Cre. This enables cell tracing and fate mapping studies, but can also mask Cre-inactivation after a Cre-expressing developmental or physiological stage.
Mice transgenic for iCre under control of the human CD2 promoter (hCD2-iCre) are a popular tool to conditionally delete genes in immature thymocytes, mature T cells and B cells without reported effects on myeloid cells [3,12,13]. The precise stage of hematopoietic development where hCD2-iCre is first active, and to what extent hCD2-iCre also deletes floxed genes in other hematopoietic cells is unknown. Testis expression shows that hCD2-iCre can be expressed in non-hematopoietic cells [3]. To better characterize the activity patterns of this important Cre-line, we analyzed EYFP expression in hematopoietic cell and progenitor subsets of hCD2-iCre +/- R26-stop-EYFP +/- mice and controls. For comparison, we also analyzed Vav-iCre +/- R26-stop-EYFP +/- mice and controls.
Our results confirm the known activity patterns for both Cre transgenes and unveil additional hCD2-iCre mediated reporter gene recombination in common lymphoid progenitors (CLP), NK cell progenitors (NKP), NK cells, all pDC and ~20% of conventional dendritic cells (cDC). This is consistent with the proposed CLP origin of NK cells and of subsets of pDC and cDC [14–18], and shows the utility of hCD2-iCre for conditional gene disruption in these cell types. Unexpected Vav-iCre activity in bone cells which do not express hematopoietic surface markers pinpoints a need to consider stromal or niche cell contributions to phenotypes of Vav-iCre mediated conditional knockout mice.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC, Assurance Number: A3194-01) of The Scripps Research Institute (TSRI). All efforts were made to minimize animal suffering. Mice were euthanized by CO2/O2 mixture or halothane volatile anesthetic overdose inhalation.
Mice
hCD2-iCre and Vav-iCre transgenic mice [3] were obtained from The Jackson Laboratory [B6.Cg-Tg(CD2-cre)4Kio/J, stock no. 008520, and B6.Cg-Tg(Vav1-cre)A2Kio/J, stock no. 008610]. Both lines were independently bred to R26-stop-EYFP mice [11] [B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J, The Jackson Laboratory, order no. 006148] and genotyped by PCR as described in [3,11] or on the Jackson Laboratory webpage. hCD2-iCre is homozygous lethal [13]. Thus, hCD2-iCre +/- R26-stop-EYFP +/-, Vav-iCre +/- R26-stop-EYFP +/-, and R26-stop-EYFP +/- mice were used for analysis. EYFP expression in thymocytes was determined in 5.5–7.5 week old mice, as the thymus starts to undergo atrophy in older mice. Otherwise, EYFP expression was determined in 6–17 week old mice. All mice were housed in the TSRI Specific Pathogen Free (SPF) facility with a 12 hr light cycle, and were given food and water ad libitum.
Cell preparation
BM, thymocyte and splenocyte single cell suspensions were prepared and BM and spleen red blood cells lysed with BD Pharmlyse (BD Biosciences) as previously described [19–21]. For isolation of bone cells, whole bones were cleaned from muscle tissue, BM was flushed out, and the bones were then chipped into little pieces and digested with collagenase as described elsewhere [22].
FACS analyses
Published surface marker staining patterns were used for the analysis of HSC [23], hematopoietic progenitor cell (HPC) subsets [24], common lymphoid progenitors (CLP) [25], endothelial cells (EC), osteoblasts (OB) and mesenchymal stem cells (MSC) [22], B cell [26], T cell [27] and NK cell precursors [21]. If not noted otherwise, all antibodies were purchased from eBiosciences, Biolegend or BD Biosciences. Unspecific antibody (AB) binding was prevented by pre-incubation with anti-CD16/32 AB (clone 93, 1:10 diluted) or, when staining for CD16/32, with 5% rat serum (Stemcell Technologies, cat. no. 19700). The cells were first stained with a Lineage cocktail (Lin) containing biotinylated AB (diluted 1:150 unless noted otherwise) against CD3ε (clone 145-2C11), CD4 (clone GK1.5), CD8α (clone 53–6.7), CD19 (clone 6D5), B220 (clone RA3-6B2), CD11b (clone M1/70), CD11c (clone N418), CD49b (clone DX5), Gr-1 (clone RB6-8C5), TER-119 (clone TER-119) and, when indicated, IL-7Rα (clone A7R34, diluted 1:75) in FACS staining buffer (PBS/3% FCS/0.1% NaN3), washed, further stained with anti-mouse c-kit PE-Cy7 (clone 2B8, diluted 1:120), anti-mouse Flk-2 PE (clone A2F10, diluted 1:120), anti-mouse Sca-1 APC-Cy7 (clone D7, diluted 1:120), anti-mouse CD34 Alexa Fluor 700 (clone RAM34, diluted 1:30, eBioscience), anti-mouse CD48 Pacific Blue (clone HM48-1, diluted 1:120), anti-mouse CD150 PerCP-Cy5.5 (clone TC15-12F12.2, diluted 1:150), anti-mouse IL-7Rα Brilliant Violet 421 (clone A7R34, diluted 1:40, Biolegend), anti-mouse CD16/32 APC (clone 93, diluted 1:60), anti-mouse CD45.2 Pacific Blue (clone 104, diluted 1:100), anti-mouse CD31 APC (clone 390, diluted 1:60), anti-mouse CD51 PE (clone RMV-7, diluted 1:60), and APC-Cy7- (diluted 1:120) or Qdot 605- (Life Technologies, cat. no. Q10101MP, diluted 1:120) conjugated streptavidin (SA). Mature hematopoietic cells were stained with anti-mouse CD11c APC (clone 418, diluted 1:12), anti-mouse CD11b Alexa Fluor 700 (clone M1/70, diluted 1:40), anti-mouse Gr-1 PE (clone RB6-8C5, diluted 1:40), anti-mouse F4/80 PerCP-Cy5.5 (clone BM8, diluted 1:40), anti-mouse NK1.1 PE-Cy7 (clone PK136, diluted 1:40), anti-mouse CD3ε APC-eFluor780 (clone 145-2C11, diluted 1:12, eBioscience), anti-mouse B220 Brilliant Violet 421 (clone RA3-6B2, diluted 1:40, Biolegend). B cell precursors were stained with anti-mouse B220 APC-Cy7 (clone RA3-6B2, diluted 1:60), anti-mouse CD43 biotin (clone S7, diluted 1:300, BD Biosciences), anti-mouse BP-1 PE (clone 6C3, diluted 1:60), anti-mouse CD24 APC (clone M1/69, diluted 1:300), anti-mouse IgM PE-Cy7 (clone RMM-1, diluted 1:60), anti-mouse IgD (clone 11-26c.2a, diluted 1:60) and SA-PerCP (diluted 1:120). T cell precursors were first stained with a Lin cocktail containing biotinylated AB (all 1:300 diluted) against CD19 (clone 6D5), B220 (clone RA3-6B2), CD11b (clone M1/70), CD11c (clone N418), CD49b (clone DX5), Gr-1 (clone RB6-8C5), TER-119 (clone TER-119) followed by staining with anti-mouse CD4 Alexa Fluor 700 (clone RM4-5, diluted 1:150, Biolegend), anti-mouse CD8α PE (clone 53–6.7, diluted 1:150), anti-mouse CD44 APC (clone 1M7, diluted 1:600), anti-mouse CD25 PerCP-Cy5.5 (clone PC61, diluted 1:600), anti-mouse CD24 Pacific Blue (clone M1/69, diluted 1:300), anti-mouse c-kit PE-Cy7 (clone 2B8, diluted 1:120), and SA-APC-Cy7 (diluted 1:120). NK cells were stained with anti-mouse CD3ε APC-eFluor780 (clone 145-2C11, diluted 1:200), anti-mouse CD122 eFluor 450 (clone TM-β1, diluted 1:60), anti-mouse NK1.1 PE-Cy7 (clone PK136, diluted 1:60), anti-mouse NKG2D APC (clone CX5, diluted 1:60) and anti-mouse CD11b Alexa Fluor 700 (clone M1/70, diluted 1:300), and with biotinylated AB (all diluted 1:600) against the lineage markers TER-119 (clone TER-119), CD19 (clone 6D5) and Gr-1 (clone RB6-8C5) followed by SA-PerCP (diluted 1:120) stain. All cells were fixed in 1.35% paraformaldehyde/PBS after staining and analyzed within 24 hr. EYFP was detected in the FITC channel. Samples were run on a Beckton Dickinson LSR-II flow cytometer and analyzed with FlowJo software (Treestar).
Results
hCD2-iCre activity in mature PBL
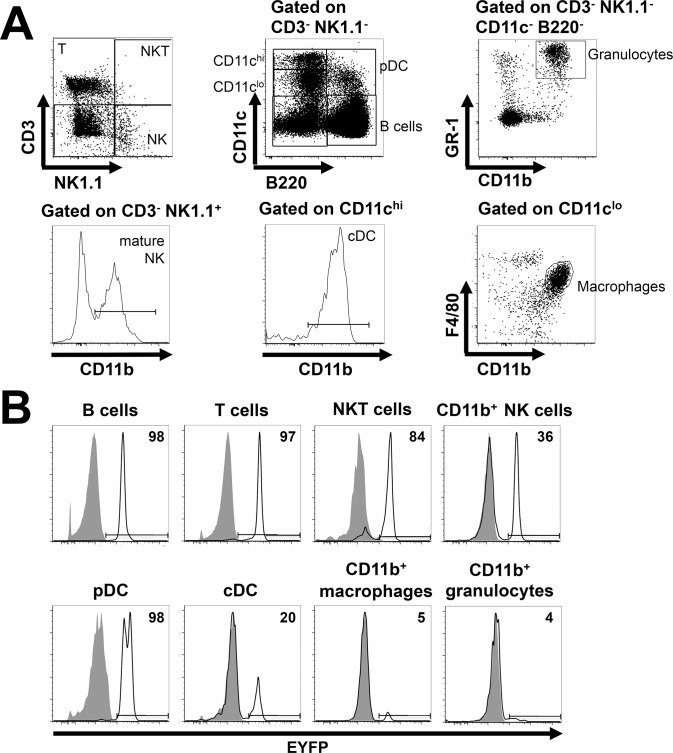
We used flow cytometry to analyze EYFP expression as a measure of Cre activity in splenic mature leukocytes from hCD2-iCre +/- R26-stop-EYFP +/- versus R26-stop-EYFP +/- mice. Consistent with the initial characterization of the hCD2-iCre mice and primarily lymphoid CD2 expression [3] (Fig 1), hCD2-iCre mediated gene deletion had occurred in essentially all mature T and B cells (>96% EYFP+ cells, Fig 2). In contrast, macrophages and granulocytes showed negligible hCD2-iCre activity (<5% EYFP+ cells).
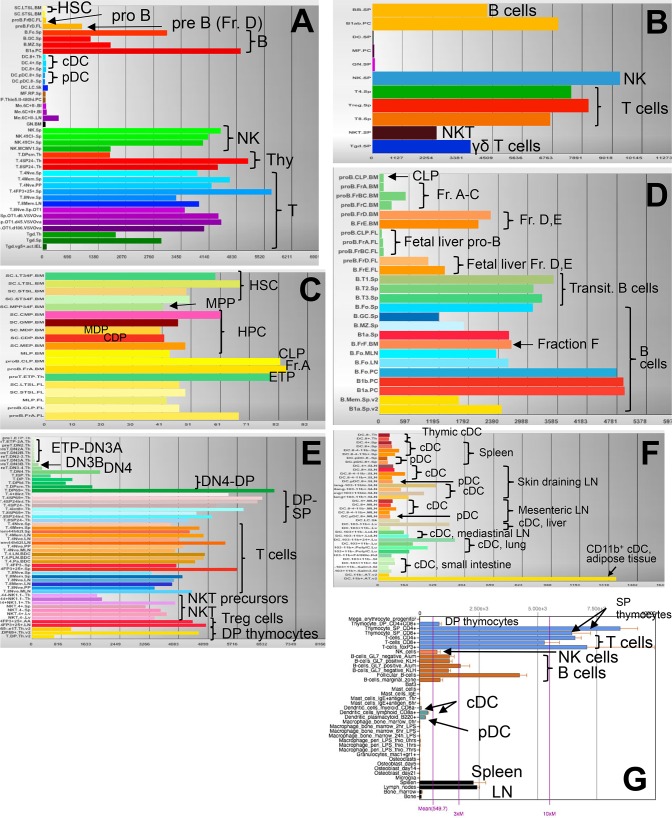
Fig 1. Murine CD2 mRNA tissue expression profiles.
Shown are ImmGen Consortium [53] (www.immgen.org) probe set 10500677 (A-F) and BioGPS [54,55] (www.biogps.org) probe set 1418770_at (G) murine CD2 mRNA expression profiles across (A, B) key hematopoietic cell populations, (C) HSC and HPC populations, (D) B cell developmental and mature populations, (E) T cell and NKT cell developmental and mature populations, (F) DC subsets and (G) multiple hematopoietic tissues and cell types. In (G), non-hematopoietic tissues are not shown because they did not express CD2. HSC, hematopoietic stem cells; HPC, hematopoietic progenitor cells; MPP, multipotent progenitors; MDP, monocyte-DC precursors; CDP, common DC precursors; CLP, common lymphoid progenitors; Fr. A, fraction A pre-pro B cells; pro B, pro B cells; Fr. B,C, fraction B and C pro- and early pre B cells; pre B (D), fraction D late pre B cells; Fr. F, fraction F recirculating mature B cells [19,34]; NK, NK cells; Thy, thymocyte; ETP, early thymocyte progenitor; DN3A, DN3B, DN4, CD4-CD8- thymocyte subsets; DP, CD4+CD8+ thymocytes; SP, CD4+ and CD8+ thymocytes; Treg cells, regulatory T cells; transit. B, transitional B cells.
Fig 2. hCD2-iCre activity in mature leukocytes.
(A) Gating strategy. Splenocytes were stained with the indicated AB and analyzed by FACS, gating on CD3+NK1.1- T cells, CD3-NK1.1+CD11b+ mature NK cells, CD3+NK1.1+ NKT cells, CD3- NK1.1-CD11c-CD11b+B220-Gr-1hi granulocytes, CD3-NK1.1-CD11cloB220+ pDC, CD3-NK1.1-CD11chi CD11b+B220- cDC, CD3-NK1.1-B220+ B cells and CD3-NK1.1-CD11cloF4/80+CD11b+ macrophages. (B) EYFP expression in the indicated mature leukocyte populations from hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) and R26-stop-EYFP +/- mice (shaded histograms). Numbers depict % EYFP+ cells in the hCD2-iCre +/- R26-stop-EYFP +/- mice. Representative of three independent experiments (n = 3 for each genotype).
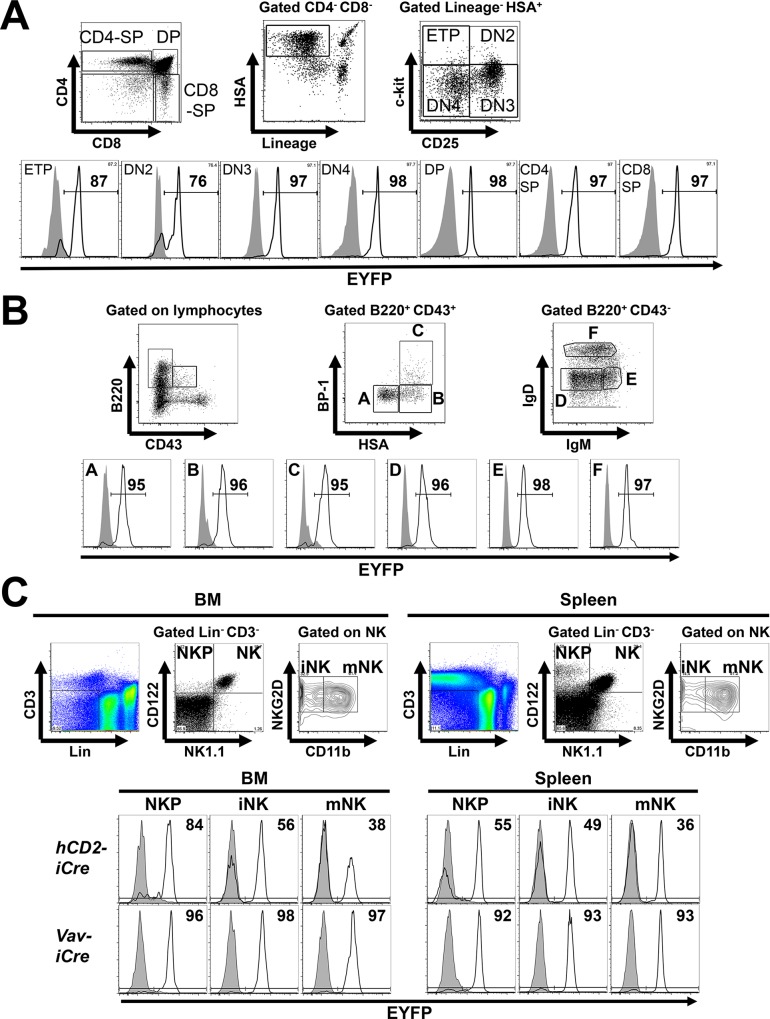
Lymphoid NKT cells develop in the thymus from CD4+CD8+ precursors and have similar signaling requirements as developing T cells [28]. Both NKT precursors and NKT cells express CD2 mRNA (Fig 1B and 1E). CD4+CD8+ thymocytes have high hCD2-iCre activity (Fig 3). Consistent with these findings, we found >83% EYFP+ NKT cells (Fig 2).
Fig 3. hCD2-iCre activity in T, B and NK cell development.
(A) Thymocyte populations were identified by the gating strategy in the upper panel [13,31–33]. Lower panel, EYFP expression in the indicated thymocyte populations from hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms). Numbers denote % EYFP+ cells within the indicated population of hCD2-iCre +/- R26-stop-EYFP +/- mice. Representative of three independent experiments (n = 3). (B) Upper panel, subsets of developing B cells in the BM were distinguished as in [19]. Lower panel, EYFP expression in the indicated BM B cell populations from hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms). Numbers denote % EYFP+ cells within the indicated population of hCD2-iCre +/- R26-stop-EYFP +/- mice. Representative of three independent experiments (n = 3). (C) Upper panels, NK cell progenitors (NKP), immature (iNK) and mature (mNK) NK cells were identified using the indicated gating strategy [18,29]. Lower panels, EYFP expression in the indicated BM and splenic NK cell populations from (top, n = 3 per genotype) hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms), or (bottom, n = 2 per genotype) from Vav-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms). Numbers indicate % EYFP+ cells in the respective Cre +/- mice. Representative of three independent experiments with hCD2-iCre transgenic mice, and of two independent experiments with Vav-iCre transgenic mice.
Innate NK cells are commonly thought to develop from CLP via Lin-CD3-CD122+NK1.1+ NK cell progenitors (NKP) [14,18,29]. However, at least in vitro, human myeloid progenitors can also produce NK cells [30]. We found ≥36% EYFP+ mature NK1.1+CD11b+ splenic NK cells (mNK), ≥49% EYFP+ immature NK cells (iNK), ≥55% EYFP+ NK cell progenitors (NKP, Figs 2 and 3C) and ≥27% EYFP+ CLP (Fig 4A) in hCD2-iCre +/- R26-stop-EYFP +/- mice. This is consistent with CD2 mRNA expression in NK cells (Fig 1A,1B and 1G) and supports derivation of many NKP and NK cells from CLP.
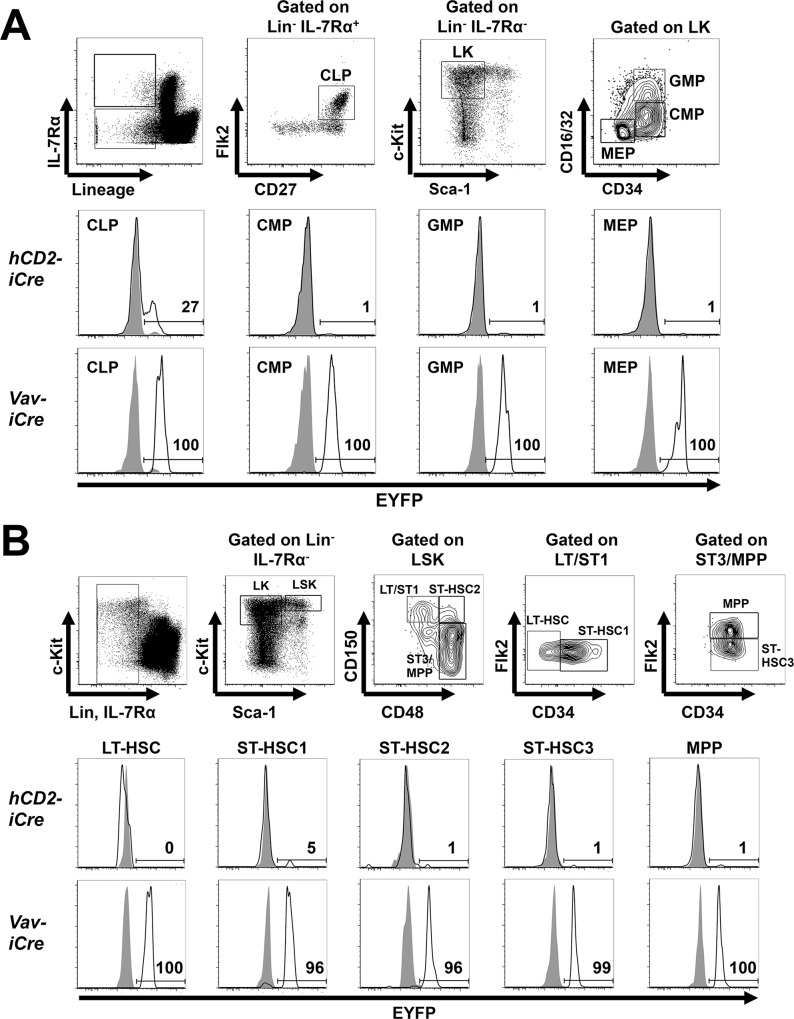
Fig 4. hCD2-iCre activity in HSC and HPC subsets.
(A) Upper panels, HPC subsets were identified using the indicated gating strategy [24,25]. Lower panels, EYFP expression in CLP (Lin-Flk2+IL-7Rα+CD27+), MEP (Lin-IL-7Rα-c-kit+CD16/32-CD34-), CMP (Lin-IL-7Rα-c-kit+CD16/32-/loCD34+) and GMP (Lin-IL-7Rα-c-kit+CD16/32+CD34+) from hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms), or from Vav-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms). Representative of three independent experiments. (B) Upper panels, phenotypic HSC/MPP subsets were identified using the indicated gating strategy [23,35,56]. Lower panels, EYFP expression in phenotypic LT-HSC (Lin-c-kit+Sca-1+CD150+CD34-CD48-Flk2-), ST-HSC1 (Lin-c-kit+Sca-1+CD150+CD34+CD48-Flk2-), ST-HSC2 (Lin-c-kit+Sca-1+CD150+CD34+CD48+Flk2-), ST-HSC3 (Lin-c-kit+Sca-1+CD150-CD34+CD48+Flk2-) and MPP (Lin-c-kit+Sca-1+CD150-CD34+CD48+Flk2+) from the mice in (A). Representative of at least two independent experiments. Numbers indicate % EYFP+ cells in the respective Cre +/- mice (n = 3 for experiments with hCD2-iCre transgenic mice, n = 2 for experiments with Vav-iCre transgenic mice).
Dendritic cells include cDC with important roles in antigen presentation and T cell activation, and pDC capable of producing large type I IFN amounts upon viral encounter. CLP and common myeloid progenitors (CMP) can each give rise to cDC and pDC, although CLP contributions are thought to play a minor role under steady state conditions in vivo, in particular for cDC development [15–17]. Interestingly, we found 98% EYFP+ pDC but only ~20% EYFP+ cDC in hCD2-iCre +/- R26-stop-EYFP +/- mice (Fig 2B). Thus, although most cDC and pDC express no or very low CD2 mRNA (Fig 1A,1F and 1G), hCD2-iCre is active in pDC and some cDC, or in their progenitors.
Altogether, our data indicate that hCD2-iCre leads to gene recombination not only in B and T cells, but also in iNKT cells, NK cells, pDC and some cDC.
hCD2-iCre activity in T and B cell precursors
We next studied at which stage in T and B cell development hCD2-iCre mediated gene deletion can occur. T cell development occurs in the thymus and proceeds from Lin-HSAhighCD4-CD8-CD44+CD25-c-Kit+ early thymocyte progenitors (ETP, a subset of DN1 cells) through successive CD44+CD25+c-Kit+ DN2, c-Kit-CD44-CD25+ DN3 and c-Kit-CD44-CD25- DN4 stages followed by CD4+CD8+ double-positive (DP) and CD4+ or CD8+ single positive (SP) more mature stages [31–33]. Consistent with, and expanding previous reports [3,13], we detected robust EYFP expression in all these stages, including 87% EYFP+ ETP, 76% EYFP+ DN2 cells, and ≥97% EYFP+ DN3, DN4, DP and SP cells (Fig 3A). The higher proportion of EYFP+ ETP and DN2 cells in our study than previously found in DN1 and DN2 cells by others [3,13] likely reflects our more stringent gating on Lin-HSAhighCD4-CD8-c-Kit+CD25- (ETP) or Lin-HSAhighCD4-CD8-c-Kit+CD25+ (DN2) cells, which defines both populations more purely [31–33]. As previously noted [13], the EYFP expression pattern is consistent with increasing CD2 mRNA expression in post-DN3 stage thymocytes and mature T cells, but contrasts with the paucity of CD2 mRNA in ETP and DN2 cells (Fig 1E and 1G).
B lymphocytes arise from CLP through a series of developmental stages in the BM [34]. A previous study reported hCD2-iCre induced EYFP expression in all peripheral B cells and BM-derived CD19+ IgD- immature B cells [3], but the precise developmental stage where EYFP is first induced remained unclear. To better characterize hCD2-iCre activity during B cell development, we thus analyzed EYFP expression on successive stage A-C pro-B and early pre-B cells, stage D late pre-B cells, stage E newly formed/immature B cells and stage F recirculating mature/follicular B cells [19,34] in the BM of hCD2-iCre +/- R26-stop-EYFP +/- or R26-stop-EYFP +/- mice (Fig 3B). Interestingly, we found >95% EYFP+ cells in all B cell developmental subpopulations of hCD2-iCre +/- R26-stop-EYFP +/- but not control mice. This activity pattern is consistent with the high CD2 mRNA expression in post-fraction C B cell developmental stages and mature B cells, but contrasts with the low CD2 mRNA expression in fractions A-C (Fig 1A,1D and 1G).
Altogether, hCD2-iCre recombines floxed genes in all B and T cell developmental subsets, including the earliest thymic T cell and BM B cell precursors despite their low CD2 mRNA expression levels.
hCD2-iCre activity in early hematopoietic progenitor cell (HPC) and phenotypic HSC subsets
All blood cell lineages develop from pluripotent long-term repopulating hematopoietic stem cells (LT-HSC) via short-term repopulating ST-HSC, multipotent progenitors (MPP) and various HPC intermediates into mature cells [10,23,35,36]. MPP give rise to CLP and CMP. CLP generate pro-B cells and NKP which ultimately produce B and NK cells, although myeloid NK cell precursors might also exist [30]. CLP may also generate ETP and thus T cells and NKT cells, although the true nature of the thymus homing T cell progenitor is still under debate and HSC/MPP subsets may contribute to ETP generation without CLP involvement [37]. CMP generate granulocyte-macrophage progenitors (GMP) and megakaryocyte-erythrocyte progenitors (MEP), which then give rise to the myeloid and erythroid lineages, respectively. Both CLP and CMP can give rise to pDC and cDC subsets [15–17].
The high EYFP expression on hCD2-iCre +/- R26-stop-EYFP +/- ETP (Fig 3A) contrasts with the reported very low CD2 expression in DN1 and DN2 cells (Fig 1E) and was hypothesized but not shown to reflect CD2 expression in earlier progenitors [13]. Moreover, the EYFP expression in hCD2-iCre +/- R26-stop-EYFP +/- NKP, NK cells, pro-B cells, pDC and few cDC identified here (Figs 2 and 3) despite low CD2 mRNA expression in pro-B cells and most DC (Fig 1A,1D,1F and 1G) would be consistent with hCD2-iCre activity in earlier hematopoietic progenitors. To test this possibility, we analyzed EYFP expression in BM HPC and phenotypic HSC subsets of hCD2-iCre +/- R26-stop-EYFP +/- and R26-stop-EYFP +/- mice (Fig 4A and 4B).
Consistent with essentially absent CD2 mRNA expression (Fig 1C), phenotypic LT-HSC, ST-HSC2/3, MPP, CMP, GMP and MEP of hCD2-iCre +/- R26-stop-EYFP +/- mice were all negative for EYFP and thus lack hCD2-iCre activity (Fig 4A and 4B). The potential significance of a low 5% EYFP+ ST-HSC2 remains to be determined. In contrast, ~27% of hCD2-iCre +/- R26-stop-EYFP +/- CLP expressed EYFP (Fig 4A). Notably, CLP, ETP and fraction A B cell CD2 mRNA levels are low but higher than in other HSC/HPC subsets (Fig 1C). This suggests that during hematopoietic development, hCD2-iCre mediated gene recombination starts in CLP and further increases in pro-B cells, ETP and NKP (Fig 3A–3C). The variable proportions of EYFP+ CLP might reflect the overall low CD2 mRNA expression in CLP (Fig 1C), differences between individual mice or the relatively poorly defined CLP surface phenotype (Lin- CD127+ Flk2+ CD27+) which might include contaminating EYFP- non-CLP cells.
CD45- bone cells harbor Vav-iCre activity
For comparison with the hCD2-iCre +/- R26-stop-EYFP +/- mice, we analyzed EYFP expression in HSC and HPC of Vav-iCre +/- R26-stop-EYFP +/- and R26-stop-EYFP +/- mice. Consistent with previous reports [3,5–9], all HSC and HPC populations in Vav-iCre +/- R26-stop-EYFP +/- mice contained ≥96% EYFP+ cells (Fig 4A and 4B).
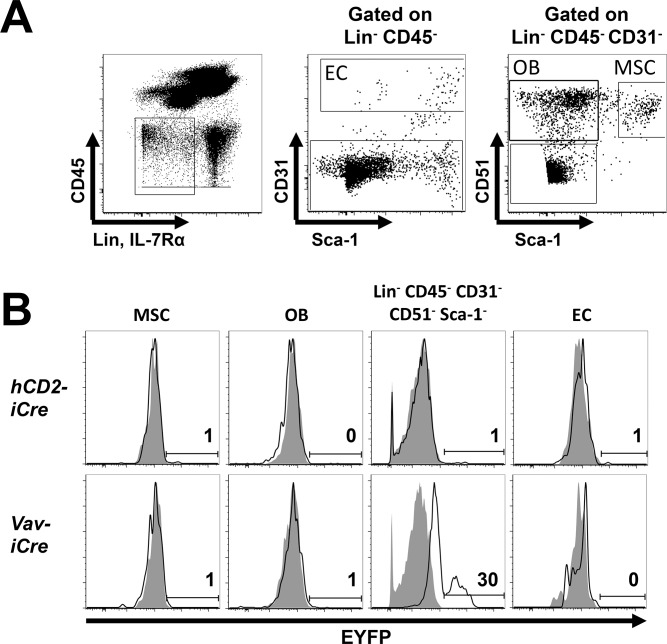
Besides hematopoietic cells, older Vav-Cre lines also recombine floxed genes in the testis, and in vascular endothelial cells (EC) or precursors which do not express Vav, possibly due to Cre-transgene or LacZ reporter insertion effects [3,6]. To assess whether this also occurs in our hCD2-iCre and Vav-iCre lines, we next analyzed EYFP expression in stromal and endothelial cells from collagenase-digested, BM depleted bones [22]. We found no EYFP expression in hCD2-iCre +/- R26-stop-EYFP +/- and Vav-iCre +/- R26-stop-EYFP +/- Lin-CD45-CD31+ EC, Lin-CD45-CD31-CD51+Sca-1- osteoblasts (OB) and Lin-CD45-CD31-CD51+Sca-1+ mesenchymal stem cells (MSC, Fig 5). As previously reported [22], most Lin-CD45- cells in collagenase-digested bones were CD31-CD51-Sca-1-. About 30% of these cells were EYFP+, the other 70% EYFPlow in Vav-iCre +/- R26-stop-EYFP +/- but not hCD2-iCre +/- R26-stop-EYFP +/- mice (Fig 5), indicating a specific Vav-iCre activity. These cells are not EC and do not bear surface markers of mast cells (FcεR) or erythroid progenitors (CD41) as assessed by FACS. Their identity requires further investigation. Altogether, these data confirm potent Vav-iCre activity in all HSC and HPC subsets and identify a novel Vav-iCre activity containing Lin-CD45-CD31-CD51-Sca-1- subset of bone cells.
Fig 5. Vav-iCre activity in non-lymphoid bone cells.
(A) Gating strategy. After flushing out the BM, bones were digested with collagenase and remaining cells stained for expression of the indicated markers. (B) EYFP expression on EC (Lin-CD45-CD31+), OB (Lin-CD45-CD31-CD51+Sca-1-), MSC (Lin-CD45-CD31-CD51+Sca-1+) and abundant Lin-CD45-CD31-CD51-Sca-1- bone cells of unknown identity [22] from (upper panels) hCD2-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms), or from (lower panels) Vav-iCre +/- R26-stop-EYFP +/- (open histograms) or R26-stop-EYFP +/- mice (shaded histograms). Numbers indicate % EYFP+ cells in the respective Cre +/- mice. Representative of three independent experiments (n = 3).
Discussion
Among several Cre-transgenes that allow conditional disruption of floxed genes in hematopoietic cells in mice, Vav-iCre is commonly used to recombine genes in HSC and all descendants, and hCD2-iCre for gene manipulation in B and T lymphocytes [2,3,12,13]. Here, we show that beyond their reported gene recombination patterns, Vav-iCre and hCD2-iCre also disrupt floxed genes in other murine cells. Besides hematopoietic cells, Vav-iCre recombined a floxed EYFP reporter gene also in a Lin-CD45-CD31-CD51-Sca-1- bone cell type that lacks hematopoietic surface markers. Its identity remains to be elucidated. hCD2-iCre recombined a floxed EYFP reporter not only in B and T cells, but also in CLP, pro-B and all other stages of B cell development, ETP and subsequent stages of T cell development, NK cell progenitors, immature and mature NK cells, pDC and subsets of cDC.
Our analysis of Vav-iCre +/- R26-stop-EYFP +/- mice confirmed the previously reported Vav-Cre or Vav-iCre activity in all hematopoietic cell subsets [3,5–9]. In one other Vav-Cre transgenic mouse model, Vav was also expressed in germ cells and endothelial cells (EC) [6]. Similarly, the Vav-iCre transgene used in our study was previously reported to express Vav in testis and ovaries [3]. But in contrast to the other Vav-Cre line, our Vav-iCre transgenic mice showed no reporter gene recombination in vascular EC. We also found no Vav-iCre activity in osteoblasts and MSC (Fig 5), alleviating concerns that Vav-iCre activity in vascular niche cells could indirectly impact early hematopoiesis in the BM. However, we observed an unexpected Vav-iCre mediated EYFP expression in a Lin-CD45-CD31-CD51-Sca-1- subset of bone cells. Although these cells have been observed elsewhere [22], their identity is unknown. Vav-iCre activity might suggest a hematopoietic origin, but their lack of hematopoietic markers (Fig 5) opens the possibility that these cells are non-hematopoietic, reminiscent of Vav-iCre expression in germ cells [3]. In the future, it will be important to determine whether these bone cells express endogenous Vav, and to elucidate their identity and function.
hCD2-iCre activity largely mirrors known patterns of CD2 mRNA expression with the notable exception of CLP, pro-B cells, ETP and DN2 cells which all express relatively little murine CD2 mRNA but display significant hCD2-iCre mediated EYFP expression. We speculate that this reflects a more efficient hCD2-iCre transgene expression combined with the high activity of the encoded improved Cre recombinase [3]. In any case, our data show that hCD2-iCre mediated gene recombination is essentially absent in HSC, MPP and myeloid progenitors, but initiates in CLP and affects early progenitors and all subsequent stages of the T cell, B cell and NK cell lineages, but no macrophages/monocytes and granulocytes. This recombination pattern strikingly resembles that of Il7r Cre, where iCre was knocked into the IL-7 receptor locus and induced flox-stop-flox ROSA26-YFP expression in CLP, pro-T and T cells, B cells, NK cells, ~10% of cDC and >90% of pDC but not in other myeloid cells [38]. Similar to the Il7r Cre R26-stop-YFP + mice, the EYFP+ CLP in hCD2-iCre +/- R26-stop-EYFP +/- mice contained a large fraction of EYFPlow cells. This suggests that these CLP have only recently undergone Cre-mediated gene recombination, consistent with an initiation of Cre activity in CLP but not earlier progenitors. Supporting this view, hCD2-iCre only partially excised a flox-stop-flox cassette from a TEL-AML1 fusion gene in CLP [39].
EYFP expression in CLP, ETP and all later stages of T cell development in both systems is consistent with a common lymphoid-primed origin for most T cells [13,38]. hCD2-iCre mediated EYFP expression in pro B cells and all later stages of B cell development, and in NKP, iNK and mNK moreover supports previously proposed common lymphoid origins of B cells and NK cells [14,40]. The existence of EYFP- ETP in hCD2-iCre +/- R26-stop-EYFP +/- mice could reflect insufficient transgene activation in the specific progenitors that gave rise to these cells, or the existence of distinct hCD2-activating and non-activating T cell progenitor subsets, reminiscent of the hypothesized origin of Il7r-reporter activating and non-activating pro T cells from distinct BM progenitors whose identity remains to be determined [38]. Maximal hCD2-iCre activity in NKP with progressive reduction in iNK and mNK might indicate partial Cre-inactivation and outgrowth of undeleting cells after the NKP stage, or simply EYFP loss during red blood cell lysis as seen in T cells [3]. In an alternative possibility, it will be interesting to study if the emergence of EYFP - mature NK cells in hCD2-iCre +/- R26-stop-EYFP +/- mice, which was also seen in another study [41], ascribes in vivo relevance to the ability of myeloid progenitors to produce NK cells in human in vitro systems [30].
One of our most interesting findings is that 98% of splenic pDC, and 20% of splenic cDC express EYFP in hCD2-iCre +/- R26-stop-EYFP +/- mice (Fig 2). The hematopoietic origin of DC has long been controversial, mainly due to the ability of both lymphoid and myeloid progenitors to generate DC in vitro and after transplantation into lymphopenic mice [17,42]. Recently, IL-7 fate mapping [38] and the identification of a CMP-derived macrophage/DC progenitor (MDP) [43] and a downstream common DC precursor (CDP) capable of producing both pDC and cDC [44–46] have suggested a primarily myeloid origin in particular for cDC. On the other hand, lymphoid progenitor contributions would be consistent with the ability of CLPs to generate pDC and cDC, with the expression of Rag1 and detection of D-J rearranged IgH genes in splenic pDC [16,47–49], and with cell-intrinsic requirements for IL-7 signaling for the development of subsets of splenic pDC and cDC [50]. The EYFP reporter expression pattern on hCD2-iCre +/- R26-stop-EYFP +/- DC again strikingly resembles that on Il7r Cre R26-stop-YFP + DC [38] and might support the view that under steady-state in vivo conditions, many pDC and a minor fraction of cDC may develop from lymphoid progenitors. Reminiscent of the situation in ETP, hCD2-iCre induced EYFP expression in pDC and cDC despite their low CD2 mRNA content (Fig 1A,1F and 1G) might possibly be explained by EYFP induction in upstream CLP (Fig 4). To further elucidate the origin of the EYFP expression in cDC from hCD2-iCre +/- R26-stop-EYFP +/- mice, it will be interesting to study if these cells emerge from EYFP+ CD11c+B220+CCR9- cDC progenitors [51]. These are included in our pDC gate, which contains 98% EYFP+ cells. In any case, our data are consistent with contributions of both myeloid and lymphoid progenitors to pDC and cDC development [17,42]. They indicate that contributions of altered DC function to any phenotypes of hCD2-iCre based conditional knockout mice need to be considered, in particular when studying T cells which are activated by antigens presented on DC.
Again similar to Il7r Cre R26-stop-YFP + mice [38], we found low-level (<5%) EYFP expression in hCD2-iCre +/- R26-stop-EYFP +/- macrophages and granulocytes (Fig 2) but not CMP, GMP and MEP (Fig 4). Although these EYFP+ myeloid cells are very rare, they could reflect the ability of CLP and T lineage precursors to generate myeloid cells under appropriate conditions, or the ability of certain neutrophil subsets to express lymphoid markers, discussed in detail elsewhere [38].
Altogether, our data confirm and expand the EYFP expression in developing and mature T cells, B cells and NK cells previously found in the same hCD2-iCre transgenic line [3,41] and the EYFP expression in developing and mature T cells and B cells in other CD2-Cre transgenics [3,12,13]. One study also reported mosaic EYFP expression in the testis of hCD2-iCre transgenic males but not in ovaries in females [3]. A hCD2 transgenic mouse line expressed hCD2 protein in T cells, but not in B cells [52]. In mice harboring multiple hCD2 transgene copies, immunohistochemistry suggested hCD2 protein expression in T cells, megakaryocytes, platelets and bone marrow cells of unknown identity with convoluted nuclei speculated to be myeloid precursors [52]. This contrasts with the lack of EYFP expression in myeloid progenitors and MEP in our mice (Fig 4) but could be consistent with the low level EYFP expression we found in macrophages and granulocytes (Fig 2), and with myeloid contributions to the EYFP+ DC in our mice (Fig 2).
The differences in transgene expression between different mouse lines in part result from differences in the precise Vav or hCD2 gene regions and supporting features included in each transgene. Moreover, during the generation of transgenic mice, the transgene integrates randomly and in variable copy numbers into the genome. Chromatin context affects transgene expression levels. During breeding, meiotic recombination might affect transgene structure and copy numbers. This, differential maternal/paternal effects and differences in genetic backgrounds such as the C57BL/6-J129 mixed background of the R26-stop-EYFP +/- mice can lead to variability in transgene expression levels between different mouse lines and even between individual mice of the same line [4]. These unavoidable limitations of transgenic mice likely explain the differences between our data and previously published data even using the same Cre-transgenic lines.
This notwithstanding, our data provide important novel insight into the Vav-iCre and hCD2-iCre transgene activity patterns in hematopoietic and non-hematopoietic cell populations. They suggest applications for these transgenes for conditional gene modulation in novel cell types, and provide a map of cell types whose extrinsic contributions to phenotypes of Vav-iCre or hCD2-iCre mediated gene modulation in a cell type of interest must be taken into account when interpreting results.
Finally, we propose that in addition to Il7r Cre R26-stop-YFP + mice [38], hCD2-iCre + R26-stop-EYFP + mice are an excellent genetic tool for lineage tracing studies of lymphoid cells in vivo that avoids the often non-physiological differentiation potential of CLP or other lymphoid precursors in vitro or upon engraftment into lymphopenic hosts [38].
Acknowledgments
We thank the TSRI vivarium for expert mouse care. This is TSRI manuscript no. 29028.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grants AI070845 and GM100785, NIH/NCATS grant UL1 TR000109, The Leukemia and Lymphoma Society Scholar Award 1440-11 to K.S., and Deutsche Forschungsgemeinschaft fellowship SI 1547/1-1 to S.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bouabe H, Okkenhaug K (2013) Gene Targeting in Mice: A Review In: Bailer SM, Lieber D, editors. Virus-Host Interactions: Humana Press; pp. 315–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abram CL, Roberge GL, Hu Y, Lowell CA (2014) Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. Journal of Immunological Methods 408: 89–100. 10.1016/j.jim.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Boer J, Williams A, Skavdis G, Harker N, Coles M, et al. (2003) Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European Journal of Immunology 33: 314–325. [DOI] [PubMed] [Google Scholar]
- 4. Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, et al. (2012) Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat Commun 3: 1218 10.1038/ncomms2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi L, Lin KK, Boles NC, Yang L, King KY, et al. (2012) Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell 11: 302–317. 10.1016/j.stem.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Georgiades P, Ogilvy S, Duval H, Licence DR, Charnock-Jones DS, et al. (2002) vavCre Transgenic mice: A tool for mutagenesis in hematopoietic and endothelial lineages. genesis 34: 251–256. [DOI] [PubMed] [Google Scholar]
- 7. Ogilvy S, Metcalf D, Gibson L, Bath ML, Harris AW, et al. (1999) Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood 94: 1855–1863. [PubMed] [Google Scholar]
- 8. Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, et al. (2012) Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 11: 415–428. 10.1016/j.stem.2012.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, et al. (2011) The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 208: 455–467. 10.1084/jem.20101145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trumpp A, Essers M, Wilson A (2010) Awakening dormant haematopoietic stem cells. Nat Rev Immunol 10: 201–209. 10.1038/nri2726 [DOI] [PubMed] [Google Scholar]
- 11. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson TJ, Cowdery HE, Xu D, Kola I, Hertzog PJ (2002) A human CD2 minigene directs CRE-mediated recombination in T cells in vivo. genesis 33: 181–184. [DOI] [PubMed] [Google Scholar]
- 13. Shi J, Petrie HT (2012) Activation kinetics and off-target effects of thymus-initiated cre transgenes. PLoS One 7: e46590 10.1371/journal.pone.0046590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, et al. (2011) Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood 118: 5439–5447. 10.1182/blood-2011-04-348912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satpathy AT, Murphy KM, Kc W (2011) Transcription factor networks in dendritic cell development. Semin Immunol 23: 388–397. 10.1016/j.smim.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sathe P, Vremec D, Wu L, Corcoran L, Shortman K (2013) Convergent differentiation: myeloid and lymphoid pathways to murine plasmacytoid dendritic cells. Blood 121: 11–19. 10.1182/blood-2012-02-413336 [DOI] [PubMed] [Google Scholar]
- 17. Moore AJ, Anderson MK (2013) Dendritic cell development: a choose-your-own-adventure story. Adv Hematol 2013: 949513 10.1155/2013/949513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carotta S, Pang SH, Nutt SL, Belz GT (2011) Identification of the earliest NK-cell precursor in the mouse BM. Blood 117: 5449–5452. 10.1182/blood-2010-11-318956 [DOI] [PubMed] [Google Scholar]
- 19. Barouch-Bentov R, Che J, Lee CC, Yang Y, Herman A, et al. (2009) A Conserved Salt Bridge in the G Loop of Multiple Protein Kinases Is Important for Catalysis and for In Vivo Lyn Function. Molecular Cell 33: 43–52. 10.1016/j.molcel.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, et al. (2007) Positive regulation of Itk PH domain function by soluble IP4. Science 316: 886–889. [DOI] [PubMed] [Google Scholar]
- 21. Sauer K, Park E, Siegemund S, French AR, Wahle JA, et al. (2013) Inositol tetrakisphosphate limits NK cell effector functions by controlling PI3K signaling. Blood 121: 286–297. 10.1182/blood-2012-05-429241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arcangeli ML, Frontera V, Bardin F, Obrados E, Adams S, et al. (2011) JAM-B regulates maintenance of hematopoietic stem cells in the bone marrow. Blood 118: 4609–4619. 10.1182/blood-2010-12-323972 [DOI] [PubMed] [Google Scholar]
- 23. Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, et al. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135: 1118–1129. 10.1016/j.cell.2008.10.048 [DOI] [PubMed] [Google Scholar]
- 24. Jia Y, Loison F, Hattori H, Li Y, Erneux C, et al. (2008) Inositol trisphosphate 3-kinase B (InsP3KB) as a physiological modulator of myelopoiesis. Proc Natl Acad Sci U S A 105: 4739–4744. 10.1073/pnas.0800218105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serwold T, Ehrlich LI, Weissman IL (2009) Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood 113: 807–815. 10.1182/blood-2008-08-173682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller AT, Sandberg M, Huang YH, Young M, Sutton S, et al. (2007) Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol 8: 514–521. [DOI] [PubMed] [Google Scholar]
- 27. Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, et al. (2004) Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity 20: 735–745. [DOI] [PubMed] [Google Scholar]
- 28. Das R, Sant'Angelo DB, Nichols KE (2010) Transcriptional control of invariant NKT cell development. Immunol Rev 238: 195–215. 10.1111/j.1600-065X.2010.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cichocki F, Sitnicka E, Bryceson YT (2014) NK cell development and function—Plasticity and redundancy unleashed. Semin Immunol 26: 114–126. 10.1016/j.smim.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 30. Grzywacz B, Kataria N, Kataria N, Blazar BR, Miller JS, et al. (2011) Natural killer-cell differentiation by myeloid progenitors. Blood 117: 3548–3558. 10.1182/blood-2010-04-281394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrie HT, Zuniga-Pflucker JC (2007) Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol 25: 649–679. [DOI] [PubMed] [Google Scholar]
- 32. Michie AM, Zuniga-Pflucker JC (2002) Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol 14: 311–323. [DOI] [PubMed] [Google Scholar]
- 33. Xiong J, Armato MA, Yankee TM (2011) Immature single-positive CD8+ thymocytes represent the transition from Notch-dependent to Notch-independent T-cell development. Int Immunol 23: 55–64. 10.1093/intimm/dxq457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hardy RR, Kincade PW, Dorshkind K (2007) The protean nature of cells in the B lymphocyte lineage. Immunity 26: 703–714. [DOI] [PubMed] [Google Scholar]
- 35. Morrison SJ, Weissman IL (1994) The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1: 661–673. [DOI] [PubMed] [Google Scholar]
- 36. Pietras EM, Warr MR, Passegue E (2011) Cell cycle regulation in hematopoietic stem cells. J Cell Biol 195: 709–720. 10.1083/jcb.201102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ceredig R (2012) Fates and potentials of thymus-seeding progenitors. Nat Immunol 13: 309–310. 10.1038/ni.2265 [DOI] [PubMed] [Google Scholar]
- 38. Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, et al. (2010) Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32: 426–436. 10.1016/j.immuni.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 39. Schindler JW, Van Buren D, Foudi A, Krejci O, Qin J, et al. (2009) TEL-AML1 corrupts hematopoietic stem cells to persist in the bone marrow and initiate leukemia. Cell Stem Cell 5: 43–53. 10.1016/j.stem.2009.04.019 [DOI] [PubMed] [Google Scholar]
- 40.Carotta S, Pang SHM, Nutt SL, Belz GT (2011) Identification of the earliest NK cell precursor in the mouse bone marrow. Blood. [DOI] [PubMed]
- 41. Sullivan RP, Leong JW, Schneider SE, Keppel CR, Germino E, et al. (2012) MicroRNA-deficient NK cells exhibit decreased survival but enhanced function. J Immunol 188: 3019–3030. 10.4049/jimmunol.1102294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shortman K, Sathe P, Vremec D, Naik S, O'Keeffe M (2013) Plasmacytoid dendritic cell development. Adv Immunol 120: 105–126. 10.1016/B978-0-12-417028-5.00004-1 [DOI] [PubMed] [Google Scholar]
- 43. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, et al. (2006) A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311: 83–87. [DOI] [PubMed] [Google Scholar]
- 44. Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, et al. (2007) Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 8: 1207–1216. [DOI] [PubMed] [Google Scholar]
- 45. Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, et al. (2007) Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8: 1217–1226. [DOI] [PubMed] [Google Scholar]
- 46. Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, et al. (2009) In vivo analysis of dendritic cell development and homeostasis. Science 324: 392–397. 10.1126/science.1170540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shortman K, Naik SH (2007) Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 7: 19–30. [DOI] [PubMed] [Google Scholar]
- 48. Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, et al. (2005) Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood 105: 4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, et al. (2003) The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol 170: 4926–4932. [DOI] [PubMed] [Google Scholar]
- 50. Vogt TK, Link A, Perrin J, Finke D, Luther SA (2009) Novel function for interleukin-7 in dendritic cell development. Blood 113: 3961–3968. 10.1182/blood-2008-08-176321 [DOI] [PubMed] [Google Scholar]
- 51. Segura E, Wong J, Villadangos JA (2009) Cutting edge: B220+CCR9- dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol 183: 1514–1517. 10.4049/jimmunol.0901524 [DOI] [PubMed] [Google Scholar]
- 52. Lang G, Wotton D, Owen MJ, Sewell WA, Brown MH, et al. (1988) The structure of the human CD2 gene and its expression in transgenic mice. EMBO J 7: 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heng TS, Painter MW, Immunological Genome Project C (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9: 1091–1094. 10.1038/ni1008-1091 [DOI] [PubMed] [Google Scholar]
- 54. Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, et al. (2008) Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res 4: 5 10.1186/1745-7580-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu C, Orozco C, Boyer J, Leglise M, Goodale J, et al. (2009) BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130 10.1186/gb-2009-10-11-r130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.