Abstract
Introduction
The number of patients requiring dialysis continues to increase worldwide imposing a substantial social and economic burden on patients, their families and healthcare systems. Compared with facility-based dialysis, dialysis performed by the patient at home is associated with higher quality of life, freedom, survival and reduced healthcare costs. International guidelines recommend suitable patients are offered a choice of dialysis modality, including home-based dialysis. Predialysis education and offering patients choice increase home dialysis uptake, yet the factors that patients and families are willing to trade off in making decisions about dialysis location are not well understood. The Home First study will explore patients’ and caregivers’ beliefs, attitudes and preferences regarding dialysis education and decision-making with regards to dialysis options; to identify key attributes which influence their decision-making, and to quantify the relative value of these attributes.
Methods and analysis
This study will use a mixed-methods approach to describe patient and caregiver preferences and views about the factors that influence their choice of home or facility-based dialysis. Face-to-face, semistructured interviews will be conducted with 30–40 patients and 10–15 caregivers. Thematic analysis of interview transcripts will be conducted. Additional to providing information on the perspectives and experiences of patients and caregivers, these analyses will also inform the design of discrete choice experiments (DCEs). We will undertake DCEs with approximately 150 patients and 150 caregivers to quantify preferences for home and facility dialysis.
Ethics and dissemination
The Hawke's Bay, Counties Manukau, and Capital Coast District Health Board Research Ethics Committees approved the study. Findings will be presented in national/international conferences and peer-reviewed journals. Dissemination to patients will take the form of presentations, newsletters and reports to support and community groups. Reports will be disseminated to funders and participating renal units and to the New Zealand Ministry of Health.
Trial registration number
ACTRN12615000314527.
Keywords: QUALITATIVE RESEARCH
Strengths and limitations of this study.
The inclusion of qualitative interviews to inform the attributes for the discrete choice experiments.
Addresses two of the top 10 research uncertainties of patients.
Explores predialysis patients perspectives which are previously not well researched.
Explores financial and cultural influences on patient and caregivers dialysis modality decision-making.
It will be conducted in one country, New Zealand. While directly relevant to inform policy and practice in this context, it is possible that patients experiences with, and preferences for, home dialysis may vary across countries.
Introduction
Chronic kidney disease (CKD) is a growing public health problem that affects 10–15% of the adult population. The number of patients on dialysis is increasing internationally by approximately 7% per annum.1 Patients on dialysis have substantially reduced survival and quality of life compared with the general population, with a 5-year mortality risk similar to some advanced cancers2 and health-related quality of life (utilities) reported between 0.39 and 0.76.3 Although renal replacement therapy only affects between 0.06 and 0.19% of the population in the UK, Europe, USA and Australasia,4–7 dialysis treatment accounts for at least 1% of the health expenditure in these countries.8–11
Home-based dialysis, with either peritoneal dialysis (PD) or haemodialysis (HD), is associated with substantially lower mortality, better quality of life and reduced costs compared to facility dialysis.12–14 These potential benefits are reflected in various international guidelines that recommend home-based dialysis as a preferred option for suitable patients.15 16 Despite this, the prevalence of home dialysis remains lower than expected in many parts of the world, ranging from 9% in the USA, to 18% in the UK, and as compared with 51.4% in New Zealand.4 Moreover, health systems with historically higher rates of home dialysis, such as New Zealand and Australia, have noted a decline in the rates of home dialysis over the last 15 years.17 18
While predialysis patient education is associated with the increased uptake of home dialysis,19–27 little is known about factors that drive patient choice of dialysis location (home or facility) despite enhanced communication between patients and providers and dialysis modality options being identified in the top 10 research uncertainties.28 Moreover, there are few studies29 30 that have incorporated the perspectives of patients’ who are in the predialysis phase, and no studies have explored the financial or cultural factors that influence patients’ and caregivers’ decisions.
The Home First study aims to assess patients and caregivers’ beliefs, attitudes and preferences when making decisions about dialysis options; identify key attributes which influence their decision-making and preferences for dialysis options; and quantify the relative values and trade-offs between these attributes.
Methods and analysis
Overview of approach and methods
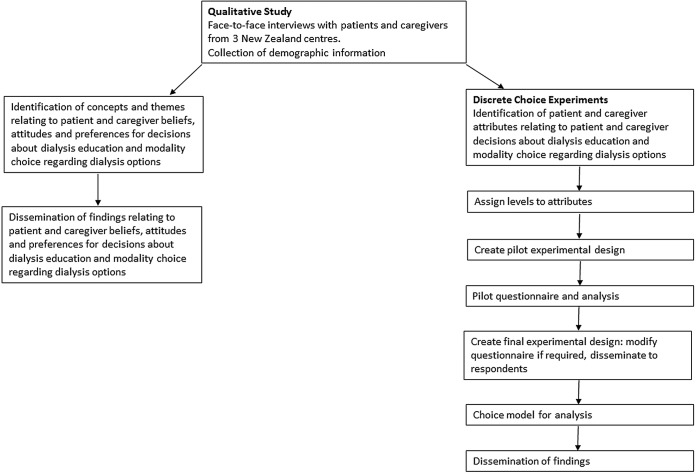
The Home First study will use a mixed-methods approach to explore patient and caregiver preferences and views on factors that influence their choice of dialysis modality, between home HD and PD, or facility-based hospital and satellite dialysis. Face-to-face, semistructured interviews will be conducted to identify and describe these preferences and attributes. These data will subsequently be used to inform the design of the discrete choice experiments (DCEs). Qualitative interviews are an effective method to identify attributes for DCEs and in doing so can improve the face validity of the DCEs.31 The DCEs will then be used to quantitatively assess patient preferences for characteristics of home and facility dialysis, and to estimate the trade-offs between characteristics that patients and caregivers are willing to accept.
DCEs utilise an attribute-based measure of benefit, based on the idea that healthcare interventions or services can be described by their attributes, or characteristics32 and that an individuals’ value of the described intervention or service depends on the levels of these attributes. DCEs are commonly used in health as a means of quantifying patient and consumer preferences for healthcare policies and programmes.31–33 In this method, the levels of attributes are varied systematically in a series of questions, respondents then choose their preferred option from two or more alternatives for each question. Respondents are assumed to choose the option of highest preference, or which has the highest ‘value’, with choices revealing an underlying utility function. This methodology is underpinned by random utility theory, consumer theory, experimental design theory and econometric analysis.31
From the choices made by the respondents within the DCEs, a mathematical function is estimated to numerically describe the value respondents attach to different choice options. Additional data collected in the survey, including sociodemographic information and dialysis modality may also enter the value functions as explanatory variables. DCEs provide rich data to determine the attributes which drive patient preferences, the trade-offs between attributes that people are willing to accept, and how changes in these attributes can lead to potential changes in preferences, which can subsequently inform service delivery and policy development. The study will follow the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Guidelines for Good Research Practices for conjoint analysis in health.33 Our study will have three stages: qualitative interviews to inform attribute development; design of the DCE survey and final survey administration, described in more detail below.
The data collection for qualitative interviews started in July 2014 and is expected to be complete by March 2015. Data collection for the DCE surveys is anticipated to take place from March until end of July 2015 with all analysis completed by June 2016 (figure 1).
Figure 1.
Study schema.
Stage 1: Qualitative interviews
Participants and recruitment
Patients and caregivers treated by one of at least three dialysis centres will be contacted through Counties Manukau, Hawke's Bay, and Capital Coast District Health Boards in northern New Zealand. These centres were chosen as they offer the complete range of dialysis modalities, and to provide services to patients who are reasonably representative of the broader NZ dialysis population. Participants will be purposively sampled to include a diverse range of demographic (age, sex, ethnicity, geographical remoteness) and clinical characteristics (CKD stage, dialysis modality—predialysis, home HD, hospital HD, PD). Qualitative interviews will be conducted until data saturation is achieved. Prior experience suggests this will be at approximately the following participant numbers: patients—predialysis/non-dialysis dependent (n=10–15); patients—home dialysis (HD and PD) (n=10–15); patients—hospital HD (n=10–15); family caregivers (n=10–15).
Data collection
Face-to-face semistructured interviews will be conducted with patients and caregivers to explore perceptions, beliefs and attitudes which influence decision-making about dialysis modality and location. Interviews will be conducted by the first author (RCW) who is a nephrology nurse practitioner with training and experience in qualitative research interviewing. Interviews will take place at the participants’ preferred location (home or clinic). The semistructured interview guide is based on current literature and discussion among the research team which includes nephrologists, qualitative research specialists and health economists. Prior to the interview, sociodemographic and clinical characteristics will be collected, including: age, sex and employment status (full-time, part-time, unemployed, beneficiary, retired), annual household income, current stage of CKD, length of time on dialysis, access formation and geographic remoteness measured in distance from home to the renal unit. Each interview will collect and explore data in three phases: (1) perceptions and views of dialysis education; (2) decision-making about dialysis modality and location; and (3) perceptions of financial and cultural influences on patient choice. All interviews will be digitally audiorecorded and transcribed verbatim.
Qualitative analysis
Transcripts will be entered into HyperRESEARCH (ResearchWare Inc, USA, V.2.8.3), by RCW. We will draw on grounded theory and thematic analysis to code transcripts line by line.34 35 RCW and AT will then independently identify concepts inductively and group similar concepts relating to patient and caregiver perceptions, beliefs and expectations of dialysis modality and location. The study group will then review and refine the coding scheme through a series of discussions to develop themes that capture the full range the depth of patient and caregiver concepts identified in interviews (investigator triangulation).
We will also use the data from the qualitative interviews to inform the development of attributes for inclusion in the DCE. The attributes will be initially developed by RCW and then discussed and refined by the research group.
Stage 2: Design of discrete choice surveys
After the identification of attributes informed by the qualitative interviews in stage 1, we will create a statistically efficient survey design for the DCEs.36 An efficient design method improves on more traditional orthogonal designs in terms of the overall reliability of the parameter estimates, provided that the design is generated with prior information (eg, parameter estimates available in the literature from similar studies, or parameter estimates from pilot studies). Using this design it is possible to generate statistically efficient designs that require significantly smaller sample sizes to obtain reliable parameter estimates.36
Initial designs using parameter estimates from current literature, where available, will be piloted face to face in a sample of 20 respondents, and preliminary models estimated. Parameter estimates from the preliminary models will be used to generate the final efficient designs for the final discrete choice study. Pilot surveys will also collect information on participant understanding of attributes, as well as sociodemographic characteristics, such as age, sex, employment status, annual household income, stage of CKD, English as a first language, family member on dialysis and distance from home to the renal unit. Quality of life using the KDQOL-36,37 EQ-5D-5L38 and self-reported health literacy, Short Test of Functional Health Literacy in Adults (STOHFLA)39 questionnaires will be collected for each respondent. Sociodemographic characteristics and quality of life values may be included as explanatory variables in the analysis of preferences for patients and caregivers.
Stage 3: Administration of DCE survey
Participants and recruitment
The DCE surveys will be administered using either a web-based survey or a paper survey with quota sampling based on dialysis modality, age, sex and ethnicity to recruit a respondent sample broadly representative of the New Zealand dialysis population. This sampling method will enable exploration of interactions between attributes and sociodemographic factors, and present subgroup analyses within the sample size.
Respondents will be either patients or caregivers recruited from the three dialysis centres included in the study. Eligible patients and caregivers will be invited to participate by the principal researcher (RCW), a nephrologist or the renal unit's CKD coordinator. Participants will receive written information regarding the voluntary nature of the study. Participants will be informed that completing questionnaires implies consent; they will be given the choice of completing their survey online or via pen and paper. Participants will return the completed surveys in a self-addressed prepaid envelope or submit their responses online.
Sample size
The final sample size required for a DCE is based on the characteristics of the design itself and includes the following factors: the number of attributes included; the attribute level range; the number of choice scenarios; the number of alternatives in each choice set; and the size and direction of prior parameters. Respondents will be presented with multiple scenarios and asked to choose between two or more dialysis options which vary across a range of attributes. Possible attributes for DCEs based on previous DCEs in dialysis patients include life expectancy, ability to travel, availability of subsidised transport.29 Based on previous DCEs within this field29 40 41 we anticipate a sample size of approximately 100–150 patients and approximately the same number of family caregivers.
Data analysis
The results from this survey will inform health policy by highlighting the factors likely to influence an individual's decision to choose home dialysis and the factors that the patients and their families perceive as most important in their decision-making process. We will use a mixed multinomial logit (MMNL) model using a panel specification to allow for non-independence of observations provided by the same respondent. MMNL models are preferred over MNL models, to better explain choice behaviour.42 In MNL choice models, parameters associated with each attribute are treated as fixed. These fixed values are the average (or point estimates) associated with a population level distribution and other information in the distribution is not considered. A MMNL, however, allows for consideration of the full distribution of a parameter estimate with each individual having an associated parameter estimate on that specified distribution. While the exact location of each individual's preferences on the distribution may not be known, estimates of ‘individual-specific preferences’ can be accommodated by deriving the individual's conditional distribution, based—within sample—on their choices (ie, prior knowledge).31 In addition other model specifications such as the generalised MNL43 and latent class model44 45 specifications will be explored.
Interactions between attributes in the discrete choice surveys, and between attributes and population characteristics will be explored in the analysis for patients and caregivers.
Model results will be expressed as parameter estimates (β), or ORs and their 95% CIs and p values for the odds of choosing one option instead of another. Trade-offs (ie, marginal rates of substitution) between attributes will also be calculated.
Ethics and dissemination
Ethical considerations
Confidentiality and anonymity of the data will be strictly maintained. Digital recording of the interviews will only take place after written informed consent is obtained from participants. Participants will not be identifiable in any transcripts, or in any publications. It will be made clear to all participants that they have the right to withdraw from the research at any point in time.
Informed consent for online surveys will be obtained by providing information for participants on the opening page of the survey which will clearly state that participation is voluntary and there is a right to withdraw participation at any stage, without reason. Participants will be advised that answers will be confidential. They will then be given a brief description of the study (with contact details of the lead researcher should they have any questions) and asked to indicate their consent to participate by ticking a box and continuing through the survey. Those completing the written survey will receive the information sheet and sign the consent form prior to completing the survey.
Dissemination
Results will be published in internal reports, peer-reviewed scientific journals and via national and international conference presentations. Results will be disseminated to nephrology clinicians through national meetings so that findings can inform practice changes in a timely manner. Dissemination to patients will be in the form of presentations and reports to patient support networks and groups such as Kidney Health New Zealand and the Auckland Kidney Society. Reports will also be disseminated to funding institutions and participating renal units.
The findings of the study will be used to inform improvements to home dialysis service delivery for payers, policymakers, providers and patients.
Footnotes
Contributors: RCW, AT, RLM, MRM and KH were responsible for the conceptual design of the study. RCW, AT, RLM, MRM, SP and KH were involved in the experimental design of the DCE. RCW drafted the manuscript; all authors revised and approved the final manuscript.
Competing interests: MRM is a consultant of Baxter Healthcare, and has previously been a consultant for Fresenius Medical Care (Asia Pacific).
Ethics approval: The Home First study protocol has been reviewed and approved by the Counties Manukau Institutional Review Board (Ref: 1771), Hawke's Bay Institutional Review Board (Ref: 14/06/160) and Capital Coast Institutional Review Board (Ref: CCDHB13/07/14). The Home First study protocol has also been reviewed and received funding from the New Zealand Lotteries Health Research Fund (Ref: 326750), and Baxter Clinical Evidence Council research programme.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Grassmann A, Gioberge S, Moeller S et al. . ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 2005;20:2587–93. 10.1093/ndt/gfi159 [DOI] [PubMed] [Google Scholar]
- 2.Nordio M, Limido A, Maggiore U et al. . Survival in patients treated by long-term dialysis compared with the general population. Am J Kidney Dis 2012;59:819–28. 10.1053/j.ajkd.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 3.Dale PL, Hutton J, Elgazzar H. Utility of health states in chronic kidney disease: a structured review of the literature. Curr Med Res Opin 2008;24:193–206. 10.1185/030079908X253410 [DOI] [PubMed] [Google Scholar]
- 4.USRDS Annual Data Report: International Comparisons. 2013. http://www.ajkd.org/issue/S0272–6386(13)X0014–9 (accessed 22 Jul 2014).
- 5.McDonald S, Clayton P, Hurst K. The Australia and New Zealand Dialysis and Transplant Registry. 35th Annual Report Adelaide, Australia, 2012. [Google Scholar]
- 6.Registry: E-E. ERA-EDTA Registry Annual Report 2012 Amsterdam, The Netherlands: Academic Medical Center, Department of Medical Informatics, 2014. [Google Scholar]
- 7.Gilg JRA, Fogarty D. UK renal registry 16th annual report: chapter 1 UK renal replacement therapy incidence in 2012: national and centre-specific analyses. Nephron Clin Pract 2013;125:1–28. 10.1159/000360020 [DOI] [PubMed] [Google Scholar]
- 8.Costs of ESRD. U.S. Renal Data System 2013 Report. http://www.usrds.org/2013/pdf/v2_ch11_13.pdf (accessed 22 Jul 2014).
- 9.Ashton T, Marshall MR. The organization and financing of dialysis and kidney transplantation services in New Zealand. Int J Health Care Finance Econ 2007;7:233–52. 10.1007/s10754-007-9023-x [DOI] [PubMed] [Google Scholar]
- 10.Cass A. The Economic Impact of End-stage Kidney Disease in Australia: Projections to 2020. Report Sydney, Australia: The George Institute for Global Health, 2010. [Google Scholar]
- 11.Manns BJ, Mendelssohn DC, Taub KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ 2007;7:149–69. 10.1007/s10754-007-9022-y [DOI] [PubMed] [Google Scholar]
- 12.Marshall MR, Hawley CM, Kerr PG et al. . Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2011;58:782–93. 10.1053/j.ajkd.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 13.Walker R, Marshall M, Morton RL et al. . The cost effectiveness of contemporary home haemodialysis modalities compared to facility haemodialysis: a systematic review of full economic evaluations. Nephrology 2014;19:459–70. 10.1111/nep.12269 [DOI] [PubMed] [Google Scholar]
- 14.Wyld M, Morton RL, Hayen A et al. . A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 2012;9:e1001307 10.1371/journal.pmed.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NICE guideline TA48—Guidance on home compared with hospital haemodialysis for patients with end-stage renal failure 2002. (5 August 2012). http://www.nice.org.uk/nicemedia/live/11472/32449/32449.pdf
- 16.Stanley M, CARI. The CARI guidelines. Peritoneal dialysis versus haemodialysis (adult). Nephrology (Carlton) 2010;15(Suppl 1):S24–31. 10.1111/j.1440-1797.2010.01228.x [DOI] [PubMed] [Google Scholar]
- 17.Briggs N, Hurst K, McDonald SP. Chapter 4: methods and location of dialysis. In: Hurst K, McDonald SP, eds. The 35th annual ANZDATA report 2012. Adelaide, South Australia: ANZDATA Registry, 2013:1–10.. [Google Scholar]
- 18.Jain AK, Blake P, Cordy P et al. . Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012;23:533–44. 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Buxo JA. Early referral and selection of peritoneal dialysis as a treatment modality. Nephrol Dial Transplant 2000;15:147–9. 10.1093/ndt/15.2.147 [DOI] [PubMed] [Google Scholar]
- 20.Elizabeth JL, Hanna L, Walker D et al. . Pre-dialysis education and patient choice. J Ren Care 2006;32:214–20. 10.1111/j.1755-6686.2006.tb00026.x [DOI] [PubMed] [Google Scholar]
- 21.Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005;20:1842–7. 10.1093/ndt/gfh905 [DOI] [PubMed] [Google Scholar]
- 22.Goovaerts T, Jadoul M, Goffin E. Influence of a predialysis education program on the choice of renal replacement therapy. Am J Kidney Dis 2012;60:499 10.1053/j.ajkd.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 23.Key SM. Optimizing dialysis modality choices around the world: a review of literature concerning the role of enhanced early pre-ESRD education in choice of renal replacement therapy modality. Nephrol Nurs J 2008;35:387–94; quiz 95. [PubMed] [Google Scholar]
- 24.Little J, Irwin A, Marshall T et al. . Predicting a patient's choice of dialysis modality: experience in a United Kingdom renal department. Am J Kidney Dis 2001;37:981–6. 10.1016/S0272-6386(05)80014-9 [DOI] [PubMed] [Google Scholar]
- 25.Manns BJ, Taub K, Vanderstraeten C et al. . The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005;68:1777–83. 10.1111/j.1523-1755.2005.00594.x [DOI] [PubMed] [Google Scholar]
- 26.Mehrotra R, Marsh D, Vonesh E et al. . Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005;68:378–90. 10.1111/j.1523-1755.2005.00453.x [DOI] [PubMed] [Google Scholar]
- 27.Morton RL, Snelling P, Webster AC et al. . Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 2012;184:E277–83. 10.1503/cmaj.111355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manns B, Hemmelgarn B, Lillie E et al. . Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014;9: 1813–21. 10.2215/CJN.01610214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton RL, Snelling P, Webster AC et al. . Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012;60:102–11. 10.1053/j.ajkd.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 30.Tong A, Palmer S, Manns B et al. . The beliefs and expectations of patients and caregivers about home haemodialysis: an interview study. BMJ Open 2013;3:e002148 10.1136/bmjopen-2012-002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145–72. 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- 32.Lancaster JK. A new approach to a consumer theory. J Political Econ 1966;74:132–57. 10.1086/259131 [DOI] [Google Scholar]
- 33.Bridges JF, Hauber AB, Marshall D et al. . Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value health 2011;14:403–13. 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Liamputtong P. Qualitative research methods. Melbourne, Australia: Oxford Univeristy Press, 2009. [Google Scholar]
- 35.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006;27:237–46. 10.1177/1098214005283748 [DOI] [Google Scholar]
- 36.Rose J, Bliemer MJ. Sample size requirements for stated choice experiments. Transportation 2013;40:1021–41. 10.1007/s11116-013-9451-z [DOI] [Google Scholar]
- 37.Kalantar-Zadeh K, Unruh M. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol 2005;37:367–78. 10.1007/s11255-004-0012-4 [DOI] [PubMed] [Google Scholar]
- 38.Herdman M, Gudex C, Lloyd A et al. . Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med 2004;36:588–94. [PubMed] [Google Scholar]
- 40.Davison SN, Kromm SK, Currie GR. Patient and health professional preferences for organ allocation and procurement, end-of-life care and organization of care for patients with chronic kidney disease using a discrete choice experiment. Nephrol Dial Transplant 2010;25:2334–41. 10.1093/ndt/gfq072 [DOI] [PubMed] [Google Scholar]
- 41.Halpern SD, Berns JS, Israni AK. Willingness of patients to switch from conventional to daily hemodialysis: looking before we leap. Am J Med 2004;116:606–12. 10.1016/j.amjmed.2003.12.025 [DOI] [PubMed] [Google Scholar]
- 42.Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. Cambridge: Cambridge University Press, 2005. [Google Scholar]
- 43.Fiebig DG, Keane MP, Louviere J et al. . The generalized multinomial logit model: accounting for scale and coefficient heterogeneity. Marketing Sci 2010;29:393–421. 10.1287/mksc.1090.0508 [DOI] [Google Scholar]
- 44.de Bekker-Grob EW, Rose JM, Donkers B et al. . Men's preferences for prostate cancer screening: a discrete choice experiment. Br J Cancer 2013;108:533–41. 10.1038/bjc.2013.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swait J. A structural equation model of latent segmentation and product choice for cross-sectional revealed preference choice data. J Retailing Consumer Serv 1994;1:77–89. 10.1016/0969-6989(94)90002-7 [DOI] [Google Scholar]