Abstract
Objective
Whether areas affected by Q fever during a large outbreak (2008–2010) had higher rates of adverse pregnancy outcomes than areas not affected by Q fever.
Design
Nationwide registry-based ecological study.
Setting
Pregnant women in areas affected and not affected by Q fever in the Netherlands, 2003–2004 and 2008–2010.
Participants
Index group (N=58 737): pregnant women in 307 areas with more than two Q fever notifications. Reference group (N=310 635): pregnant women in 921 areas without Q fever notifications. As a baseline, pregnant women in index and reference areas in the years 2003–2004 were also included in the reference group to estimate the effect of Q fever in 2008–2010, and not the already existing differences before the outbreak.
Main outcome measures
Preterm delivery, small for gestational age, perinatal mortality.
Results
In 2008–2010, there was no association between residing in a Q fever-affected area and both preterm delivery (adjusted OR 1.01 (95% CI 0.94 to 1.08)), and perinatal mortality (adjusted OR 0.87 (95% CI 0.72 to 1.05)). In contrast, we found a weak significant association between residing in a Q fever-affected area in 2008–2010 and small for gestational age (adjusted OR 1.06 (95% CI 1.01 to 1.12)), with a population-attributable fraction of 0.70% (95% CI 0.07% to 1.34%). We observed no dose–response relation for this outcome with increasing Q fever notifications, and we did not find a stronger association for women who were in their first trimester of pregnancy during the months of high human Q fever incidence.
Conclusions
This study found a weak association between residing in a Q fever-affected area and the pregnancy outcome small for gestational age. Early detection of infection would require mass screening of pregnant women; this does not seem to be justified considering these results, and the uncertainties about its efficacy and the adverse effects of antibiotic treatment.
Keywords: Epidemiology < INFECTIOUS DISEASES, Public health < INFECTIOUS DISEASES, OBSTETRICS
Strengths and limitations of this study.
The registry-based approach with nationwide coverage of Q fever notifications and pregnancies allowed for accurate estimation of regional differences in Q fever incidence and adverse pregnancy outcome.
By using a multivariable model with an interaction term, we were able to estimate the effect of residing in a Q fever-affected area in 2008–2010, and not the already existing differences before the outbreak.
The classification ‘Q fever-affected area’ or ‘area not affected by Q fever’ was based on notifications of acute Q fever. Such notification requires a positive laboratory result indicating a recent Coxiella burnetii infection with a matching clinical presentation (fever, pneumonia or hepatitis). Cases could be over-reported because laboratory criteria cannot always discriminate between acute or past resolved infection because of long-lasting persistence of IgM antibodies and aspecific clinical symptoms.
The notifications of acute Q fever could also have led to under-reporting, as people with illness might not seek medical care or the attending physician might not request microbiological tests.
Some misclassification might have occurred, as people might have acquired the infection in a different postal code area as in which they live.
Introduction
Q fever, caused by Coxiella burnetii, is a worldwide occurring zoonosis, with goats and sheep as primary sources of human infections.1 Infected goat and sheep herds can have high abortion rates, with massive contamination of the environment from infectious birth products.2 The Netherlands faced the world's largest reported outbreak of Q fever, starting in 2007 and reaching a peak in 2009.3 There are indications that the increase in acute Q fever had already started before 2007.4
A number of case descriptions and case series reports of pregnant women have documented that untreated acute or chronic C. burnetii infections may result in adverse pregnancy outcome in up to 81% of the cases.5 The risk of adverse events on the fetus is highest when infection occurs during first trimester.5 In addition, reactivation of a latent C. burnetii infection might also cause an adverse pregnancy outcome.6 These adverse outcomes include spontaneous abortion, perinatal death, preterm delivery, and low birth weight.5 7–11 These reports were supported by community-based studies among pregnant women in Canada and Spain in whom serological titres consistent with an acute or recent Q fever infection were found to have a twofold higher risk for poor obstetrical outcomes.6 12 However, studies in France, the Netherlands and Denmark found no evidence of adverse pregnancy outcome among women with a serological indication of a C. burnetii infection.13–15 Moreover, a prospective controlled clinical trial conducted during the outbreak in the Netherlands showed no benefits of screening for antibodies against C. burnetii during pregnancy.16
The inconsistent findings from studies analysing the risks of potentially serious obstetric complications and the lack of an accurate algorithm to identify pregnancies at risk preclude the implementation of evidence-based preventive public health measures in case of increased exposure. Therefore, during the Dutch outbreak, large-scale preventive screening was not implemented. The aim of this study was to assess whether Q fever-affected areas had higher rates of adverse pregnancy outcome than areas not affected by Q fever and thus, to evaluate the policy of not implementing large-scale screening among pregnant women during the outbreak.
Methods
Study design and setting
This was a nationwide registry-based ecological study. Data on Q fever incidence and pregnancy outcome were obtained for the 3 years with the highest Q fever incidence in the Netherlands (2008–2010). In addition, data were obtained for the years 2003–2004, which preceded this large outbreak.
Defining areas affected and not affected by Q fever in 2008–2010
As in most other European countries, acute Q fever is a notifiable disease in the Netherlands. For notification, requirements include a positive laboratory result indicating a recent C. burnetii infection and a matching clinical presentation of fever, pneumonia or hepatitis. The laboratory criteria were a fourfold IgG titre rise or more measured by immunofluorescence assay, ELISA or complement fixation test, a positive IgM phase II antibody test or detection by PCR of C. burnetii DNA in blood or respiratory material. These data as well as patient age, gender, and four-digit postal-code area are recorded in the national infectious diseases database.3
For this study, postal-code areas were divided into those without notifications of Q fever and those with two or more notifications (Q fever-affected areas) in one of the outbreak years 2008–2010. Population numbers for the year 2009 were used to calculate Q fever incidence for Q fever-affected areas. The Q fever-affected areas were subdivided into four quartiles: <4.59, 4.59–10.61, 10.62–21.50 and ≥21.51 notifications per 10 000 inhabitants. Areas with only one notification in a year were excluded to minimise the risk of falsely identifying an area as Q fever affected, as isolated infections are more likely to have occurred outside the area of residence.
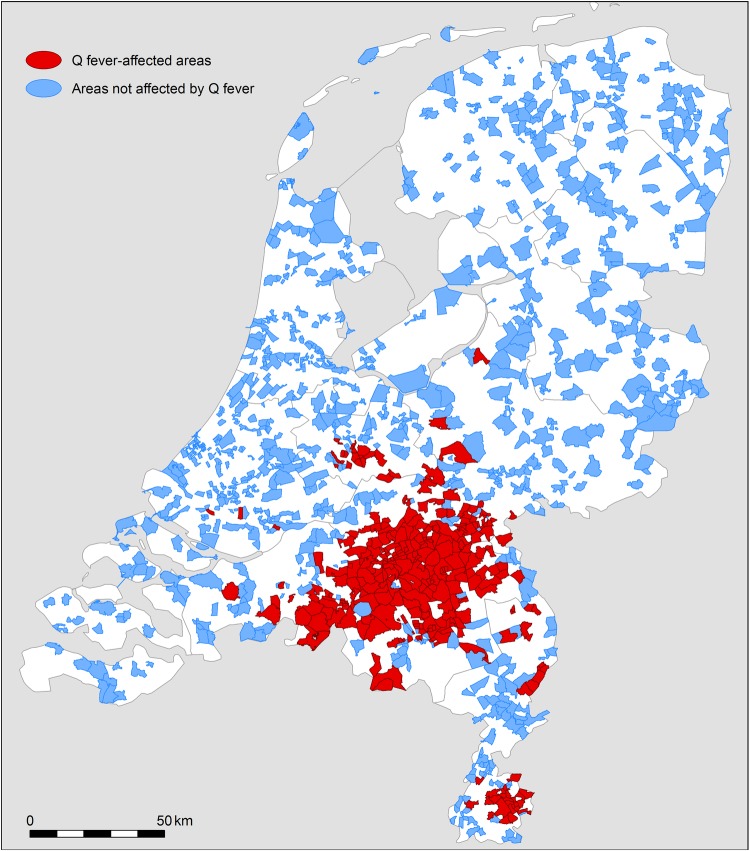
Areas not affected by Q fever were selected in two stages. First, four-digit postal-code areas with zero Q fever notifications in 2008 through 2010 were identified. Then, for each Q fever-affected area, we selected three postal-code areas not affected by Q fever (figure 1), which resembled the affected area in their proportion of adverse pregnancy outcomes in 2003 through 2004 (before the Q fever outbreak). With this method, we created two area types (areas affected and not affected by Q fever) which were comparable with respect to the risk of obstetric complications before the start of the Q fever outbreak.
Figure 1.
Postal-code areas affected by Q fever (2 or more notifications in 1 year during the years 2008, 2009 and 2010) and postal-code areas not affected by Q fever.
Pregnancy outcome
Information on pregnancy outcome at the individual level was obtained from The Netherlands Perinatal Registry (PRN). This registry is a joint effort of the professional organisations of midwives, gynaecologists, obstetrically trained general practitioners and paediatricians in the Netherlands. The PRN covers 96% of all births in the Netherlands.17
Our analyses included singleton births only, from 20 weeks of gestation onwards, for which the mother's postal-code was known. Births of children with congenital malformations were excluded, because these malformations could lead to termination of the pregnancy. Therefore, this could introduce bias for preterm delivery and perinatal mortality. Additionally, a congenital malformation is often accompanied with the outcome child small for gestational age; this could also introduce bias.
We investigated three outcome variables, namely preterm delivery, a child small for gestational age and perinatal mortality. Preterm delivery was defined as a delivery before a gestational age of 37 weeks. Small for gestational age was defined as a birth weight below the 10th centile, as derived from sex-specific, parity-specific and ethnic background-specific reference curves.18 Finally, perinatal mortality was defined as fetal (from 20 weeks of gestation onwards) or neonatal (up to 7 days after birth).
Confounding variables
Information was collected on a number of a priori determined confounding variables known to be associated with both C. burnetii infection and adverse pregnancy outcome. At the individual level, these included maternal age, ethnic background and smoking behaviour. Additionally, socioeconomic status (SES), degree of urbanisation and animal densities (goat, sheep, and cattle) were included at the level of the four-digit postal-code area. Maternal age was categorised as younger than 20, 20–34 and 35 years or older.19 Ethnic background of the mother was classified by the healthcare provider as Western or non-Western, the latter consisting largely of ethnic groups from Surinam, Morocco and Turkey.19 Smoking behaviour was classified by the healthcare provider as heavy (>20 cigarettes per day) and non-heavy.20
At four-digit postal-code area level, SES, degree of urbanisation and animal densities (goat, sheep and cattle) were included in the analysis as confounding variables. SES was estimated from the woman’s postal-code (four-digits) using mean income level, employment and education level.21 22 The SES was categorised as low (≤25th centile), average (26–74th centile), and high (≥75th centile). Degree of urbanisation was based on information at municipality level supplied by Statistics Netherlands and was translated to four-digit postal-code area.23 For the year 2003, no degree of urbanisation was available from Statistics Netherlands. We assumed that it differed little from the following year and therefore, applied figures for 2004 to the year 2003. Degree of urbanisation was categorised into five categories ranging from highly urbanised (≥2500 addresses per km2) to not urbanised (<500 addresses per km2). As Q fever-affected areas generally have high livestock densities, we assumed that zoonotic infections other than Q fever might occur in these areas. Some of the infections that can cause adverse pregnancy outcome in humans are brucellosis, toxoplasmosis, and infections with the bacteria Chlamydia psittaci and Chlamydia abortus.24–28 Therefore, as a proxy for those zoonotic infections, we considered the animal densities as confounders. The number of goats, sheep and cattle was based on information at municipality level supplied by Statistics Netherlands and was translated to four-digit postal-code area. In the number of cattle, we excluded veal calves, because they skewed the expected distribution of cattle. The animal densities per square kilometre were calculated per four-digit postal-code area and divided into three categories of equal size for all postal-code areas in the Netherlands.
Data analysis
To investigate whether there were statistically significant differences in characteristics between the areas affected and not affected by Q fever, for the periods 2003–2004 and 2008–2010, and to investigate differences for the three pregnancy outcomes between the areas affected and not affected by Q fever in different years, we used the χ2 test. To determine the association between residing in a Q fever-affected area and the three adverse pregnancy outcomes, we performed multivariable multilevel analyses, using the four-digit postal-code number as cluster variable, adjusting for maternal age, ethnic background, smoking behaviour, SES, urbanisation degree, and animal densities (goat, sheep and cattle). An interaction term consisting of period (before and during the Q fever outbreak) with residing in a Q fever-affected area (yes or no) was included in the model. The index group was defined as the pregnancy outcomes in 2008–2010 in the areas affected by Q fever. The reference group was defined as the pregnancy outcomes in the areas not affected by Q fever in 2008–2010 combined with outcomes in areas affected and unaffected in the preoutbreak years of 2003–2004. In our study, the interaction term was the most important result, as we were able to estimate only the effect of residing in a Q fever-affected area during 2008–2010, and not the already existing differences before the outbreak. Next, this analysis was repeated with a Q fever incidence variable in which incidence was divided into four categories to determine whether there was a dose–response relationship. In addition, we estimated for the statistically significant associations the population-attributable fraction (PAF) with accompanying 95% CIs,29 which represents the estimated proportion of the adverse pregnancy outcomes that is attributable to residing in a Q fever-affected area, if in fact there is a causal relation. With the PAF, we estimated the number of women who had a negative pregnancy outcome due to residing in a Q fever-affected area in the worst-case scenario. The worst-case scenario was based on the published estimate that one acute Q fever notification represents 12.6 incident infections.30 Finally, we performed a stratified analysis for women who were in their first trimester or in their second to third trimester of pregnancy in April and May, the months with highest Q fever transmission to humans.3 A p value <0.05 indicated statistical significance. Missing values occurred for only 1% of the outcome variable and for all confounders. These were imputed once with single imputation, using R software.31 32 The other analyses were performed using SAS software V.9.3 (SAS institute, Cary, North Carolina, USA).
Results
We identified 307 postal-code areas with two or more Q fever notifications in one of the outbreak years, and 921 areas not affected by any Q fever notification (figure 1). There was a statistically significant difference in all recorded characteristics between the areas affected and not affected by Q fever, for the periods 2003–2004 and 2008–2010. We found the largest differences for ethnic background, SES, urbanisation, goat density and cattle density (table 1).
Table 1.
Descriptive statistics of all births in Q fever-affected areas and areas not affected by Q fever, in the years 2003 through 2004 and 2008 through 2010
| Category | 2003–2004 |
2008–2010 |
||
|---|---|---|---|---|
| Q fever-affected area N (%) | Area not affected by Q fever N (%) | Q fever-affected area N (%) | Area not affected by Q fever N (%) | |
| Maternal age (years) | ||||
| <20 | 604 (1.4) | 2561 (2.3) | 767 (1.3) | 2821 (1.7) |
| 20–34 | 34 311 (79.8) | 86 984 (78.5) | 46 146 (78.1) | 124 123 (77.4) |
| ≥35 | 8094 (18.8) | 21 231 (19.2) | 12 136 (20.6) | 33 474 (20.9) |
| Data missing | 0 (0.0) | 7 (<0.1) | 0 (0.0) | 3 (<0.1) |
| Ethnic background | ||||
| Western | 38 482 (89.5) | 87 754 (79.2) | 52 121 (88.3) | 125 101 (78.0) |
| Non-Western | 4247 (9.9) | 22 194 (20.0) | 6745 (11.4) | 34 350 (21.4) |
| Data missing | 280 (0.6) | 835 (0.8) | 183 (0.3) | 970 (0.6) |
| Smoking | ||||
| Heavy smokers | 244 (0.6) | 344 (0.3) | 210 (0.4) | 373 (0.2) |
| Non-heavy smokers | 42 765 (99.4) | 110 439 (99.7) | 58 839 (99.6) | 160 048 (99.8) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Socioeconomic status | ||||
| Low | 9248 (21.5) | 46 882 (42.3) | 15 214 (25.8) | 68 886 (42.9) |
| Average | 23 225 (54.0) | 45 733 (41.3) | 30 085 (50.9) | 62 389 (38.9) |
| High | 10 536 (24.5) | 18 168 (16.4) | 13 750 (23.3) | 29 146 (18.2) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urbanisation degree | ||||
| Very high urban area | 3126 (7.3) | 30 643 (27.7) | 4922 (8.3) | 48 714 (30.4) |
| High urban area | 7429 (17.3) | 26 779 (24.2) | 11 679 (19.8) | 39 855 (24.8) |
| Moderate urban area | 14 330 (33.3) | 16 632 (15.0) | 18 803 (31.8) | 24 013 (15.0) |
| Minor urban area | 9285 (21.6) | 18 339 (16.5) | 12 689 (21.5) | 23 622 (14.7) |
| Rural area | 8839 (20.5) | 18 390 (16.6) | 10 956 (18.6) | 24 197 (15.1) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (<0.1) |
| Goat density | ||||
| Low | 11 649 (27.1) | 46 963 (42.4) | 18 829 (31.9) | 73 678 (45.9) |
| Medium | 9577 (22.3) | 39 763 (35.9) | 8812 (14.9) | 51 179 (31.9) |
| High | 21 783 (50.6) | 24 057 (21.7) | 31 408 (53.2) | 35 564 (22.2) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sheep density | ||||
| Low | 17 893 (41.6) | 51 111 (46.1) | 28 589 (48.4) | 75 135 (46.8) |
| Medium | 17 810 (41.4) | 36 943 (33.4) | 20 016 (33.9) | 51 945 (32.4) |
| High | 7306 (17.0) | 22 729 (20.5) | 10 444 (17.7) | 33 341 (20.8) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cattle density | ||||
| Low | 10 057 (23.4) | 61 176 (55.2) | 17 202 (29.1) | 92 118 (57.4) |
| Medium | 19 006 (44.2) | 29 694 (26.8) | 23 571 (39.9) | 40 774 (25.4) |
| High | 13 946 (32.4) | 19 913 (18.0) | 18 276 (31.0) | 27 529 (17.2) |
| Data missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
In 2003 and 2004, the proportions preterm delivery, child small for gestational age, and perinatal mortality were not statistically significant different in Q fever-affected areas compared with unaffected areas (table 2). In 2008 and 2009, the proportion of child small for gestational age was statistically significantly higher in Q fever-affected areas compared to unaffected areas. There was no statistically significant difference in preterm delivery between the areas affected and not affected by Q fever in all years. The proportion of perinatal mortality was higher in areas not affected by Q fever except for 2010.
Table 2.
Pregnancy outcome in Q fever-affected areas and areas not affected by Q fever
| Year | Area | 4-Digit postal- code areas (n) | Birth (n) | Preterm delivery (%)† | Child small for gestational age (%)‡ | Perinatal mortality (%) |
|---|---|---|---|---|---|---|
| 2003/2004 | Q fever | 307 | 42 686 | 6.1 | 10.4 | 0.9 |
| No Q fever | 921 | 109 012 | 6.3 | 10.2 | 1.0 | |
| 2008 | Q fever | 307 | 19 735 | 5.9 | 9.2* | 0.7* |
| No Q fever | 921 | 52 503 | 6.1 | 8.5* | 0.9* | |
| 2009 | Q fever | 307 | 19 936 | 5.9 | 9.3* | 0.6* |
| No Q fever | 921 | 53 221 | 6.1 | 8.6* | 0.7* | |
| 2010 | Q fever | 307 | 19 066 | 6.3 | 8.9 | 0.7 |
| No Q fever | 921 | 53 213 | 6.0 | 8.5 | 0.7 |
*p<0.05.
†Preterm delivery outcome was missing for: 2003/2004 (n=2095), 2008 (n=523), 2009 (n=656) and 2010 (n=537).
‡Child small for gestational age outcome was missing for: 2003/2004 (n=1918), 2008 (n=55), 2009 (n=549) and 2010 (n=545).
The multivariable analysis confirmed these results (table 3), in which we adjusted for maternal age, ethnic background, smoking behaviour, SES, urbanisation degree, cattle density, goat density and sheep density. For the pregnancy outcome child small for gestational age, the variable residing in a Q fever-affected area had an adjusted OR of 1.10 (95% CI 1.05 to 1.14) and the interaction term residing in a Q fever-affected area×period had an adjusted OR of 1.06 (95% CI 1.01 to 1.12). The first OR of 1.10 reflects that the affected areas and not affected areas already differed before the Q fever outbreak (2003–2004). The second OR of 1.06 implies that the differences between areas were further increased in the period 2008–2010. This means that the selection of the three not affected areas per affected area was not perfect in this study. Therefore, the interaction term is our term of interest.
Table 3.
Multivariable adjusted association between residing in a Q fever-affected area in 2008–2010 and three adverse pregnancy outcomes
| Variable | Category | Preterm delivery, OR (95% CI) | Child small for gestational age, OR (95% CI) | Perinatal mortality, OR (95% CI) |
|---|---|---|---|---|
| Residing in a Q fever-affected area | Yes | 1.00 (0.95 to 1.05) | 1.10 (1.05 to 1.14) | 0.96 (0.85 to 1.09) |
| No | Reference | Reference | Reference | |
| Period | 2003–2004 | Reference | Reference | Reference |
| 2008–2010 | 0.92 (0.89 to 0.95) | 0.82 (0.80 to 0.84) | 0.71 (0.65 to 0.77) | |
| Interaction term: residing in a Q fever-affected area×period† | Yes, in 2008–2010 | 1.01 (0.94 to 1.08) | 1.06 (1.01 to 1.12) | 0.87 (0.72 to 1.05) |
| Reference group* | Reference | Reference | Reference | |
| Age of the mother (years) | <20 | 1.47 (1.34 to 1.61) | 1.26 (1.17 to 1.36) | 1.17 (0.91 to 1.51) |
| 20–35 | Reference | Reference | Reference | |
| ≥35 | 1.02 (0.98 to 1.06) | 1.06 (1.03 to 1.09) | 1.33 (1.21 to 1.45) | |
| Ethnic background | Western | Reference | Reference | Reference |
| Non-Western | 1.02 (0.98 to 1.06) | 1.22 (1.18 to 1.26) | 1.68 (1.53 to 1.85) | |
| Smoking | Heavy smoking | 1.71 (1.39 to 2.09) | 3.36 (2.92 to 3.87) | 1.67 (0.98 to 2.84) |
| Non-heavy smoking | Reference | Reference | Reference | |
| Socioeconomic status | Low | 1.17 (1.11 to 1.23) | 1.33 (1.27 to 1.39) | 1.19 (1.05 to 1.35) |
| Average | 1.08 (1.03 to 1.14) | 1.13 (1.09 to 1.18) | 1.09 (0.96 to 1.22) | |
| High | Reference | Reference | Reference | |
| Urbanisation degree | Very high urban area | 0.94 (0.88 to 1.00) | 1.05 (0.99 to 1.10) | 0.89 (0.77 to 1.04) |
| High urban area | 0.97 (0.92 to 1.03) | 1.13 (1.08 to 1.19) | 0.94 (0.81 to 1.08) | |
| Moderate urban area | 0.98 (0.93 to 1.04) | 1.08 (1.02 to 1.13) | 0.97 (0.85 to 1.12) | |
| Minor urban area | 0.92 (0.87 to 0.98) | 1.01 (0.97 to 1.06) | 0.86 (0.74 to 0.99) | |
| Rural area | Reference | Reference | Reference | |
| Cattle density | Low | Reference | Reference | Reference |
| Medium | 1.00 (0.95 to 1.05) | 1.02 (0.98 to 1.06) | 1.05 (0.93 to 1.19) | |
| High | 1.03 (0.97 to 1.10) | 1.00 (0.95 to 1.05) | 1.03 (0.89 to 1.20) | |
| Goat density | Low | Reference | Reference | Reference |
| Medium | 1.01 (0.97 to 1.05) | 0.98 (0.95 to 1.02) | 0.98 (0.88 to 1.09) | |
| High | 0.94 (0.89 to 0.99) | 0.97 (0.93 to 1.02) | 0.99 (0.88 to 1.13) | |
| Sheep density | Low | Reference | Reference | Reference |
| Medium | 0.97 (0.92 to 1.01) | 0.95 (0.92 to 0.98) | 1.00 (0.92 to 1.14) | |
| High | 0.97 (0.93 to 1.02) | 0.92 (0.88 to 0.96) | 1.01 (0.89 to 1.10) |
Number of observations used: 312 420.
*Reference group included pregnancy outcomes in areas not affected by Q fever in 2008–2010 combined with outcomes in areas affected and unaffected by Q fever in the preoutbreak years of 2003–2004.
†Interaction term of interest, adjusted for confounders age of the mother, ethnic background, smoking, socioeconomic status, urbanisation degree, cattle density, goat density and sheep density.
In contrast, we found no statistically significant association for preterm delivery and perinatal mortality in the Q fever-affected areas in 2008–2010, compared to the reference group.
As expected, there were stronger associated factors for the three adverse pregnancy outcomes in the multivariable analyses compared to residing in a Q fever-affected area, notably heavy smoking, young maternal age, non-Western ethnic background of the mother, and residence in an area with low SES. The PAF for the significant relationship with the outcome child small for gestational age was 0.70% (95% CI 0.07% to 1.34%). This implies that—if there is a causal relation between residing in a Q fever-affected area and adverse pregnancy outcome and if Q fever had not occurred—0.7% of the children small for gestational age in the Q fever-affected areas could have been prevented. Accordingly, of 5381 children small for gestational age for women residing in a Q fever-affected area in 2008–2010, 38 could have been attributable to residing in the area and in the worst-case scenario, whereby each notified case represents 12.6 infected people, 475 could have been attributable to residing in that area.
However, we found no clear dose–response relation between a higher incidence of Q fever notifications and all three adverse pregnancy outcomes (table 4 for the main outcomes and see online supplementary table S1 for the detailed information).
Table 4.
Multivariable adjusted association between Q fever incidence in 2008–2010 and three adverse pregnancy outcomes
| Variable | Category | Preterm delivery, OR (95% CI) | Child small for gestational age, OR (95% CI) | Perinatal mortality, OR (95% CI) |
|---|---|---|---|---|
| Interaction term: incidence Q fever postal-code area×period† | <4.59 notifications/10 000 inhabitants, in 2008–2010 | 1.01 (0.91 to 1.11) | 1.04 (0.96 to 1.13) | 0.86 (0.65 to 1.14) |
| 4.59–10.61 notifications/10 000 inhabitants, in 2008–2010 | 1.04 (0.92 to 1.16) | 1.13 (1.03 to 1.24) | 0.84 (0.62 to 1.14) | |
| 10.62–21.50 notifications/10 000 inhabitants, in 2008–2010 | 0.87 (0.76 to 1.00) | 1.03 (0.92 to 1.15) | 0.80 (0.54 to 1.19) | |
| ≥21.51 notifications/10 000 inhabitants, in 2008–2010 | 1.13 (0.98 to 1.31) | 1.04 (0.92 to 1.16) | 1.04 (0.70 to 1.57) | |
| Reference group* | Reference | Reference | Reference |
Number of observations used: 312 420.
*Reference group included pregnancy outcomes in areas not affected by Q fever in 2008–2010 combined with outcomes in areas affected and unaffected by Q fever in the preoutbreak years of 2003–2004.
†Adjusted for confounders age of the mother, ethnic background, smoking, socioeconomic status, urbanisation degree, cattle density, goat density and sheep density.
Lastly, we found no evidence for a stronger association between residing in a Q fever-affected area and adverse pregnancy outcomes for women who were in their first trimester of pregnancy during months of high human Q fever incidence, compared to women who were in their second or third trimester (see online supplementary tables 2–4).
Discussion
During the years 2008–2010 of the Q fever outbreak, pregnant women residing in a Q fever-affected area had slightly higher rates of having children small for gestational age compared to the reference group. There were no differences between the two groups in rates of preterm delivery and perinatal mortality. A higher incidence of Q fever notifications was not associated with higher rates of the three adverse pregnancy outcomes. Of the 5381 children small for gestational age of women residing in a Q fever area in 2008–2010, 38 could have been attributable to residing in that area. In a worst-case scenario, this could have been 475 children. However, this is conditional given the causal relationship between residing in a Q fever-affected area and adverse pregnancy outcome, an assumption for which the present ecological study design can provide no evidence.
Strengths and limitations
Our study has several strengths. First, the registry-based approach with nationwide coverage of Q fever notifications and pregnancies allowed for accurate estimation of regional differences in Q fever incidence and adverse pregnancy outcome. Second, by using a multivariable model with an interaction term, we were able to compare pregnancy outcomes in Q fever areas in 2008–2010 with both the outcomes of the areas without Q fever in 2008–2010 and the outcomes in the period before the Q fever outbreak (in areas with and without Q fever). Therefore, we were able to estimate the effect of residing in a Q fever-affected area in 2008–2010, and not the already existing differences before the outbreak. Finally, adjustment for potential confounding variables at the individual level was possible for those variables that are routinely recorded in PRN.
However, our study has some limitations. First, maternal smoking behaviour is an established risk factor for adverse pregnancy outcome. However, as the PRN records only heavy smoking, the role of smoking may have been underestimated. Second, information on some other potential confounding variables were available only at postal-code area level. For instance, we used livestock animal densities at a postal-code area level, as an indicator for individual exposure to livestock animals. Third, other well-known risk factors for an adverse pregnancy outcome, like body mass index, were not included in the PRN database; therefore, we were not able to adjust for these.33 Fourth, as the PRN registry does not contain information on early pregnancies, we could not study spontaneous abortion as a possible adverse outcome from acute Q fever infection. Results from previous studies on spontaneous abortion, as an adverse outcome from a C. burnetii infection, are inconclusive.12 34 Fifth, there were statistically significant differences between areas affected and not affected by Q fever for all characteristics in the periods 2003–2004 and 2008–2010. However, we added these factors to the multivariable model and therefore, adjusted for these differences between the cohorts. Sixth, the classification as ‘Q fever-affected area’ or ‘area not affected by Q fever’ was based on notifications of acute Q fever. Such notification requires a positive laboratory result indicating a recent C. burnetii infection with a matching clinical presentation (fever, pneumonia or hepatitis). Cases could be over-reported because laboratory criteria cannot always discriminate between acute or past resolved infection because of long-lasting persistence of IgM antibodies and aspecific clinical symptoms.35 The opposite, that is, under-reporting, applies to Q fever as well as to many other infectious diseases, because people with illness might not seek medical care or the attending physician might not request microbiological tests. To compensate for under-reporting, we performed a worst case scenario analysis. Next, some misclassification might have occurred, as people might acquire the infection in a different postal code area as in which they live. We assumed this is the case for only a small proportion of infected people as previous studies have shown that residential address is a good proxy for environmental exposure.36 37 Lastly, any ecological study is subject to bias when it is used to make inferences about individual effects and is a weaker design compared with an observational study with individual data.
Interpretation of findings
Several case reports indicate a high risk for adverse pregnancy outcome after Q fever infection during pregnancy,5 7–11 but several large community-based studies could not find such a relationship.13–15 The percentage of people with C. burnetii antibodies in the Dutch population increased from 2.4% before the Q fever outbreak to about 12.2% after the outbreak.38 39 Despite this very high-attack rate, the present study found that the large Q fever outbreak in the Netherlands posed no major public health threat to pregnant women. However, individual cases with an adverse pregnancy outcome, especially children small for gestational age, might have occurred.
Early detection of infected pregnant women would require nationwide screening, and a previous trial showed that screening to detect acute Q fever infection was not clinically effective.16 Given the difficulty of making a consistent diagnosis of an acute infection because of the lack of discriminating factors or even absence of clinical factors, repeated serological screening of all pregnant women would be needed to identify a case at risk. In addition, there are uncertainties about the efficacy and adverse effects of antibiotic treatment, as only observational studies have been performed on this subject. In retrospect, these findings justify the Dutch approach of not implementing nationwide screening during the 2007–2010 outbreak.
Conclusions and implications
We report a weak association between residing in a Q fever-affected area and the pregnancy outcome of having a child small for gestational age. Early detection of infection would require mass screening of pregnant women and this seems not to be justified based on the results of the present study, the difficulty of making a consistent diagnosis of an acute infection, the lack of discriminating factors or even the absence of clinical factors, and uncertainties about efficacy and adverse effects of antibiotic treatment. However, a case-by-case approach, that is, early diagnosis and treatment of pregnant women with acute Q fever, is recommended.
Acknowledgments
The authors would like to acknowledge Ben Bom for providing data and for compiling the map; all midwives, obstetricians, paediatricians, nurses, and residents who took the time to collect data that are available in The Netherlands Perinatal Registry; the municipal health services and diagnostic microbiology laboratories for reporting data about the notifiable Q fever patients; and Jan van de Kassteele and Hendriek Boshuizen for their statistical support. Final editorial review was provided by Lucy D Phillips.
Footnotes
Contributors: CWPMH, JMM, PMS and WvdH designed the study. CWPMH, JMM and MMAdL conducted the data analyses. MMAdL drafted the final manuscript. All authors contributed to the analysis of results and writing of the manuscript.
Funding: This study was financed from the regular budget of the National Institute for Public Health and the Environment, Centre for Infectious Disease Control Netherlands made available by the Ministry of Health, Welfare and Sport, project number V/151503/01/RZ.
Competing interests: None declared.
Ethics approval: The Board of the PRN approved the study; this included an assessment by a privacy commission.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999;12:518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer NC, Kierstead M, Key DW et al. . Placentitis and abortion in goats and sheep in Ontario caused by Coxiella burnetii. Can Vet J 1983;24:60–1. [PMC free article] [PubMed] [Google Scholar]
- 3.Dijkstra F, van der Hoek W, Wijers N et al. . The 2007–2010 Q fever epidemic in the Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol 2012;64:3–12. 10.1111/j.1574-695X.2011.00876.x [DOI] [PubMed] [Google Scholar]
- 4.van den Wijngaard CC, Dijkstra F, van Pelt W et al. . In search of hidden Q-fever outbreaks: linking syndromic hospital clusters to infected goat farms. Epidemiol Infect 2011;139:19–26. 10.1017/S0950268810001032 [DOI] [PubMed] [Google Scholar]
- 5.Carcopino X, Raoult D, Bretelle F et al. . Managing Q fever during pregnancy: the benefits of long-term cotrimoxazole therapy. Clin Infect Dis 2007;45:548–55. 10.1086/520661 [DOI] [PubMed] [Google Scholar]
- 6.Langley JM, Marrie TJ, Leblanc JC et al. . Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes Am J Obstet Gynecol 2003;189:228–32. 10.1067/mob.2003.448 [DOI] [PubMed] [Google Scholar]
- 7.Ellis ME, Smith CC, Moffat MAJ. Chronic or fatal Q-fever infection: a review of 16 patients seen in North-East Scotland (1967–80). Q J Med 1983;52:54–66. [PubMed] [Google Scholar]
- 8.Riechman N, Raz R, Keysary A et al. . Chronic Q fever and severe thrombocytopenia in a pregnant woman. Am J Med 1988;85:253–4. 10.1016/S0002-9343(88)80355-3 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen SY, Mølbak K, Henriksen TB et al. . Adverse pregnancy outcomes and Coxiella burnetii antibodies in pregnant women, Denmark. Emerg Infect Dis 2014;20:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Million M, Roblot F, Carles D et al. . Reevaluation of the risk of fetal death and malformation after Q fever. Clin Infect Dis 2014;59:256–60. 10.1093/cid/ciu259 [DOI] [PubMed] [Google Scholar]
- 11.Stein A, Raoult D. Q fever during pregnancy: a public health problem in southern France. Clin Infect Dis 1998;27:592–6. 10.1086/514698 [DOI] [PubMed] [Google Scholar]
- 12.Quijada SG, Terán BM, Murias PS et al. . Q fever and spontaneous abortion. Clin Microbiol Infect 2012;18:533–8. 10.1111/j.1469-0691.2011.03562.x [DOI] [PubMed] [Google Scholar]
- 13.van der Hoek W, Meekelenkamp JCE, Leenders ACAP et al. . Antibodies against Coxiella burnetii and pregnancy outcome during the 2007–2008 Q fever outbreaks in the Netherlands. BMC Infect Dis 2011;11:44 10.1186/1471-2334-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rey D, Obadia Y, Tissot-Dupont H et al. . Seroprevalence of antibodies to Coxiella burnetti among pregnant women in South Eastern France. Eur J Obstet Gynecol Reprod Biol 2000;93:151–6. 10.1016/S0301-2115(00)00276-1 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen SY, Andersen AM, Mølbak K et al. . No excess risk of adverse pregnancy outcomes among women with serological markers of previous infection with Coxiella burnetii: evidence from the Danish National Birth Cohort. BMC Infect Dis 2013;13:87 10.1186/1471-2334-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munster JM, Leenders AC, Hamilton CJ et al. . Routine screening for Coxiella burnetii infection during pregnancy: a clustered randomised controlled trial during an outbreak, the Netherlands, 2010. Euro Surveill 2013;18:20504. [PubMed] [Google Scholar]
- 17.The Netherlands Perinatal Registry. Yearbook The Netherlands Perinatal Registry 2012. http://www.perinatreg.nl/uploads/150/150/Jaarboek_Zorg_in_Nederland_2012_Tabellen_13032014.pdf (accessed Oct 2013). Dutch.
- 18.Visser GHA, Eilers PHC, Elferink-Stinkens PM et al. . New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–44. 10.1016/j.earlhumdev.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 19.Ravelli ACJ, Eskes M, Tromp M et al. . Perinatal mortality in the Netherlands 2000–2006; risk factors and risk selection. Ned Tijdschr Geneeskd 2008;152:2728–33. Dutch. [PubMed] [Google Scholar]
- 20.Chiolero A, Bovet P, Paccaud F. Association between maternal smoking and low birth weight in Switzerland: the EDEN study. Swiss Med Wkly 2005;135:525–30. [DOI] [PubMed] [Google Scholar]
- 21.The Netherlands Institute for Social Research. State score. http://www.scp.nl/Onderzoek/Lopend_onderzoek/A_Z_alle_lopende_onderzoeken/Statusscores. (accessed Oct 2013). Dutch.
- 22.Agyemang C, Vrijkotte TGM, Droomers M et al. . The effect of neighbourhood income and deprivation on pregnancy outcomes in Amsterdam, the Netherlands. J Epidemiol Community Health 2009;63:755–60. 10.1136/jech.2008.080408 [DOI] [PubMed] [Google Scholar]
- 23.Statistics Netherlands. Urbanisation degree of years 2003, 2004, 2008, 2009, 2010. http://www.cbs.nl (accessed Oct 2013). Dutch.
- 24.Freeman K, Oakley L, Pollak A et al. . Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG 2005;112:31–7. 10.1111/j.1471-0528.2004.00299.x [DOI] [PubMed] [Google Scholar]
- 25.Kampinga GA, Schröder FP, Visser IJR et al. . Lambing sheep as a source of severe psittacosis in a pregnant woman. Ned Tijdschr Geneeskd 2000;144:2500–4. Dutch. [PubMed] [Google Scholar]
- 26.Malone FD, Athanassiou A, Nores LA et al. . Poor perinatal outcome associated with maternal Brucella abortus infection. Obstet Gynecol 1997;90:674–6. 10.1016/S0029-7844(97)00345-1 [DOI] [PubMed] [Google Scholar]
- 27.Pospischil A, Thoma R, Hilbe M et al. . Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1). Swiss Med Wkly 2002;132:64–6. [DOI] [PubMed] [Google Scholar]
- 28.Gulsun S, Aslan S, Satici O et al. . Brucellosis in pregnancy. Trop Doct 2011;41:82–4. 10.1258/td.2011.100386 [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993;49:865–72. 10.2307/2532206 [DOI] [PubMed] [Google Scholar]
- 30.van der Hoek W, Hogema BM, Dijkstra F et al. . Relation between Q fever notifications and Coxiella burnetii infections during the 2009 outbreak in the Netherlands. Euro Surveill 2012;17:20058. [PubMed] [Google Scholar]
- 31.Moons KGM, Donders RART, Stijnen T et al. . Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemioly 2006;59:1092–101. 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 32.Little RJA. Regression with missing X's: a review. J Am Stat Assoc 1992;87:1227–37. [Google Scholar]
- 33.Aune D, Saugstad OD, Henriksen T et al. . Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536–46. 10.1001/jama.2014.2269 [DOI] [PubMed] [Google Scholar]
- 34.Nielsen SY, Hjøllund NH, Andersen AMN et al. . Presence of antibodies against Coxiella burnetii and risk of spontaneous abortion: a nested case-control study. PLoS ONE 2012;7:e31909 10.1371/journal.pone.0031909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long-term follow-up of acute Q fever patients after a large epidemic [PhD thesis]. Utrecht University, 2014. [Google Scholar]
- 36.van Leuken JPG, Havelaar AH, van der Hoek W et al. . A model for the early identification of sources of airborne pathogens in an outdoor environment. PLoS ONE 2013;8:e80412 10.1371/journal.pone.0080412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Hoek W, Meekelenkamp JCE, Dijkstra F et al. . Proximity to goat farms and Coxiella burnetii seroprevalence among pregnant women. Emerg Infect Dis 2011;17:2360–3. 10.3201/eid1712.110738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schimmer B, Notermans DW, Harms MG et al. . Low seroprevalence of Q fever in the Netherlands prior to a series of large outbreaks. Epidemiol Infect 2012;140:27–35. 10.1017/S0950268811000136 [DOI] [PubMed] [Google Scholar]
- 39.Hogema BM, Slot E, Molier M et al. . Coxiella burnetii infection among blood donors during the 2009 Q-fever outbreak in the Netherlands Transfusion 2012;52:144–50. 10.1111/j.1537-2995.2011.03250.x [DOI] [PubMed] [Google Scholar]