Abstract
Background:
Hyperglycemia and gestational diabetes mellitus are complications of pregnancy. Both mothers and newborns are typically at increased risk for complications. This study sought to determine effect of zinc supplementation on serum glucose levels, insulin resistance, energy and macronutrients intakes in pregnant women with impaired glucose tolerance.
Methods:
In this clinical trial 44 pregnant women with impaired glucose tolerance, from December 2012 –April 2013 were randomly divided into zinc (n=22) and placebo (n=22) groups and recived 30mg/day zinc gluconate and (n=22), and placebo for eight consecutive weeks respectively. Dietary food intake was estimated from 3-days diet records. Serum levels of zinc, fasting blood sugar, and insulin were measured by conventional methods. Also homeostatic model assessment of insulin resistance was calculated.
Results:
Serumlevels of fasting blood sugar, insulin and homeostatic model assessment of insulin resistance slightly decreased in zinc group, but these changes were not statistically significant. Serum zinc levels (P =0.012), energy (P=0.037), protein (P=0.019) and fat (P=0.017) intakes increased statistically significant in the zinc group after intervention but not in the placebo group.
Conclusion:
Oral supplementation with zinc could be effective in increasing serum zinc levels and energy intake with no effects on fasting blood sugar, homeostatic model assessment of insulin resistance and insulin levels.
Keywords: Zinc, Insulin resistance, Impaired glucose tolerance, Energy
Introduction
Pregnant women with impaired glucose tolerance (IGT) is prone to gestational diabetes mellitus (GDM) and is characterized by impaired insulin sensitivity. Insulin resistance and impaired pancreatic beta-cell function are important factors for GDM. Undesirable effects of hyperglycemia and GDM include emberyonic mortality, premature delivery, dystocia, macrosomia, neonatal hypoglycemia, hyper bilirobinemia, hypocalcemia (1).The overall reported incidence of an abnormal IGT in pregnant women was 3.6% (2).
IGT is more critical especially at the beginning of the second trimester of pregnancy. In healthy pregnant women, insulin resistance is compensated by pancreas, while it is not responsive with women with GDM and impaired beta-cell function (3).
There are many accumulative evidences on association between hyperglycemia and metabolism of minerals. Majority of the papers have provided evidences that impaired insulin secretion, and increased insulin resistance associated with impaired levels of many elements such as chromium, magnesium, selenium, vanadium, zinc and copper (4).
As a mineral, zinc affects pancreatic function as well as insulin secretion. Prenatal iron and folic acid supplementation has negative correlation with zinc absorption (5). In addition, pregnant women, require higher levels of zinc (5), on the other hand zinc deficiency reported in many provinces of Iran (6).
Zinc deficiency may aggravate carbohydrate intolerance. Studies indicated the role of zinc deficiency in glucose intolerance, diabetes mellitus, insulin resistance and cardiovascular disease (7). Maternal zinc supplementation has been suggested as a potential intervention to reduce the incidence of some pregnancy complications (8).
Women with IGT, i.e. suffering high amount of blood glucose level, are in the high risk of prenatal complications although they are not thought to result in GDM (9–11). The prevalence of GDM has increased rapidly and this condition has adverse effects on maternal and fetal outcomes. To manage a pregnancy with healthier outcome it is necessary to implement appropriate intervention strategies. In particular, GDM possess a big share in total burden of pregnancy related health risks. Therefore, the current study sought to determine effect of zinc supplementation on insulin resistance, energy and macronutrients intakes in pregnant women with IGT.
Materials and Methods
In this matched, controlled clinical trial, pregnant women referred to Rohzendeh health center in Shabestar, Iran from December 2012 –April 2013 have been selected as our subjects. The subjects visited for the 24–28 weeks screening in order to receive the oral glucose challenge test (OGCT). Each subject was administered 50g glucose orally, and blood glucose measured after an hour. If the blood sugar levels were ≥130mg/dL, the OGTT as follows performed in order to excluding whom that had GDM.
FBS≥92 mg/dL
1 h ≥180 mg/dL following a 75 g oral glucose load
2 h ≥153 mg/dL following a 75 g oral glucose load (12).
The exclusion criteria include history of diagnosis of diabetes or chronic disease and specific infections, alcohol consumption, and cigarette smoking at recruitment. The informed written consent was obtained from all participants, with ethical clearance for the study obtained from the ethics committee of Tabriz University of Medical Sciences. This study is registered at the Iranian Registry of Clinical Trials (Irct registration number: IRCT 201212265670N6).
In this clinical trial 44 pregnant women with IGT that meet inclusion and exclusion criteria were randomly assigned to 2 intervention groups: zinc group (n=22), and placebo group (n=22). Zinc group received 30mg zinc tablet (in the form of zinc gluconate, NatureMed,USA) daily between meals and not together with other vitamin or mineral supplements. Second group received placebo (starch) with the same manner. In addition all the participants received a dietary plan according to their gestational status by a professional dietitian. A research staff made call interviews every week to all subjects to ensure full compliance of the pills. All participants were observed via a monthly visit during the trial. The dietary intakes were estimated from 3-days food records before and after the study. Nutrients intake were analyzed by nutritionist IV software. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared based on pre-pregnancy weight. To measure levels of serum zinc, fasting blood sugar (FBS) and insulin, 5 ml fasting blood sample was taken. FBS was measured enzymatically by auto-analyzer (Hitachi, Tokyo, Japan). Serum fasting insulin was measured using a chemiluminescent immunoassay method (DiaSorin, Liaison, Italy). Insulin resistances were calculated using the following formula according to the Homeostatic model assessment of insulin resistance (HOMAIR) method; Fasting Glucose (mg/dL) × fasting insulin (mU/L)/450. To measure zinc, blood samples were centrifuged for 5 min at 3000rpm and the serum was poured into metal-free plastic tubes. The levels of zinc from fresh serum samples were examined by conventional photometric: (Pars Azmoon co ltd; Iran).
Statistical analysis
Kolmogorov-Smirnov goodness of fit test was used to test distribution of the data. Results are expressed as median and upper and lower quartiles for non-parametric data. Paired t-test was used to compare before and after scores. The statistical software SPSS version21 (SPSS Inc. IL, Chicago, USA) was employed for data entry and analysis. P value <0.05 was considered statistically significant.
Results
As shown in Table 1, at the beginning of the study none of the variables showed any statistically significant difference between the two groups on the baseline characteristics. Serum levels of zinc, FBS, Insulin, and HOMA-IR before and after supplementation are presented in Table 2. According to the results shown in this table, serum zinc increased (P =0.012) significantly, but there were no significant statistical changes in FBS, serum insulin levels and HOMA-IR (P ≥ 0.05).
Table 1:
Baseline characteristics of subjects in two groups
| Variables | Zinc (N=22) mean ± SD | Placebo (N=22) mean ± SD | P* |
|---|---|---|---|
| Age (yr) | 29.45± 4.21 | 29.82± 5.41 | 0.805 |
| Weight(Kg) | 70.05± 11.23 | 68.43± 11.33 | 0.638 |
| Height (m) | 1.58± 0.05 | 1.60± 0.03 | 0.057 |
| BMI (Kg/m2) | 28.34± 4.17 | 26.82± 3.73 | 0.210 |
Abbreviations: SD: standard deviation, BMI: Body Mass Index /
Independent Sapmple t-test
Table 2:
Biochemical markers before and after intervention
| Variables | Zinc (n=22) | Placebo (n=22) | ||||
|---|---|---|---|---|---|---|
| Before (mean ± SD) | After (mean ± SD) | pa | Before (mean ± SD) | After (mean ± SD) | pa | |
| FBS (mg/dL) | 81± 13.76 | 74.73± 8.20 | 0.094 | 81.18± 9.15 | 80± 8.07 | 0.572 |
| Insulin (μU/mL) | 14.73±8.76 | 13.47±6.19 | 0.208 | 10.75±7.83 | 11.75±7.31 | 0.286 |
| HOMA-IR | 2.84±1.65 | 2.70±1.61 | 0.354 | 2.23±1.74 | 2.32±1.50 | 0.401 |
| Serum zinc (μg/dL) | 77.77± 22.28 | 87.54± 19.03 | 0.012* | 64.09± 18.44 | 71.81± 19.36 | 0.067 |
Abbreviations: SD: standard deviation, FBS: Fasting Blood Glucose, HOMA-IR: Homeostasis model assessment of insulin resistance;
Statistically significant,
: Paired t-test
Daily total energy and macronutrients intakes are depicted in Table 3. Supplementation with 30mg of zinc significantly increased energy, protein, and fat and zinc intake in zinc group (P < 0.05). However, no change was observed in the placebo group (P >0.05). Flowchart of design and protocol of the study is shown (Fig. 1).
Table 3:
Dietary intakes before and after intervention
| Variables | Zinc (n=22) | Placebo (n=22) | ||||
|---|---|---|---|---|---|---|
| Before (mean ± SD) | After (mean ± SD) | pa | Before (mean ± SD) | After (mean ± SD) | pa | |
| Energy (Kcal/day) | 1909± 485.14 | 2547± 939.20 | 0.037* | 1939± 328.60 | 2300± 753.05 | 0.134 |
| Cho (gr/day) | 290.29± 78.64 | 409.91± 194.93 | 0.071 | 285.04± 55.80 | 317.08±101.14 | 0.341 |
| Pro (gr/day) | 77.61± 22.43 | 103.18±32.35 | 0.019* | 76.53± 17.40 | 85.06± 26.36 | 0.394 |
| Fat (gr/day) | 51.30± 17.17 | 70.06± 27.56 | 0.017* | 56.19± 15.65 | 81.99± 44.61 | 0.092 |
| Dietary zinc(mg/day) | 7.94± 2.48 | 11.68± 3.49 | 0.008* | 8.32± 1.64 | 9.70± 3.89 | 0.313 |
Abbreviations: SD: standard deviation, Cho: Carbohydrate, Pro: protein;
Statistically significant,
: Paired t-test.
Fig. 1:
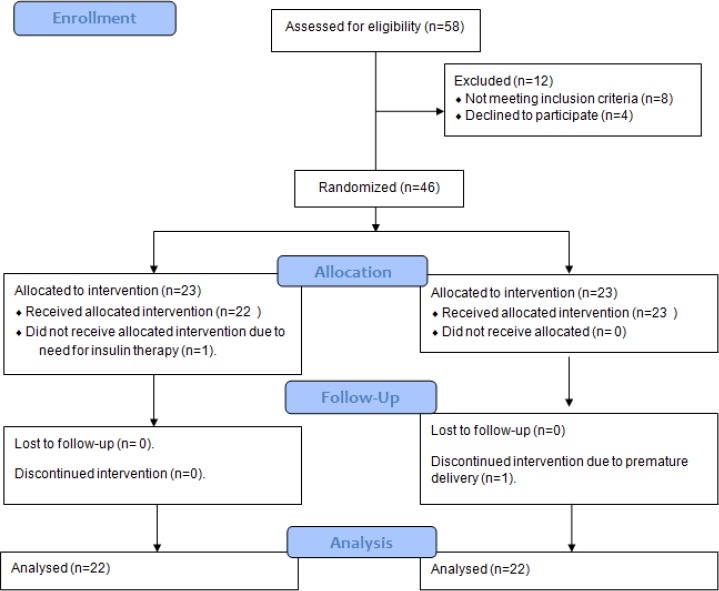
Flowchart of design and protocol of the study
Discussion
To the best of our knowledge, this is one of the first randomized controlled trials evaluating the effects of zinc supplementation in IGT women. We evaluated the effect of zinc supplementation on insulin resistance, energy and macronutrients intakes in pregnant women with IGT. In this study zinc supplementation increased serum zinc significantly and decreased fasting glucose, insulin and HOMA-IR too, but not statistically significant. To date, it has been obvious that oxidative stress has an important role in the pathogenesis of diabetes mellitus. Interestingly, antioxidant feature of zinc (13) protects insulin and pancreatic cells against free radicals (14). The element is also shown to be effective for insulin synthesis, its storage and secretion (13). It also up regulates insulin function via stimulation of insulin tyrosine kinase receptors and increasing the phosphorylation of tyrosine kinase (15). Besides, zinc is important for stability of insulin hexamer and in turn pancreatic storage of the hormone (13).
Zinc deficiency reported in pregnant women and in the patients with GDM (5, 16). Effect of zinc supplementation on insulin resistance and FBS has been studied in diverse group of populations suggesting that zinc supplementation could be effective in controlling the disease (17–19).
The results of our study with respect to serum zinc is similar to Kim and Lee study that showed zinc supplementation increased serum zinc but HOMA-IR values and fasting insulin were unaffected by this supplementation (20). FBS and zinc concentration in plasma did not change in obese women after 4 weeks zinc supplementation, but they showed a significant decrease in fasting insulin level in supplementation group (21). The results of this study are consistent with the findings of payahoo, et al. They concluded that serum zinc concentration increased significantly but FBS did not change in the healthy obese adults after one month of zinc supplementation (22). Our findings were in contrast to hashemipour et al results. In that study after receiving zinc supplementation, the mean FBS, insulin and HOMA-IR decreased significantly in prepubertal obese children (23). In addition, zinc deficiency reduced the body's response to insulin and administered zinc supplements could have beneficial effects on glucose homeostasis (24).
In a randomized controlled trial in 40 diabetic patients after supplementation with 660 mg zinc sul-fate for 6 weeks, FBS level did not change significantly, but after 12 weeks, there was a significant decrease in HbA1C with zinc sulfate consumption (25). In subjects who received zinc sulfate 220mg (50 mg elemental zinc) daily for 12 weeks, insulin level and HOMA-IR index similar to our studies did not show significant changes (19). Four weeks of 50 mg zinc supplementation as zinc gluconate in type 2 diabetic patients showed reduction in fasting blood glucose and improve glycemic control in patients with marginal zinc status (26).
Conflicting results in different studies could be due to variation in doses, supplementation period and difference in chemical form of zinc. On the other hand, the supplementations have been conducted in different population groups. Further baseline serum zinc status and blood glucose level can effect on results of various studies.
Regarding the potential benefite of zinc on the appetite, this supplementation caused a significant increase in energy level. This agrees with the findings of most studies in the literature review (27–29).
The strengths of our study were that all women participating in the study were almost at the same gestational age (24–28 weeks of pregnancy). The main limitation of the present study might be its low sample size.
Conclusion
Zinc supplementation at 30 mg daily for 8 weeks improved serum zinc concentrations, energy, macronutrients and dietary zinc intakes in pregnant women with IGT, although the overall differences for FBS were non-significant. Another study with a larger sample size, and longer period of study might lead to more reliable results.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We are grateful and thankful to Nutrition Research Center, Tabriz University of Medical Sciences, Dr Saeid Amniati, Mrs Talebi, Mrs Sayadi, Mrs Nezafati and all would-be mothers who participated in this research project. The authors declare that there is no conflict of interest.
References
- 1. Test OGT. ( 2008). Hyperglycemia and adverse pregnancy outcomes. N Engl J Med, 358: 1991– 2002. [DOI] [PubMed] [Google Scholar]
- 2. Farah N, McGoldrick A, Fattah C, et al. ( 2012). Body Mass Index (BMI) and Glucose Intolerance during Pregnancy in White European Women. J Reprod Infertil, 13( 2): pp. 95– 99. [PMC free article] [PubMed] [Google Scholar]
- 3. O'Brien KO, Zavaleta N, Caulfield LE, et al. ( 2000). Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr, 130( 9): pp. 2251– 2255. [DOI] [PubMed] [Google Scholar]
- 4. Abdolsamadi H, Zamani M, Goodarzi M, et al. ( 2012). Comparative Evaluation of Chromium and Cadmium in Gestational diabetes and healthy pregnant women. Iran J Endocrinol Metab, 13( 6): pp. 666– 672. [Google Scholar]
- 5. Salimi S, Yaghmaei M, Joshaghani HR, Mansourian AR. ( 2004). Study of zinc deficiency in pregnant women. Iran J Public Health, 33( 3): pp. 15– 18. [Google Scholar]
- 6. Sharifi F, Hedayati M, Mirmiran P, et al. ( 1999). The serum level of Zinc, Cu, iron in elementary students of 23 province of Iran in 1996. Iran J Endocrinol Metab, 1( 4): pp. 275– 85. [Google Scholar]
- 7. Nokhostin F, Bavar A, Hedayati M, et al. ( 2005). The frequency of diabetes in pregnant women with pyelonephritis in Imam Khomeini hospital of Ahwaz. Iran J Endocrinol Metab, 7( 4): pp. 325– 329. [Google Scholar]
- 8. Mahmoodi MR, Hedayati M, Mehrabi Y, et al. ( 2010). The effects of omega-3 fatty acids plus vitamin E and vitamin C plus zinc supplementations on glycemic control in postmenopausal women with type 2 diabetes. Iran J Nutr Food Sci Food Technol, 4 ( 4), pp. 9– 20. [Google Scholar]
- 9. Hossein-Nezhad A, Maghbooli Z, Vassigh AR, Larijani B. ( 2007). Prevalence of gestational diabetes mellitus and pregnancy outcomes in Iranian women. Taiwan J Obstet Gynecol, 46( 3): pp. 236– 241. [DOI] [PubMed] [Google Scholar]
- 10. Åberg A, Rydhstroem H, Frid A. ( 2001). Impaired glucose tolerance associated with adverse pregnancy outcome: a population-based study in southern Sweden. Am J Obstet Gynecol, 184( 2): pp. 77– 83. [DOI] [PubMed] [Google Scholar]
- 11. Jensen DM, Damm P, Sørensen B, et al. ( 2001). Clinical impact of mild carbohydrate intolerance in pregnancy: a study of 2904 nondiabetic Danish women with risk factors for gestational diabetes mellitus. Am J Obstet Gynecol, 185( 2): pp. 413– 419. [DOI] [PubMed] [Google Scholar]
- 12. Association AD ( 2011). Executive summary: standards of medical care in diabetes--2011. Diabetes Care, 34: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiernsperger N, Rapin J. ( 2010). Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr, 19( 2): pp. 70– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Q, van Dam RM, Willett WC, Hu FB. ( 2009). Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care, 32( 4): pp. 629– 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konukoglu D, Turhan MS, Ercan M, Serin O. ( 2004). Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem, 15( 12): pp. 757– 760. [DOI] [PubMed] [Google Scholar]
- 16. Al-Saleh E, Nandakumaran M, Al-Rashdan I, et al. ( 2007). Maternal-foetal status of copper, iron, molybdenum, selenium and zinc in obese gestational diabetic pregnancies. Acta Diabetol, 44( 3): pp. 106– 113. [DOI] [PubMed] [Google Scholar]
- 17. Chaffee BW, King JC. ( 2012). Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr Perinat Epidemiol, 26( s1): pp. 118– 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh RB, Niaz MA, Rastogi SS, et al. ( 1998). Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr, 17( 6): pp. 564– 570. [DOI] [PubMed] [Google Scholar]
- 19. Soheylikhah S, Dehestani MR, Mohammadi SM, et al. ( 2012). The Effect of Zinc Supplementation on Serum Adiponectin Concentration and Insulin Resistance in First Degree Relatives of Diabetic Patients. Iran J Diabetes Obes, 4( 2): pp. 57– 62. [Google Scholar]
- 20. Kim J, Lee S. ( 2012). Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutr Res Pract, 6( 3): pp. 221– 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marreiro DN, Geloneze B, Tambascia MA, et al. ( 2006). Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res, 112( 2): pp. 109– 118. [DOI] [PubMed] [Google Scholar]
- 22. Payahoo L, Ostadrahimi A, Mobasseri M, et al. ( 2013). Effects of Zinc Supplementation on the Anthropometric Measurements, Lipid Profiles and Fasting Blood Glucose in the Healthy Obese Adults. Adv Pharm Bull, 3( 1): pp. 161– 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashemipour M, Kelishadi R, Shapouri J, et al. ( 2009). Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones (Athens), 8( 4): pp. 279– 285. [DOI] [PubMed] [Google Scholar]
- 24. Zabihi S, Wentzel P, Eriksson UJ. ( 2008). Maternal blood glucose levels determine the severity of diabetic embryopathy in mice with different expression of copper-zinc superoxide dismutase (CuZnSOD). Toxicol Sci, 105( 1): pp. 166– 172. [DOI] [PubMed] [Google Scholar]
- 25. Afkhami-Ardekani M, Karimi M, Mohammadi SM, Nourani F. ( 2008). Effect of zinc sulfate supplementation on lipid and glucose in type 2 diabetic patients. Pak J Nutr, 7( 4): pp. 550– 553. [Google Scholar]
- 26. Oh HM, Yoon JS. ( 2008). Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr Res Pract, 2( 4): pp. 283– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClain C, Stuart M, Kasarskis E, Humphries L. ( 1993). Zinc, appetite regulation and eating disorders. Prog Clin Biol Res, pp. 380: 47. [PubMed] [Google Scholar]
- 28. Ninh NX, Thissen JP, Collette L, et al. ( 1996). Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am J Clin Nutr, 63( 4): pp. 514– 519. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki H, Asakawa A, Li JB, et al. ( 2011). Zinc as an appetite stimulator-the possible role of zinc in the progression of diseases such as cachexia and sarcopenia. Recent Pat Food Nutr Agric, 3( 3): pp. 226– 231. [DOI] [PubMed] [Google Scholar]


