Abstract
Background:
Diabetes is a global health problem in the world. Probiotic food has anti-diabetic property. The aim of this trial was to determine the effect of probiotic fermented milk (kefir) on glucose and lipid profile control in patients with type 2 diabetes mellitus.
Methods:
This randomized double-blind placebo-controlled clinical trial was conducted on 60 diabetic patients aged 35 to 65 years.Patients were randomly and equally (n=30) assigned to consume either probiotic fermented milk (kefir) or conventional fermented milk (dough) for 8 weeks. Probiotic group consumed 600 ml/day probiotic fermented milk containing Lactobacillus casei, Lactobacillus acidophilus and Bifidobacteria and control group consumed 600 ml/day conventional fermented milk.Blood samples tested for fasting blood glucose, HbA1C, triglyceride (TG), total cholesterol, HDL-C and LDL-C at the baseline and end of the study.
Results:
The comparison of fasting blood glucose between two groups after intervention was statistically significant (P=0.01). After intervention, reduced HbA1C compared with the baseline value in probiotic fermented milk group was statistically significant (P=0.001), also the HbA1C level significantly decreased in probiotic group in comparison with control group (P=0.02) adjusting for serum levels of glucose, baseline values of HbA1c and energy intake according to ANCOVA model. Serum triglyceride, total cholesterol, LDL-cholesterol and HDL- cholesterol levels were not shown significant differences between and within the groups after intervention.
Conclusion:
Probiotic fermented milk can be useful as a complementary or adjuvant therapy in the treatment of diabetes.
Keywords: Probiotic fermented milk, Kefir, Diabetes, Glucose, Lipid profile
Introduction
Diabetes is global health problem in the world.Long-term damage, dysfunction, and failure of different organs especially eyes, kidneys, nerves, heart and blood vessels is related to chronic hyperglycemia in diabetes patients. The incidence of type 2 diabetes is increasing worldwide. (1). The prevalence of diabetes among adults aged 25 to 64 years is 7.7 percent in Iran. An additional 16.8%, or 4.4 million of Iranian adults suffer impaired fasting glucose (2).
Beside drug treatment for diabetes; in recent years, many efforts have been made on traditional medicines as a complementary or adjuvant therapy in the treatment of diabetes. In this regards, probiotics has been considered in diabetic patients. Probiotics are live microorganisms, which induce a health benefit when administered in adequate amount (3). These health benefits are performed by stimulating beneficial gastrointestinal indigenous microfloraprolifration. Several strain of theses microorganisms show health benefits. However, Lactobacillus and Bifidobacteriumare the most common probiotic bacteria used in food (4). Approved probiotics with lactic acid producing property in human origin include Bifidobacteria and Lactobacilli. The consumption of probiotics may decrease the serum level of glucose and glucose tolerance in diabetes (5, 6).
Many experimental models in chemical or diet and genetically mutated animals (db/db mice) have been shown Lactobacillus is effective for preventing and delaying of diabetes onset (7).).
Probiotics may improve insulin resistance by reducing the inflammatory response in diabetes (4). Oxidative stress condition in diabetes can lead to insulin resistance and consequently the glucose uptake by peripheral tissue reduction (8). In recent years, probiotics were used as alternative supplements, and theses microorganisms can apply health benefits including decrease of serum/plasma total cholesterol, LDL-cholesterol and triglycerides and increase HDL-cholesterol (9–12).
Following positive results in animals, numerous short-term randomized controlled trials demonstrated the benefit of prebiotics and probiotics on insulin sensitivity, inflammatory markers and glucose tolerance (13). The previous studies (14, 15) in Iran reported the effect of probiotic enrichment food such as yoghurt or probiotic supplements in diabetes. Therefore, the purpose of the present study was to investigate the effect of probiotic fermented milk (kefir) containing L. acidophilus and Bifidobacterium on glucose and lipid profile control in patients with type 2 diabetes mellitus.
Materials and Methods
Participants
The randomized double-blind placebo-controlled clinical trial was conducted on patients with type 2 diabetes in Tabriz, Iran. The research was approved by the Ethics committee of Tabriz University of Medical Sciences. Written informed consent was obtained from all patients’ prior beginning of the study. Clinical trial number of this study was IRCT201307092017N14.
The sample size was determined based on the data obtained from the study by Ejtahed for Hb A1C (16). We considered α value equal to 0.05 and a power of 80 percent for calculating the sample size. The sample size was computed as 23 per groups. For accommodating the anticipated dropout rate, this number was increased to 35 persons per group. Flow chart of the study is shown in Fig. 1. Patients recruited from Association of diabetes patients in Tabriz. Recruitment was done by call and announcement.
Fig.1:
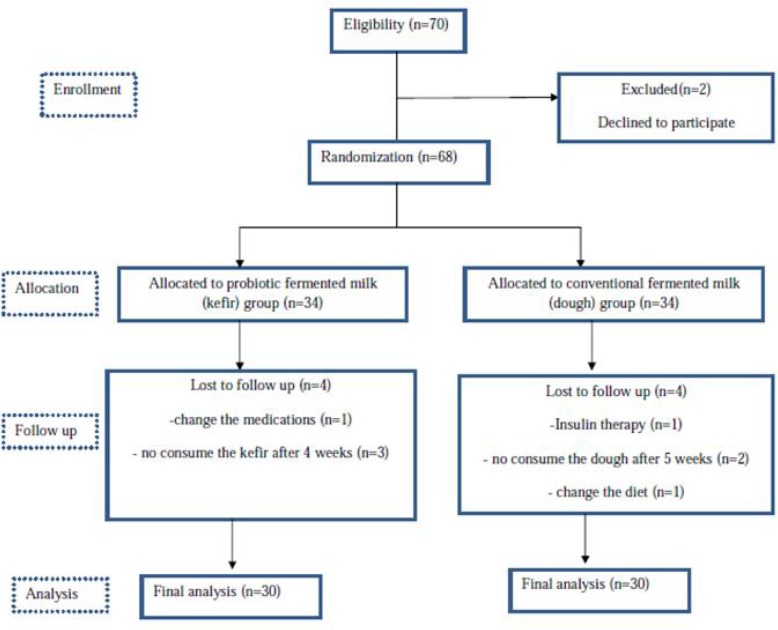
Flow chart of the study
Inclusion criteria were diabetic patients with fasting blood glucose ≥125 mg/dl, aged from 35 to 65 years, no insulin therapy and duration of illness less than 20 years. Exclusion criteria included conventional cigarette smoking, pregnancy, breast feeding, patients with conventional medical complications such as thyroid, liver, gastrointestinal, cardiovascular, kidney and defective immune systems disease, vitamin and mineral use, subjects on non-steroidal anti-inflammatory drugs and hormone replacement therapy.
Study design and variables assessment
The eligible participants were randomly assigned to intervention or placebo groups based on random block procedure produced by Random Allocation Software (RAS). Subjects in each group were matched on sex, age and duration of diseases.Each group consisted of 30 patients. Two week before the beginning the study, all patients had refrained period from consuming probiotic fermented milk and any probiotic food. During the eight weeks of intervention, patients in the intervention group received 600ml fermented milk (kefir) containing probiotics twice a day (in lunch and dinner), and placebo group received 600 ml conventional fermented milk(dough) twice daily(with lunch and dinner). All the participants were asked not to change their usual dietary intakes, life style, other vitamin and minerals supplement consumption, medication and traditional medicine as an adjuvant therapy during the study.
In addition, the patients were asked to notify the researcher, if medical changes accrued during the intervention.
To ensure that the participants would use according to prescription, patients received supply of fermented milk-kefir and conventional fermented milk every week. All information containing 3 days dietary records, anthropometric measurements, and fasting blood samples were collected at the beginning and end of the study. The Nutritionist IV software was used for analyzing 3 days averages of macronutrients and micronutrients intakes. Personal and demographic information were obtained by questionnaire. Body weight was measured using a scale (Seca, Hamburg, Germany) with 0.1 kg accuracy by wearing light clothing and without shoes. Height was measured using a stadiometer (Seca) with 0.1 cm accuracy.Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2).
Fasting blood samples were drawn for biochemical analyses from the antecubital vein. They were collected from all participants after 10 to 12 hours of overnight fasting and were centrifuged within 30–45 min of collection and serum was stored at −70°C (SANYO, mdf-u33v, Japan, 2010).
Serum concentrations of fasting blood glucose (FBS), triglyceride (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were determined using kit (Parsazmon, Tehran, Iran) and enzymatic method by Auto-analyzer Bio-Systems (Authoanalyzer, BS-200, MINDRAY chemistry analyzer, Germany, 2009). Low-density lipoprotein cholesterol (LDL-C) was calculated according to the procedure of Friede-Wald formula. Glycated hemoglobin (HbA1c) was measured immediately by cation exchange chromatography in the whole blood (Bio system, Spain).
Characteristics of intervention
The conventional fermented milk (dough) contained Streptococcus thermophiles and Lactobacillus bulgaricus according to standard Goldam factory protocol. The probiotic-fermented milk (kefir) contained Streptococcus thermophiles; also, it was enriched with Lactobacillus casei, Lactobacillus acidophilus and Bifidobacterium lactis (DSM. Co, Australia). Lactobacillus acidophilus was cultured on clindamycin and MRS PH6.2 medium, Lactobacillus casei on vancomycin and MRS PH6.2 medium and finally Bifidobacterium lactis on strictly anaerobic medium with L- cysteine according to manufacturer instructions. Both types of fermented milk were produced weekly and both types were refrigerated at 4°C. Microbiological analysis for kefir was performed on first day and then was refrigerated for subsequent analyzing on 7th, 14th and 21th day of storage. Microbiological analysis of probiotic fermented milk (kefir) on 7th, 14th and 21th day are shown in Table 1. Fat content of both types of fermented milk was 0.3%. Both types of fermented milk (kefir and conventional dough) were prepared for this study by Goldam Dairy Company (Tabriz, Iran).
Table 1:
Colony count of probiotic strains in probiotic fermented milk
| Probitic strain | Colony count/ml | Colony count/ml | Colony count/ml | Colony count/ml |
|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | |
| Lactobacillus acidophilus | 25×10 6 | 12×10 6 | 5×10 6 | 3×10 6 |
| Lactobacillus casei | 15×10 6 | 10×10 6 | 4×10 6 | 2×10 6 |
| Bifidobacterium lactis | 8×10 6 | 6×10 6 | 2×10 6 | 0.5×10 6 |
Statistical analysis
The data were analyzed by SPSS 11.5 (Chicago, IL, USA). Normality of variables distribution was evaluated using Kolmogorov-Smirnov test.Quantitative data were stated as mean ± standard deviation (SD).Paired t-test was used to compare within group changes before and after the intervention. Comparison of mean changes between two groups was carried out by independent samples t- test. Analysis of Covariance (ANCOVA) test was used to adjust baseline values covariates. P<0.05 was considered statistically significant.
Results
Demographic characteristics of the participants are shown in Table 2. This randomized double-blind placebo-controlled clinical trial was conducted on 60 patients. In the study population, 43.33% were female and 56.66%were male. Body mass index in two groups at the baseline of study are presented in Table 2. There is no significant difference in BMI between probiotic and conventional fermented milk (P=0.23).
Table 2:
The demographic data in the probiotic fermented milk(kefir) and conventional fermented milk (dough) groups
| Variables | Probiotic fermented milk (kefir) mean±SD (n=30) | Conventional fermented milk (dough) mean±SD (n=30) | P-value |
|---|---|---|---|
| Sex | 0.84 | ||
| Female n (%) | 12(40) | 14(46.66) | |
| Male n(%) | 18(60) | 16(53.33) | |
| Body Mass Index (kg/m 2 ) | 28.89±4.77 | 27.47±3.55 | 0.23 |
| Duration of Diseases | 6.47±0.90 | 7.36±0.84 | 0.10 |
Weight and dietary intake data before and after intervention in two groups are presented in Table 3. There were no statistically significant differences in weight, energy, carbohydrate and protein values between or within groups at the beginning and at the end of the study. The intake of total fat was significantly different between the probiotic and conventional fermented milk groups at beginning of the study (P=0.01), and also there was the difference in total fat intake between two groups at the end of the study but this difference was not significant.
Table 3:
Weight and dietary intake of subjects before and after intervention
| Variables | Probiotic fermented milk(kefir) mean±SD (n=30) | conventional fermented milk(dough) mean±SD (n=30) | P-value |
|---|---|---|---|
| Weight (kg) | |||
| Before | 77.46±13.26 | 74.92±11.48 | 0.46 |
| After intervention | 77.78±12.78 | 75.40±11.27 | 0.47 |
| Energy (kcal) | |||
| Before | 1994.13±405.02 | 1806.13±380.89 | 0.13 |
| After intervention | 2015.13±402.45 | 1927.65±402.25 | 0.39 |
| Carbohydrate(g) | |||
| Before | 225.13±69.78 | 252.20±52.30 | 0.87 |
| After intervention | 246.25±60.56 | 248.98±54.51 | 0.88 |
| Protein(g) | |||
| Before | 67.72±19.13 | 61.17±18.54 | 0.26 |
| After intervention | 66.01±18.66 | 66.54±20.62 | 0.93 |
| Total Fat (g) | |||
| Before | 80.10±22.33 | 63.27±17.13 | 0.01 * |
| After intervention | 82.09±29.19 | 66.23±19.47 | 0.08 |
Significant difference between groups at baseline (P=0.01, independent sample t -test )
Table 4 shows the biochemical variables assessment in two groups at the beginning and at the end of the study with the comparison within-groups changes and the between-groups changes. After removing the outlier data, there was no statistically significant differences in fasting blood glucose at the beginning of the study between two groups (P=0.22). After intervention with fermented milk, fasting blood glucose were decreased in probiotic fermented milk (P=0.05), but this decrease was not statistically significant. The comparison of fasting blood glucose between two groups after intervention was statistically significant (P=0.01) and after adjusting for baseline value according to ANCOVA model (P=0.03). After intervention, HbA1C reduced within probiotic fermented milk was statistically significant (P=0.001). As well as this decreased HbA1C between two groups was significant after adjusting for serum levels of glucose, baseline values of HbA1c and energy intake according to ANCOVA model (P=0.02).
Table 4:
Blood levels of serum glucose, HbA1Cand lipid profiles at baseline and at the end of the study
| Variables | Probiotic fermented milk(kefir)mean±SD (n=30) | Conventional fermented mean±SD (n=30) | P-value |
|---|---|---|---|
| Serum Glucose (mg/dl) | |||
| Before | 161.63±57.71 | 183.42±74.76 | 0.22 |
| After intervention | 139.22±46.66 a | 182.16±73.78 | 0.01 b |
| HbA1C | |||
| Before | 7.61±1.22 | 6.98±1.63 | 0.12 |
| After intervention | 6.40±1.91 c | 7.00±1.98 | 0.26 d |
| Total Cholestrol(mg/dl) | |||
| Before | 197.86±51.99 | 204.56±42.85 | 0.60 |
| After intervention | 186.07±61.03 e | 195.96±54.85 | 0.52 f |
| TG (mg/dl) | |||
| Before | 179.25±87.84 | 176.67±98.65 | 0.92 |
| After intervention | 170.11±118.66 | 171.76±78.47 | 0.95 |
| LDL-Cholestrol(mg/dl) | |||
| Before | 102.65±30.04 | 102.78±31.56 | 0.92 |
| After intervention | 98.19±39.23 | 92.80±34.43 | 0.74 |
| HDL- Cholestrol(mg/dl) | |||
| Before | 45.36±11.14 | 43.37±13.03 | 0.53 |
| After intervention | 44.00±13.30 | 43.64±11.44 | 0.96 |
differences within the group (P=0.05, paired t-test)
difference between two groups (P=0.03), adjusting for baseline value according to ANCOVA model
difference within group (P=0.001, paired t-test).
difference between two groups (P=0.02), adjusting for serum levels of glucose, baseline values of HbA1c and energy intake according to ANCOVA model
differences within the group (P=0.07, paired t-test), analysis was performed for 27 patients.
difference between two groups (P=0.08). Near to significance. Adjusting for baseline values, energy and fat intake according to ANCOVA model
As presented in Table 4, serum triglyceride, LDL-cholesterol and HDL- cholesterol and total cholesterol levels have no significant changes within the groups in comparison with the baseline values (P>0.05). Although total cholesterol, triglyceride and LDL- cholesterol in probiotic fermented milk group decreased but these changes was not statistically significant. Between two groups, comparison of serum triglyceride, total cholesterol, LDL-cholesterol and HDL- cholesterol levels was not shown significant differences. Serum total cholesterol level in probiotic fermented milk diminished in comparison with conventional fermented milk but this comparison was not statistically significant (P=0.08) after adjusting for baseline values, energy and fat intake by ANCOVA model.
Discussion
Management of diabetes without any side effects by natural food is a challenge for medical nutrition therapy of diabetes. In this study, the probiotic fermented milk consumption causes the decline of fasting blood glucose and HbA1C in comparison with conventional fermented milk. As well as, in consistent with previous studies total cholesterol, LDL cholesterol and triglyceride in probiotic-fermented milk (kefir) decreased toward to conventional fermented milk but these changes were not statistically significant.
Antidiabetic effect of Lactobacillus and Bifidobacteria has been investigated in several animal and human studies (7, 17–19). Some studies have expressed probiotic treatment can reduce blood glucose levels in diabetic status (20, 21). Several possible mechanisms of this effect are expressed. A possible explanation for hypoglycemic effect is that probiotics affected gut bacteria to produce insulinotropic polypeptides and glucagon-like peptide-l so induce uptake of glucose by muscle. As well as liver stimulates the absorption of more blood glucose in the form of glycogen (22). Oral administration of Lactobacillus johnsonii strain La1 for two weeks diminished the elevation of blood glucose and glucagon levels after an oral glucose load in streptozotocin-diabetic rats (23). Probiotic use in diabetic patients was not shown significant difference in FBS levels (14). In Iran, Ejtahed et al. (16) declared the consumption of probiotic yogurt improved fasting blood glucose, Hb A1C and antioxidant status in type 2 diabetic patients. Oxidative stress caused by hyperglycemia occurs before other clinical disorders (24). In diabetes or insulin resistance status, failure of insulin stimulated glucose uptake by fat and muscle causes high concentrations of glucose in blood, so the glucose uptake in insulin-independent tissues increases (25). Consequently this condition causes the elevated oxidant products and the damage of antioxidant defenses in diabetes mellitus by multiple interacting pathways (26, 27). In our study, antioxidant indexes have not measured, but the decrease of fasting blood glucose and HbA1C may be related to antioxidant activity of probiotic-fermented milk by several interacting pathways, which eventually are leading to blood sugar regulation. In addition, probiotics could be effective for reducing glucose absorption from intestine tract and could be alter the metabolic use of glucose.
Various studies (28–30) have expressed probiotic bacteria especially Lactobacillus and Bofidobacteria can reduce the serum cholesterol levels and subsequently these probiotics can be useful in hyper-cholesterolemia medical management. In one study probiotic treatment in patients with type 2 diabetes had no effects on FBS, triglyceride, total cholesterol and LDL- cholesterol in comparison with placebo group, however after probiotic treatment HDL-cholesterol and hs-CRP levels were slightly increased (14). Kefir consumption in hyperlipidemic men during 4 week was not effective in reducing lipid profile (31). The consumption of probiotic yogurt containing L.acidophilus and B. lactis in type 2 diabetes mellitus patients improved total cholesterol and LDL-C concentrations, the authors declared this product can be the appropriate management in controlling the cardiovascular diseases risk factors in diabetic patients (15). In this study the lipid profile in probiotic group from the clinical perspective decreased but these decreases were not statistically significant and total cholesterol changes were near the statistically significant within and between groups.
The mechanistic approach for hypocholesterolmic properties implies that probiotic strains use cholesterol for their own metabolism. Probiotics bind to cholesterol and convert the binding cholesterol to its catabolic products. Therefore, the cholesterol levels decreased by the deconjugating cholesterol to the bile acids. This mechanism causes the reduction of total body pool of cholesterol. L.acidophilus inhibits the 3-hydroxy 3-methyl glutamyl CoA reductase, which is a rate-limiting enzyme responsible for the endogenous cholesterol biosynthesis in the body and this enzyme can de-conjugate bile acids in the gut and, eventually this process causes the reducing cholesterol concentration (30, 32). As previously mentioned, these significant changes were not shown in this study; the possible reasons, which could be noted, are differences in probiotic strains and genetic differences in our patients.
Some probiotic strains can improve intestinal barrier function and can cause the decrease of micro-organism and their products derived from them such as lipopolysaccharide (LPS). The elevated LPS can release circulating pro-inflammatory cytokines. Subsequently increasing the pro-inflammatory factors may involve in the pathogenesis of insulin resistance in the type 2 diabetes mellitus patients (18, 33, 34). The specific strain of probiotics has anti-inflammatory properties (18, 35, 36) and can cause the reducing inflammatory condition, regulating glucose and lipid metabolism in diabetic patients.
The limitation of this study included the absence of a control group that consumed no fermented milk. If there was a control group without fermented milk consumption, we could determine the net effect of fermented milk on biochemical parameters in type 2 diabetic patients. The sample size of the studied population was limiting agent for obtaining more power of statistical analysis. Duration of the study could be prolonged; short duration of intervention was another limitation of this study. Therefore, further studies with long-term duration are needed to mark the other probiotic fermented milk (kefir) effects in diabetic patients. As well as intestinal microflora assessment and colonization of bacteria in gut has not been survey in this study.
The strength of this study was the intense desire of kefir consumption by diabetic patients. As well as, weekly monitoring of patients at the time of delivery-fermented milk was strength of this study.
Conclusion
Consumption of probiotic-fermented milk (kefir) in diabetic patients in comparison with conventional fermented milk decreased the fasting blood glucose and HbA1C levels. Compared with two groups, serum level of total cholesterol declined but this reduction was not statistically significant. These findings suggest that probiotic fermented milk can be useful in medical nutrition management of diabetic patients.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgment
We thank all the patients who participated in this study. The authors are grateful to Nutrition Research Center of Tabriz University of Medical Sciences and Association of Diabetic Patients in Tabriz. The authors declare that there is no conflict of interests.
References
- 1. Leahy JL. ( 2005). Pathogenesis of type 2 diabetes mellitus. Arch Med Res, 36 ( 3): 197– 209. [DOI] [PubMed] [Google Scholar]
- 2. Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. ( 2008). Prevalence of Diabetes and Impaired Fasting Glucose in the Adult Population of Iran National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care, 31 ( 1): 96– 98. [DOI] [PubMed] [Google Scholar]
- 3. Guarner F, Perdigon G, Corthier Gr, Salminen S, Koletzko B, Morelli L. ( 2005). Should yoghurt cultures be considered probiotic? Br J Nutr, 93 ( 6): 783– 86. [DOI] [PubMed] [Google Scholar]
- 4. Lye H-S, Kuan C-Y, Ewe J-A, Fung W-Y, Liong M-T. ( 2009). The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci, 10 ( 9): 3755– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davari S, Talaei SA, Alaei H. ( 2013). Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience, Epub Mar 7. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Wang L, Zhang J, Li Y, He Q, Li H, et al. ( 2013). Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur J Nutr, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7. Yun SI, Park HO, Kang JH. ( 2009). Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol, 107 ( 5): 1681– 86. [DOI] [PubMed] [Google Scholar]
- 8. Greenfield JR, Campbell LV. ( 2006). Relationship Between Inflammation, Insulin Resistance and Type 2 Diabetes: Cause or Effect? Curr Diabetes Rev. 2 ( 2): 195– 211. [DOI] [PubMed] [Google Scholar]
- 9. Lin M-Y, Chen T-W. ( 2000). Reduction of cholesterol by Lactobacillus acidophilus in culture broth. J Food Drug Anal, 8 ( 2): 97– 102. [Google Scholar]
- 10. Kumar R, Grover S, Batish VK. ( 2011). Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. British J Nutr, 105 ( 4): 561– 573. [DOI] [PubMed] [Google Scholar]
- 11. Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, et al. ( 2012). Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res, 2012: 902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ooi L-G, Liong M-T. ( 2010). Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci, 11 ( 6): 2499– 2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Musso G, Gambino R, Cassader M. ( 2010). Obesity, Diabetes, and Gut Microbiota The hygiene hypothesis expanded? Diabetes Care, 33 ( 10): 2277– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. ( 2013). Effect of Probiotics on Lipid Profile, Glycemic Control, Insulin Action, Oxidative Stress, and Inflammatory Markers in Patients with Type 2 Diabetes: A Clinical Trial. Iran J Med Sci, 38 ( 1): 38– 43. [PMC free article] [PubMed] [Google Scholar]
- 15. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, et al. ( 2011). Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci, 94 ( 7): 3288– 94. [DOI] [PubMed] [Google Scholar]
- 16. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. ( 2012). Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition, 28 ( 5): 539– 543. [DOI] [PubMed] [Google Scholar]
- 17. Moroti C, Magri LFS, de Rezende Costa M, Cavallini DCU, Sivieri K. ( 2012). Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids In Health And Disease, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RMG, Moller K, Svendsen KD, et al. ( 2010). Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr, 104 ( 12): 1831– 1838. [DOI] [PubMed] [Google Scholar]
- 19. Yadav H, Jain S, Sinha PR. ( 2007). Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition, 23 ( 1): 62– 68. [DOI] [PubMed] [Google Scholar]
- 20. Lin C-H, Lin C-C, Shibu MA, Liu C-S, Kuo C-H, Tsai F-J, et al. ( 2014). Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Br J Nutr, 111 ( 4): 598– 605. [DOI] [PubMed] [Google Scholar]
- 21. Yun S, Park H, Kang J. ( 2009). Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol, 107 ( 5): 1681– 86. [DOI] [PubMed] [Google Scholar]
- 22. Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. ( 2008). Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet, 33 ( 2): 101– 106. [DOI] [PubMed] [Google Scholar]
- 23. Yamano T, Tanida M, Niijima A, Maeda K, Okumura N, Fukushima Y, et al. ( 2006). Effects of the probiotic strain Lactobacillus johnsonii strain La1 on autonomic nerves and blood glucose in rats. Life Sci, 79 ( 20): 1963– 1967. [DOI] [PubMed] [Google Scholar]
- 24. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. ( 2003). Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes, 52 ( 1): 1– 8. [DOI] [PubMed] [Google Scholar]
- 25. King GL, Loeken MR. ( 2004). Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol, 122 ( 4): 333– 8. [DOI] [PubMed] [Google Scholar]
- 26. Dunlop M. ( 2000). Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney International, 77 S3– S12. [DOI] [PubMed] [Google Scholar]
- 27. Nishikawa T, Edelstein D, Du XL, Yamagishi S-i, Matsumura T, Kaneda Y, et al. ( 2000). Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature, 404 ( 677): 787– 90. [DOI] [PubMed] [Google Scholar]
- 28. Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. ( 2011). Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis, 21 ( 11): 844– 850. [DOI] [PubMed] [Google Scholar]
- 29. Wang L-X, Liu K, Gao D-W, Hao J-K. ( 2013). Protective effects of two Lactobacillus plantarumstrains in hyperlipidemic mice. World J Gastroenterol, 19 ( 20): 3150– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sudha MR, Chauhan P, Dixit K, Babu S, Jamil K. ( 2009). Probiotics as complementary therapy for hypercholesterolemia. Biology and Medicine, 1 ( 4). [Google Scholar]
- 31. St-Onge M-P, Farnworth ER, Savard T, Chabot D, Mafu A, Jones PJH. ( 2002). Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: a randomized controlled trial [ISRCTN10820810]. BMC Complement Altern Med, 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motawee MM, ElBehairy S, Suzan FIES, Abd El-All S. Effect of some probiotic strains on lipid metabolism National organization for drug control and research (NODCAR) Giza- Egypt: 1–20. [Google Scholar]
- 33. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. ( 2007). Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia, 50 ( 11): 2374– 2383. [DOI] [PubMed] [Google Scholar]
- 34. Pickup JC. ( 2004). Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care, 27 ( 3): 813– 23. [DOI] [PubMed] [Google Scholar]
- 35. Chiu Y-H, Lu Y-C, Ou C-C, Lin S-L, Tsai C-C, Huang C-T, et al. ( 2013). Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol, 13: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, et al. ( 2005). Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia, 48 ( 8): 1565– 75. [DOI] [PubMed] [Google Scholar]


