Abstract
Background:
This study was conducted with the aim of determining surface water contamination with cysts of Entamoeba histolytica using PCR in Rasht City, Northern Iran.
Methods:
In this cross-sectional study, 49 water samples including 18 rivers and 6 wetlands were collected from different regions near the city of Rasht in autumn of 2012. After filtration using 0.22 μm nitrate cellulose membrane filters, the samples were examined using microscope and PCR method.
Results:
In microscopic examination, four samples of the 49 samples were positive for cysts of E. (histolytica / dispar / muschkovskii). By using PCR method and molecular analysis, one sample was positive for E. histolytica.
Conclusion:
In the molecular analysis, contamination by E. histolytica was proved in the waters of Rasht City. Further investigations including more samples and necessary preparations must be applied to prevent contamination.
Keywords: Entamoeba histolytica, PCR, Surface water, Iran
Introduction
Entamoeba histolytica is an enteric anaerobic protozoan parasite with about 50 million infections and over 100,000 deaths worldwide annually (1–4). Developing countries have the highest prevalence of amoebiasis because of human faeces have not been properly separated from food and water supplies. However, socio-economic factors, including poor education, poverty, overcrowding, and un-sanitary conditions are also involved in fecal-oral transmission (5). The travellers to endemic areas with low standards of hygiene and sanitation are at risk (6). E. histolytica may also be transmitted by food like uncooked vegetables, and salads. Contaminated hands of food handlers are important in transmission, too. The swimming pools are a potential source, although it has not been proved (7). The cysts of E. histolytica are very resistant and can survive for several months in water with temperature of 0 °C, 3 days at 30 °C, 30 minutes at 45 °C, 5 minutes at 50 °C, and are extremely resistant to chlorination (8).
The genus Entamoeba contains six species (E. histolytica, E. dispar, E. moshkovskii, E. poleki, E. coli, and E. hartmanni) in the human intestinal lumen (9–13). E. moshkovskii is a free-living amoeba found in anoxic sediments (10) and E. dispar is considered as a commensal of the human gut. Although E. histolytica is proved a pathogen, we still cannot definitely determine that other two species do not cause diseases (11, 12). E. histolytica, E. dispar, and E. moshkovskii are morphologically similar, but have differences in genetic and biochemistry characteristics (9–13). Since these three species cannot be differentiated by microscopy that is the most frequently used diagnostic method predominantly in tropical countries where resources are limited and can only be differentiated by the use of molecular methods such as the polymerase chain reaction based methodologies (1, 9, 14).
There are few studies on surface water contamination with Entamoeba and the most studies have been done on fecal samples in epidemiological surveillance. In Turkey two out of six water samples (32%) collected from the Ankara River were positive for E. histolytca by PCR (15). Based on a study in Thailand, 27% of surface and wastewater samples were positive for Entamoeba spp. (16). Using direct method and Gram staining on water samples in Mazandaran showed the contamination rate of E. histolytica and E. coli were 2.3%. and 0.7%, respectively (17) and Mahmoudi et al., detected Acanthamoeba species in 14 out of 27 samples by PCR method in surface water of Rasht, Guilan, Iran (18).
Considering the high level of ground water in the northern parts of Iran, the lack of adequate sanitation in rural areas, integration of surface water with domestic and industrial wastewater, especially in the rainy season, and also the fact that contaminated water is one of the transmission ways for E. histolytica, this study was conducted with the aim of determining surface water contamination with cysts of E. histolytica using PCR method in Rasht City.
Materials and Methods
In this cross-sectional study, 49 water samples were randomly taken from 18 rivers and 6 wetlands from different regions near Rasht City in autumn of 2012. Rasht City, in the southern of the Caspian Sea and capital of Guilan province, is one of the wettest regions in Iran, which can also be very humid. It is Seven meters below sea level and 15 km inland from the Anzali Lagoon. The samples were collected from 30 cm depth in one-liter bulk and transferred to the laboratory in sterile containers. After centrifugation and filtration using 0.22 μm nitrate cellulose membrane filters, the samples were examined and analysed using microscope in direct method. Positive samples for Entamoeba spp. were examined by PCR method and sequencing. Genomic DNA of E. histolytica (HM-1: IMSS) was kindly provided by Dr. Haghighi, Department of Parasitology, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Freeze-Thaw method was used for lysing cysts wall before DNA extraction. In the first phase, cysts were subjected to five freeze-and-thaw cycles to facilitate the breakage of cyst wall, followed by 20–25 minutes of sonication (30-second pulse followed by 30-second rest) electrical shock (seven shocks every 15 s) was given to cysts by Sonificator system (Hielscher, Germany). Then, DNA was extracted using a DNA isolation kit (DNP kit, Cina gene, Iran) and Phenol- Chloroform extraction method.
PCR primers were designed based on small-subunit rRNA (ribosomal RNA) of E. histolytica using Invitrogen site. Primers sequences were as follows: forward primer 5′CCCGAGAATAGAAAACTCTT3′ and reverse primer 5′TCAAGTATAGTGCACCATCT 3′. PCR amplifications were performed in a final volume of 25 μl containing one-time PCR buffer 2.5 μl, 1.5 mM MgCl2 0.8 μl, 200 μM of each dNTP, 2 U Taq DNA polymerase (Takapoo Zist, Iran), 1 μl of each primer (10 mM, Takapoo Zist) and DNA Template 2.5 μl(100–200 ng). Reactions were carried out in a Thermocycler (Eppendorf, Germany) PCR System and set as follows: 35 cycles contain denaturation at 94 ˚C, annealing at 43.5 ˚C, extension at 72˚C, every stage for 30 s and finally the PCR products were analyzed on 1.8% agarose gel after electrophoresis. PCR generates 220 bp amplicon. The Sequencing was used on PCR product (by Pishgam co., Iran) for controlling of the specificity of the result for E. histolytica.
Results
In microscopic examination, four samples of the 49 samples were positive for cysts of Entamoeba (histolytica / dispar / muschkovskii). These three species cannot be differentiated by microscopy and can only be differentiated by the use of molecular methods. By using PCR method, one sample was positive for E. histolytica. Just as we expected, in one sample in addition to positive control that was Genomic DNA of E. histolytica (HM-1: IMSS), had a band with 220 bp weight (Fig. 1).
Fig. 1:
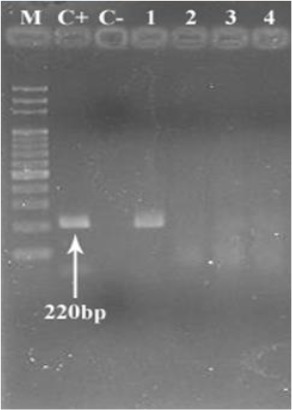
PCR amplification of samples DNA with the Entamoeba histolytica specific primers
Lane M: molecular marker (100 bp) ladders, C+: positive control (E. histolytica DNA), C−: negative control (H2O), Lane 1: amplified product (220 bp) indicating positive sample, Lanes 2–4: Positive Samples in microscopic examination that were not amplified by PCR.
In molecular analysis and sequencing (by Pishgam co., Iran) as shown in Table 1., the gene sequence had 94%, 94% and 93% homology with 18s ribosomal RNA(rRNA), 5.8s rRNA (in plasmid) and small-subunit 1 gene E. histolytica, respectively.
Table 1:
Sequences producing significant algnments (by Pishgam co., Iran)
| Gene | Accession Number* | Homology (%) |
|---|---|---|
| Entamoeba histolytica rRNA**(18s rRNA) | X65163 | 94 |
| E.histolytica plasmid genes for 5.8s rRNA and heoly sins HLY1, HLY5mc1 HLY5mc2 HLY4 | Z29969 | 94 |
| E. histolytica ss*** 1 gene | Y11271 | 93 |
GenBank
ribosomal RNA
small subunite
Discussion
The differentiation between the amoeba species is not possible using light microscopic methods and WHO has put emphasis on the need to develop improved techniques for the species-specific diagnosis of E. histolytica infection (3).
Many epidemiological surveys on the prevalence of intestinal amoeba based on microscopic methods were performed in Iran and almost all of them show a high prevalence of infection in different parts of Iran. It is essential to note that the majority of them have not used molecular methods (19–23). The recently recognized distinction among the E. histolytica, E. dispar, and E. moshkovskii has led to some confusion in epidemiological studies of amoebiasis (24).
A study in stool samples by direct and formalin-ether concentration methods in Iran proved the prevalence of infection with E. histolytica/ E. dispar was 0.78%, 3.9% and 4.6% for the central, northern and southern part of Iran, respectively (21).
A molecular method for differential diagnosis of E. histolytica and E. dispar (PCR-RFLP method) showed that in different regions of Iran, 92.1% of the isolates were E. dispar and 7.9% were E. histolytica or mixed infections. In the northern areas, 5.9% and 94.1% of isolates were E. histolytica and E. dispar, respectively (20). Many studies using molecular methods confirm that E. histolytica is a rare species in Iran and E. dispar is the predominant species (12, 20, 21, 24–30).
The only molecular study on amoeba in Iran suggesting that E. histolytica as more prevalent than E. dispar, was conducted by PCR, where 10 of 11 positive samples in microscopic examination were E. histolytica and only one of them was E. dispar (31)
Water is a possible source for transmission of Entamoeba to human host. Cysts can survive for prolonged periods in the environment, because of the protection by their cell wall (32, 33).
Bakir et al. indicated that two out of six water samples (32%) collected from the Ankara River in Turkey were positive for E. histolytca by PCR (15). Phuc et al., suggested in northern of Vietnam where Livestock and domestic sewage are used in agriculture, infection with E. histolytica depends on hygiene-related behaviors and socio-economic factors (34).
In Mazandaran (Iran) by direct method and Gram staining, 197(19.9%) out of 989 samples were contaminated with parasites. From 197 parasitic contaminated samples, 53 cases (26.9%) were pathogenic parasites. The contamination rate of E. histolytica was 2.3%. Overall, 100 cases (50.8%) were nonpathogenic and the contamination rate of E. coli was 0.7% (17). Furthermore, Mahmoudi et al., detected Acanthamoeba species in 14 out of 27 samples by PCR method in surface water of Rasht, Guilan, Iran (18).
In this study, by microscopic examination, four samples of surface water of Rasht were positive for Entamoeba, but we had not any suggestion about the species. By PCR method in these four samples, one sample was positive for E. histolytica. We had a positive control for E. histolytica, but had not any positive controls for E. dispar and E. moshkovskii, therefore could not identify the species of other three samples. For confirmation the result of PCR, we used sequencing on the PCR production. In sequencing, the gene sequence had 94%, 94% and 93% homology with 18s rRNA, 5.8s rRNA (in plasmid) and small-subunit 1 gene E. histolytica, respectively that is a confirmation for PCR examination.
Conclusion
Contamination by E. histolytica was proved in the surface water of Rasht City and this is the first report of detection of E. histolytica in surface water in Iran by molecular method.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors would like to express their special thanks to B. Rezavand, School of Medicine, Baqiyatallah University, Tehran, Iran, Dr. N. Ranji, Islamic Azad University, Rasht Branch, Rasht, Iran and T. Taheri, Tehran Kharazmi University, Tehran, Iran for support, helpful comments and editing on this manuscript. The authors declare that there is no conflict of interests.
References
- 1. Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J. ( 2007). Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev, 20: 511– 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson TF. ( 1998). Entamoeba histolytica and Entamoeba dispar are distinct species; clinical, epidemiological and serological evidence. Int J Parasitol, 28: 181– 186. [DOI] [PubMed] [Google Scholar]
- 3. WHO: World Health Organization ( 1997). Amoebiasis. WHO Weekly Epidemiological Record, 72: 97– 100. [Google Scholar]
- 4. Zlobl TL. ( 2001). Amebiasis. Primary Care Update for OB/GYNS, 8 ( 2): 65– 68. [DOI] [PubMed] [Google Scholar]
- 5. Stanley SL., Jr ( 2003). Amoebiasis. Lancet, 361 ( 9362): 1025– 34. [DOI] [PubMed] [Google Scholar]
- 6. Tan ZN, Wong WK, Nik Zairi Z, Abdullah B, Rahmah N, Zeehaida M, Rumaizi S, Lalitha P, Tan GC, Olivos-Garcia A, Lim BH. ( 2010). Identification of Entamoeba histolytica trophozoites in fresh stool sample: comparison of three staining techniques and study on the viability period of the trophozoites. Tropic Biomed, 27 ( 1): 79– 88. [PubMed] [Google Scholar]
- 7. WHO ( 1987). Prevention and control of intestinal parasitic infections: Report of a WHO Expert Committee, Geneva. WHO Technical Report Series, 749: 25. [PubMed] [Google Scholar]
- 8. WHO ( 1996). Guidelines for Drinking-Water Quality, V.2: Health Criteria and Other Supporting Information. International Programme on Chemical Safety: WHO Library Cataloguing in Publication Data. 2nd ed. Mastercom/Wiener Verlag; Austria, pp.: 15, 51. [Google Scholar]
- 9. Ali IK, Hossain MB, Roy S, Ayeh-Kumi PF, Petri WA, Jr, Haque R, Clark CG. ( 2003). Entamoeba moshkovskii infection in children, Bangladesh. Emerg Infect Dis, 9: 580– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark CG, Diamond LS. ( 1991). The Laredo strain and other ‘ Entamoeba histolytica like’ amoebae are Entamoeba moshkovskii. Mol Biochem Parasitol, 46: 11– 18. [DOI] [PubMed] [Google Scholar]
- 11. Diamond LS, Clark CG. ( 1993). A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol, 40: 340– 344. [DOI] [PubMed] [Google Scholar]
- 12. Hamzah Z, Petmitr S, Mungthin M, Leelayoova S, Chavalitshewinkoon-Petmitr P. ( 2006). Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J Clin Microbiol, 4: 3196– 3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haque R, Ali IK, Clark CG, Petri WA., Jr ( 1998). A case report of Entamoeba moshkovskii infection in a Bangladeshi child. Parasitol Int, 47: 201– 202. [Google Scholar]
- 14. Khairnar K, Parija SC, Palaniappan R. ( 2007). Diagnosis of intestinal amoebiasis by using rested polymerase chain reaction-restriction fragment length polymorphism assay. J Gastroenterol, 42: 631– 640. [DOI] [PubMed] [Google Scholar]
- 15. Bakir B, Tanyuksel M, Saylam F. ( 2003). Investigation of waterborne parasites in drinking water sources of Ankara. J Microbiol, 41 ( 2): 148– 51. [Google Scholar]
- 16. Sukprasert S, Rattaprasert P, Hamzah Z, Oleg V Shipin, Porntip Chavalitshewinkoon-Petmitr. ( 2008). Detection of Entamoeba spp. from surface and waste water samples by PCR using genus-specific primers. Southeast Asian J Trop Med Publ Health, 39 ( 1): 6– 9. [Google Scholar]
- 17. Yousefi Z, Ziaei-hezarjaribi H, Enayati AA, Mohammadpoor RA. ( 2009). Parasitic contamination of wells drinking water in Mazandaran province, Iran. J Environ Health Sci Eng, 6 ( 4): 241– 246. [Google Scholar]
- 18. Mahmoudi MR, Taghipour N, Eftekhar M, Haghighi A, Karanis P. ( 2012). Isolation of Acanthamoeba species in surface waters of Gilan province-north of Iran. Parasitol Res, 110 ( 1): 473– 477. [DOI] [PubMed] [Google Scholar]
- 19. Bairami Kuzehkanani A, Rezaei S, Babaei Z, Niyyati M, Hashemi SN, Rezaeian M. ( 2011). Enteric Protozoan Parasites in Rural Areas of Bandar-Abbas, Southern Iran: Comparison of Past and Present Situation. Iran J Public Health, 40 ( 1): 80– 85. [PMC free article] [PubMed] [Google Scholar]
- 20. Hooshyar H, Rezaian M, Kazemi B, Jeddi-Tehrani M, Solaymani-Mohammadi S. ( 2004). The distribution of Entamoeba histolytica and Entamoeba dispar in northern, central, and southern Iran. Parasitol Res, 94: 96– 100. [DOI] [PubMed] [Google Scholar]
- 21. Hooshyar H, Rezaian M, Mahmoodi M, Farnia Sh, Solaymani-Mohammadi Sh. ( 2004). A Field Study of the Distribution of Entamoeba histolytica/E. dispar Cyst Passers in Northern, Central, and Southern Iran. Iran J Public Health, 33 ( 2): 28– 32. [Google Scholar]
- 22. Khousheh-Mehri G, Mobedi I, Kia EB, Kaviani A, Ahmadi SA, Mehdi-Miri SR, Yousefi H, Gholami E. ( 2009). Study on the intestinal parasites in mazandaran province (northern of Iran). Iran J Publ Health, 29 ( 1–4): 155– 164. [Google Scholar]
- 23. Rezaiian M, Hooshyar H. ( 1996). The prevalence of intestinal parasitic infection in rural areas of Tonekabon, Iran. Iran J Public Health, 25 ( 3–4): 47– 58. [Google Scholar]
- 24. Hooshyar H, Rostamkhani P, Rezaiian M. ( 2012). Molecular Epidemiology of Human Intestinal Amoebas in Iran. Iran J Public Health, 41 ( 9): 10– 17. [PMC free article] [PubMed] [Google Scholar]
- 25. Fallah M, Haghighi A, Tachibana H. ( 2001). Preliminary comparative study of Entamoeba histolytica and Entamoeba dispar by PCR technique in Iran. Med J Islamic Rep Iran, 14 ( 4): 369– 372. [Google Scholar]
- 26. Haghighi A, Salimi Khorashad A, Nazemalhosseini Mojarad E, Kazemi B, Rostami Nejad M, Rasti S. ( 2009). Frequency of enteric protozoan parasites among patients with gastrointestinal complaints in medical centers of Zahedan, Iran. R Soc of Trop Med and Hyg, 103: 452– 54. [DOI] [PubMed] [Google Scholar]
- 27. Hooshyar H, Rezaiian M, Kazemi B. ( 2003). Distribution and differential diagnosis of Entamoeba histolytica from Entamoeba dispar by the PCR-RFLP method in central Iran. Ann Saudi Med, 23 ( 6): 363– 366. [DOI] [PubMed] [Google Scholar]
- 28. Nazemalhoseini Mojarad E, Haghighi A, Azimi Rad M, Mesgarian F, Rostami Nejad M, Zali MR. ( 2007). Prevalence of Entamoeba histolytica and Entamoeba dispar in Gonbad City, 2006. Iran J Parasitol, 2 ( 2): 48– 52. [Google Scholar]
- 29. Nazemalhosseini Mojarad E, Nochi Z, Sahebekhtiari N, Rostami Nejad M, Dabiri H, Haghighi A. ( 2010). Characterization of Entamoeba histolytica and Entamoeba dispar in fresh stool by PCR. Gastro Hepath, 3 ( 1): 37– 41. [Google Scholar]
- 30. Rezaiian M, Hooshyar H. ( 2006). Differential diagnosis of Entamoeba histolytica from Entamoeba dispar and a study on the intestinal parasites in rural areas of Ahwaz and Hamidieh. J Sch Publ Health Ins Publ Health Res, 4 ( 4): 33– 38. [Google Scholar]
- 31. Pestehchian N, Nazary M, Haghighi A, Salehi A, Yosefi H. ( 2011). Frequency of Entamoeba histolytica and Entamoeba dispar prevalence among patients with gastrointestinal complaints inchelgerd city, southwest of Iran. J Res Med Sci, 16 ( 11): 1436– 1440. [PMC free article] [PubMed] [Google Scholar]
- 32. Markell EK. ( 1999). Lumen-dwelling protozoa. In: Markell and Voge's Medical Parasitology. Eds, John, Krotoski 8th edition W. B. Saunders, Philadelphia, pp.: 24– 89. [Google Scholar]
- 33. Mohammad K, Zalie MR, Sirous S. ( 1995). Intestinal parasites in Iran. Iran J Public Health, 24 ( 3–4): 9– 26. [Google Scholar]
- 34. Phuc PD, Nguyen-Viet H, Hattendorf J, Zinsstag J, Cam PD, Odermatt P. ( 2011). Risk factors for Entamoeba histolytica infection in an agricultural community in Hanam province, Vietnam. Parasites & Vectors, 4: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]


