Abstract
A patient assessed by heart rate variability (HRV) methodology, beginning just after the completion of brain death (BD) diagnosis, showed remaining very low frequency (VLF) waves for approximately 10 min. A time-varying spectral analysis showed that during the first 550 s, a significant power spectral density remained in the high-frequency (HF), low-frequency (LF) and VLF bands. From 550 to 675 s, the HF oscillations totally vanished, and a marked progressive decay of the LF and VLF power density occurred. After 700 s the VLF undulations stopped and remaining small amplitude oscillations at 0.2 Hz coincided with the ventilator frequency. The VLF oscillations recorded in our case might be related to residual sympathetic vasomotor activity that progressively disappeared due to the extension of necrosis affecting the nervous centres of the lower part of the medulla and the first 2–3 cervical spine segments.
Background
Several papers have appeared over the past decades regarding the assessment of coma and brain death (BD) by a heart rate variability (HRV) technique. HRV can easily be calculated on ECG records by using time and/or frequency domain methods of analysis.1–8 The HRV power spectral analysis has been proposed as a tool to diagnose brainstem damage through the assessment of the functional integrity of the autonomic nervous system vital nuclei in this area.9–11 Several authors have also suggested the use of an HRV time-frequency analysis as an indicator of the progressive loss of different HRV bands during the clinical evolution from coma to BD.2 3 9 For example, Baillard et al2 found that the spectral power in the low-frequency (LF) range reached zero, whereas spectral power in the high-frequency (HF) band persisted weakly. We recently published a paper comparing comatose patients classified in two subgroups according to the Glasgow Coma Scale (GCS) scores, and found a significant decrement for absolute power of the very low frequency (VLF), LF and HF bands in the subgroup of GCS=3.9
This case was part of a protocol in which we are studying patients fully diagnosed as BD by the HRV methodology. This patient showed remaining VLF waves during approximately 10 min, following the completion of BD diagnosis.
Case presentation
A 45-year-old woman with a history of arterial hypertension developed a severe and acute headache without focal neurological signs. In the subsequent days, her condition deteriorated, and a transcranial Doppler (TCD) study showed severe vasospasm. A second CT scan ruled out rebleeding, but demonstrated bilateral infarction of the anterior cerebral arteries territories. On day 12, the patient suffered a cardiac arrest, and after reanimation the GCS decreased to a score of 3 points; a second TCD study showed a systolic spike pattern, demonstrating the absence of cerebral blood flow, and diabetes insipidus was diagnosed.
BD was certified based on the Cuban Criteria for the Determination of and Certification of Death BD diagnosis.7 12–14 These criteria are included in a national law, describing a precise protocol, which should be followed step by step, applying a neurological examination for assessing the loss of brainstem reflexes, the apnoea test and the ancillary test to demonstrate the total absence of bioelectrical activity (EEG and evoked potentials) and cerebral blood flow (TCD). After a period of observation of 6 h, the neurological examinations and ancillary tests are repeated. According to these criteria, the last examination to complete and certify the BD diagnosis is the end of the second apnoea test, which is the official time of death.
Although the ECG had been recorded for several hours in this patient, as part of the general monitoring, the results presented here were considered from the ECG record immediately after BD diagnosis was entirely finished. The pattern of the HRV analysis before the diagnosis of BD corresponded to that described by us in comatose patients with GCS=3, but not BD.9
According to the aforementioned protocol, a bipolar CM2–V5 chest lead was used to record the ECG during 20 min, beginning just after the completion of BD diagnosis. Technical details have been described elsewhere.9 In this study, for the time-varying spectral analysis (TVSPA), spectral estimations included 300 s and a progression step of 5 s. The total number of estimations was 196.
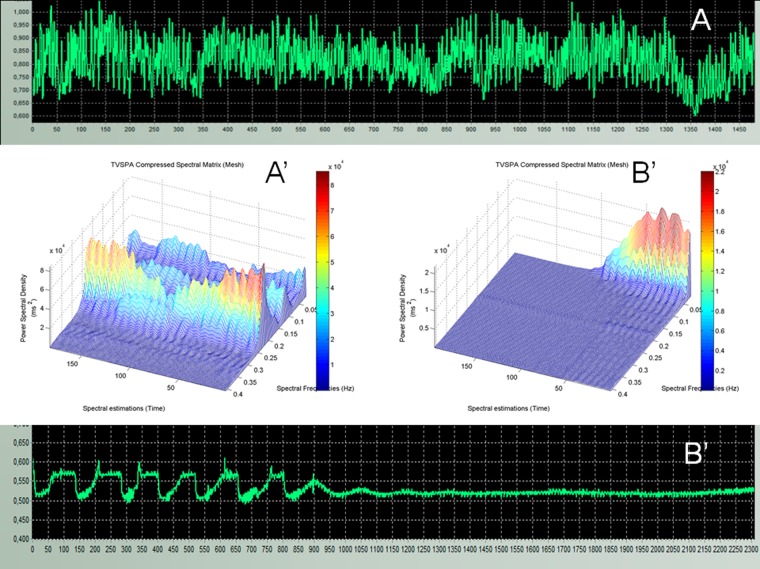
In figure 1, the tachogram of RR sequences and the spectrogram of a control healthy subject (panels A and A′), and the patient (panels B and B′), matched by age and gender, are presented. In figure 1A, clear oscillations of beat-to-beat values were observed, as typically expected for a healthy subject in supine position and spontaneously breathing. In the spectrogram (figure 1A′), the HF components (from 0.15 to 0.40 Hz) were dominant during the whole ECG record, showing the characteristic parasympathetic preponderance of this functional state. The VLF and LF spectral components (from 0.02 to 0.15 Hz) were significantly less evident, indicating a slight predominance of the vagal tone during the control ECG recording in this participant. In figure 1B, it was possible to observe eight long period oscillations in the RR intervals tachogram of the BD patient, corresponding to the VLF band frequencies, and with an amplitude (peak to peak) of about 100 ms. These waves progressively reduced their amplitude, and vanished approximately 10 min after the ECG record was begun.
Figure 1.
(A) A tachogram showing RR cardio-intervals from a healthy subject, obtained from a 20 min ECG record. (A′) Spectral matrices arranged in three-dimensional surface diagrams to represent the time-varying spectral analysis (TVSPA) analysis of the RR cardio-intervals tachogram shown in A. (B) The tachogram from the brain-dead is shown, and the corresponding TVSPA analysis is labelled as B′.
In figure 1B′, it is shown that during the first 50 spectral estimations (550 s) there was still a well-defined power spectral density in the range of the VLF (0.023–0.04 Hz), and the LF (0.04–0.15 Hz) bands, in the BD case. In the range of the HFs (>0.15 Hz), it could be observed a low power spectral density of about 0.2 Hz which was slightly larger than the power observed after the first 50 spectral estimations, at that same discrete spectral frequency. Between the 50 and the 75 spectral estimations (550–675 s), a total reduction of the HF power density and a progressive decay of LF and VLF power density were evident. The VLF spectral frequencies were the last to disappear, and after 100 spectral estimations (800 s), oscillations of 0.2 Hz only remained, coinciding with the life support ventilator frequency (12 cycles per minute).
Investigations
For assessing HRV, the spectral resolution was 1/300 s=0.003 Hz. The selected processing parameters allowed the study of spectral frequencies including the VLF band from 0.023 to 0.04 Hz, the LF band from 0.04 to 0.085 Hz, the mid-frequency band from 0.085 to 0.15 Hz and the HF band from 0.15 to 0.40 Hz.
To represent the TVSPA analysis, spectral matrices were arranged in three-dimensional surface diagrams (figure 1), representing the PSD intensity, the corresponding discrete spectral frequencies and the consecutive time, always considering that the first spectral estimation included the first 300 s, and later on, each one was displaced for 5 s from the preceding one.
Discussion
When we observed in this patient's record long-period oscillations within the range of the VLF band, our first task was to exclude the possibility that these undulations were related to any biological or technical artefact on the ECG record.
The HF component is considered to be a marker of the parasympathethic activity.15–18 Hereafter, the initial complete loss of HF component in our case might be an expression of the progressive destruction of the lower region of the medulla, affecting the vagal nuclei.9 19 A remaining oscillation within the HF band coincided with the life support ventilator frequency. Baillard et al2 demonstrated that during the apnoea test in BD cases, these HF frequencies disappeared. Therefore, the artificial ventilator was in fact the driving input with a pure mechanical origin for the generation of this HF peak.2 9
The LF band is related to vagal and sympathetic influences.20–22 Therefore, the destruction and dysfunction of the lower medulla affecting vagal, sympathetic nuclei and descending tracts might explain the progressive disappearance of the LF band in our case.
The most interesting finding to discuss was the remaining VLF spectral frequencies for approximately 10 min after the completion of BD diagnosis. Regarding the VLF band, there are still discussions about its origin.23 24 The VLF power probably represents several factors influencing the heart, such as the renin–angiotensin system, thermoregulation and sympathetic vasomotor activity.
Concerning the renin–angiotensin system, Bonaduce et al reported that after captopril medication in patients with acute myocardial infarction, a significant increase was observed in the VLF power band. Therefore, these authors support the hypothesis that the renin–angiotensin system modulates the amplitude VLF band by reducing its power, not enhancing it.25 Other authors have also commented that a blockade of ACE increases HRV in the VLF range.23 Hence, the preservation in our case of the VLF oscillations cannot be explained due to the direct effect of the renin–angiotensin system.
The hypothalamic contribution to the thermoregulatory mechanisms might be excluded to explain the presence of those VLF oscillations, because the corresponding ECG record was performed after demonstrating the loss of cerebral blood flow by a TCD study, and the patient reported a diabetes insipidus. The only possible correlate to a thermoregulation might be a vascular mechanism coming from sympathetic vasomotor activation.9
Some authors have reported that VLF oscillations are primarily derived from parasympathetic outflow.23 26 As has been previously discussed, the HF and LF bands disappeared before the remaining VLF oscillations. Therefore, the destruction of the nucleus ambiguous had already occurred, excluding that the VLF component in our case had a parasympathetic drive.
On the contrary, several authors affirm that the VLF is closely related to a sympathetic outflow. Palma et al27 reported that patients with Parkinson's disease consistently had a significantly decreased LF and VLF during rapid eye movement sleep, indicating a reduced sympathetic influence on HRV. Marthol et al28 reported a prominent sympathetic storm shortly before BD, characterised by an increase of diastolic and systolic blood pressure, by a sympathetically mediated blood pressure–LF mechanism. Montemurro et al reported that patients with severe obstructive sleep apnoea, but without excessive daytime sleepiness (EDS), have higher VLF activity than those with EDS, suggesting a main sympathetic modulation of the HRV band.29 Ryan et al10 demonstrated that the VLF band is an independent predictor of mortality and morbidity in haemodynamically stable traumatic brain-injury patients. We recently studied comatose patients with different GCS scores by the HRV technique and found that a significant decrement occurred for the VLF band absolute power in the subgroup of GCS=3. We speculated that these findings might be explained by the destruction of brain-stem sympathetic structures, mainly those related to the rostral ventrolateral medulla oblongata area.9
Therefore, according to these results, it might be considered that there is a primary sympathetic contribution to the VLF band. Several authors have suggested that VLF frequencies originate in supraspinal vasomotor centres.19 30–33 Kuo and Yang19 have demonstrated that the VLF power of arterial pressure variability are related to vasomotor reactivity in response to control signals from the rostral ventrolateral medulla via the sympathetic system in the rat. A recent review describes different neuronal structures playing an important role in the regulation of the vasomotor tone at the medullar brainstem level that extends from the inferior pole of the inferior olive to the C1-C2 cervical spinal segments.34 35
Walker et al36 reported that the cervicomedullary junction was necrotic in about half of their BD patients. Moreover, Goldie et al37 emphasised that the region of the cervicomedullary junction is considered an interphase between extracranial and intracranial blood supply, which might explain functional activity in this region for some time period after the onset of BD syndrome.
We reported a BD case, assessed by median nerve short latency somatosensory evoked potential (SEPs), and e found no somatosensory components after P9-N9–Erb potential in any of the cephalic and non-cephalic derivations. This patient suffered an intracerebral haemorrhage and had been diagnosed as BD 26 h before the SEPs study. On the basis of the SEPs assessment, we stated that the somatosensory electrical conduction was blocked at the level of the brachial plexus, and that no impulse transmission passed through the cervical spine segments.38
We might speculate that the remaining VLF oscillations in our case were related to residual sympathetic vasomotor activity that progressively disappeared due to the extension of necrosis from the lower part of the medulla, up to the cervicomedullary junction, and the first 2–3 cervical spine segments.
Patient's perspective.
This patient was dead based on neurological grounds (brain death).
Learning points.
We studied a patient after completion of brain death testing and found the persistence of very low frequency (VLF) waves for approximately 10 minutes before disappearing.
The VLF spectral frequencies were the last heart rate variability (HRV) oscillations to disappear, and after 700 s, only very small amplitude undulations at 0.2 Hz remained, coinciding with the ventilator frequency (12 cycles/min).
We might speculate that the remaining VLF oscillations in our case were related to the residual sympathetic vasomotor that progressively disappeared due to the extension of necrosis from the lower part of the medulla, up to the cervicomedullary junction, and the first 2–3 cervical spine segments.
Footnotes
Contributors: CM was the main contributor to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work. ME provided substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work. JP-N contributed to the analysis or interpretation of data for the work; acquisition, analysis, or interpretation of data for the work. AS contributed to the analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Machado C, Garcia OD, Gutierrez J et al. Heart rate variability in comatose and brain-dead patients. Clin Neurophysiol 2005;116:2859–60. 10.1016/j.clinph.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 2.Baillard C, Vivien B, Mansier P et al. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med 2002;30:306–10. 10.1097/00003246-200202000-00007 [DOI] [PubMed] [Google Scholar]
- 3.Baillard C, Goncalves P, Mangin L et al. Use of time frequency analysis to follow transitory modulation of the cardiac autonomic system in clinical studies. Auton Neurosci 2001;90:24–8. 10.1016/S1566-0702(01)00263-6 [DOI] [PubMed] [Google Scholar]
- 4.Conci F, Di RM, Castiglioni P. Blood pressure and heart rate variability and baroreflex sensitivity before and after brain death. J Neurol Neurosurg Psychiatry 2001;71:621–31. 10.1136/jnnp.71.5.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crenna P, Conci F, Boselli L. Changes in spinal reflex excitability in brain-dead humans. Electroencephalogr Clin Neurophysiol 1989;73:206–14. 10.1016/0013-4694(89)90121-1 [DOI] [PubMed] [Google Scholar]
- 6.Rapenne T, Moreau D, Lenfant F et al. Could heart rate variability predict outcome in patients with severe head injury? A pilot study. J Neurosurg Anesthesiol 2001;13:260–8. 10.1097/00008506-200107000-00016 [DOI] [PubMed] [Google Scholar]
- 7.Machado C. Brain death: a reappraisal. New York: Springer, 2007:1–126. [Google Scholar]
- 8.Su CF, Kuo TB, Kuo JS et al. Sympathetic and parasympathetic activities evaluated by heart-rate variability in head injury of various severities. Clin Neurophysiol 2005;116:1273–9. 10.1016/j.clinph.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 9.Machado-Ferrer Y, Estevez M, Machado C et al. Heart rate variability for assessing comatose patients with different Glasgow Coma Scale scores. Clin Neurophysiol 2013;124:589–97. 10.1016/j.clinph.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Ryan ML, Thorson CM, Otero CA et al. Clinical applications of heart rate variability in the triage and assessment of traumatically injured patients. Anesthesiol Res Pract 2011;2011:416590 10.1155/2011/416590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan ML, Ogilvie MP, Pereira BM et al. Heart rate variability is an independent predictor of morbidity and mortality in hemodynamically stable trauma patients. J Trauma 2011;70:1371–80. 10.1097/TA.0b013e31821858e6 [DOI] [PubMed] [Google Scholar]
- 12.Machado C. [Resolution for the determination and certification of death in Cuba]. Rev Neurol 2003;36:763–70. [PubMed] [Google Scholar]
- 13.Machado C. Determination of death. Acta Anaesthesiol Scand 2005;49:592–3. 10.1111/j.1399-6576.2004.00584.x [DOI] [PubMed] [Google Scholar]
- 14.Machado C, Abeledo M, Alvarez C et al. Cuba has passed a law for the determination and certification of death. Adv Exp Med Biol 2004;550:139–42. 10.1007/978-0-306-48526-8_11 [DOI] [PubMed] [Google Scholar]
- 15.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med 2009;76(Suppl 2):S86–90. 10.3949/ccjm.76.s2.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav 2003;79:503–13. 10.1016/S0031-9384(03)00156-2 [DOI] [PubMed] [Google Scholar]
- 17.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol 2001;42:123–46. 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- 18.Reed SF, Ohel G, David R et al. A neural explanation of fetal heart rate patterns: a test of the polyvagal theory. Dev Psychobiol 1999;35:108–18. [DOI] [PubMed] [Google Scholar]
- 19.Kuo TB, Yang CC. Altered frequency characteristic of central vasomotor control in SHR. Am J Physiol Heart Circ Physiol 2000;278:H201–7. [DOI] [PubMed] [Google Scholar]
- 20.No authors listed Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–81. 10.1093/oxfordjournals.eurheartj.a014868 [DOI] [PubMed] [Google Scholar]
- 21.Akselrod S, Gordon D, Ubel FA et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981;213:220–2. 10.1126/science.6166045 [DOI] [PubMed] [Google Scholar]
- 22.Appel ML, Berger RD, Saul JP et al. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol 1989;14:1139–48. 10.1016/0735-1097(89)90408-7 [DOI] [PubMed] [Google Scholar]
- 23.Tripathi KK. Very low frequency oscillations in the power spectra of heart rate variability during dry supine immersion and exposure to non-hypoxic hypobaria. Physiol Meas 2011;32:717–29. 10.1088/0967-3334/32/6/008 [DOI] [PubMed] [Google Scholar]
- 24.Bilgin S, Colak OH, Polat O et al. Determination of a new VLF band in HRV for ventricular tachyarrhythmia patients. J Med Syst 2010;34:155–60. 10.1007/s10916-008-9227-8 [DOI] [PubMed] [Google Scholar]
- 25.Bonaduce D, Marciano F, Petretta M et al. Effects of converting enzyme inhibition on heart period variability in patients with acute myocardial infarction. Circulation 1994;90:108–13. 10.1161/01.CIR.90.1.108 [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Carr DL, Myers CW et al. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 1998;98:547–55. 10.1161/01.CIR.98.6.547 [DOI] [PubMed] [Google Scholar]
- 27.Palma JA, Urrestarazu E, Alegre M et al. Cardiac autonomic impairment during sleep is linked with disease severity in Parkinson's disease. Clin Neurophysiol 2013;124:1163–8. 10.1016/j.clinph.2012.12.042 [DOI] [PubMed] [Google Scholar]
- 28.Marthol H, Intravooth T, Bardutzky J et al. Sympathetic cardiovascular hyperactivity precedes brain death. Clin Auton Res 2010;20:363–9. 10.1007/s10286-010-0072-8 [DOI] [PubMed] [Google Scholar]
- 29.Taranto ML, Floras JS, Picton P et al. Relationship of heart rate variability to sleepiness in patients with obstructive sleep apnea with and without heart failure. J Clin Sleep Med 2014;10:271–6. 10.5664/jcsm.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K, Miyake S, Kumashiro M et al. Power spectral analysis of blood pressure variability in traumatic quadriplegic humans. Am J Physiol 1991;260:H842–7. [DOI] [PubMed] [Google Scholar]
- 31.Cooley RL, Montano N, Cogliati C et al. Evidence for a central origin of the low-frequency oscillation in RR-interval variability. Circulation 1998;98:556–61. 10.1161/01.CIR.98.6.556 [DOI] [PubMed] [Google Scholar]
- 32.Montano N, Gnecchi-Ruscone T, Porta A et al. Presence of vasomotor and respiratory rhythms in the discharge of single medullary neurons involved in the regulation of cardiovascular system. J Auton Nerv Syst 1996;57:116–22. 10.1016/0165-1838(95)00113-1 [DOI] [PubMed] [Google Scholar]
- 33.Leor-Librach RJ, Eliash S, Kaplinsky E et al. Very low-frequency heart rate variability wave amplitude and sympathetic stimulation—characterization and modeling. IEEE Trans Biomed Eng 2003;50:797–803. 10.1109/TBME.2003.813547 [DOI] [PubMed] [Google Scholar]
- 34.Goodchild AK, Moon EA. Maps of cardiovascular and respiratory regions of rat ventral medulla: focus on the caudal medulla. J Chem Neuroanat 2009;38:209–21. 10.1016/j.jchemneu.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 35.Goodchild AK, Moon EA, Dampney RA et al. Evidence that adrenaline neurons in the rostral ventrolateral medulla have a vasopressor function. Neurosci Lett 1984;45:267–72. 10.1016/0304-3940(84)90237-4 [DOI] [PubMed] [Google Scholar]
- 36.Walker AE, Diamond EL, Moseley J. The neuropathological findings in irreversible coma. A critque of the “respirator”. J Neuropathol Exp Neurol 1975;34:295–323. 10.1097/00005072-197507000-00001 [DOI] [PubMed] [Google Scholar]
- 37.Goldie WD, Chiappa KH, Young RR et al. Brainstem auditory and short-latency somatosensory evoked responses in brain death. Neurology 1981;31:248–56. 10.1212/WNL.31.3.248 [DOI] [PubMed] [Google Scholar]
- 38.Machado C, Valdes P, Garcia O et al. Short latency somatosensory evoked potentials in brain-dead patients using restricted low cut filter setting. J Neurosurg Sci 1993;37:133–40. [PubMed] [Google Scholar]