Abstract
We describe the rare occurrence of an Actinomyces meyeri cerebral abscess in a 55-year-old woman following a dental extraction. This patient presented with a 2-day history of hemisensory loss, hyper-reflexia and retro-orbital headache, 7 days following a dental extraction for apical peridonitis. Neuroimaging showed a large left parietal abscess with surrounding empyema. The patient underwent craniotomy and drainage of the abscess. A. meyeri was cultured. Actinomycosis is a rare cause of cerebral abscess. The A. meyeri subtype is particularly rare, accounting for less than 1% of specimens. This case describes an unusually brief course of the disease, which is usually insidious. Parietal lobe involvement is unusual as cerebral abscesses usually have a predilection for the frontal and temporal regions of the brain. Although there are no randomised trials to guide therapy, current consensus is to use a prolonged course of intravenous antibiotics, followed by 6–12 months of oral therapy.
Background
Actinomyces spp as a cause of brain abscess is rare. The rate of symptom progression in this particular case to time of presentation was unusually rapid. The patient's neurological symptoms occurred 5 days following dental extraction. It is probable that some pre-existing underlying dental pathology led to direct cranial extension of the Actinomyces spp prior to the extraction. The location of the abscess in the parietal lobe is unusual as cerebral abscesses usually have a predilection for the temporal or frontal regions.
The Actinomyces meyeri organism itself is very rare, accounting for 1% of all Actinomyces spp involved in human pathogenesis. Optimal duration of therapy is not clearly defined, and requires further cases to be reported to allow evidence to accumulate.
Case presentation
A 55-year-old woman presented with a 2-day history of left retro-orbital headache, right hemisensory loss and unsteady gait. She initially experienced subacute onset of left retro-orbital pain and headache, which worsened over the following hours, and subsequently developed gradual onset of right arm and leg numbness with associated unsteady gait. Her medical history was only remarkable for chronic right-sided hearing impairment and dental extraction 7 days prior to presentation. She had been on no regular medications before admission and had no known drug allergies. She had a history of dental extraction of her left lower molar 7 days prior to presentation. Her dental records documented a finding of apical periodontitis. She did not have antibiotic prophylaxis prior to the extraction as there was no evidence of dental abscess. She also had a history of six dental implants. On the evening of the dental extraction she developed fever of 38°C at home followed by recurrent temperatures the following morning. She presented to the hospital 6 days later.
Examination revealed normal tone and power in all limbs, hyper-reflexia in the right lower limb (knee and ankle), bilateral downgoing plantars, and decreased right upper and lower limb sensation. Truncal sensation was normal. Cranial nerve examination was normal apart from chronic hearing loss in the right ear. Cerebellar examination was unremarkable. Gait was unsteady to the right side and heel-toe gait was impaired. Dental examination was normal.
Investigations
Blood tests on admission showed a raised C reactive protein of 92 mg/dL, white cell count 11.1×109/L and neutrophils 7.8×109/L. Blood cultures were negative. Chest X-ray was normal. CT of the brain showed a left parietal mass initially reported as a likely primary glial tumour but revised to results consistent with brain abscess and empyema (figure 1). The patient proceeded to have an MRI of the brain with gadolinium contrast to further delineate the nature of the lesion, which showed a peripherally enhancing lesion in the posterior left parietal lobe measuring 4×3×4 cm with significant adjacent oedema with mild local mass effect, and restricted diffusion centrally (figures 2 and 3). There was an accompanying small subdural empyema. CT of sinuses showed mucoperiosteal thickening in the sphenoid sinus but nothing else of significance. Transthoracic echocardiography did not show any valvular abnormalities.
Figure 1.
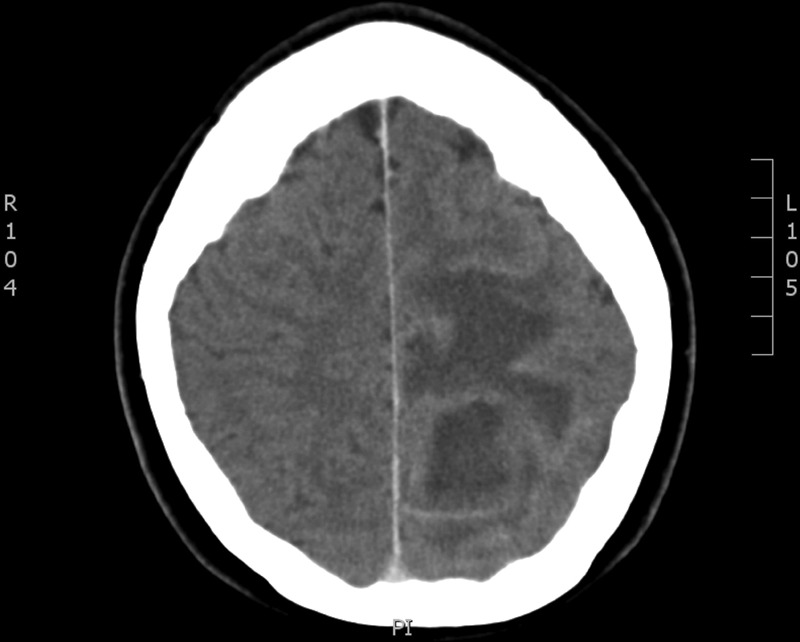
Initial CT of the brain with contrast.
Figure 2.
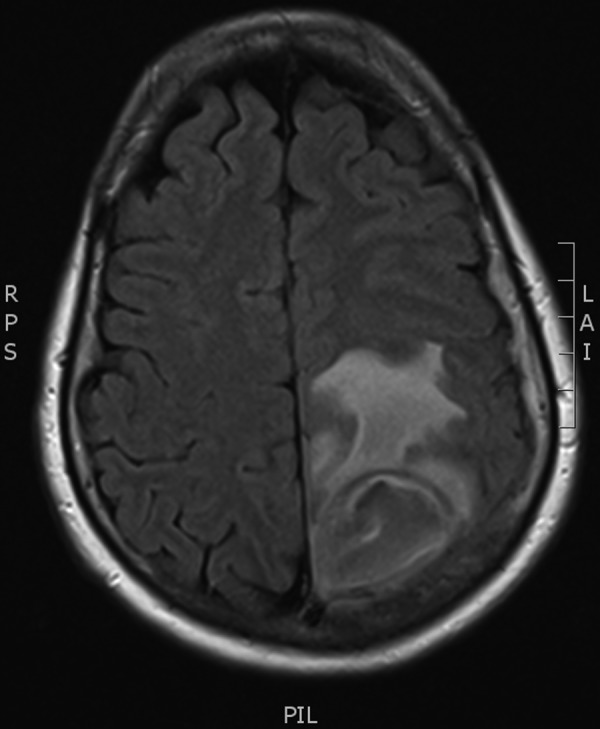
MRI of the brain with gadolinium contrast.
Figure 3.
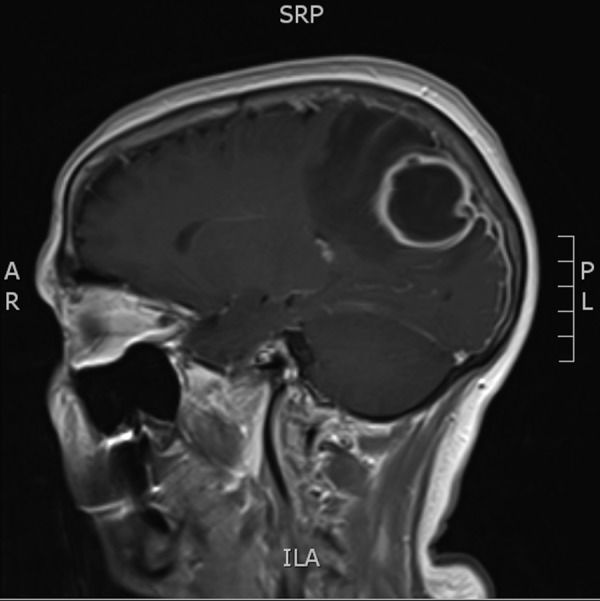
MRI post-gadolinium—sagittal view.
The patient underwent craniotomy and drainage of the abscess. Her neurological symptoms resolved.
Pus from the abscess initially grew Gram-positive cocci. The sample underwent extended incubation and further culture grew Streptococcus mitis and a metronidazole-resistant anaerobic organism after 5 days. This organism was subsequently identified as A. meyeri by MALDI-TOF (Matrix-assisted laser desorption/ionisation time of flight) mass spectrometry (MALDI Biotyper, Bruker), which was confirmed by the reference laboratory using the same methodology.
Differential diagnosis
Postoperatively, the diagnosis of abscess was clear, but at initial presentation the differential diagnoses had included primary or metastatic brain tumour (including central nervous system lymphoma), haematoma (although no history of head injury) and demyelinating disease.
Treatment
A peripherally inserted central catheter line was placed to facilitate a predicted long duration of antimicrobial therapy.
Initially, the patient was started on vancomycin, ceftriaxone and metronidazole, prior to full availability of sensitivities. Vancomycin was stopped after 11 days. Intravenous metronidazole was stopped after 1 month and ceftriaxone continued (discussed later). The patient was treated with intravenous therapy on an outpatient parenteral antimicrobial therapy (OPAT) programme as she was resident in a relatively remote region over 2 h away from the specialist hospital and requested discharge.
Outcome and follow-up
The patient had an interval follow-up CT of the brain 8 days following her first surgery, which showed decreased size of the original abscess cavity in the left parietal lobe. However, it did show a new, small left frontal lobe subdural collection, not evident on prior imaging. She was discharged on OPAT therapy for re-scanning as an outpatient. Three weeks later, reimaging showed an increase in size of the left frontal lobe lesion (figure 4). She underwent a left frontal craniotomy and further evacuation. The pus drained from this abscess isolated a group B Streptococcus and Staphylococcus capitis. Extended incubation for 10 days did not yield any further isolates.
Figure 4.
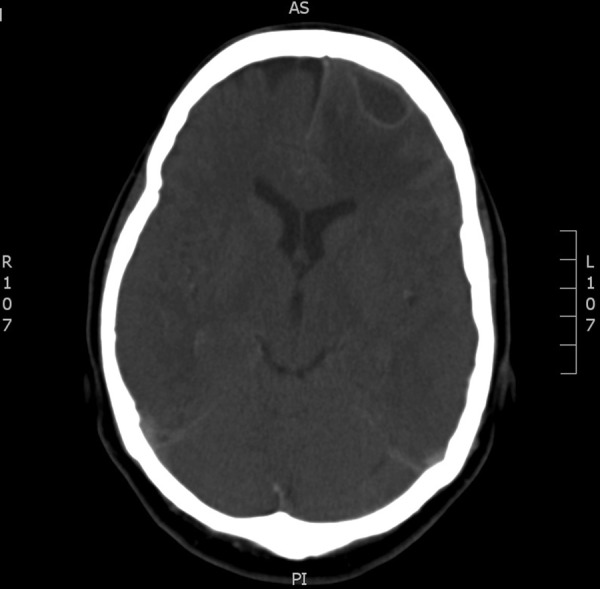
Interval CT of the brain with new frontal collection.
The patient remained on intravenous ceftriaxone for a total of 4 months with excellent clinical response and was subsequently placed on oral amoxicillin therapy to complete a total of 12 months of treatment. Therapy is ongoing with interval neurosurgical reviews and CTs of the brain. The patient remains asymptomatic. She was also prescribed levetiracetam for seizure prophylaxis.
Discussion
Actinomycosis was first named as the causative agent of bovine actinomycosis when it was described in 1876 by the German pathologist Otto Bollinger, who identified the species in cattle mandibular specimens and termed the condition ‘lumpy jaw disease of cattle’.1
Actinomyces is a species composed of anaerobic, non-acid fast, branching filamentous rods.2 It gives rise to ‘sulfur granules’ that form due to conglomeration of the organism.3 These granules tend to advance through, rather than along, soft tissue planes, as demonstrated by the cervicofacial extension to the cerebrum in this case.2
It is an oral commensal organism that rarely causes disease but is implicated in chronic, insidious infections of the cervicofacial, gastrointestinal and respiratory tracts.4 Most infections that do arise are caused by the subtype Actinomyces israeli, which accounts for over 40% of all Actinomyces infections. The A. meyeri subtype is particularly rare; accounting for less than 1% of cultures.5 Dental extraction procedures are thought to be a risk factor for cervicofacial Actinomyces due to penetration and microtrauma of the oral mucosa; they are particularly associated with mandibular molar extractions.2 They are reported to have a predilection for the frontal and temporal regions of the brain.5
This patient's infection had probably been forming for some time prior to her dental procedure. Actinomyces infections tend to have an insidious/progressive onset with a chronic course, with a median symptom duration of 2.1 months prior to presentation.5 The proximity of dental extraction to the onset of symptoms is likely to be coincidental, that is, the abscess was already formed prior to dental extraction. The rapidity of clinical progression and time to initial presentation in this case is unusual.
Actinomyces is commonly isolated in combination with other organisms, for example, streptococcal species, as in this case. Actinomyces organisms are particularly sensitive to penicillin, and have also been shown to be susceptible to Erythromycin, Cephalosporins and Clindamycin.6
The decision was made to treat the patient with intravenous ceftriaxone because 24 h infusion pumps for penicillin for OPAT were not available in our region. Ceftriaxone has previously been used successfully to treat significant Actinomyces infections.7 Another reason for the choice of ceftriaxone was the presence of polymicrobial infection (all organisms susceptible to ceftriaxone in vitro), and because of its excellent central nervous system (CNS) penetration. The patient was counselled regarding the risks of Clostridium difficile infection while on therapy.
Previous case reports have suggested a duration of 3–12 months of therapy for the most severe infections.8 One case report describes a patient with a history of chronic ethanol abuse who was non-compliant with oral antibiotics following a 6-week course of parenteral antibiotics. Despite this, CT imaging 6 months later showed resolution of multiple cerebral abscesses.8 A case series reviewing 26 cases of A. meyeri infection reported between 1960 and 1995 found that the mean duration of therapy was 6 months. No recurrences were reported in 10 patients treated for a shorter period of 3–4 months.9 Some experts suggest using intravenous antibiotics for 2–6 weeks, followed by 6–12 months of oral therapy, although we felt a longer course of intravenous therapy was warranted in this instance, given CNS infection and the use of ceftriaxone instead of penicillin.
This organism is a rare cause of cerebral abscesses. There are 58 reported cases of A. meyeri infections; primarily thoracic in nature. Three further case reports describe A. meyeri brain abscesses secondary to haematogenous dissemination from pulmonary Actinomyces infection.
Learning points.
This case describes an unusually brief clinical course of cerebral abscess secondary to actinomycosis—it usually follows a slowly progressive course.
The parietal lobe is an unusual location for these abscesses, as they are reported to have a predilection for the frontal and temporal regions of the brain.
The Actinomyces meyeri species is particularly rare, accounting for less than 1% of all actinomycosis.
Ceftriaxone may be a treatment option for Actinomyces infections requiring outpatient parenteral antimicrobial therapy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Miller M, Haddad AJ. Cervicofacial actinomycosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:496–508. 10.1016/S1079-2104(98)90280-3 [DOI] [PubMed] [Google Scholar]
- 2.Bartels LJ, Vrabec DP. Cervicofacial actinomycosis. Arch Otolaryngol 1978;104:705–8. 10.1001/archotol.1978.00790120031005 [DOI] [PubMed] [Google Scholar]
- 3.Crislip M. Infectious disease compendium. http://www.pusware.com/dkasdk234lszclsv/Infectious%20Disease%20Compendium%2014.pdf (accessed 6 Sep 2014).
- 4.Smego RA, Foglia G. Actinomycosis. Clin Infect Dis 1998;26:1255–61. 10.1086/516337 [DOI] [PubMed] [Google Scholar]
- 5.Smego RA., Jr Actinomycosis of the central nervous system. Rev Infect Dis 1987;9:855–65. 10.1093/clinids/9.5.855 [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Hall V, Thakker B et al. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J Antimicrob Chemother 2005;56:407–9. 10.1093/jac/dki206 [DOI] [PubMed] [Google Scholar]
- 7.Skoutelis A, Petrochilos J, Bassaris H. Successful treatment of thoracic actinomycosis with ceftriaxone. Clin Infect Dis 1994;19:161–2. 10.1093/clinids/19.1.161 [DOI] [PubMed] [Google Scholar]
- 8.Haggerty CJ, Tender GC. Actinomycotic brain abscess and subdural empyema of odontogenic origin: case report and review of the literature. J Oral Maxillofac Surg 2012;70:e210–13. 10.1016/j.joms.2011.09.035 [DOI] [PubMed] [Google Scholar]
- 9.Apotheloz C, Regamey C. Dissemininated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis 1996;22:621–5. 10.1093/clinids/22.4.621 [DOI] [PubMed] [Google Scholar]


