Abstract
The drug rapamycin is the only pharmacological agent thus far shown to reproducibly extend lifespan and delay a subset of age-associated pathologies in multiple strains of mice. Unfortunately, the vast majority of aging-related studies on rapamycin in mice have been performed at a single dose of the drug delivered in encapsulated form through the diet. Recently, the National Institute on Aging Interventions Testing Program reported that a three-fold higher dose of dietary rapamycin results in a significantly greater increase in lifespan. This observation demonstrates that current studies of the effects of rapamycin on lifespan and healthspan in mice are being performed under conditions that are sub-optimal. Here I argue that the failure to properly determine the dose and timing response profile for rapamycin with respect to healthy aging represents a major barrier for the field. This barrier continues to hamper our ability to gain mechanistic insights and may threaten efforts to translate these findings into interventions that promote healthy aging in people.
Keywords: aging, mechanistic target of rapamycin, mTOR, anti-aging, rejuvenation, stem cells, mitochondria, dose response
Introduction
The mechanistic target of rapamycin (mTOR) is a nutrient and growth factor responsive kinase that modulates lifespan in species from yeast to mice (Johnson et al., 2013b). mTOR exists in two complexes within cells, mTOR complex I (mTORC1) and mTOR complex 2 (mTORC2) (Laplante and Sabatini, 2012). Abundant evidence suggests that mTORC1 is the primary mTOR complex involved in regulating longevity: mutations that reduce the activity of mTORC1 have been shown to extend lifespan in yeast (Kaeberlein et al., 2005; Powers et al., 2006), nematode worms (Jia et al., 2004; Vellai et al., 2003), fruit flies (Kapahi et al., 2004), and mice (Lamming et al., 2012), as has deletion of the mTORC1 substrate ribosomal S6 kinase (Fabrizio et al., 2004; Fabrizio et al., 2001; Kapahi et al., 2004; Pan et al., 2007; Selman et al., 2009). Consistent with these genetic data, treatment with the mTORC1 inhibitor rapamycin has also been found to increase lifespan in yeast (Medvedik et al., 2007; Powers et al., 2006), worms (Robida-Stubbs et al., 2012), fruit flies (Bjedov et al., 2010), and mice (Harrison et al., 2009).
mTOR inhibition is believed to play a central role in mediating the beneficial effects of dietary restriction (DR) on healthy aging (Kapahi and Zid, 2004; Kennedy et al., 2007). DR, which can be defined as a reduction in nutrient availability in the absence of malnutrition, is the most studied intervention for extending lifespan and enhancing healthy aging across a diverse range of model organisms (Anderson and Weindruch, 2012; Masoro, 2005). DR is sufficient to reduce mTORC1 activity in each of the organisms where it has been shown to increase lifespan, and epistasis studies have placed DR in the same genetic pathway as mTORC1 with respect to lifespan in yeast (Kaeberlein et al., 2005; Steffen et al., 2008), nematodes (Ching et al., 2010), and fruit flies (Kapahi et al., 2004; Zid et al., 2009). These observations, along with the fact that mTORC1 inhibition is sufficient to extend lifespan in each of these species, has led to the general consensus that inhibition of mTORC1 plays a direct role in promoting longevity and healthspan in response to DR (Kaeberlein, 2013a; Kapahi et al., 2010).
As of early 2014, at least seven independent studies have reported lifespan extension from rapamycin in wild type mice (Table 1), with most studies using a dietary formulation where rapamycin is encapsulated for enteric release (Nadon et al., 2008). The first report, published in 2009, demonstrated that UMHET3 mice fed a diet containing encapsulated rapamycin at 14 ppm (∼2.24 mg/kg/day) beginning at 600 days of age is sufficient to increase lifespan in both male and female animals (Harrison et al., 2009). Subsequent reports where rapamycin feeding was initiated in young adulthood showed a similar magnitude of lifespan extension in UMHET3 mice (Miller et al., 2011). Rapamycin feeding has also been shown to extend lifespan in C57BL/6J mice when initiated at mixed ages (Neff et al., 2013) or as late as 19 months of age in C57BL/6N mice (Zhang et al., 2014). Recently, a partial dose response study was performed in UMHET3 mice treated with either 4.7, 14, or 42 ppm rapamycin in the diet, with the striking result that animals fed the highest dose of rapamycin lived the longest (Miller et al., 2014,Miller et al., 2014). Thus, it seems likely that all of the prior studies examining effects of rapamycin on lifespan and age-related health measures have been performed at doses of the drug that are sub-optimal for longevity.
Table 1. Published studies showing lifespan extension from rapamycin in mice.
| Mouse strain | Rapamycin dosing/delivery | Rapamycin in blood (ng/mL) | Lifespan effect | Reference |
|---|---|---|---|---|
| UMHET3 | 14 ppm (∼2.24 mg/kg/day) encapsulated in food beginning at 600 days | 60-70 | Maximum lifespan (90th percentile) increase of 9% and 14% for males and females, respectively. | Harrison et al., 2009 |
| UMHET3 | 14 ppm encapsulated in food beginning at 9 months | NR | Mean lifespan increase of 10% and 18% for males and females, respectively. Maximum lifespan increase of 16% and 13% for males and females, respectively. | Miller et al., 2011 |
| UMHET3 | 4.7 ppm encapsulated in food beginning at 9 months | 7 (females); 6 (males) | Median lifespan increase of 3% and 16% for males and females, respectively. Maximum lifespan increase of 6% and 5% for males and females, respectively. | Miller et al., 2014,Miller et al., 2014 |
| UMHET3 | 14 ppm encapsulated in food beginning at 9 months | 16 (females); 9 (males) | Median lifespan increase of 13% and 21% for males and females, respectively. Maximum lifespan increase of 8% and 11% for males and females, respectively. | Miller et al., 2014,Miller et al., 2014 |
| UMHET3 | 42 ppm encapsulated in food beginning at 9 months | 80 (females);23 (males) | Median lifespan increase of 23% and 26% for males and females, respectively. Maximum lifespan increase of 8% and 11% for males and females, respectively. | Miller et al., 2014,Miller et al., 2014 |
| C57BL/6N | 14 ppm encapsulated in food beginning at 19 months | 3-4 | Increased in females. No percentage given. | Zhang et al., 2014 |
| C57BL/6N | 4 mg/kg i.p. injection every other day for 6 weeks beginning at 20-22 months | NR | 80% survival in treated group compared to 20% survival in controls at 30 months | |
| C57BL/6J | 14 ppm encapsulated in food initiated at 4, 15, or 20-22 months | 4.6 | Increased median lifespan in males; no percentage given; survival study not completed | Neff et al., 2013 |
| 129/Sv | 1.5 mg/kg rapamycin three times per week for two weeks out of every month beginning at 2 months of age | NR | At 800 days 54% of rapamycin treated animals were alive compared to 36% of controls. At 900 days of age 31% rapamycin treated were alive and 10% of controls were alive. | Anisimov et al., 2011 |
NR = not reported.
In addition to the partial dose response study reported by the National Institute on Aging Interventions Testing Program (ITP)in UMHET3 mice (Miller et al., 2014,Miller et al., 2014), one additional study suggests that even higher doses of rapamycin could result in greater improvements in longevity and healthspan than have thus far been observed (Chen et al., 2009). Treating C57BL/6N mice with 4 mg/kg rapamycin by intraperitoneal injection every other day for 6 weeks beginning at 20-22 months of age resulted in significant improvements in hematopoietic stem cell function, as assessed by successful vaccination against influenza virus. Strikingly, a partial survival analysis reported in the same study showed that a similar rapamycin treatment regimen significantly enhanced survival at 30 months of age from around 20% for the control cohort to around 80% for the rapamycin treated cohort. Unfortunately, the full survival analysis was not reported, so it remains unclear what the actual magnitude of lifespan extension from this transient higher-dose rapamycin treatment might have been.
Challenges with attempting to assess whether rapamycin “slows aging” at sub-optimal doses
One major reason why it is critical that we obtain a better understanding of the dose response profile for rapamycin with respect to longevity is that it is difficult, if not impossible, to rigorously assess the full impact of rapamycin on healthspan using sub-optimal doses. To illustrate this point, we should consider two recent studies which aimed at assessing whether rapamycin slows aging in mice by quantifying the effects of the 14 ppm rapamycin diet on age-related phenotypes (Wilkinson et al., 2012; Neff et al., 2013). Both studies detected improvements in some, but not all, of the age-sensitive parameters they measured in the rapamycin treated animals compared to untreated controls. Yet, the two studies reached opposite conclusions as evidenced by their titles: “Rapamycin slows aging in mice” (Wilkinson et al., 2012) and “Rapamycin extends murine lifespan but has limited effects on aging” (Neff et al., 2013). Clearly, both interpretations cannot be correct.
These studies, and others like them, are based on the idea that if rapamycin is extending lifespan by slowing aging, then most age-related declines in function should also be delayed by rapamycin. While this is a logical assumption, it does not necessarily follow that all age-sensitive traits will show a similar magnitude of response to a given dose of rapamycin. In other words, just because median lifespan is increased by 10% when mice are fed a diet containing 14 ppm rapamycin, it need not be the case that all age-associated cancers, age-associated cardiac dysfunction, age-associated cognitive decline, etc. will each also be attenuated by 10%. Instead, it is almost certain that different age-associated phenotypes will have differential responses to any given “anti-aging” intervention, and it is overly simplistic to think that a single, sub-optimal dose of rapamycin would yield detectable effects on all age-sensitive traits, even if rapamycin does, in fact, attenuate age-dependent changes in those traits.
If the goal is to determine which age-sensitive traits are responsive to rapamycin, then it is necessary to first optimize the treatment regimen in order to provide the most robust changes and enhance the likelihood of being able to detect those changes. It is by no means guaranteed that the dose of rapamycin that yields the largest positive effect on lifespan will also yield the largest effects on all age-associated phenotypes of interest; however, it is certain that a sub-optimal dose of rapamycin won't. Much of the current confusion over whether rapamycin generally slows aging or not may very well be due to over-interpretation of negative results based on treatment regimens that are not optimized to detect changes in the phenotypes being assessed (Johnson et al., 2013a).
High dose rapamycin impacts mitochondria and metabolism
We recently reported that daily intraperitoneal (i.p.) injection of 8 mg/kg rapamycin can dramatically attenuate progression of severe mitochondrial disease in the Ndufs4-/- mouse model of Leigh Syndrome (Johnson et al., 2013c). Survival was more than doubled, and disease symptoms were absent in roughly half of the treated animals. In addition to suppressing mitochondrial disease, this dose of rapamycin also resulted in striking metabolic changes in both knockout and wild type C57BL/6N animals, including increases in percent body fat and an apparent metabolic shift toward enhanced amino acid and fatty acid catabolism, based on metabolomic profiling of both brain and liver (Johnson et al., 2013c). We hypothesize that this metabolic shift, which resembles the metabolic response to fasting in both mice and people, contributed to improved outcome in the Ndufs4-/- animals by alleviating the accumulation of glycolytic intermediates and lactic acid, which indicate a depletion of NAD+. This suggests the possibility that a similar metabolic shift, and corresponding improvements in mitochondrial function, could underlie benefits of rapamycin on normative aging.
There is little direct evidence that the doses of rapamycin thus far associated with enhanced lifespan in wild type UMHET3 and C57BL/6 mice results in changes in mitochondrial metabolism similar to what we have reported from daily i.p. injection. Our observation that body fat percentage is increased by rapamycin differs from another study where it was reported no effect on body fat percentage from rapamycin feeding at 14 ppm in aged C57BL/6N mice (Zhang et al., 2014). Of course, these differences in the metabolic response to rapamycin could be due to dosage, age of initiation of treatment, or both. Recent work from the ITP concluded that rapamycin at 42 ppm does not result in similar metabolic changes as DR based on hepatic gene expression profiles (Miller et al., 2014,Miller et al., 2014), although transcriptional changes may not reflect metabolite levels, and the extent to which even high dose rapamycin treatment would recapitulate transcriptional responses to DR is unclear. Indeed, the metabolomic changes we observed in wild type mice treated with 8 mg/kg/day of rapamycin may be more akin to a fasting response than chronic DR (Johnson et al., 2013c). In this regard, a comparative metabolomic analysis of liver and serum, as well as other tissues, from animals subjected to a short-term fast, chronic DR, or different doses of rapamycin would be particularly informative.
Analysis of rapamycin levels in blood suggests that daily i.p. injection of 8 mg/kg yields blood levels of around 1800 ng/mL one hour after injection and 45 ng/mL 24 hours after injection (Johnson et al., 2013c). For comparison, blood levels of 3-4 ng/mL rapamycin were measured following dietary delivery at 14 ppm in the same mouse strain (Zhang et al., 2014) and studies from the ITP have reported between 9-16 ng/mL at this dose and 23-80 ng/mL in animals receiving the 42 ppm rapamycin diet (Miller et al., 2011). Thus, daily i.p. injection of 8 mg/kg rapamycin, which appears to be well tolerated in wild type mice (Johnson et al., 2013c), yields circulating levels of the drug that are at least 20-fold higher than the highest concentration that has been carefully tested for effects on normative aging.
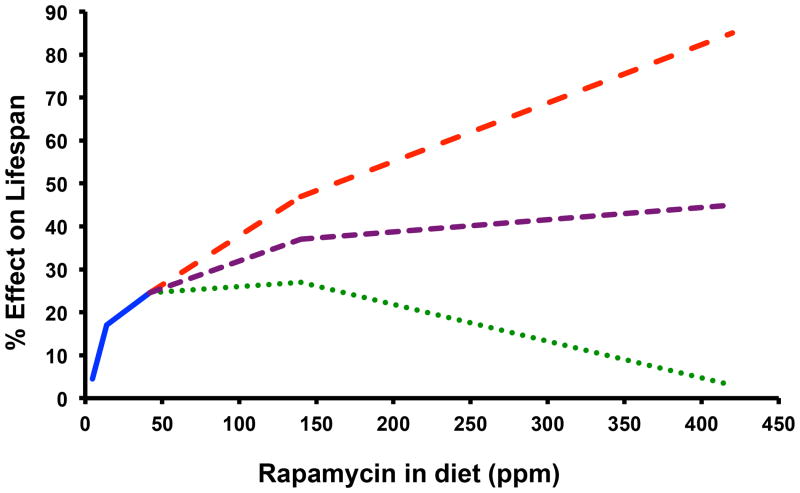
These observations, taken in combination with prior evidence that 6 weeks of rapamycin injection at 4 mg/kg may be sufficient to enhance survival in aged mice (Chen et al., 2009), clearly indicates a lack of clarity regarding optimal dosing and length of treatment needed to maximize beneficial effects of rapamycin on lifespan and healthspan (Fig. 1). Further, it remains unclear whether the low doses of rapamycin used for most aging studies are sufficient to induce the profound metabolic changes associated with higher doses of rapamycin and whether those changes would impact, either positively or negatively, age-associated diseases or lifespan.
Figure 1.
Three potential outcomes of a rapamycin dose response trial for effects on lifespan. The solid blue line represents the published data from the Interventions Testing Program for the effect of encapsulated rapamycin on mean lifespan of UMHET3 mice when provided in the diet beginning at 9 months of age (average of effects in males and females combined). Dashed lines represent a few possible outcomes of a dose response trial for rapamycin at higher doses.
A late life rapamycin intervention trial
Although it may be impractical to measure lifespan in mice fed rapamycin across a full range of doses, I believe that it is feasible to perform this type of dose response experiment if rapamycin treatment is initiated late in life. Such trials should attempt to answer two questions: (1) what dose(s) of rapamycin maximally extends lifespan and healthspan and (2) is transient rapamycin treatment sufficient to obtain benefits similar to continuous treatment. Current evidence suggests that initiating rapamycin delivery at 600 days of age is nearly as effective as beginning treatment at 9 months, at least for the 14 ppm rapamycin diet (Harrison et al., 2009; Miller et al., 2011), and there is growing evidence that several measures of healthspan can be positively impacted from rapamycin treatment starting between 15 and 24 months of age in mice (Chen et al., 2009; Flynn et al., 2013; Neff et al., 2013; Zhang et al., 2014).
As a first pass, I propose measuring the effects of three doses of rapamycin delivered in the diet on survival when treatment is initiated at 20-22 months of age: 42 ppm, 140 ppm, and 420 ppm. The highest dose is based on our unpublished studies, which indicate that 8 mg/kg/day of rapamycin delivered by i.p. injection results in biological activity roughly comparable to 420 ppm delivered in the diet. The lowest dose represents the rapamycin diet that has, so far, been shown to provide the greatest magnitude of lifespan extension when treatment is initiated in young mice. I also propose two different treatment regimens: a continuous treatment regimen until end of life and a transient 3 month treatment regimen after which animals will be returned to a control diet. Non-invasive measures of healthspan could be monitored prior to and during the survival experiment in order to assess, at least in part, the impact of rapamycin dosage on healthy aging. A smaller number of animals added to each cohort for gene and protein expression, metabolomic, and histopathological assessments would provide important additional data on the similar and different effects that each dose of rapamycin is having on relevant molecular processes which can then be correlated to survival and healthspan outcomes. This would equate to six treatment groups plus a control group: a large survival study, but not unrealistic for a lab or group of labs that routinely perform such experiments.
Completion of a late life intervention trial for rapamycin similar to this would have several important outcomes. First, we would begin to have an idea of optimal dosing for rapamycin to maximize longevity and healthspan. If the highest dose tested yields effects that are less beneficial than either lower dose, we will know where to focus for future studies. If the positive effects are biggest at the highest dose tested, we will realize that all of the prior studies have been performed at doses at least 10-fold too low and be able to interpret them in that context while guiding future studies toward more informative designs. Additionally, we would gain a better understanding of the benefits that can be obtained from starting rapamycin treatment later in life. Clearly, a mid- or late-life intervention is preferable when considering potential translation of any anti-aging therapy to humans. It is somewhat surprising that greater emphasis has not been put on intervention studies that begin in mid-to-late life in mice, especially since rapamycin has twice already been shown to increase lifespan when treatment is started in aged mice (Harrison et al., 2009; Zhang et al., 2014). This is a remarkable accomplishment that, while achieving some acclaim in terms of prizes and popular press, seems to have had a minimal impact on how we think about and perform longevity and healthspan studies in mice.
Another important outcome of a trial as I've outlined above would be to definitively determine whether transient treatment with rapamycin is also effective at improving lifespan and healthspan. As with later age of onset, an optimal therapy for use in otherwise healthy people would be transient in nature, perhaps lasting a few months, rather than something that needs to be taken for the rest of an individual's life. In the case of rapamycin, this might alleviate concerns about potential side effects, such as increased risk of certain infections and impaired wound healing (Kaeberlein, 2013b).
Conclusion
The identification of a drug, rapamycin, that can consistently and robustly extend lifespan in mice is a major accomplishment. Available evidence indicates that beginning treatment late in life is sufficient to extend lifespan, and that transient treatment late in life may yield similar benefits. Available data also demonstrate that prior studies examining effects of rapamycin on lifespan and healthspan have likely been performed using dosing and delivery regimens that are sub-optimal. This is a significant shortcoming that is limiting our ability to define mechanisms by which rapamycin impacts the aging process and may also be slowing the translation of these remarkable discoveries into therapies to improve healthy aging in humans.
Acknowledgments
Work in the Kaeberlein lab related to this topic is supported by NIH grant R01AG039390 and by the UW Nathan Shock Center of Excellence in the Basic Biology of Aging NIH grant P30AG013280.
References
- Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching TT, Paal AB, Mehta A, Zhong L, Hsu AL. drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell. 2010;9:545–557. doi: 10.1111/j.1474-9726.2010.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557:136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013 doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving youth: does rapamycin deliver? Sci Transl Med. 2013a;5:211fs240. doi: 10.1126/scitranslmed.3007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013b;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013c;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. Longevity and aging. F1000prime Reports. 2013a;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. mTOR inhibition: from aging to autism and beyond. Scientifica (Cairo) 2013b;2013:849186. doi: 10.1155/2013/849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Science of aging knowledge environment : SAGE KE. 2004;2004:PE34. doi: 10.1126/sageke.2004.36.pe34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL, Strong R, Miller RA, Nelson J, Javors M, Sharp ZD, Peralba JM, Harrison DE. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) 2008;30:187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Holter SM, Moreth K, Prehn C, Puk O, Racz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Hofler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. The Journal of clinical investigation. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, Kaeberlein M. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]