Abstract
We have previously demonstrated that protein restriction throughout gestation and lactation reduced liver triglyceride content in adult rat offspring. The mechanism(s) mediating the decrease in liver triglyceride content are not understood. The objective of the current study was to use a new group of pregnant animals and their offspring and determine the contribution of increased triglyceride utilization via the hepatic fatty acid oxidation and triglyceride secretory pathways to the reduction in liver triglyceride content. Pregnant Sprague-Dawley rats received either a control or a low protein diet throughout pregnancy and lactation. Pups were weaned onto laboratory chow on day 28 and sacrificed on day 65. Liver triglyceride content was reduced in male, but not female, low protein offspring both in the fed and fasted states. The reduction was accompanied by a trend towards higher liver carnitine palmitoyltransferase-1a activity suggesting increased fatty acid transport into the mitochondrial matrix. However, medium chain acyl CoA dehydrogenase activity within the mitochondrial matrix, expression of nuclear peroxisome proliferator activated receptor-α, and plasma levels of β-hydroxybutyrate were similar between low protein and control offspring indicating a lack of change in fatty acid oxidation. Hepatic triglyceride secretion, assessed by blocking peripheral triglyceride utilization and measuring serum triglyceride accumulation rate, and the activity of microsomal transfer protein were similar between low protein and control offspring. Since enhanced triglyceride utilization is not a significant contributor, the decrease in liver triglyceride content in male low protein offspring is likely due to alterations in liver fatty acid transport or triglyceride biosynthesis.
Keywords: Diet protein restricted, Programming, Liver triglyceride content, Liver glycogen content, Fatty acid oxidation, Hepatic triglyceride secretion
INTRODUCTION
The liver plays a central role in whole body lipid homeostasis and the triglyceride (TG) content of the liver has important physiological and metabolic functions. Excessive accumulation of TG in the liver is a characteristic of several metabolic diseases including insulin resistance and non-alcoholic fatty liver disease. Over the past decade there has been great interest in identifying factors that affect liver TG content as a possible prelude to the development of therapeutic strategies to reduce liver TG accumulation. As reviewed recently, a variety of factors are known to affect liver TG content including nutritional status, genetics, and chronic inflammation (1).
Rodent studies have now identified the perinatal nutrition environment as a novel factor that can imprint long term alterations in hepatic TG content. We have reported that protein restriction throughout gestation and lactation decreased liver TG content in 65 day old male, but not female, offspring and this decrease persisted at least until day 150, when the study was terminated (2). To the best of our knowledge, this is the first study to show that protein restriction throughout gestation and lactation can imprint long term reductions in liver TG content of the adult offspring. Interestingly, other authors have shown that the paradigm is associated with favorable metabolic outcomes in the adult offspring as evidenced in their lower body weight, leaner phenotype, improved insulin sensitivity, hypoleptinemia, and decreased susceptibility to diet induced obesity (3–7) as a possible result of which they have a longer lifespan (8).
The specific mechanisms mediating the decrease in hepatic TG content in adult low protein rat offspring are not known. A reduction in liver TG content can occur either due to a decrease in TG synthesis or an increase in TG utilization. In adult rats, perinatal (throughout gestation and lactation) protein restriction increases sympathetic nervous system (SNS) drive (9, 10), which would be expected to increase lipid utilization. Suggestive evidence of such an increase in lipid utilization is observed in decreased plasma levels of TG and cholesterol (11), decreased epididymal fat pad weight (9), and decreased whole body lipid content (6) in perinatally protein restricted offspring. Collectively, these data form the basis of our hypothesis that maternal protein restriction decreases liver TG content in the adult offspring by increasing lipid utilization. Therefore, the objective of the present study was to use a new group of pregnant rats and their offspring and specifically determine the contribution of increased hepatic TG utilization to the gender specific decrease in liver TG content imprinted by protein restriction throughout gestation and lactation. Hepatic TG utilization occurs by two major pathways, namely oxidation of free fatty acids and export of hepatic TG to adipose tissue and muscle in the form of very low density lipoprotein (VLDL) particles. In this study, the status of fatty acid oxidation was assessed by: i) measuring the activities of carnitine palmitoyltransferase-1a (CPT-1a) and medium chain acyl CoA dehydrogenase (MCAD), critical enzymes in the fatty acid oxidation pathway, ii) quantitating the expression of nuclear peroxisome proliferator activated receptor-α (PPARα), the predominant transcription factor regulating the activity of CPT-1a and MCAD, and iii) determining the plasma levels of ketone bodies, the end product of fatty acid oxidation. The status of TG export from the liver via VLDL particles was assessed by: i) measuring the in vivo hepatic TG secretion rate and ii) determining the in vitro activity of microsomal transfer protein (MTP), a key enzyme required for the assembly and secretion of VLDL particles. Finally, since alterations in TG homeostasis can also affect carbohydrate metabolism, we assessed the status of carbohydrate metabolism by measuring liver glycogen content. Since TG and glycogen utilization are low in the fed state and are markedly enhanced in the fasted state, we measured most of these parameters both in the fed and fasted states.
RESULTS
Protein restriction during gestation decreased birth weight and continued restriction during lactation prevented catch up growth in the offspring
Table 1 shows that maternal low protein diet during pregnancy and lactation reduced body weight in 65 day old male and female offspring. Absolute liver weight was reduced in low protein offspring, although body weight normalized liver weight was not affected by maternal diet. We recently reported growth trajectories of offspring in the current study and showed that the paradigm lowered birth weight and prevented catch up growth during the lactation period (9).
Table 1.
Body weight, liver weight, and liver cholesterol content in control and low protein offspring in the fed and fasted states.
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Control offspring | Low Protein offspring | Control offspring | Low Protein offspring | |||||
| Parameters | Fed State | Fasted State | Fed State | Fasted State | Fed State | Fasted State | Fed State | Fasted State |
| Body weight (g) | 330 ± 7 | 304 ± 10 | 240 ± 6* | 220 ± 7* | 223 ± 10 | 203 ± 5 | 173 ± 5* | 157 ± 3* |
| Liver weight (g) | 12.2 ± 0.4 | 8.2 ± 0.6# | 9.5 ± 0.3* | 6.3 ± 0.2*# | 7.1 ± 0.3 | 5.4 ± 0.2# | 6.5 ± 0.1* | 4.4 ± 0.2*# |
| % Liver weight$ | 3.7 ± 0.1 | 2.7 ± 0.1# | 3.9 ± 0.1 | 2.9 ± 0.1# | 3.4 ± 0.1 | 2.7 ± 0.1# | 3.7 ± 0.1 | 2.8 ± 0.1# |
| Liver cholesterol content (µg/g liver tissue) | 2540 ± 115 | 3027 ± 72# | 2412 ± 67 | 3047 ± 100# | 2549 ± 116 | 3724 ± 179# | 2459 ± 65 | 4389 ± 322# |
Significantly different from control group within the same feed status, p<0.05.
Significantly different from fed state within the same group, p<0.05.
percent of body weight
Values are mean ± SEM, n = 9 – 10
Protein restriction throughout gestation and lactation decreases liver triglyceride content in the male, but not in the female, offspring
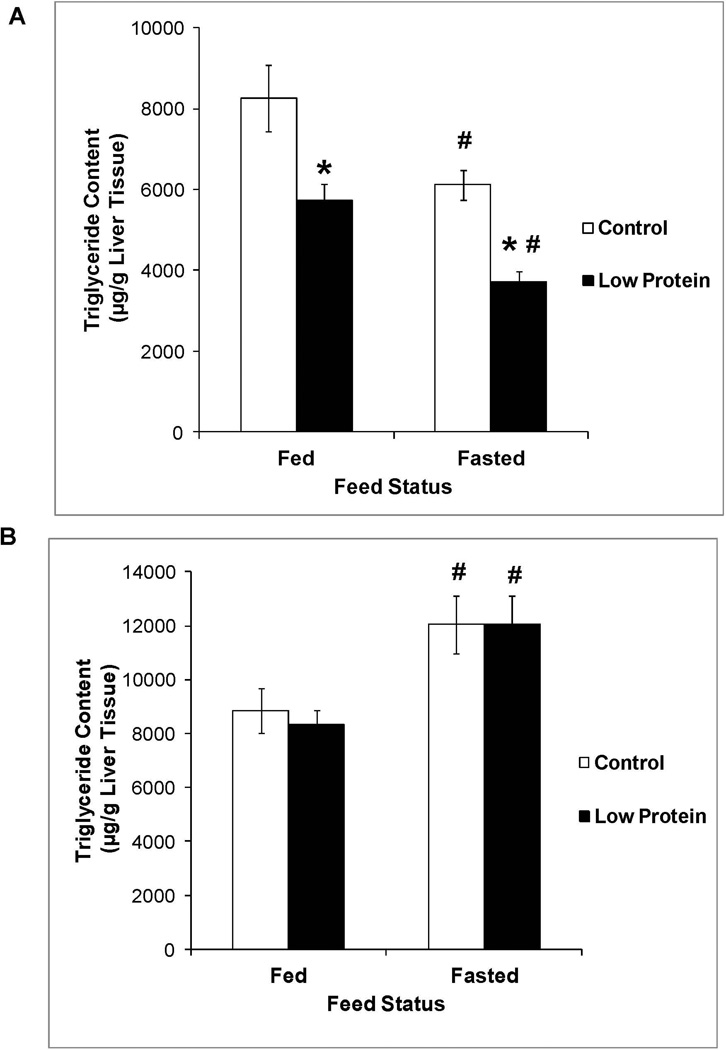
Figure 1 shows that male low protein offspring exhibited lower liver TG content than their controls while liver TG content in the female low protein offspring were similar to controls. These results are observed both in the fed state when TG utilization is low and after a 24 hour fast when TG utilization is high. Fasting increased liver TG content in the female offspring but decreased it in the male offspring. Protein restriction did not affect liver cholesterol content in the fed or fasted state (Table 1).
Figure 1.
Liver triglyceride content in 65 day old control and low protein offspring in the fed and fasted states. A. Males B. Females. Data were analyzed by two-way split plot ANOVA using maternal diet (control or low protein) and feed status (fed or fasted) as the main factors followed by Bonferroni multiple comparison test. n = 9 – 10.
* P<0.05 compared to liver triglyceride content in control group within the same feed status.
# P<0.05 compared to liver triglyceride content in the fed state within the same group.
Plasma hormone and lipid levels
Table 2 shows that plasma insulin levels in the fed state were similar between the control and low protein offspring. However, compared to their corresponding controls, fasting insulin levels were 46% and 55% lower in the male and female low protein offspring respectively, though the differences were not statistically significant. Plasma leptin levels in the fed and fasted states were lower in male low protein offspring and tended to be lower in female low protein offspring compared to their respective controls. There were no differences in glucagon levels between the control and low protein groups in the fed or fasted states. The glucagon to insulin ratio, an indicator of the switch to lipid utilization in the fed to fasting transition was similar between the control and low protein offspring in the fed state. As expected, fasting produced a marked increase in the ratio. Male, but not female, low protein offspring exhibited a higher fasting glucagon to insulin ratio.
Table 2.
Plasma hormones and plasma lipids in control and low protein offspring in the fed and fasted states.
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Control offspring | Low Protein offspring | Control offspring | Low Protein offspring | |||||
| Parameters | Fed State | Fasted State | Fed State | Fasted State | Fed State | Fasted State | Fed State | Fasted State |
| Insulin (ng/ml) | 1.13 ± 0.1 | 0.13 ± 0.02# | 0.84 ± 0.05 | 0.07 ± 0.01# | 0.72 ± 0.07 | 0.09 ± 0.02# | 0.64 ± 0.07 | 0.04 ± 0.01# |
| Leptin (pg/ml) | 387 ± 33 | 56 ± 10# | 215 ± 37* | 15 ± 5*# | 229 ± 33 | 46 ± 11# | 154 ± 15 | 17 ± 3# |
| Glucagon (pg/ml) | 61 ± 7 | 58 ± 4 | 56 ± 4 | 67 ± 5 | 74 ± 4 | 80 ± 6 | 84 ± 8 | 82 ± 2 |
| Glucagon / Insulin ratio | 0.055 ± 0.004 | 0.611 ± 0.156# | 0.065 ± 0.005 | 1.081 ± 0.17*# | 0.108 ± 0.009 | 1.652 ± 0.511# | 0.147 ± 0.023 | 2.185 ± 0.382# |
| Triglycerides (mg/dl) | 137 ± 16 | 39 ± 5# | 79 ± 8* | 25 ± 3*# | 64 ± 8 | 23 ± 2# | 57 ± 5 | 13 ± 1*# |
| Free fatty acids (µM) | 320 ± 26 | 586 ± 39# | 293 ± 17 | 603 ± 28# | 328 ± 29 | 639 ± 33# | 283 ± 19 | 528 ± 51# |
| β-Hydroxybutyrate (µM) | 96 ± 9 | 689 ± 109# | 90 ± 4 | 688 ± 67# | 144 ± 33 | 803 ± 71# | 104 ± 8 | 783 ± 77# |
| Cholesterol (mg/dl) | 61 ± 3 | 60 ± 3 | 60 ± 3 | 58 ± 3 | 70 ± 2 | 65 ± 7 | 74 ± 4 | 64 ± 4 |
Significantly different from control group within the same feed status, p<0.05.
Significantly different from fed state within the same group, p<0.05.
Values are mean ± SEM, n = 9 – 10.
To determine if the higher glucagon to insulin ratio in the male low protein offspring enhanced lipid utilization, we measured plasma levels of various lipid fuels. Consistent with the putative increase of lipid utilization in the male low protein offspring, Table 2 shows that male low protein offspring had lower plasma TG levels compared to their controls in the fed and fasted states while female low protein offspring exhibited decreases only in the fasted state. Fasting reduced plasma TG levels in all groups consistent with its effect of increasing lipid utilization. Plasma free fatty acid and cholesterol levels were similar between low protein and control offspring in the fed and fasted states.
Status of hepatic fatty acid oxidation
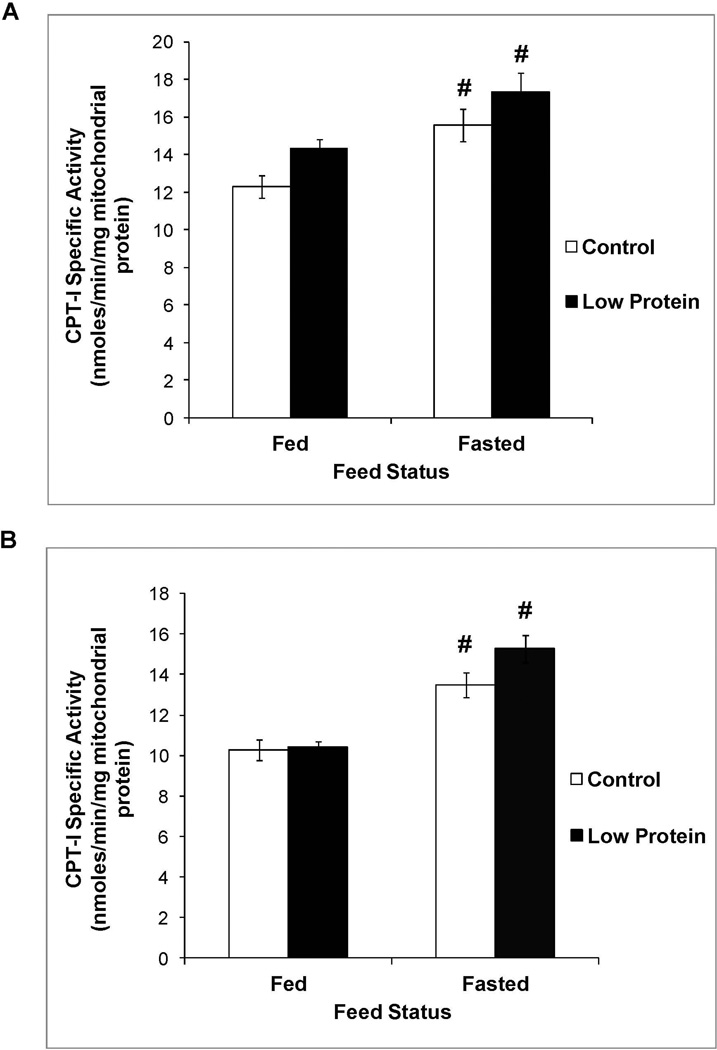
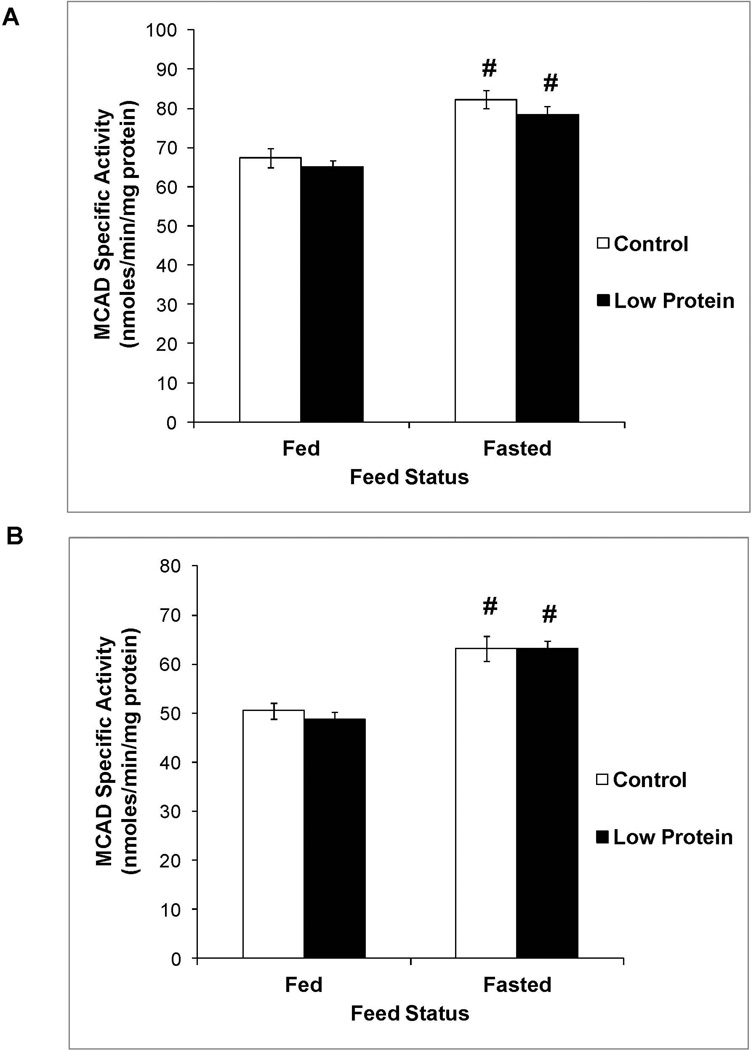
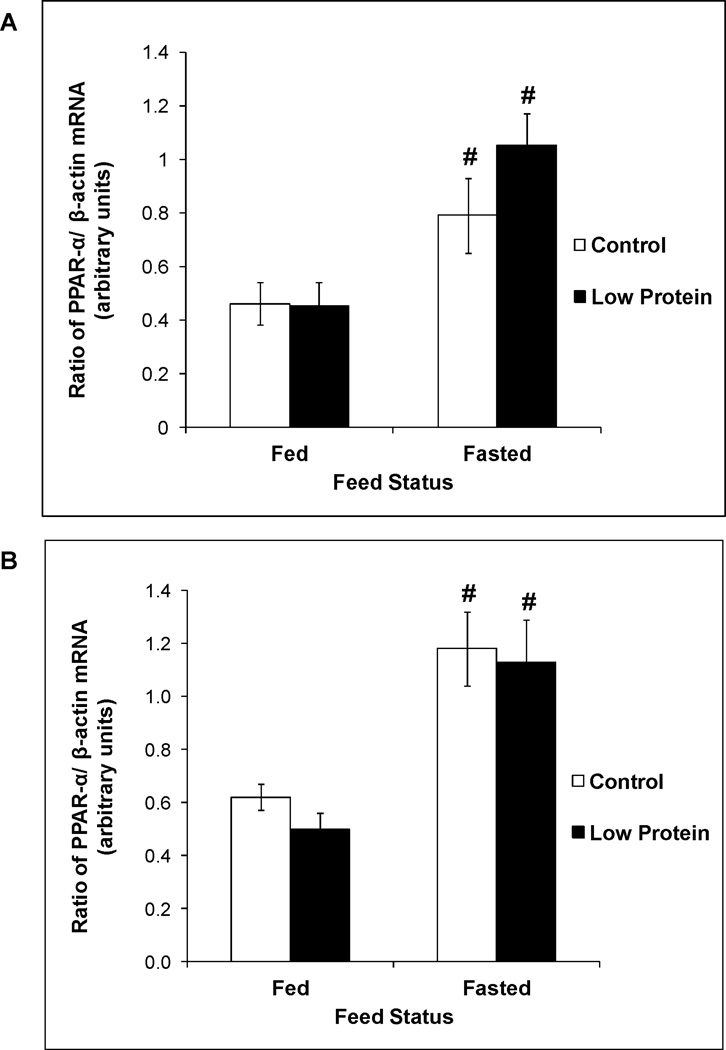
Figure 2 shows that male low protein offspring tended to exhibit a small increase in CPT-1a activity compared to controls (P value for main effect of maternal diet = 0.0535). CPT-1a activity in the fed and fasted states was similar between female low protein offspring and their controls (P value for main effect of maternal diet = 0.2). However, MCAD activity (Figure 3), PPAR-α expression (Figure 4) and plasma levels of β-hydroxybutyrate (Table 2) in the fed and fasted states was similar between low protein offspring and their controls.
Figure 2.
Liver CPT-1a activity in 65 day old control and low protein offspring in the fed and fasted states. A. Males B. Females.
For details of statistical analyses refer methods and legend of Figure 1. n = 9 – 10.
# P<0.05 compared to activity in the fed state within the same group.
Figure 3.
Liver MCAD activity in 65 day old control and low protein offspring in the fed and fasted states. A. Males B. Females.
For details of statistical analyses refer methods and legend of Figure 1. n = 9 – 10.
# P<0.05 compared to activity in the fed state within the same group.
Figure 4.
mRNA expression of PPAR-α in 65 day old control and low protein male offspring in the fed and fasted states. A. Males B. Females.
For details of statistical analyses refer methods and legend of Figure 1. n = 8 – 10.
# P<0.05 compared to activity in the fed state within the same group.
As expected, in both control and protein restricted groups, fasting produced a 1.7 – 2.3 fold increase in PPAR-α expression (Figure 4). Increased expression of this transcription factor in the fasted state resulted in increased activity of its downstream targets in the fatty acid oxidation pathway, namely CPT-1a (Figure 2) and MCAD (Figure 3), and resulted in a marked increase in fasted plasma levels of β-hydroxybutyrate, the functional output of the β oxidation pathway (Table 2).
Status of triglyceride export from the liver
To determine the effect of protein restriction throughout gestation and lactation on TG utilization via the secretory pathway, we measured the in vivo TG secretion rate in litter mates of animals used in the previous experiments. Table 3 shows that basal plasma TG levels prior to the administration of tyloxapol tended to be lower in the low protein offspring. Serum TG accumulation rates were similar between low protein offspring and their respective controls. TG secretion is mediated by microsomal transfer protein. Consistent with the similar TG accumulation rates, the activity of MTP was also similar between male low protein offspring and controls.
Table 3.
Plasma triglyceride concentrations and triglyceride secretion parameters in control and low protein offspring
| Males | Females | |||
|---|---|---|---|---|
| Parameters | Control offspring | Low Protein offspring | Control offspring | Low Protein offspring |
| Triglycerides (mg/dL) | 136 ± 29 | 88 ± 9 | 77 ± 15 | 42 ± 8 |
| Triglyceride accumulation rate (mg/dL.min) | 3.8 ± 0.3 | 3.9 ± 0.2 | 7.1 ± 0.5 | 7.0 ± 0.3 |
| MTP activity (% lipid transfer) | 20.7 ± 1.8 | 20.4 ± 2.0 | N.D.+ | N.D. |
N.D. Not Determined
Values are mean ± SEM, n = 9 – 10
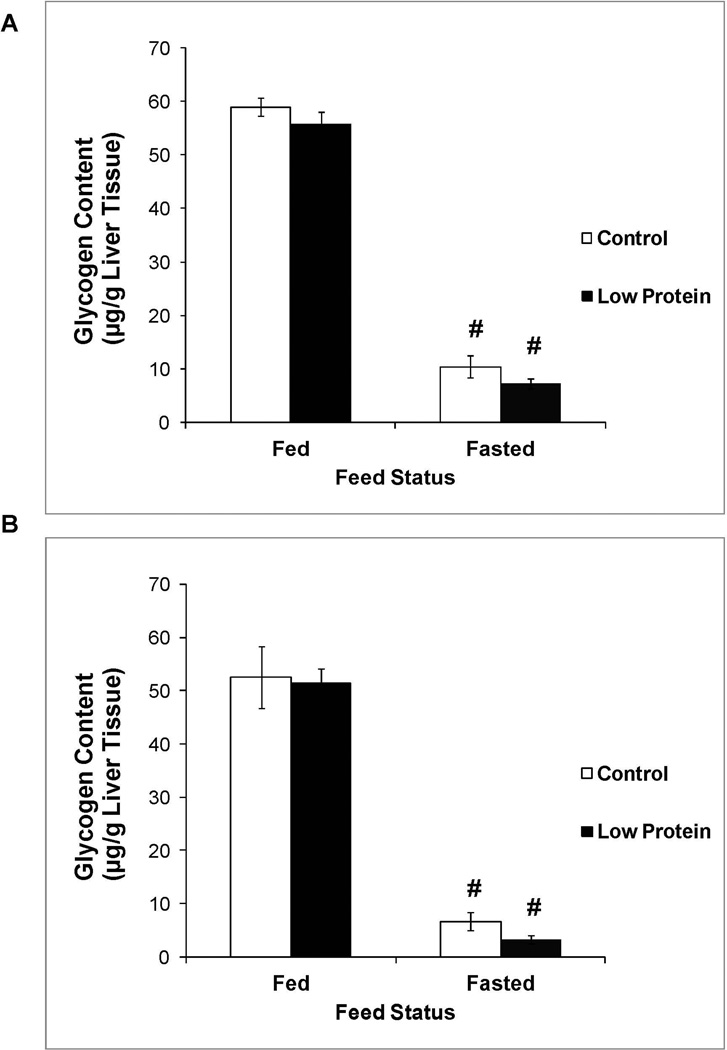
Status of liver glycogen content
Figure 5 shows that liver glycogen content in the fed state was similar between low protein offspring and their controls. A 24 hour fast increased glycogen utilization and reduced liver glycogen content in all groups. Liver glycogen content in the fasted state was also similar between low protein offspring and their controls.
Figure 5.
Liver glycogen content in 65 day old control and low protein offspring in the fed and fasted states. A. Males B. Females.
For details of statistical analyses refer methods and legend of Figure 1. n = 9 – 10.
# P<0.05 compared to liver glycogen content in the fed state within the same group.
DISCUSSION
Protein restriction throughout gestation and lactation decreases liver TG content selectively in the male offspring. The decrease is observed in the fed state when TG utilization is low and in the fasted state when TG utilization is high. The paradigm did not affect liver cholesterol content either in the male or female offspring. The results of the present study conducted in a new group of pregnant animals and their offspring in the fed and fasted states is testimony to the robust nature of this lipid and gender specific imprinting. We have recently published birth weight and growth curve data for offspring in the current study and demonstrated that protein restriction during gestation produces intrauterine growth restriction and continued protein restriction during lactation prevents catch up growth and results in long term reductions in body weight due to a decrease in both lean and fat mass (9).
Protein restriction throughout gestation and lactation imprints increased SNS drive in the adult offspring that would be expected to increase lipid utilization (9, 10). In the current study, measurement of plasma hormones revealed that fasting produced a greater increase in the glucagon to insulin ratio selectively in the male low protein offspring and thereby created a hormonal milieu that is expected to increase their TG utilization. Therefore, we determined the contribution of TG utilization to the specific decrease in liver TG content in the male low protein offspring. Hepatic TG utilization occurs, in part, via the fatty acid oxidation pathway. CPT-1a is a critical enzyme in the pathway that is responsible for carnitine dependent shuttling of long chain fatty acyl CoA esters from the cytosol into the mitochondrial matrix for subsequent oxidation. The trend towards higher CPT-1a activity in the male, but not female, low protein offspring suggested enhanced transport of fatty acids into the mitochondrial matrix in male protein restricted offspring and provided a possible explanation for their lower liver TG content. Similar to our results, Guzman et al showed that protein restriction throughout gestation and lactation increased CPT-1a activity in the offspring (12). However, their measurements were conducted in 21 day old offspring and did not permit evaluation of the longer term, imprinting effects of perinatal protein restriction. We next determined if increased fatty acid transport into the mitochondrial matrix was accompanied by elevations in fatty acid oxidation within the mitochondrial matrix. MCAD catalyzes the oxidation of medium chain (C6 – C12) fatty acids that are formed as a consequence of mitochondrial or peroxisomal β-oxidation of long chain fatty acids. Due to its critical importance for complete fatty acid oxidation, MCAD occupies a pivotal step in fatty acid oxidation. Interestingly, protein restriction did not alter the activity of MCAD. PPARα is a well-established transcriptional regulator of genes involved in hepatic fatty acid oxidation including MCAD and CPT-1a (13). Hepatic PPARα expression is upregulated during fasting and it plays an important role in the switch from glucose to lipid catabolism during the fed to fasting transition (14). In our studies fasting produced the expected increase in its expression, however perinatal protein restriction did not alter PPARα expression in the fed or fasted state. Consistent with these collective findings, fed and fasted plasma levels of β-hydroxybutyrate, the functional output of the fatty acid oxidation pathway, were also similar between low protein offspring and their controls. Thus, in our study the modest increase in CPT-1a activity is not supported by other indices of enhanced fatty acid oxidation leading to the conclusion that the fatty acid oxidation pathway cannot account for the decrease in liver TG content in male low protein offspring. While a comprehensive evaluation of fatty acid oxidation in this paradigm is not available in the literature, there is support for some of our critical findings. Ozanne et al showed that protein restriction throughout gestation and lactation produced small decreases in fed and fasted β-hydroxybutyrate levels in the adult offspring and did not alter their acetoacetate levels (15). We also determined CPT-1 and MCAD activity in the two other major lipid utilizing tissues namely, skeletal muscle and heart, to determine if preferential utilization in these tissues might have diverted lipids away from the liver and contributed to the decrease in liver TG content. We observed no changes in the status of fatty acid oxidation in muscle and heart (data not shown). In support of these findings, using a similar low protein paradigm and respiratory exchange ratio as an overall whole body marker of fuel metabolism, Lim et al recently concluded that there was no preferential utilization of fats in low protein offspring (3).
We next determined if enhanced hepatic TG secretion was a possible contributor to the decreased liver TG content in the male low protein offspring. Tyloxapol induced TG accumulation rate in serum, a measure of in vivo hepatic TG secretion, was similar between control and low protein offspring. MTP is a protein responsible for the assembly and secretion of VLDL particles. In support of the in vivo findings, MTP activity was also similar between the two groups. To the best of our knowledge, this is the first study that has evaluated hepatic TG secretion status in adult offspring exposed to protein restriction throughout gestation and lactation. The regulation of MTP and hepatic TG secretion is multifactorial and complex and includes PPARα and free fatty acids (16). As shown in the present study and by other authors (3, 5, 6, 17), protein restriction throughout gestation and lactation does not affect these key regulators of MTP activity which likely accounts for the similar hepatic TG secretion rate in control and low protein offspring. Thus, hepatic TG secretion does not contribute to the decrease in TG content in the male low protein offspring. Hepatic TG secretion is a critical regulator of plasma TG levels. Low protein offspring exhibit lower fed (male) and fasted (male and female) plasma TG levels despite possessing a hepatic TG secretion rate similar to controls. Recent reports show that plasma TG clearance is enhanced by elevated SNS activity via increased TG utilization in brown adipose tissue (18). Elevated SNS activity increases brown adipose tissue lipoprotein lipase activity with the resultant increase in the uptake and oxidation of free fatty acids. Protein restriction throughout gestation and lactation enhances SNS activity in the adult offspring as evidenced by increased SNS firing rates (19), plasma catecholamine levels (10), beta-adrenergic receptor expression (10), core body temperature and decreased bone mineral density (9). It is therefore conceivable that elevated SNS activity in low protein offspring facilitates brown adipose tissue TG utilization and reduces plasma TG levels.
Our results add to the growing body of evidence that firmly establishes a role for the perinatal nutritional environment in imprinting long term changes in liver TG content. Comparing our results to this literature allow for interesting insights in the area of developmental programming. In rats, protein or calorie restriction during gestation followed by regular ad libitum diet during lactation produces fetal growth restriction followed by catch up growth in the neonatal period and increases liver TG content during adulthood (20, 21). The increase in liver TG content is partially mediated by decreases in liver PPARα expression and consequent decreases in liver CPT-1 and MCAD expression (21–23). These offspring also exhibit a range of metabolic abnormalities (4, 6, 17) as a possible consequence of which they have a shorter lifespan (8, 24). In contrast, protein restriction started at gestation and sustained throughout lactation produces fetal growth restriction but does not result in catch up growth. Our results suggest that the paradigm reverses the increase in liver TG content produced by intrauterine growth restriction possibly by normalizing PPARα, CPT-1, and MCAD expression. Supporting this provocative suggestion, several studies in intrauterine growth restricted offspring show that continued calorie or protein restriction during lactation normalizes several hormonal, metabolic and physiological indices including whole body adiposity, plasma insulin and leptin levels, and life span (3–8). These data suggest that the lactation period represents an opportune period for initiating judicious nutritional interventions that can reverse the long term detrimental effect of intrauterine growth restriction. Confirmation of the potentially normalizing effects of protein restriction during lactation on elevated liver TG content of the intrauterine growth restricted offspring will require a more comprehensive experimental design with simultaneous inclusion of groups that are exposed to protein restriction during gestation alone and protein restriction throughout gestation and lactation.
In our final studies, we determined if the reduction in liver TG content in the male low protein offspring was accompanied by alterations in liver carbohydrate metabolism as measured by liver glycogen content. Protein restriction throughout gestation and lactation did not affect glycogen storage capacity as demonstrated by similar fed state liver glycogen content nor did it affect glycogen utilization as shown by similar fasted state liver glycogen content in the low protein and control offspring. Liver glycogen content is partially regulated by insulin and leptin that act on the liver to stimulate glycogen deposition in the fed state and spare glycogen stores during a fast (25, 26). Male low protein offspring exhibited lower fed and fasted plasma leptin levels and a trend towards lower plasma insulin levels. However, the magnitude of the decrease in plasma levels of these regulators did not affect liver glycogen content. Few studies have explored the effect of protein restriction throughout gestation and lactation on offspring liver glycogen content. A singular reference reported that the paradigm did not affect fed state liver glycogen content in three month old offspring but the effect on fasting state content was not examined (27). In contrast, intrauterine growth restriction followed by post-natal catch up growth produces increases in liver glycogen content in the adult offspring (28, 29), though the mechanism(s) mediating the increase are not completely elucidated. Collectively, these results emphasize the critical importance of timing of early life growth restriction in programming liver glycogen content.
A final interesting observation in our study was the gender specific imprinting effect of perinatal protein restriction on liver TG content. In support of our findings, other studies have also reported gender differences in the imprinting of liver TG content. Calorie restriction selectively during gestation increases liver TG content only in the male offspring (20) while a high unsaturated fat, high protein diet during gestation and lactation imprints decreases in liver TG content only in the female offspring (30). As reviewed by Aiken and Ozanne, numerous studies in a variety of developmental programming models report gender differences in imprinting of blood pressure, nephrogenesis, insulin sensitivity, and hypothalamic pituitary adrenal axis responses to stress (31). The reasons for these gender differences remain elusive and, as hypothesized by these authors, are likely to result from differences in the patterns and timing of development and to the differential pattern of hormone exposure during in utero and postnatal life.
In conclusion, our results firmly establish a role for protein restriction throughout gestation and lactation in programming long term and gender specific decreases in liver TG content in rats. They conclusively demonstrate that the decrease in liver TG content in male low protein offspring is not mediated by changes in liver fatty acid oxidation or TG secretion. It is conceivable that the decrease in liver TG content is due to decreased fatty acid transport into the liver or decreased de novo fatty acid synthesis and/or TG synthesis within the liver. Ongoing studies in our laboratory are exploring these possibilities.
METHODS
Animals and experimental design
The study was approved by the Institutional Animal Care and Use Committee of the University of the Sciences in Philadelphia. From day one of pregnancy, rats were randomly assigned to be fed a modified version of the AIN 76A purified diet containing 19% protein (Control group) or its corresponding isoenergetic low protein formulation AIN M76 A containing 8% protein (Low protein group) as described previously (2). Pregnant rats (9–10 per group) were fed the appropriate diet throughout pregnancy and lactation. At birth, pups were weighed, sexed and randomly culled to 12 pups per dam. At 72 hours post-birth, litters were randomly culled to 8 pups (4 males and 4 females) to ensure a standard litter size for each dam. On day 28 post-birth, offspring were weaned onto normal laboratory chow and subsequently kept on this diet for the entire duration of the study. Body weight of the pups was periodically measured throughout the duration of the study. Our previous study showed that decreases in liver TG content first occurred in 65 day old low protein offspring (2). Therefore, in the current study, one male and female offspring from each litter of both groups were sacrificed on day 65 by decapitation in the fed state and another set sacrificed after a 24 hour fast. Blood was collected into cold aprotinin-lined polypropylene tubes and serum stored at −80°C. Liver was dissected, blotted and snap frozen in liquid nitrogen and stored at −80°C.
Measurement of triglyceride secretion rate in adult offspring
TG secretion rate in whole animals was measured as described by Huang et al (32). Briefly, one male and one female offspring from each litter in both groups were fasted from 7:00 am to 1:00 pm in order to minimize the confounding influence of dietary chylomicrons. At 1:00 pm, 300 mg/kg of triton WR-1339 (tyloxapol), a non-ionic detergent that blocks peripheral utilization of TG, was injected through the saphenous vein under brief isoflorane anesthesia. Blood samples were collected via tail nicks before and 30 min, 60 min and 90 min after the tyloxapol injection and serum stored at −80°C. Serum TG accumulation rate, a quantitative measure of hepatic TG secretion, was obtained from the slope of the serum TG concentration versus time plot.
Extraction of liver triglycerides and cholesterol
Liver TG and cholesterol were extracted from 50 mg of liver tissue in a chloroform / methanol (2:1 by vol) mixture using the Folch method as previously described (2). TG and cholesterol content in the extracts were determined using commercial kits from Wako Chemicals (Richmond, VA).
Measurement of liver glycogen levels
Hepatic glycogen content was measured in 50 mg of liver tissue by aminoglycosidase mediated digestion of glycogen to free glucose as previously described (2). Glucose content was measured using a kit supplied by Cayman Chemicals (Ann Arbor, Michigan).
Measurement of plasma lipids and hormones
Plasma triglycerides, β-hydroxybutyrate, free fatty acids and cholesterol were assayed using kits from Wako Chemicals (Richmond, VA). Plasma insulin and leptin levels were determined by ELISA (Alpco, Salem, NH) and plasma glucagon levels were measured by radioimmunoassay (Linco Research, St. Charles, MO). The inter-day coefficient of variation of each assay was less than 5%.
Measurement of carnitine palmitoyl transferase-1a activity
Liver mitochondria were isolated from 200 mg of liver tissue and the activity of CPT-1a was measured using a spectrophotometric method (33). The assay measured the rate of formation of coenzyme A (CoA) from the reaction of palmitoyl CoA and L-carnitine catalyzed by CPT-1a. The assay was conducted at 250C in a vial containing 25 mM Tris-HCl buffer (pH 6.8), 60mM KCl, 1 mM EDTA, 1.3 mg/mL fatty acid free bovine serum albumin, 0.1 mM 4,4’-dithiopyridine, and 90 micrograms of mitochondrial protein. The reaction was initiated by the addition of palmitoyl CoA and L-carnitine at final concentrations of 100 µM and 0.4 mM, respectively. The liberated CoA reacts with 4,4’-dithiopyridine to produce 4-thiopyridone that was quantitated at 325 nm using an extinction coefficient of 19800 M−1cm−1. The CPT-1a independent formation of CoA was quantified by omitting L-carnitine from the assay mixture. CPT-1a activity was calculated as the difference in rate of formation of CoA in the presence and absence of L-carnitine and expressed as nmoles/min/mg protein.
Measurement of medium chain acyl CoA dehydrogenase activity
MCAD activity was determined in liver homogenates as described by Lehman et al (34). MCAD catalyzed oxidation of octanoyl CoA results in the formation of reducing equivalents that are transferred to ferricenium ion which in turn is reduced to ferrocene. The reduction in the absorbance of the ferricenium ion was monitored. The assay was conducted at 37oC in a vial containing 100 mM HEPES buffer (pH 7.6), 0.1 mM EDTA, 50 µM octanoyl CoA, 200 µM ferricenium hexafluorophosphate, and 0.5 mM sodium tetrathionate. The reaction was initiated by the addition of 50 µg of homogenate protein. The decrease in absorbance of the ferricenium ion at 300 nm was followed for 10 min and the slope used to calculate MCAD activity and expressed as nmoles/min/mg protein.
Measurement of peroxisome proliferator activated receptor-α mRNA expression
Total RNA was isolated from liver samples with TRIzol (Invitrogen,Carlsbad, CA) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). The concentration of RNA was determined spectrophotometrically at 260 nm.
One µg of total RNA was reverse transcribed in a total volume of 20 µL with the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time quantitative PCR was performed with Power SYBR Green PCR MasterMix (Applied Biosystems) and a universal thermal cycling condition for 40 cycles on the StepOne Plus instrument (Applied Biosystems). The specificity of the assay was verified by performing a melting curve analysis.
Expression levels were quantified using the relative standard curve method with β-actin as the housekeeping control. Results are expressed as the ratio of mRNA expression of PPARα to the mRNA expression of actin. Primer sequences were as follows: PPARα; forward: GACAAGGCCTCAGGATACCA and reverse: ACTTGTCGTACGCCAGCTTT; β-actin; forward: CTAAGGCCAACCGTGAAAAG and reverse: GCATACAGGGACAACACAGC.
Microsomal transfer protein activity assay
MTP activity was measured in supernatants of liver homogenate (100 mg) with an MTP assay kit (Chylos, Inc., Woodbury, NY). The assay measures the transfer of lipids between donor and acceptor vesicles. The assay was conducted in Microfluor 2, U bottom, black 96 well microtiter plate (Thermo Scientific, Palm Beach, FL) at 30° C in a solution consisting of a 10 µL mixture of donor and acceptor vesicles and 85µL water. Transfer of lipids was initiated by the addition of 17 µg of supernatant protein. The extent of transfer was assessed at equilibrium conditions by serially measuring fluorescence for 100 min at an excitation wavelength of 460 nm and an emission wavelength of 528 nm. MTP activity was calculated as fluorescence of the sample divided by total fluorescence and expressed as percent lipid transfer.
Statistical analyses
All data are expressed as mean ± SEM. In each experiment, we used one pup of each gender from all litters. Consequently the offspring sample size for each group never exceeded the number of treated dams for that group. Such a conservative experimental design is recommended for experiments in multiparous species since it minimizes inflation of the α level and the spurious statistical significance that results as a consequence (35, 36). Measurements conducted on more than two groups were analyzed by two-way split plot ANOVA using maternal diet (control or low protein) and feed status (fed or fasted) as the main factors. Wherever appropriate, multiple comparisons were conducted using the Bonferroni post-hoc test. Direct comparisons between two groups were conducted using the Student’s t-test. All statistical tests were conducted at a significance level of 0.05.
ACKNOWLEDGEMENTS
We thank Dr Diane Morel for critical review of the manuscript and Dr Kelleen Flaherty for editorial assistance.
This work was supported by a research grant from Jenrin Discovery and by the National Institutes of Child Health and Human Development Grant R15-HD-066267 to Anil D’mello
REFERENCES
- 1.Sanal MG. The blind men 'see' the elephant-the many faces of fatty liver disease. World J Gastroenterol. 2008;14:831–844. doi: 10.3748/wjg.14.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qasem RJ, Cherala G, D'Mello AP. Maternal protein restriction during pregnancy and lactation in rats imprints long-term reduction in hepatic lipid content selectively in the male offspring. Nutr.Res. 2010;30:410–417. doi: 10.1016/j.nutres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Lim K, Armitage JA, Stefanidis A, Oldfield BJ, Black MJ. IUGR in the absence of postnatal "catch-up" growth leads to improved whole body insulin sensitivity in rat offspring. Pediatr. Res. 2011;70:339–344. doi: 10.1203/PDR.0b013e31822a65a3. [DOI] [PubMed] [Google Scholar]
- 4.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am. J. Physiol. Regu.l Integr. Comp. Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 5.Garg M, Thamotharan M, Dai Y, et al. Early postnatal caloric restriction protects adult male intrauterine growth-restricted offspring from obesity. Diabetes. 2012;61:1391–1398. doi: 10.2337/db11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano E, Bautista CJ, Deas M, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J. Physiol. (Lond) 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem. Soc. Trans. 1996;24:341–350. doi: 10.1042/bst0240341. [DOI] [PubMed] [Google Scholar]
- 9.Qasem RJ, Yablonski E, Li J, Tang HM, Pontiggia L, D'Mello AP. Elucidation of thrifty features in adult rats exposed to protein restriction during gestation and lactation. Physiol. Behav. 2012;105:1182–1193. doi: 10.1016/j.physbeh.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE. Catecholamine levels and receptor expression in low protein rat offspring. Diabetic medicine : a journal of the British Diabetic Association. 2000;17:848–853. doi: 10.1046/j.1464-5491.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 11.Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. The British journal of nutrition. 1996;76:605–612. doi: 10.1079/bjn19960066. [DOI] [PubMed] [Google Scholar]
- 12.Guzman M, Azzolin IR, Moulin CC, Perry ML. Pre- and postnatal protein undernutrition increases hepatic carnitine palmitoyltransferase I activity and decreases enzyme sensitivity to inhibitors in the suckling rat. Horm. Metab. Res. 1992;24:471–473. doi: 10.1055/s-2007-1003365. [DOI] [PubMed] [Google Scholar]
- 13.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 14.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozanne SE, Wang CL, Petry CJ, Smith JM, Hales CN. Ketosis resistance in the male offspring of protein-malnourished rat dams. Metabolism. 1998;47:1450–1454. doi: 10.1016/s0026-0495(98)90068-3. [DOI] [PubMed] [Google Scholar]
- 16.Blasiole DA, Davis RA, Attie AD. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol Biosyst. 2007;3:608–619. doi: 10.1039/b700706j. [DOI] [PubMed] [Google Scholar]
- 17.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am. J. Obstet. Gynecol. 2007;196:555, e1–e7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 19.Leon-Quinto T, Magnan C, Portha B. Altered activity of the autonomous nervous system as a determinant of the impaired beta-cell secretory response after protein-energy restriction in the rat. Endocrinology. 1998;139:3382–3389. doi: 10.1210/endo.139.8.6149. [DOI] [PubMed] [Google Scholar]
- 20.Choi GY, Tosh DN, Garg A, Mansano R, Ross MG, Desai M. Gender-specific programmed hepatic lipid dysregulation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 2007;196:477, e1–e7. doi: 10.1016/j.ajog.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1702–E1714. doi: 10.1152/ajpendo.00605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am. J. Physiol. Regu.l Integr. Comp. Physiol. 2001;280:R183–R190. doi: 10.1152/ajpregu.2001.280.1.R183. [DOI] [PubMed] [Google Scholar]
- 23.Magee TR, Han G, Cherian B, Khorram O, Ross MG, Desai M. Down-regulation of transcription factor peroxisome proliferator-activated receptor in programmed hepatic lipid dysregulation and inflammation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 2008;199:271, e1–e5. doi: 10.1016/j.ajog.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aihie Sayer A, Dunn R, Langley-Evans S, Cooper C. Prenatal exposure to a maternal low protein diet shortens life span in rats. Gerontology. 2001;47:9–14. doi: 10.1159/000052764. [DOI] [PubMed] [Google Scholar]
- 25.Edgerton DS, Cardin S, Emshwiller M, et al. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes. 2001;50:1872–1882. doi: 10.2337/diabetes.50.8.1872. [DOI] [PubMed] [Google Scholar]
- 26.O'Doherty RM, Anderson PR, Zhao AZ, Bornfeldt KE, Newgard CB. Sparing effect of leptin on liver glycogen stores in rats during the fed-to-fasted transition. Am. J. Physiol. 1999;277:E544–E550. doi: 10.1152/ajpendo.1999.277.3.E544. [DOI] [PubMed] [Google Scholar]
- 27.Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am. J. Physiol. 1996;270:E559–E564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- 28.Miles JL, Huber K, Thompson NM, Davison M, Breier BH. Moderate daily exercise activates metabolic flexibility to prevent prenatally induced obesity. Endocrinology. 2009;150:179–186. doi: 10.1210/en.2008-1035. [DOI] [PubMed] [Google Scholar]
- 29.Thompson NM, Norman AM, Donkin SS, et al. Prenatal and postnatal pathways to obesity: different underlying mechanisms, different metabolic outcomes. Endocrinology. 2007;148:2345–2354. doi: 10.1210/en.2006-1641. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Wang C, Terroni PL, Cagampang FR, Hanson M, Byrne CD. High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am. J. Physiol. Regu.l Integr. Comp. Physiol. 2005;288:R112–R118. doi: 10.1152/ajpregu.00351.2004. [DOI] [PubMed] [Google Scholar]
- 31.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145:R1–R13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O'Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 33.Ghadiminejad I, Saggerson ED. The relationship of rat liver overt carnitine palmitoyltransferase to the mitochondrial malonyl-CoA binding entity and to the latent palmitoyltransferase. Biochem. J. 1990;270:787–794. doi: 10.1042/bj2700787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman TC, Hale DE, Bhala A, Thorpe C. An acyl-coenzyme A dehydrogenase assay utilizing the ferricenium ion. Anal. Biochem. 1990;186:280–284. doi: 10.1016/0003-2697(90)90080-s. [DOI] [PubMed] [Google Scholar]
- 35.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol. Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 36.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev. Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]