Abstract
Both arthropods and large grazing herbivores are important components and drivers of biodiversity in grassland ecosystems, but a synthesis of how arthropod diversity is affected by large herbivores has been largely missing. To fill this gap, we conducted a literature search, which yielded 141 studies on this topic of which 24 simultaneously investigated plant and arthropod diversity. Using the data from these 24 studies, we compared the responses of plant and arthropod diversity to an increase in grazing intensity. This quantitative assessment showed no overall significant effect of increasing grazing intensity on plant diversity, while arthropod diversity was generally negatively affected. To understand these negative effects, we explored the mechanisms by which large herbivores affect arthropod communities: direct effects, changes in vegetation structure, changes in plant community composition, changes in soil conditions, and cascading effects within the arthropod interaction web. We identify three main factors determining the effects of large herbivores on arthropod diversity: (i) unintentional predation and increased disturbance, (ii) decreases in total resource abundance for arthropods (biomass) and (iii) changes in plant diversity, vegetation structure and abiotic conditions. In general, heterogeneity in vegetation structure and abiotic conditions increases at intermediate grazing intensity, but declines at both low and high grazing intensity. We conclude that large herbivores can only increase arthropod diversity if they cause an increase in (a)biotic heterogeneity, and then only if this increase is large enough to compensate for the loss of total resource abundance and the increased mortality rate. This is expected to occur only at low herbivore densities or with spatio-temporal variation in herbivore densities. As we demonstrate that arthropod diversity is often more negatively affected by grazing than plant diversity, we strongly recommend considering the specific requirements of arthropods when applying grazing management and to include arthropods in monitoring schemes. Conservation strategies aiming at maximizing heterogeneity, including regulation of herbivore densities (through human interventions or top-down control), maintenance of different types of management in close proximity and rotational grazing regimes, are the most promising options to conserve arthropod diversity.
Keywords: grazing, insects, invertebrates, plants, large grazers, ungulates, management, species richness, defoliation, soil compaction
I. INTRODUCTION
Large grazing herbivores exert major influences on their habitat and are abundant and important in all grassland ecosystems (Hobbs, 1996; Olff, Ritchie & Prins, 2002). Populations and communities of large herbivores have been under human influence for millennia, with humans causing extinctions (Owensmith, 1989; Lorenzen et al., 2011; Rule et al., 2012) and changes in abundances (Owensmith, 1989). Additionally, ever since the first goats and sheep were domesticated over 11000 years ago (Zeder, 2008) agricultural livestock practices have intensified, culminating in the year 2000 in 26% of the terrestrial biome being used for livestock production as pasture or fodder crops (FAO, 2008). This may pose a threat to biodiversity through overgrazing (e.g. Smith, 1940), and habitat loss and fragmentation (e.g. Kruess & Tscharntke, 1994; Fahrig, 2003). Conversely, in many semi-natural types of grassland, especially in Europe, the maintenance or reintroduction of large herbivores is a widely applied management tool, aiming to preserve an open, species-rich landscape (Ostermann, 1998; WallisDeVries, 1998). In these systems, livestock is thought to replace ecological functions of now-extinct native herbivores such as aurochs and tarpan (Bakker et al., 2004). Grazing thus has a large impact on a global scale and in many areas grazing regimes have recently changed due to agricultural intensification (increased stocking rates), agricultural abandonment (EEA, 2004) and changes in wild herbivore assemblages (Campbell & Borner, 1995; Donlan et al., 2006). It is therefore imperative to understand the influence of large grazing herbivores on the biodiversity of various plant and animal groups.
Effects of grazing on plant diversity are variable, with literature supporting both positive and negative effects (Milchunas, Sala & Lauenroth, 1988; Olff & Ritchie, 1998). Reported effects on arthropod diversity are equally diverse, with studies reporting negative (e.g. Kruess & Tscharntke, 2002a,b,; Pöyry et al., 2004), positive (Joern, 2005; Woodcock & Pywell, 2009), or neutral (Bestelmeyer & Wiens, 2001; Hofmann & Mason, 2006) effects of large herbivores. Intuitively, a strong positive relationship between the diversity of resources (plants) and consumers (arthropods) would be expected (Murdoch, Peterson & Evans, 1972; Tilman, 1986), but evidence is mounting that the response of arthropod diversity to grazing deviates from that of plant diversity (e.g. Kruess & Tscharntke, a2002; Pöyry et al., 2006; Zhu et al., 2012). For plants, a number of mechanisms underlying the effects of grazing on diversity have been identified, and general frameworks bringing these mechanisms together have been proposed (Milchunas et al., 1988; Olff & Ritchie, 1998). Such a framework is largely missing for understanding effects of large herbivores on arthropod diversity (but see e.g. Morris, 2000; Bell, Wheater & Cullen, 2001), despite the fact that arthropods constitute the most species-rich eukaryotic group on earth, are responsible for myriad ecosystem services (Prather et al., 2013) and take a central place in all terrestrial food webs (Seastedt & Crossley, 1984).
In this review we explore the patterns and processes of grassland arthropod responses to large herbivores. First, we present an overview of published literature in terms of taxonomic, geographic and experimental focus in published research, and perform a quantitative review in which we compare the responses of arthropod and plant diversity to grazing. Next, we classify the mechanisms through which large herbivores affect arthropod diversity. The resulting framework includes both direct effects (such as disturbance and incidental predation) and indirect effects (through modifications of soil and vegetation properties) of large herbivores on arthropod communities. Finally, we synthesise these effects, discuss the implications for conservation of arthropod diversity and identify remaining questions.
We focus this review on the effects of large herbivores on aboveground arthropod communities in open landscapes and on ecological time scales. Obviously, large herbivores also affect belowground communities (as reviewed by Bardgett & Wardle, 2003), play a role in forested landscapes (included in the review by Suominen & Danell, 2006) and have co-evolutionary relations with grassland plants (McNaughton, 1984; Milchunas et al., 1988) and arthropods (e.g. Siegfried, 1990). Given these earlier syntheses, these habitats, ecosystem compartments and evolutionary timescales fall outside the scope of this review. Other potentially important drivers of the diversity of grassland arthropods, such as burning and hay-making have been included in reviews by Morris (2000), Littlewood, Stewart & Woodcock (2013bb) and Joern & Laws (2013), and are, therefore, not considered here either. Large-scale patterns and processes, such as landscape characteristics and meta-community dynamics have recently been reviewed and synthesized by Tscharntke et al. (2012).
II. QUANTITATIVE RESPONSE OF ARTHROPOD DIVERSITY TO GRAZING
In order to get an overview of taxonomic spread, geographic location, and experimental design in studies reporting on the impact of large herbivores on arthropod diversity, we searched published literature for publications on this topic. Of the publications found, we used a sub selection (those that simultaneously assessed response of arthropod and plant diversity to grazing) to quantitatively assess (i) whether the response of arthropod diversity to grazing differs from that of plant diversity, and (ii) whether the response of arthropod diversity is related to the response of the plant community, ecosystem productivity or differences in experimental design among studies.
(1) Literature search
We performed a systematic search (Pullin & Stewart, 2006) for papers on effects of grazing by large herbivores on arthropod species richness, comparing different grazing intensities, species or breeds, or which compared grazing to other forms of conservation management such as burning, hay-making or abandonment. Only studies meeting the following three criteria were assessed: (i) published or in press in international, peer-reviewed scientific journals in Thomson Reuters Web of Science, accessible to the University of Groningen; (ii) performed in (semi)-natural grass- or heathland ecosystems; (iii) with arthropods identified to species level. Studies in which grazing effects were potentially confounded with other variables (such as soil type or climate) were omitted. We initially used cross-referencing to get an overview of the groups of arthropods commonly assessed, and finally performed searches on each of these groups, as well using general search terms ‘insects’, ‘arthropods’ and ‘invertebrates’ (see online Table S1) in combination with ‘graz*’ in Web of Science.
(2) Dataset description
Our search yielded 141 studies assessing the effects of large herbivores on arthropod communities published between 1940 and May 2013, sometimes in combination with other management types (see online Table S1). An overview of the taxonomic and geographic focus of all 141 studies is given in Fig. 1. Ground beetles, butterflies and grasshoppers have been studied most extensively, while other, sometimes extremely species-rich groups, such as parasitic Hymenoptera, (non-syrphid) flies and aphids have received virtually no attention (Fig. 1A). More than half of the studies assessed only one taxonomic group, with less than 25% of studies assessing more than two arthropod taxa (Fig. 1B). The number of years that arthropods were sampled during these studies varied: in about half of the studies arthropods were sampled for only 1 year while only during two studies were data collected for 8 years or more (Fig. 1C).
Fig 1.
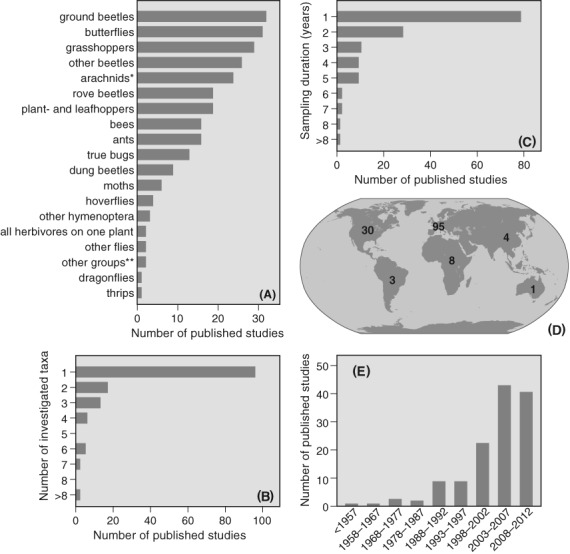
Research focus of 141 published studies assessing the effects of large herbivores on arthropod diversity, conducted in open landscapes (grass- or heathlands) with arthropods identified to species level. (A) Studied taxa, (B) taxonomic spread (number of investigated taxa), (C) duration of sampling, (D) geographic location, and (E) year of publication (until 2012). We documented the identity of the most commonly assessed taxonomic groups (usually to order level, but sometimes to family or class level). A complete list of the analysed studies and definitions of arthropod groups can be found in Tables S1 and S3. *Arachnids: spiders, harvestmen, pseudoscorpions; **other groups: Mantodea, Phasmatodea, Neuroptera, Dermaptera.
The majority of grazing studies were conducted in Europe (>65%; Fig. 1D), where domestic grazer populations are often managed for nature-conservation purposes. In North America (21%) and Africa (5%) grazing studies are also regularly conducted, often focusing on the effects of wild herbivores, sometimes in comparison to domestic livestock. Studies from Oceania, Asia and South America are rare, although several studies from these continents have been published on grazing effects in wood- or scrublands (see online Table S2). More than half of the studies were published after 2002 (Fig. 1E).
Studies of the effects of large herbivores on arthropod diversity could roughly be divided into two types: controlled experimental approaches and historic studies. In controlled experiments, a comparison was made between experimental plots receiving (randomly assigned) treatments differing in stocking density or grazing species (e.g. Gibson et al., 1992a,b,; Dennis et al., 1997; Joern, 2005; Rickert et al., 2012). These include studies using exclosures to exclude some or all vertebrate herbivores within sites (e.g. Morris, 1967; Fisher, Barham & Stewart, 2005; Gómez & González-Megías, 2007). The controlled experiments usually ran for less than 10 years (although some impressive examples of long-term experimental grazing research exist, see online Table S1) and had a relatively small number of replicates (maximum three). In the historical studies, effects of grazing were compared among a number of sites that differed historically in densities or species of herbivore (e.g. Smith, 1940; Kruess & Tscharntke, 2002a,b,; Nickel & Hildebrandt, 2003). Here, the number of replicate sites and the geographical extent were usually larger, but the sites did not necessarily have a constant grazing pressure or identical starting conditions. In our database, experimental and historical studies were represented approximately equally.
(3) Statistical analysis
For the quantitative assessment of grazing effects on arthropod diversity we used all studies that reported the response of both arthropod and plant diversity to different grazing intensities, including no grazing (24 of the initial 141 studies). This selection included 21 studies conducted in Europe, one in Africa, and two in the Americas. Ecosystems ranged from prairies and savannahs to coastal salt marshes and alpine grasslands, all of which had a history of grazing of at least several decades. Both experimental and descriptive approaches were represented. From these studies we extracted the reported numbers of plant and arthropod species found under each grazing treatment. In three cases effects on plant diversity were extracted from other publications about the same experiment, and in four cases effects on plant diversity were obtained directly from the authors. For studies where plant or arthropod richness responses to grazing were only reported in graphs, we used ImageJ software (Abramoff, Magalhaes & Ram, 2004) to extract accurate estimates of richness.
We performed two separate linear mixed-model analyses to analyse the relation between plant and arthropod diversity in response to grazing. As the response variable, we used untransformed response ratios of the change in richness with an increase in grazing intensity (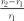 , where r1 = richness at lower grazing intensity and r2 = richness at higher grazing intensity), because these better approximated a normal distribution than log-transformed response ratios (Hedges, Gurevitch & Curtis, 1999; see also Milchunas & Lauenroth, 1993; Wardle et al., 2001). When responses of multiple arthropod taxa were reported (10 studies), we used the response ratio averaged over all taxa so that changes in comparatively species-poor taxa (e.g. butterflies) would not be overshadowed by changes in species-rich taxa (e.g. beetles). Therefore, only one data point per comparison between two grazing levels was included per study. When more than two grazing intensities were reported in a study, all pairwise comparisons were included as separate data points, as were multiple sites per study (whenever reported separately). This resulted in a total of 61 data points. A complete list of the analysed studies and definitions of arthropod groups can be found in Tables S1 and S3.
, where r1 = richness at lower grazing intensity and r2 = richness at higher grazing intensity), because these better approximated a normal distribution than log-transformed response ratios (Hedges, Gurevitch & Curtis, 1999; see also Milchunas & Lauenroth, 1993; Wardle et al., 2001). When responses of multiple arthropod taxa were reported (10 studies), we used the response ratio averaged over all taxa so that changes in comparatively species-poor taxa (e.g. butterflies) would not be overshadowed by changes in species-rich taxa (e.g. beetles). Therefore, only one data point per comparison between two grazing levels was included per study. When more than two grazing intensities were reported in a study, all pairwise comparisons were included as separate data points, as were multiple sites per study (whenever reported separately). This resulted in a total of 61 data points. A complete list of the analysed studies and definitions of arthropod groups can be found in Tables S1 and S3.
First, we tested whether plant and arthropod diversity responded differently to grazing management, using taxonomic group (arthropod/plant) as a fixed factor and ‘data point’ nested in ‘publication’ as random factors. Secondly, we analysed which variables explained the response of arthropod diversity to an increase in grazing intensity. For this analysis we used the same response variable for arthropods described above and ‘publication’ was again used as a random factor. As explanatory variables we included response ratio of plant diversity and productivity of the study system, and as covariates we included the type of experimental design [duration of the grazing treatment, nature of the study (experimental or descriptive), and the difference in grazing intensity studied]. These variables were included as they are known to affect the response of plant diversity to grazing (Milchunas et al., 1988; Olff & Ritchie, 1998; Proulx & Mazumder, 1998; Bakker et al., 2006). Duration of treatment was included as the number of years since the most recent management change. Productivity and difference in grazing intensity between compared treatments were included as ordinal variables and estimated from the site descriptions (productivity: ‘1’ for unproductive systems such as steppes and heathlands, ‘2’ for mesotrophic grasslands and ‘3’ for productive systems such as savannahs, floodplains and salt marshes; difference in grazing intensity: ‘1’ indicates a small difference in herbivore density, for instance low versus moderate density, whereas ‘3’ indicates a large difference in density e.g. ungrazed versus intensively grazed, ‘2’ was used for intermediate differences). Interaction terms were not included, as there was no a priori biological reason to assume any of these to be of particular relevance. To obtain an estimate of the variation explained by this second model, we obtained a pseudo-r2 using the recently published method for mixed models (Nakagawa & Schielzeth, 2013) using the MuMIn package for R (Barton, 2013). This gives the ‘marginal r2’, which represents the variance explained by the fixed factors, and the ‘conditional r2’, representing the variance of both the random and the fixed factors. All analyses were performed in R 2.14.1 (R Core Team, 2013), with use of the lme4 package (Bates, Maechler & Bolker, 2013).
(4) Results
There was large variation in response of both plant and arthropod diversity to grazing (Fig. 2A). Across all studies, arthropod diversity responded significantly negatively to an increase in grazing intensity (GLMM: μ = −0.14 ± 0.04, t = −3.36, P = 0.002, Fig. 2A), with over 80% of the data points showing a decrease in richness. Plant diversity, however, did not show a significant response to grazing (GLMM: μ = 0.04 ± 0.04, t = 0.98, P = 0.33), with approximately as many positive responses as negative ones (Fig. 2B). When the two effects were compared, the response of arthropod diversity was significantly more negative than that of plant diversity (GLMM: μ = −0.15 ± 0.03, t = 4.54, P < 0.001, Fig. 2A). The second mixed model, including multiple explanatory variables, revealed a significant, but weak positive relationship between the responses of plant and arthropod diversity to grazing (β = 0.41 ± 0.13, t = 3.28, P = 0.004, model fit: χ2 = 9.65, P = 0.002, Fig. 2B), with a negative intercept (μ = −0.15 ± 0.04). We found no significant effect of ecosystem productivity (χ2 = 1.21, P = 0.55), study duration (χ2 = 6.98, P = 0.14), experimental type (χ2 = 0.56, P = 0.45), or difference in grazing intensity (χ2 = 3.94, P = 0.27). The variation explained by the model was relatively low. The fixed variables (marginal r2) explained only 14% of the variation, but the fixed and random variables combined (conditional r2) explained 55% of the total variation, indicating large variation in response to grazing among studies.
Fig 2.
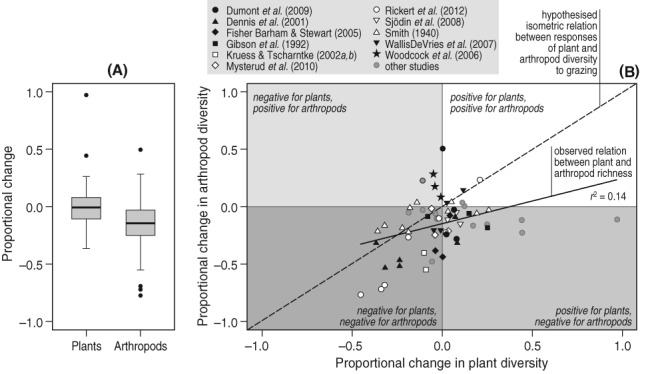
Comparison of the response ratios of plant and arthropod diversity to an increase in grazing intensity (median ± interquartile range, whiskers represent 1.5 × interquartile range, dots represent outliers) (A) and the relationship between these response ratios (B) Data were extracted from 24 studies published between 1940 and 2013 reporting on the effects of grazing on both plant and arthropod diversity, supplemented with data obtained from several authors (see online Table S1).
III. MECHANISMS UNDERLYING GRAZING EFFECTS ON ARTHROPOD DIVERSITY
The quantitative analysis in Section II2004 showed that (i) the prevailing effect of large-herbivore grazing on arthropod diversity is negative, (ii) within studies, arthropod diversity responds more negatively to grazing than does plant diversity, (iii) the response of plant diversity to grazing is a poor predictor for the response of arthropod diversity, and (iv) there is large variation in the effects of grazing on arthropod diversity. None of the covariates included in our model [productivity of the study system, duration of the grazing treatment, nature of the study (experimental or descriptive) and the difference in grazing intensity studied] proved significant. This may indicate that these factors are not of major importance in determining arthropod richness changes in response to grazing. However, because of the size of the dataset and the frequently limited accuracy of estimates (especially for productivity) caution is advised when drawing conclusions and more research may be required. The majority of variation explained by our mixed model was due to the differences between studies (random effects). Differences between focal arthropod groups might be one of the main sources of this random variation. Arthropods form a large, heterogeneous group with a broad diversity in life-history traits and different groups have repeatedly been shown to differ in their sensitivity to changes in habitat characteristics (Dauber et al., 2005; Oertli et al., 2005).
In order to understand these patterns, we will focus on the potential mechanisms by which large herbivores affect arthropod species. Figure 3 shows a conceptual framework of direct and indirect pathways through which herbivores can affect arthropods. The impact of these pathways on arthropod diversity is mediated by the three ecological determinants of the populations that constitute a community: (i) abiotic conditions of the environment (including non-trophic use of biotic structures), (ii) trophic resource availability and (iii) predation (Chase & Leibold, 2003). We use these determinants to classify the mechanisms by which arthropods are affected.
Fig 3.
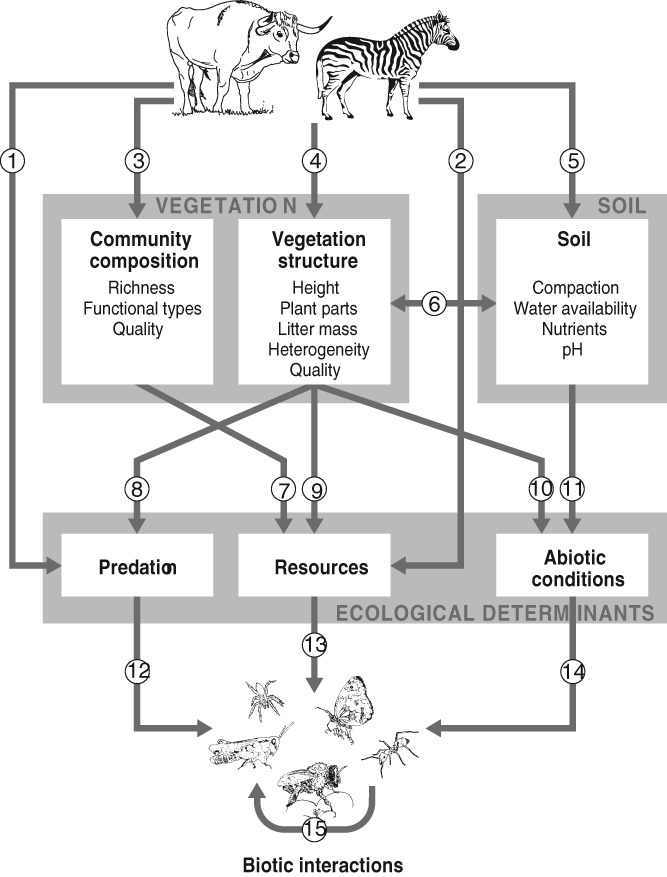
A conceptual framework of the mechanistic pathways by which large herbivores directly and indirectly affect arthropod diversity. Arrows represent mechanisms. The first row of boxes represents biotic and abiotic conditions that are modified by large herbivores; the second row of boxes represents the mechanisms operating on arthropod individuals, populations and communities. (1) Direct effects: trampling and unintentional predation (Section III.12009); (2) direct effects: dung, carcasses, blood, live tissue (Section III.12009); (3) increase or decrease in plant species richness and changes in functional groups, the direction of which depends on large herbivore density and ecosystem properties (Section III.32006); (4) changes in vegetation structure: lowering of vegetation height through defoliation and changes in horizontal heterogeneity resulting from herbivore selectivity (Section III.22004); (5) changes in soil conditions (pH, bulk density) (Section III.42013); (6) changes in soil conditions can affect vegetation characteristics (Section III.42013); (7) changes in plant species richness can affect species richness of associated insect herbivores (Section III.32006); (8) a reduction in vegetation height can increase predation risk by vertebrate predators (Section III.22004); (9) direct competition for resources between the base of the arthropod food web and large herbivores (Section III.22004); (10) a reduction in vegetation height increases surface temperatures, but decreases shelter from climatic extremes and essential structures for egg deposition or web construction (Section III.22004); (11) changing soil properties may affect insects that spend part of their lives below ground (Section III.42013); (12–14) the combined changes in abiotic conditions, resources and predation determine the effects on each arthropod species, thereby affecting species richness; (15) due to the interactions between arthropod species, changes in species' abundances may have cascading effects on other species, with ultimate effects on total arthropod species richness.
(1) Direct effects
Large herbivores can affect arthropod diversity directly through unintentional ingestion or trampling (Fig. 3, Path 1), but also by supplying resources for specialised groups such as dung feeders and scavengers (Fig. 3, Path 2).
Large herbivores frequently ingest arthropods as a by-product of their forage intake. Such unintentional predation can lead to reduced arthropod population sizes (Bonal & Muñoz, 2007; Gómez & González-Megías, 2007; Van Noordwijk et al., 2012b2012b). The potential consequences at the community level have rarely been measured, but defoliation by mowing is known to cause high direct arthropod mortality (reviewed by Humbert, Ghazoul & Walter, 2009). Gómez & González-Megías (2007) demonstrated large differences between guilds of herbivorous insects in susceptibility to unintentional predation. While endophagous insects (living within plant structures) often were ingested by large herbivores, ectophagous insects (living on plants) were generally not affected. Aphids and ladybirds, for example have been shown to avoid ingestion by dropping off the plant when detecting the breath of large vertebrates (Gish, Dafni & Inbar, 2010; Ben-Ari & Inbar, 2013). However, in short vegetation with limited possibilities to escape, and during immobile life stages (eggs and larvae) ectophagous species also may be ingested (Van Noordwijk et al., 2012b2012b). These differences in vulnerability to incidental ingestion among arthropod guilds suggest a large potential for shifts in arthropod communities.
Large herbivores also cause direct disturbance while moving through their habitats; most evident in the form of trampling living vegetation, litter and soil (Cumming & Cumming, 2003; Hobbs, 2006; Fig. 3, Path 1). Knowledge on the extent to which this affects arthropods is limited, but there is some observational (Chappell et al., 1971; Bayfield, 1979; Bonte & Maes, 2008; Woodcock & Pywell, 2009) and experimental (Duffey, 1975) evidence that trampling by herbivores or humans mostly negatively affects population sizes and diversity of arthropods. It is not always clear, however, whether these effects resulted directly from direct trampling on arthropods, or indirectly, through changes in soil, litter or plant characteristics (see also Sections III.2 and III.32004). Duffey (1975) demonstrated convincingly that even low frequencies of 5–10 treads per month on litterbags were highly detrimental to the arthropod fauna, and Chappell et al. (1971) showed large decreases in faunal abundance between lightly and heavily trampled calcareous grasslands. For less-mobile arthropods, such as caterpillars, but also for large dung beetles (Negro, Rolando & Palestrini, 2011) trampling could be an underestimated direct source of mortality (Fig. 3, Path 1). Additionally, frequent disturbance by large herbivores may decrease habitat suitability for arthropods. This may again be of greater importance for less-mobile species that could experience difficulties in returning to their host plants, like many larval insects (Dennis, Young & Gordon, 1998; Kruess & Tscharntke, a), and may even be evident at low herbivore density, when no measurable effect on vegetation characteristics is documented (Kruess & Tscharntke, 2002b,2002).
Conversely, large herbivores may have positive effects by directly supplying resources to arthropods in the form of dung, carcasses, blood and living tissue (Fig. 3, Path 2). Studies investigating the effect of dung on arthropod communities mostly focused on dung beetles, despite the fact that termites (Freymann et al., 2008) and various fly families also feed on dung. Not surprisingly, these studies often report positive effects of large-herbivore presence on dung beetle diversity and abundance (Lumaret, Kadiri & Bertrand, 1992; Verdu et al., 2007; Jay-Robert et al., 2008), but high herbivore densities may be detrimental to dung beetle abundance and diversity (Jankielsohn, Scholtz & Louw, 2001; Negro et al., 2011). Differences in dung beetle diversity between livestock grazing and natural herbivore assemblages have been reported to be small, although community composition can differ between areas with different herbivore assemblages (Jankielsohn et al., 2001; Numa et al., 2012). Effects of livestock management on dung-feeding fauna is also strongly influenced by the use of antiparasitic medication, which has highly detrimental effects on dung-feeding fauna (Wall & Strong, 1987; Madsen et al., 1990) and dung decomposition rates (Wall & Strong, 1987; Beynon et al., 2012).
Although it is intuitive that the presence of herbivores may enhance the diversity of scavenging and parasitic arthropods, field studies showing such patterns are scarce (Barton et al., 2013). Evidence has been presented that a deer carcass can be a hotspot for biodiversity compared to the surrounding forest (Melis et al., 2004) and that the presence of large herbivores can increase tick populations (Keesing et al., 2013), but decrease populations of mice and their fleas (McCauley et al., 2008). For these arthropod groups, human influence may be of extra importance, because in many grazed ecosystems, the resources that these species depend on are highly managed. For instance, removal of carcasses and treatment with anti-parasitic medication are very common in European semi-natural grasslands. Also targeted extermination of livestock parasites has large impacts on parasite populations. For example, the presence of cattle treated with acaricides reduces tick abundance (Keesing et al., 2013), and several species of parasites have been eradicated from parts of their former range (e.g. Wilson, 1986; Vreysen et al., 2000). Nevertheless, introductions of livestock outside their native range have probably enhanced the spread of their parasites even more (e.g. Scholl, 1993). Anthropogenic causes of changes in large herbivore densities, with in its most dramatic form extinctions of species, will almost certainly lead to co-extinctions of their parasites (Dunn et al., 2009) and scavengers.
In conclusion, the direct effects of large herbivores on arthropod diversity are potentially manifold and sometimes obvious, but are, with the exception of dung beetles, poorly quantified. Nevertheless, the overall impact on arthropod diversity of these direct effects is probably small in comparison to the indirect effects, as we will see below.
(2) Vegetation-structure-mediated effects
The most prominent effect caused by large herbivores is defoliation, leading to a decrease in vegetation height and structural complexity (Fig. 3, Path 4). Most plants can tolerate defoliation to some extent by resorting to dwarf growth, vegetative spread, or by fast regrowth. Repeated defoliation and trampling can lead to changes in plant species composition (Fig. 3, Path 3), which will be discussed in Section III.3. For arthropods, short and tall vegetation types provide different abiotic conditions, food resources and predation risk (Fig. 3, Paths 8–10). Currently emerging insights into how these differences affect arthropod diversity are outlined below.
The abiotic conditions arthropods are exposed to differ vastly between short and tall vegetation (Fig. 3, Path 10). When vegetation is permanently grazed short and bare soil is exposed, this often leads to a warmer microclimate in the vegetation and higher soil temperatures, which are essential for the larval development of various thermophilous arthropods such as many grasshopper and butterfly species (e.g. Thomas et al., 1986; Cherrill & Brown, 1992; Bourn & Thomas, 2002; Roy & Thomas, 2003). Moreover, several species require bare, exposed soil for egg deposition (e.g. tiger beetles) or nesting (e.g. solitary bees). Tall and dense vegetation, on the other hand, can act as a temperature buffer, with relatively cool temperatures during the day and benign temperatures at night or in winter (Luff, 1966; Dennis, Thomas & Sotherton, 1994), or provide shelter from extreme climatic conditions such as droughts or (periodical) floods (Pétillon et al., 2008). It also offers complex three-dimensional structures for web-building spiders (Gibson, Hambler & Brown, 1992b), for species that pupate (many parasitoid Hymenoptera) or deposit eggs (e.g. some grasshopper species) in or on plants, and offers hiding and stalking opportunities for predatory arthropods in the canopy (e.g. crab spiders, praying mantes).
Resource availability also differs between tall and short vegetation (Fig. 3, Path 9). Tall vegetation possesses aerial structures, like flowers and stems, and the removal of these structures is logically detrimental to their consumers, such as pollinators (Gómez, 2003) and insects developing in flowerheads and fruits (Morris, 1967, 1971bb; Völkl et al., 1993; Gómez & González-Megías, 2007). Tall, ungrazed, vegetation is usually also accompanied by a dense litter layer, providing food for detritivores and their predators. Large herbivores consume large quantities of plant biomass that will therefore not enter the detrital food web. Litter additions have indeed been shown to increase abundance of predatory arthropods (Langellotto & Denno, 2004).
Conversely, short-grazed vegetation offers resources in the form of short-statured plants, that many specialised herbivorous insects depend upon (Thomas et al., 1986; Van Klink et al., 2013), but also in the form of nutrient-rich regrowth. After defoliation, the young leaves often have higher nutrient contents than older plant parts (McNaughton, 1976; Ydenberg & Prins, 2008). All else being equal, herbivorous insects react positively to an increase in resource quality (White, 1993; Ritchie, 2000), which sometimes leads to species attaining plague densities (Onsager, 2000). Positive effects on arthropod diversity, however, have thus far not been shown. Other plant species, especially in dry, unproductive systems, respond to defoliation by producing secondary compounds that are unattractive to large herbivores, but usually also for herbivorous arthropods (Vicari & Bazely, 1993; Nykanen & Koricheva, 2004). Specialist arthropods, however, have often co-evolved with their host plants in such a way that they tolerate or even profit from the secondary compounds that are produced after defoliation by large herbivores (Poelman et al., 2009).
Furthermore, predation risk is modulated by vegetation height (Fig. 3, Path 8). Large-eyed predators, such as some ground beetle species (Morris, 2000), but also vertebrate predators, such as birds (Belovsky, Slade & Stockhoff, 1990), hunt more efficiently in short vegetation or on bare ground. Tall vegetation may thus protect arthropods from predation, although the densities of arthropod predators, such as spiders, are known to increase with vegetation complexity (Langellotto & Denno, 2004).
Taken together, tall, complex vegetation should generally provide more food resources (Lawton, 1983), lower predation risk (Belovsky et al., 1990) and more opportunities for coexistence of arthropods than short vegetation, for instance through vertical niche differentiation (Denno, 1980). Indeed, a positive relation between vegetation biomass and arthropod diversity is often reported (Duffey, 1962; Luff, 1966; Woodcock et al., 2007; but see Joern, 2005; Woodcock & Pywell, 2009). Consequently, arthropod diversity has often been found to decrease with increasing densities of large herbivores (Dennis et al., 1997; Kruess & Tscharntke, 2002a,b,; Pöyry et al., 2004). Some arthropod species, however, depend on short vegetation with patches of bare soil (e.g. Joern & Lawlor, 1981). It is therefore likely that heterogeneous vegetation, consisting of a patchwork of short and tall vegetation will generally harbour the highest arthropod diversity.
Large herbivores can, under specific circumstances, enhance vegetation heterogeneity. They are usually not distributed homogeneously over the landscape, and exhibit spatial selectivity in their behaviour, such as feeding, defaecation and wallowing [dust-bathing, which creates sparsely vegetated patches (Collins & Barber, 1985)]. Spatial heterogeneity in feeding behaviour can lead to a patchy vegetation structure of short and tall vegetation if (i) herbivores forage selectively, with smaller herbivore species usually being more selective than large species (Jarman, 1974), (ii) herbivore density is too low to consume all vegetation and (iii) there is a positive feedback between large herbivores and the quality of their food (Adler, Raff & Lauenroth, 2001). Resulting heterogeneity in vegetation structure can then lead to heterogeneity of other ecosystem processes (McNaughton, 1984; Hobbs, 1996). This is most likely to occur in productive ecosystems (Hobbs & Swift, 1988). Conversely, if these conditions are not met, or when high underlying abiotic heterogeneity is already present, grazing is more likely to decrease heterogeneity (Adler et al., 2001).
Although arthropod diversity would be expected to be highest in heterogeneous grasslands, evidence for this relationship is remarkably scarce. Joern (2005) showed a positive relationship between grasshopper diversity and grazing-induced heterogeneity in vegetation height. However, this is not corroborated by other studies searching for such a relationship (Dennis et al., 1998; Van Klink et al., 2013). Moreover, some studies report highest vegetation heterogeneity to occur after cessation of grazing, and consequently find highest arthropod diversity under these conditions (e.g. Kruess & Tscharntke, a2002; Pöyry et al., 2006).
To complicate matters, the effects of grazing on vegetation structure vary across spatial scales (WallisDeVries, Laca & Demment, 1999; Adler et al., 2001). Grazing may, for example, lead to a more homogenous vegetation structure at a small scale, while simultaneously leading to heterogeneity at a larger scale (Adler et al., 2001). Such divergent effects of herbivores on vegetation heterogeneity may obscure general effects on arthropods.
Heterogeneity in vegetation structure caused by large herbivores may not only be expressed spatially, temporal heterogeneity is also likely to occur. This may be caused by seasonal variation in plant growth, but also by temporal variation in grazing pressure due to seasonal herbivore migrations or active management (Fryxell & Sinclair, 1988; Bischof et al., 2012). The range of spatial and temporal scales at which grazers can affect heterogeneity severely complicates field measurements of the effects on arthropod diversity. An increased understanding of the spatial and temporal scales at which grazing affects vegetation heterogeneity and knowledge of how scale affects the availability of resources and abiotic conditions for arthropods will greatly enhance our understanding of the impact of large herbivores on arthropod diversity.
(3) Vegetation-community-mediated effects
Large herbivores often have profound effects on plant diversity (Fig. 3, Path 3) and plant ecologists have a long history of studying these (Olff & Ritchie, 1998). In general, effects of herbivores on plant diversity tend to be positive in wet, productive systems and negative in dry, infertile ones (Olff & Ritchie, 1998; Proulx & Mazumder, 1998; Bakker et al., 2006; Lezama et al., 2014). Moreover, some of the most plant-species-rich ecosystems in the world are traditionally grazed grasslands in Europe (Wilson et al., 2012). A decrease of grazing, therefore, often leads to a decrease in plant diversity, as light competition causes exclusion of short-statured plant species (Grime, 1973).
Arthropod (consumer) diversity has been hypothesised to be correlated with plant (producer) diversity (Murdoch et al., 1972; Tilman, 1986), and experimental increases of plant diversity have indeed been shown to increase arthropod diversity (Siemann et al., 1998; Haddad et al., 2009), abundance (Haddad et al., 2001), functional group richness (Siemann et al., 1998; Rzanny & Voigt, 2012) and food-web complexity (Scherber et al., 2010; Rzanny & Voigt, 2012). Moreover, this relation was not only found for diversity of herbivorous insects, but also for predators (Haddad et al., 2009) and parasitoids (Ebeling et al., 2012). However, in experimental grazing research this interrelation between plant and arthropod diversity has rarely been supported. In fact, several researchers showed a negative response of arthropod diversity to grazing even when plant diversity increased (Kruess & Tscharntke, a2002; Pöyry et al., 2004), and the generality of these results is corroborated by our quantitative review (Section II2004). The response of plant diversity to grazing therefore seems to be a poor predictor for the response of arthropod diversity.
Obviously, the loss of host plants due to grazing or a lack thereof will lead to the co-extinction of their specialist herbivores. However, the presence of a plant species does not guarantee suitable conditions for its specialist herbivores. This may be due to the presence or absence of certain required plant parts (Morris, 1967) or the size of the plant (Lawton, 1983), but also to microclimate (Thomas et al., 1986), or isolation from the closest source population (Kruess & Tscharntke, 1994). Moreover, tall-statured and widespread plant species generally harbour a richer fauna of specialist insect herbivores than short-statured plant species (Lawton & Schroder, 1977; Strong, Lawton & Southwood, 1984; Tscharntke, 1997). This implies that with a lack of grazing, replacement of a short-statured host plant will cause a relatively small loss in diversity, while the gain of tall-statured species can potentially cause a large increase.
Another obvious way by which large herbivores modify the composition of plant communities is by changing the relative abundance of different plant functional groups (Fig. 3, Path 3). For instance, in wet, productive systems, grazing can increase the cover of palatable, grazing-tolerant plant species (often grasses) (McNaughton, 1984), whereas in arid systems it can increase the abundance of unpalatable shrubs (Archer, Schimel & Holland, 1995). In temperate systems, both intensive grazing and cessation of grazing can cause an increase in the relative cover of grasses (McNaughton, 1986; Milchunas & Lauenroth, 1993). Consistent with these observations, species richness of polyphagous and grass-feeding insects was found to be highest under intensive grazing (Nickel & Hildebrandt, 2003) as well as after cessation (Littlewood, 2008). Similarly, the diversity of both insect-pollinated plants and flower-visiting insects can be affected positively (Vulliamy, Potts & Willmer, 2006), negatively (Potts et al., 2009) or not at all (Batáry et al., 2010) by large herbivores. This suggests that shifting abundances of different functional plant groups as a result of grazing can have a large impact on herbivorous and flower-visiting insects and that these shifts may better explain changes in arthropod communities in response to grazing than plant diversity per se.
(4) Soil-mediated effects
Large herbivores can have a strong impact on soil properties, with some of the most consistent outcomes being altered levels of soil nutrients, pH values, water availability (Milchunas & Lauenroth, 1993; Bakker, Olff & Gleichman, 2009) and increased soil compaction (Trimble & Mendel, 1995) (Fig. 3, Path 5). Changes in soil conditions can lead to changes in plant communities (Liddle, 1997) (Fig. 3, Path 6), but can potentially also have direct effects on aboveground arthropods (Fig. 3, Path 11).
Although the effects of grazing on belowground fauna are strong (Bardgett & Wardle, 2003; Beylich et al., 2010), few studies report soil-mediated effects of herbivores on aboveground arthropods. Many species best known for their aboveground appearance, for example clickbeetles and crane flies, spend part of their life cycle below ground, as eggs or larvae. During these developmental stages, arthropods have been shown to react to changes in soil nutrients (Larsen et al., 1996; Goulet, 2003; Oliver et al., 2005), pH (Van Straalen & Verhoef, 1997; Goulet, 2003) and moisture level (Goulet, 2003), which can all be altered by large herbivores. Indications that herbivore-mediated changes in soil properties may affect aboveground fauna have so far only been reported for rove beetle communities (Hofmann & Mason, 2006) and some ant species (Bestelmeyer & Wiens, 2001). The generality of these effects is, however, as yet poorly known.
(5) Effects on interactions among arthropod species
Like all organisms, co-occurring arthropod species interact in myriad ways, including resource competition, predation and mutualistic interactions (Fig. 3, Path 15). Food webs are complex in nature, and often changes in one trophic level can have unforeseen consequences for another trophic level or guild (Schmitz, 2011). Experimental evidence for the way in which large herbivores can alter relations among arthropod species is scarce. It has been suggested that large grazers have an especially negative impact on parasitoids through direct disturbance and fragmentation of resources, thereby shortening arthropod food chains in grazed grasslands (Tscharntke, 1997). The general dearth of knowledge on the response of parasitoid Hymenoptera to habitat change (Shaw & Hochberg, 2001; Shaw, 2006), however, inhibits generalisation, and in fact positive effects of large herbivores on parasitoid abundance in experimental thistle patches have been reported (Vanbergen et al., 2006).
There is, however, a great potential for bottom-up-driven diversity control in grasslands, as suggested by the strong relationship between vegetation complexity and arthropod diversity (Section III.22004). An increase in abundance or diversity of herbivorous insects and detritivores can potentially increase the diversity of higher trophic levels, as was shown in plant diversity manipulation experiments (e.g. Scherber et al., 2010). From grazing experiments, so far only correlative evidence is available, showing similar changes in the diversity of herbivorous and predatory taxa to changes in grazing pressure (Gibson et al., a1992; Kruess & Tscharntke, 2002b2002b; Báldi, Batáry & Kleijn, 2013). Moreover, the diversity of parasitic Hymenoptera was found to correlate well with overall diversity (Anderson et al., 2011), suggesting that these potentially respond indirectly to herbivore-mediated changes in diversity of lower trophic levels. Still, causal relations explaining these changes have not yet been mapped in the context of grazing.
There is also potential for changes in top-down processes controlling diversity, since large herbivores can affect the abundance and diversity of predatory arthropods, which then might affect the diversity of lower trophic levels. Evidence for the importance of this process in grasslands is, however, extremely limited, and increased predator abundance may in fact enhance the diversity of lower trophic levels (Sanders & Platner, 2007). To understand these complex relations better, there is a strong need for food-web approaches in grazing research, with a good potential for path analysis (e.g. Scherber et al., 2010).
Finally, it is possible that grazing alters competitive outcomes between arthropod species from the same trophic level. For plants, it is well established that grazing strongly alters competitive relationships (Hobbs & Huenneke, 1992; Olff & Ritchie, 1998), but for arthropods, evidence is scarce. The importance of competitive exclusion in arthropod communities has been debated for decades (Lawton & Hassell, 1981; Denno, 1995). Although there is now ample evidence that resource competition and competitive exclusion do occur between herbivorous insects (White, 1993; Denno, 1995; Reitz & Trumble, 2002; Kaplan & Denno, 2007), it remains unclear how important these processes are in structuring natural communities in a field setting. Since the vast majority of arthropod species exploit different resource bases, the importance of competition among species in limiting diversity is probably small (Strong et al., 1984). Therefore, the disruption of competitive hierarchies by large herbivores is unlikely to have great impacts on arthropod diversity (Fuentes & Jaksic, 1988). Disentangling the relative importance of all these processes remains a formidable future challenge.
IV. SYNTHESIS
(1) Why is arthropod diversity so often negatively affected by grazing?
Ultimately, the mechanisms through which large herbivores affect arthropods are mediated by three key main components of arthropod population regulation: predation, trophic resource availability and abiotic conditions (Fig. 3). In the presence of large herbivores, (unintentional) predation and direct mortality of arthropods are likely to increase, which is especially likely to affect sedentary arthropods (Section III.1). These direct effects will be negative for diversity if mortality rates are high, but not detrimental if arthropod populations can be maintained.
The total trophic resource availability for arthropods will be reduced as herbivores consume plants and litter, which form the base of the arthropod food web (Section III.22004). Therefore, overall arthropod abundance is likely to be reduced under grazing. Given the large body of theoretical (Fisher, Corbet & Williams, 1943) and empirical evidence (Kruess & Tscharntke, a2002; Pöyry et al., 2006) showing a positive relationship between abundance and diversity of organisms, defoliation by large herbivores can be expected to be negative for arthropod diversity. However, plant diversity is often increased by grazing (Olff & Ritchie, 1998), creating opportunities for a wider group of specialist herbivores (Section III.32006). Also for species such as dung beetles and parasites resource abundance will increase with grazing (Section III.12009).
Large herbivores often strongly modify the abiotic environment experienced by arthropods (Section III.22004). Such modifications will be positive for some species and negative for others. Overall effects of changes in microclimatic conditions on diversity therefore depend on the habitat requirements of the species present in the regional species pool and the interactions of large herbivores with prevailing (microclimatic) conditions.
Taking all these effects together, the variation in biotic (e.g. dung and plant species) and abiotic (e.g. microclimate and habitat complexity) conditions may be enhanced by large herbivores (Section III2012). Therefore, arthropod diversity can be augmented by large herbivores if the following conditions are met: (i) grazing causes an increase in biotic and abiotic heterogeneity, (ii) this increase in heterogeneity occurs at such a spatial and temporal scale that it can be exploited by new species immigrating from the regional species pool and (iii) this positive effect of increased heterogeneity is large enough to compensate for the negative effects of direct mortality and resource competition between arthropods and large herbivores. This combination of conditions is most likely to occur at low densities of herbivores, because direct mortality and resource competition are minimal, while variation in (a)biotic conditions is most likely to increase (see Section III.22004).
High densities of large herbivores are likely always to be detrimental to arthropod diversity, although some arthropod species or groups may profit. This is indeed supported by most empirical studies (e.g. Gibson et al., a1992; Kruess & Tscharntke, 2002a,b,; Nickel & Hildebrandt, 2003; Rickert et al., 2012). Studies reporting otherwise (Vulliamy et al., 2006; Yoshihara et al., 2008) have all studied flower-visiting insects, which may not spend their whole life cycle in the study environment and may not represent overall arthropod diversity (Vessby et al., 2002; Oertli et al., 2005).
(2) Why is arthropod diversity affected more negatively by grazing than is plant diversity?
The difference between plants and arthropods in response to grazing can be understood by considering the mechanisms by which both groups are affected. Three differences between plants and arthropods emerge to explain the contrasting response to grazing.
First, plant diversity is generally increased by grazing through a decrease in light competition and an increase in colonisation by new species (Olff & Ritchie, 1998). Since there is no evidence for an important role of competition in limiting arthropod diversity (Section III.52000), it is unlikely that large herbivores can cause any type of competitive release on arthropod communities. Conversely, the majority of species at the base of the arthropod food web (herbivores and detritivores) compete directly for resources with large herbivores, as outlined in Section IV.12013. This competition is highly asymmetrical, and can lead to competitive exclusion and decreased population sizes (Gómez & González-Megías, 2002), which is likely to reduce arthropod diversity.
Secondly, the habitat requirements of plants and arthropods operate at different spatial and temporal scales (Bourn & Thomas, 2002). Plants are sedentary and need a specific set of conditions that are all met at one site. Arthropods generally have distinct phases in their life cycle, which often need different site conditions (e.g. warm microclimate and abundant host plants for larval development and nectar for adult life stages). In particular, during immature stages many species have a narrow niche and limited ability to actively find suitable habitat patches (Bourn & Thomas, 2002). For arthropods to survive, the requirements of all life-cycle stages must be met within the area an individual can travel. This means that single arthropod species often need a certain level of habitat heterogeneity (creating favourable microclimatic conditions and food resources for all life stages) at a specific spatial scale to survive. Plant species, on the contrary, can thrive in fairly homogeneous grasslands as long as their specific habitat requirements are met. As more intensive grazing management generally decreases habitat heterogeneity (see Section III.22004) this is inevitably detrimental to many arthropod species, even if the requirements of individual life stages are still met. In addition, the life cycle of many arthropod species is strictly synchronized (Zaslavski, 1988). This means that the habitat conditions for each life-cycle stage must be present at exactly the right time of year, making arthropods especially sensitive to the timing of grazing (Carvell, 2002; Lenoir & Lennartsson, 2010; Van Noordwijk et al., a2012)
Third, plants are more plastic in their response to grazing than are arthropods. Plants can often survive (periodical) high trampling and defoliation through dwarf growth, vegetative spread and belowground storage of resources. Arthropods generally do not have such back-up strategies. Some arthropods can attempt to escape unfavourable conditions by dispersal (Berggren, 2004), but they can only disperse over limited distances where they have to find favourable conditions again. This difference in vulnerability to grazing between plants and arthropods has strong implications for nature conservation.
(3) Implications for arthropod conservation management
Most grassland types worldwide depend on the presence of large herbivores to prevent succession to scrub or forest (Hobbs & Huenneke, 1992). In most of these grasslands herbivore densities are (strongly) influenced by human intervention including active management, exploitation, agricultural activities and abandonment of former agricultural practices. This will have profound impacts on these grasslands and their biodiversity, including arthropod diversity. Conservation goals, and hence decisions on stocking densities and other human interventions, vary widely over grazed ecosystems. A major part of grazed systems is being used for livestock grazing, where production of meat or other animal products, rather than nature conservation, is the primary goal. In a much smaller area of global grasslands, conservation purposes prevail. Here, management priorities may vary from a focus on maintaining diverse herbivore assemblages in African savannahs (Mbano et al., 1995), to the restoration of natural processes on the North American prairies (Sanderson et al., 2008) and a focus on preserving high (plant) diversity in European semi-natural grasslands (Ostermann, 1998; WallisDeVries, 1998). In agricultural grazing systems, management effects on (arthropod) diversity are generally not considered in decision making. Indeed, studies investigating the effects of livestock grazing in agricultural systems usually report negative impacts on diversity (Smith, 1940; Forbes et al., 2005; Xie, Williams & Tang, 2008) and abundances (Hutchinson & King, 1980) of arthropods. Also, in natural and semi-natural grasslands, arthropods are not always given high priority, but awareness of the importance of arthropods is growing among conservationists, as is attention for arthropods in conservation and restoration research (Fig. 1E). Our review highlights that specific attention for arthropods is essential for their conservation, as arthropods are generally more sensitive to grazing than plants. Therefore we highly recommend that arthropod species richness is monitored in addition to botanical composition when evaluating grazing management.
Although grazing is essential to conserve species-rich grasslands in the long run, we have shown that increased grazing intensity quickly becomes detrimental to overall arthropod diversity. On the other hand, high plant species richness is often best attained under moderate grazing regimes (Olff & Ritchie, 1998; Wilson et al., 2012) and many thermophilous insects, including many butterflies depend on favourable microclimates (Bourn & Thomas, 2002) created by more intensive grazing (see Section III.22004). Both plants and thermophilous butterflies characteristic of semi-natural grasslands have become severely threatened due to increased eutrophication and abandonment of traditional farming practices (Ostermann, 1998; Van Swaay et al., 2010) and, hence, are of special conservation interest (Van Swaay et al., 2010). This creates potential for conflict between the requirements of plant diversity, threatened arthropod species and maintenance of high overall arthropod diversity (see for example Negro et al., 2013). In habitat restoration, where arthropod populations of high conservation value are absent, a focus on plant restoration in the first few years may be justified, as this is a prerequisite for the establishment of many arthropod species (Woodcock et al., 2010, 2012). However, in a conservation context, solutions should be sought to meet the requirements of as many species as possible by conserving or promoting a heterogeneous habitat. Low densities of herbivores provide the best chance of attaining this objective (see Section IV.12013), but so far no evidence has been presented that a single management regime can accommodate all species in a local species pool (Dennis et al., 1997, Dennis, Young & Bentley, 2001). Therefore, it has been suggested that arthropod diversity can best be conserved at the landscape scale by maintaining grasslands under different types of management in close proximity (Dennis et al., 1997; Morris, 2000; Kruess & Tscharntke, 2002b2002b; Rickert et al., 2012). In addition to such spatial variation, temporal heterogeneity can be created by using rotational grazing with periods (weeks to decades) of grazing alternated with periods of cessation. This creates periods in which the negative effects of grazing (direct mortality and resource competition) are absent (Morris, 1967), while still providing opportunities for high plant diversity and an open vegetation structure. Rotational grazing has been shown to be successful for arthropod conservation in several ecosystems (Morris, Clarke & Rispin, 2005; Farruggia et al., 2012), but needs additional research in many others. Especially the duration of the different rotations may be of importance, since several weeks of grazing exclusion may already benefit flower-visiting insects (Farruggia et al., 2012), but endophagous grass-feeders may require multiple years before their populations increase (Rothenwöhrer, Scherber & Tscharntke, 2013). Offering variation in grazing intensity and timing on a landscape scale may also offer a feasible approach to increase arthropod diversity in agricultural landscapes, especially where agricultural fields are interspersed with semi-natural habitats (Tscharntke et al., 2012).
Whether specific species survive under a given grazing regime inevitably depends on the match between their habitat requirements and the timing, scale and intensity of grazing. While low-intensity grazing and variation of grazing intensities at the landscape scale will benefit overall arthropod diversity, more detailed grazing regimes will be required in cases where a specific suite of target species has been set. In these cases, a fruitful approach to finding the optimal grazing regime is to analyse the life cycles of these species (Williams et al., 2010; Verberk, van Noordwijk & Hildrew, 2013). This approach has been advocated for conservation purposes (Van Noordwijk et al., a2012), but can also be used actively to suppress populations of pest species (Onsager, 2000).
V. NEXT STEPS
From this review, clear patterns explaining the patterns of arthropod diversity in grazed ecosystems have emerged. Analysing the mechanisms affecting arthropod diversity responses to grazing has revealed why generally arthropod diversity responds negatively to (intensive) grazing and how the variation in these responses can be explained. Our study has also identified a number of issues that remain poorly understood and require further research. Although we have argued that a positive effect of large herbivores on arthropod diversity can mostly be expected at low herbivore densities, empirical evidence remains scarce, and more experimental testing is needed. In particular we need to expand our knowledge of the specific conditions under which large herbivores have a positive effect on arthropod diversity, for example by directly comparing a number of promising low-intensity grazing regimes. As we have demonstrated that spatial and temporal heterogeneity in (a)biotic conditions are crucial to arthropod diversity, these aspects need special attention. It has become apparent that there are large differences between arthropod taxa in their response to grazing. Therefore, multi-taxon studies are highly desirable, preferably conducted over multiple years to account for weather effects and population dynamics. In addition, a great deal can be learnt from smaller experimental studies targeting single mechanisms (e.g. incidental ingestion, effects of soil compaction or effects of plant diversity). To add to our current knowledge, these experiments should especially focus on effects of these mechanisms at the community level (the extent to which diversity and composition are affected). Helpful approaches in this respect include (i) trait-based approaches, demonstrating which traits determine to what extent arthropod species are affected by certain mechanisms and (ii) integrated food-web studies, demonstrating the importance of bottom-up, top-down and competitive interactions in shaping arthropod communities in grazed ecosystems. A food-web approach could also be used to link above- and belowground effects of large herbivores. Finally, to understand differences in responses of arthropod diversity to grazing between ecosystems, it is important to be able to compare in situ grazing pressure between studies and ecosystems. Such comparisons are currently hampered by, for example differences in ecosystem productivity and land-use history. An account of the percentage net primary productivity consumed by large herbivores should improve comparability, and aid future syntheses.
VI. CONCLUSIONS
(1) The vast majority of published studies on the effects of grazing on arthropods were conducted in Europe and North America, and focus on a small number of arthropod taxa. Studies demonstrating effects on overall arthropod diversity are lacking.
(2) Responses of arthropod diversity to grazing are highly variable, but arthropod diversity is often more negatively affected than plant diversity. Moreover, plant diversity is a poor predictor for arthropod diversity in grazed ecosystems. Therefore, we strongly recommend considering the specific requirements of arthropods and including arthropods in monitoring schemes evaluating the effects of grazing.
(3) Unintentional predation and disturbance have a negative effect on population sizes and diversity of most arthropod groups. Positive direct effects, like availability of resources such as dung and carrion, will only benefit a small number of arthropod species.
(4) Defoliation by large herbivores will cause a reduction of resource abundance for the base of the arthropod food web (herbivores and detritivores) and also reduces habitable space for species dependent on tall vegetation structures. This will generally have a negative effect on diversity.
(5) Large herbivores can, under specific conditions, increase both plant diversity and structural heterogeneity of the vegetation. This increase in resource heterogeneity may increase arthropod diversity, but only if its positive effects are large enough to compensate for the above-mentioned negative effects of large herbivores.
(6) Conservation strategies aiming at maximising heterogeneity, such as low-intensity grazing, maintenance of different types of management in close proximity, or rotational grazing regimes, are most likely to conserve or restore arthropod diversity.
VII. ACKNOWLEDGEMENTS
This manuscript benefitted greatly from valuable discussions with Maarten Schrama and Marijn Nijssen. We thank Jan P. Bakker, Corinna Rickert and two reviewers for helpful comments on earlier versions. We thank Wanda Floor-Zwart for drawing large herbivores and arthropods. Finally, we would like to thank Alan Stewart, Atle Mysterud and Thomas Frank for granting us access to their diversity measurements. R. v. K. was funded by Het Waddenfonds (project WF 200451), C. G. E. v. N. was funded by the O+BN research program financed by the Dutch ministry of Economic affairs (project no.: O+BN/2009/dk 118) and received financial support from Ghent University (BOF joint PhD grant) and H. O. was supported by PIONIER grant 833.02.001 from the Netherlands Organization for Scientific Research (NWO).
IX. SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article.
Table S1. Geographic location, ecosystem, arthropod taxa, experimental setup and duration of the studies used for Figs 1 and 2.
Table S2. Studies on effects of large herbivores on arthropod diversity in wood- and scrublands.
Table S3. Definitions of taxonomic groups.
VIII. REFERENCES
- *Abensperg-Traun M, Smith GT, Arnold GW. Steven DE. The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. 1. Arthropods. Journal of Applied Ecology. 1996;33:1281–1301. [Google Scholar]
- Abramoff MD, Magalhaes PJ. Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–52. [Google Scholar]
- Adler PB, Raff DA. Lauenroth WK. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia. 2001;128:465–479. doi: 10.1007/s004420100737. [DOI] [PubMed] [Google Scholar]
- Anderson A, McCormack S, Helden A, Sheridan H, Kinsella A. Purvis G. The potential of parasitoid Hymenoptera as bioindicators of arthropod diversity in agricultural grasslands. Journal of Applied Ecology. 2011;48:382–390. [Google Scholar]
- *Andresen H, Bakker J, Brongers M, Heydemann B. Irmler U. Long-term changes of salt marsh communities by cattle grazing. Vegetatio. 1990;89:137–148. [Google Scholar]
- Archer S, Schimel DS. Holland EA. Mechanisms of shrubland expansion: land use, climate or CO2. Climatic Change. 1995;29:91–99. [Google Scholar]
- *Azcaráte FM. Peco B. Abandonment of grazing in a mediterranean grassland area: consequences for ant assemblages. Insect Conservation and Diversity. 2012;5:279–288. [Google Scholar]
- Bakker ES, Olff H. Gleichman JM. Contrasting effects of large herbivore grazing on smaller herbivores. Basic and Applied Ecology. 2009;10:141–150. [Google Scholar]
- Bakker ES, Olff H, Vandenberghe C, De Maeyer K, Smit R, Gleichman JM. Vera FWM. Ecological anachronisms in the recruitment of temperate light-demanding tree species in wooded pastures. Journal of Applied Ecology. 2004;41:571–582. [Google Scholar]
- Bakker ES, Ritchie ME, Olff H, Milchunas DG. Knops JMH. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecology Letters. 2006;9:780–788. doi: 10.1111/j.1461-0248.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- Báldi A, Batáry P. Kleijn D. Effects of grazing and biogeographic regions on grassland biodiversity in Hungary – analysing assemblages of 1200 species. Agriculture, Ecosystems & Environment. 2013;166:28–34. [Google Scholar]
- *Balmer O. Erhardt A. Consequences of succession on extensively grazed grasslands for central European butterfly communities: rethinking conservation practices. Conservation Biology. 2000;14:746–757. [Google Scholar]
- Bardgett RD. Wardle DA. Herbivore-mediated linkages between aboveground and belowground communities. Ecology. 2003;84:2258–2268. [Google Scholar]
- Barton K. 2013. MuMIn: multi-model inference. R package version 1.9.11. Available at http://cran.r-project.org/package=MuMIn.
- Barton PS, Cunningham SA, Lindenmayer DB. Manning AD. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia. 2013;171:761–772. doi: 10.1007/s00442-012-2460-3. [DOI] [PubMed] [Google Scholar]
- Batáry P, Báldi A, Sárospataki M, Kohler F, Verhulst J, Knop E, Herzog F. Kleijn D. Effect of conservation management on bees and insect-pollinated grassland plant communities in three European countries. Agriculture, Ecosystems & Environment. 2010;136:35–39. [Google Scholar]
- *Batáry P, Orci KM, Báldi A, Kleijn D, Kisbenedek T. Erdős S. Effects of local and landscape scale and cattle grazing intensity on Orthoptera assemblages of the Hungarian Great Plain. Basic and Applied Ecology. 2007a;8:280–290. [Google Scholar]
- *Batáry P, Báldi A, Szel G, Podlussany A, Rozner I. Erdos S. Responses of grassland specialist and generalist beetles to management and landscape complexity. Diversity and Distributions. 2007b;13:196–202. [Google Scholar]
- Bates D, Maechler M. Bolker B. 2013. lme4: linear mixed-effects models using S4 classes. R package version 0.999999-2. Available at http://cran.r-project.org/package=lme4.
- *Bates AJ, Sadler JP. Fowles AP. Livestock trampling reduces the conservation value of beetle communities on high quality exposed riverine sediments. Biodiversity and Conservation. 2007;16:1491–1509. [Google Scholar]
- Bayfield N. Some effects of trampling on Molophilus ater (Meigen). (Diptera, Tipulidae) Biological Conservation. 1979;16:219–232. [Google Scholar]
- Bell JR, Wheater CP. Cullen WR. The implications of grassland and heathland management for the conservation of spider communities: a review. Journal of Zoology. 2001;255:377–387. [Google Scholar]
- Belovsky GE, Slade JB. Stockhoff BA. Susceptibility to predation for different grasshoppers – an experimental-study. Ecology. 1990;71:624–634. [Google Scholar]
- Ben-Ari M. Inbar M. When herbivores eat predators: predatory insects effectively avoid incidental ingestion by mammalian herbivores. PLoS One. 2013;8:e56748. doi: 10.1371/journal.pone.0056748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren Å. Impact of grazing on individual male movement in Roesel's Bush-cricket Metrioptera roeseli: one possible clue to species range expansion. Journal of Insect Behavior. 2004;17:419–429. [Google Scholar]
- *Bestelmeyer BT. Wiens JA. The effects of land use on the structure of ground-foraging ant communities in the Argentine Chaco. Ecological Applications. 1996;6:1225–1240. [Google Scholar]
- Bestelmeyer BT. Wiens JA. Ant biodiversity in semiarid landscape mosaics: the consequences of grazing vs. natural heterogeneity. Ecological Applications. 2001;11:1123–1140. [Google Scholar]
- Beylich A, Oberholzer H-R, Schrader S, Hoeper H. Wilke B-M. Evaluation of soil compaction effects on soil biota and soil biological processes in soils. Soil & Tillage Research. 2010;109:133–143. [Google Scholar]
- Beynon SA, Peck M, Mann DJ. Lewis OT. Consequences of alternative and conventional endoparasite control in cattle for dung-associated invertebrates and ecosystem functioning. Agriculture, Ecosystems & Environment. 2012;162:36–44. [Google Scholar]
- Bischof R, Loe LE, Meisingset EL, Zimmermann B, Van Moorter B. Mysterud A. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave? The American Naturalist. 2012;180:407–424. doi: 10.1086/667590. [DOI] [PubMed] [Google Scholar]
- *Blake S, Foster GN, Eyre MD. Luff ML. Effects of habitat type and grassland management-practices on the body-size distribution of carabid beetles. Pedobiologia. 1994;38:502–512. [Google Scholar]
- *Blight O, Fadda S, Orgeas J, Ponel P, Buisson E. Dutoit T. Using stone cover patches and grazing exclusion to restore ground-active beetle communities in a degraded pseudo-steppe. Journal of Insect Conservation. 2011;15:561–572. [Google Scholar]
- *Bock CE, Bailowitz RA, Danforth DW, Jones ZF. Bock JH. Butterflies and exurban development in southeastern Arizona. Landscape and Urban Planning. 2007;80:34–44. [Google Scholar]
- Bonal R. Muñoz A. Multi-trophic effects of ungulate intraguild predation on acorn weevils. Oecologia. 2007;152:533–540. doi: 10.1007/s00442-007-0672-8. [DOI] [PubMed] [Google Scholar]
- *Bonte D, Maelfait JP. Hoffmann M. The impact of grazing on spider communities in a mesophytic calcareous dune grassland. Journal of Coastal Conservation. 2000;6:135–144. [Google Scholar]
- Bonte D. Maes D. Trampling affects the distribution of specialised coastal dune arthropods. Basic and Applied Ecology. 2008;9:726–734. [Google Scholar]
- *Botes A, McGeoch MA. van Rensburg BJ. Elephant- and human-induced changes to dung beetle (Coleoptera: Scarabaeidae) assemblages in the Maputaland Centre of Endemism. Biological Conservation. 2006;130:573–583. [Google Scholar]
- *Boulton AM, Davies KF. Ward PS. Species richness, abundance, and composition of ground-dwelling ants in northern California grasslands: role of plants, soil, and grazing. Environmental Entomology. 2005;34:96–104. [Google Scholar]
- Bourn NA. Thomas J. The challenge of conserving grassland insects at the margins of their range in Europe. Biological Conservation. 2002;104:285–292. [Google Scholar]
- *Branson DH. Sword GA. An experimental analysis of grasshopper community responses to fire and livestock grazing in a northern mixed-grass prairie. Environmental Entomology. 2010;39:1441–1446. doi: 10.1603/EN09378. [DOI] [PubMed] [Google Scholar]
- *Brown VK, Gibson CWD. Kathirithamby J. Community organization in leaf hoppers. Oikos. 1992;65:97–106. [Google Scholar]
- *Cagnolo L, Molina SI. Valladares GR. Diversity and guild structure of insect assemblages under grazing and exclusion regimes in a montane grassland from Central Argentina. Biodiversity and Conservation. 2002;11:407–420. [Google Scholar]
- *Calcaterra LA, Cabrera SM, Cuezzo F, Jimenez Perez I. Briano JA. Habitat and grazing influence on terrestrial ants in subtropical grasslands and savannas of Argentina. Annals of the Entomological Society of America. 2010;103:635–646. [Google Scholar]
- Campbell K. Borner M. Population trends and distribution of Serengeti herbivores: implications for management. In: Sinclair ARE, Arcese P, editors; Serengeti II Dynamics, Management, and Conservation of an Ecosystem. Chicago and London: University of Chicago Press; 1995. pp. 117–145. [Google Scholar]
- Carvell C. Habitat use and conservation of bumblebees (Bombus spp.) under different grassland management regimes. Biological Conservation. 2002;103:33–49. [Google Scholar]
- Chappell HG, Ainsworth JF, Cameron RAD. Redfern M. The effect of trampling on a chalk grassland ecosystem. Journal of Applied Ecology. 1971;8:869–882. [Google Scholar]
- Chase JM. Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago and London: University of Chicago Press; 2003. [Google Scholar]
- Cherrill AJ. Brown VK. Ontogenic changes in the microhabitat preferences of Decticus verrucivorus (Orthoptera, Tettigoniidae) at the edge of its range. Ecography. 1992;15:37–44. [Google Scholar]
- Collins SL. Barber SC. Effects of disturbance on diversity in mixed-grass prairie. Vegetatio. 1985;64:87–94. [Google Scholar]
- Cumming DHM. Cumming GS. Ungulate community structure and ecological processes: body size, hoof area and trampling in African savannas. Oecologia. 2003;134:560–568. doi: 10.1007/s00442-002-1149-4. [DOI] [PubMed] [Google Scholar]
- *Dahms H, Lenoir L, Lindborg R, Wolters V. Dauber J. Restoration of seminatural grasslands: what is the impact on ants? Restoration Ecology. 2010;18:330–337. [Google Scholar]
- *Danell K. Huss-Danell K. Feeding by insects and hares on birches earlier affected by moose browsing. Oikos. 1985;44:75–81. [Google Scholar]
- D'Aniello B, Stanislao I, Bonelli S. Balletto E. Haying and grazing effects on the butterfly communities of two Mediterranean-area grasslands. Biodiversity and Conservation. 2011;20:1731–1744. [Google Scholar]
- Dauber J, Purtauf T, Allspach A, Frisch J, Voigtländer K. Wolters V. Local vs. landscape controls on diversity: a test using surface-dwelling soil macroinvertebrates of differing mobility. Global Ecology and Biogeography. 2005;14:213–221. [Google Scholar]
- *Debinski DM, Moranz RA, Delaney JT, Miller JR, Engle DM, Winkler LB, McGranahan DA, Barney RJ, Trager JC, Stephenson AL. Gillespie MK. A cross-taxonomic comparison of insect responses to grassland management and land-use legacies. Ecosphere. 2011;2:art131. [Google Scholar]
- *Den Herder M, Virtanen R. Roininen H. Effects of reindeer browsing on tundra willow and its associated insect herbivores. Journal of Applied Ecology. 2004;41:870–879. [Google Scholar]
- *Dennis P, Aspinall R. Gordon IJ. Spatial distribution of upland beetles in relation to landform, vegetation and grazing management. Basic and Applied Ecology. 2002;3:183–193. [Google Scholar]
- *Dennis P, Doering J, Stockan JA, Jones JR, Rees ME, Vale JE. Sibbald AR. Consequences for biodiversity of reducing inputs to upland temperate pastures: effects on beetles (Coleoptera) of cessation of nitrogen fertilizer application and reductions in stocking rates of sheep. Grass and Forage Science. 2004;59:121–135. [Google Scholar]
- Dennis P, Thomas MB. Sotherton NW. Structural features of field boundaries which influence the overwintering densities of beneficial arthropod predators. Journal of Applied Ecology. 1994;31:361–370. [Google Scholar]
- Dennis P, Young MR. Bentley C. The effects of varied grazing management on epigeal spiders, harvestmen and pseudoscorpions of Nardus stricta grassland in upland Scotland. Agriculture, Ecosystems & Environment. 2001;86:39–57. [Google Scholar]
- Dennis P, Young MR. Gordon IJ. Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecological Entomology. 1998;23:253–264. [Google Scholar]
- Dennis P, Young MR, Howard CL. Gordon IJ. The response of epigeal beetles (Col: Carabidae, Staphylinidae) to varied grazing regimes on upland Nardus stricta grasslands. Journal of Applied Ecology. 1997;34:433–443. [Google Scholar]
- Denno RF. Ecotope differentiation in a guild of sap-feeding insects on the salt-marsh grass, Spartina patens. Ecology. 1980;61:702–714. [Google Scholar]
- Denno RF. Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Annual Review of Entomology. 1995;40:297–331. [Google Scholar]
- *Desender K, Baert L, Maelfait J-P. Verdyck P. Conservation on Volcán Alcedo (Galápagos): terrestrial invertebrates and the impact of introduced feral goats. Biological Conservation. 1999;87:303–310. [Google Scholar]
- *Dolek M. Geyer A. Influence of management on butterflies of rare grassland ecosystems in Germany. Journal of Insect Conservation. 1997;1:125–130. [Google Scholar]
- Donlan JC, Berger J, Bock CE, Bock JH, Burney DA, Estes JA, Foreman D, Martin PS, Roemer GW, Smith FA, Soulé ME. Greene HW. Pleistocene rewilding: an optimistic agenda for twenty-first century conservation. The American Naturalist. 2006;168:660–681. doi: 10.1086/508027. [DOI] [PubMed] [Google Scholar]
- Duffey E. A population study of spiders in limestone grassland – Field-layer fauna. Oikos. 1962;13:15–34. [Google Scholar]
- Duffey E. The effects of human trampling on the fauna of grassland litter. Biological Conservation. 1975;7:255–274. [Google Scholar]
- *Dumont B, Farruggia A, Garel J-P, Bachelard P, Boitier E. Frain M. How does grazing intensity influence the diversity of plants and insects in a species-rich upland grassland on basalt soils? Grass and Forage Science. 2009;64:92–105. [Google Scholar]
- Dunn RR, Harris NC, Colwell RK, Koh LP. Sodhi NS. The sixth mass coextinction: are most endangered species parasites and mutualists? Proceedings of the Royal Society B: Biological Sciences. 2009;276:3037–3045. doi: 10.1098/rspb.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A, Klein A-M, Weisser WW. Tscharntke T. Multitrophic effects of experimental changes in plant diversity on cavity-nesting bees, wasps, and their parasitoids. Oecologia. 2012;169:453–465. doi: 10.1007/s00442-011-2205-8. [DOI] [PubMed] [Google Scholar]
- EEA. 2004. High nature value farmland – Characteristics, trends and policy challenges. European Environment Agency Report No. 1/2004. European Environment Agency, Copenhagen. Available at http://www.eea.europa.eu/publications/report_2004_1.
- *Eyre MD, Luff ML. Woodward JC. Grouse moor management: habitat and conservation implications for invertebrates in southern Scotland. Journal of Insect Conservation. 2003;7:21–32. [Google Scholar]
- *Fabriciusova V, Kanuch P. Kristin A. Response of Orthoptera assemblages to management of montane grasslands in the Western Carpathians. Biologia. 2011;66:1127–1133. [Google Scholar]
- *Fadda S, Henry F, Orgeas J, Ponel P, Buisson E. Dutoit T. Consequences of the cessation of 3000 years of grazing on dry Mediterranean grassland ground-active beetle assemblages. Comptes Rendus Biologies. 2008;331:532–546. doi: 10.1016/j.crvi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Fahrig L. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2003;34:487–515. [Google Scholar]
- FAO. 2008. Are grasslands under threat? Brief analysis of FAO statistical data on pasture and fodder crops. Available at http://www.fao.org/uploads/media/grass_stats_1.pdf Accessed 1.6.2013.
- Farruggia A, Dumont B, Scohier A, Leroy T, Pradel P. Garel J-P. An alternative rotational stocking management designed to favour butterflies in permanent grasslands. Grass and Forage Science. 2012;67:136–149. [Google Scholar]
- Fisher ARA, Corbet AS. Williams CB. The number of animals in a random sample of an animal population. Journal of Animal Ecology. 1943;12:42–58. [Google Scholar]
- Fisher Barham D. Stewart AJA. Differential indirect effects of excluding livestock and rabbits from chalk heath on the associated leafhopper (Hemiptera: Auchenorrhyncha) fauna. Journal of Insect Conservation. 2005;9:351–361. [Google Scholar]
- *Foote AL. Rice Hornung CL. Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecological Entomology. 2005;30:273–283. [Google Scholar]
- Forbes GS, Van Zee JW, Smith W. Whitford WG. Desert grassland canopy arthropod species richness: temporal patterns and effects of intense, short-duration livestock grazing. Journal of Arid Environments. 2005;60:627–646. [Google Scholar]
- *Ford H, Garbutt A, Jones L. Jones DL. Grazing management in saltmarsh ecosystems drives invertebrate diversity, abundance and functional group structure. Insect Conservation and Diversity. 2013;6:189–200. [Google Scholar]
- *Franzen M. Nilsson SG. How can we preserve and restore species richness of pollinating insects on agricultural land? Ecography. 2008;31:698–708. [Google Scholar]
- Frenette-Dussault C, Shipley B. Hingrat Y. Linking plant and insect traits to understand multitrophic community structure in arid steppes. Functional Ecology. 2013;27:786–792. [Google Scholar]
- Freymann BP, Buitenwerf R, Desouza O. Olff H. The importance of termites (Isoptera) for the recycling of herbivore dung in tropical ecosystems: a review. European Journal of Entomology. 2008;105:165–173. [Google Scholar]
- Fryxell JM. Sinclair ARE. Causes and consequences of migration by large herbivores. Trends in Ecology & Evolution. 1988;3:237–241. doi: 10.1016/0169-5347(88)90166-8. [DOI] [PubMed] [Google Scholar]
- Fuentes ER. Jaksic FM. The hump-backed species-diversity curve – Why has it not been found among land animals. Oikos. 1988;53:139–143. [Google Scholar]
- *Gardner SMM, Hartley SEE, Davies A. Palmer SCF. Carabid communities on heather moorlands in northeast Scotland: the consequences of grazing pressure for community diversity. Biological Conservation. 1997;81:275–286. [Google Scholar]
- *Gebeyehu S. Samways MJ. Grasshopper assemblage response to a restored national park (Mountain Zebra National Park, South Africa) Biodiversity and Conservation. 2002;11:283–304. [Google Scholar]
- *Gebeyehu S. Samways MJ. Responses of grasshopper assemblages to long-term grazing management in a semi-arid African savanna. Agriculture, Ecosystems & Environment. 2003;95:613–622. [Google Scholar]
- Gibson CWD, Brown VK, Losito L. McGavin GC. The response of invertebrate assemblies to grazing. Ecography. 1992a;15:166–176. [Google Scholar]
- Gibson CWD, Hambler C. Brown VK. Changes in spider (Araneae) assemblages in relation to succession and grazing management. Journal of Applied Ecology. 1992b;29:132–142. [Google Scholar]
- Gish M, Dafni A. Inbar M. Mammalian herbivore breath alerts aphids to flee host plant. Current Biology. 2010;20:628–629. doi: 10.1016/j.cub.2010.06.065. [DOI] [PubMed] [Google Scholar]
- Gómez JM. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysimum mediohispanicum: consequences for plant specialization. American Naturalist. 2003;162:242–256. doi: 10.1086/376574. [DOI] [PubMed] [Google Scholar]
- Gómez JM. González-Megías A. Asymmetrical interactions between ungulates and phytophagous insects: being different matters. Ecology. 2002;83:203–211. [Google Scholar]
- Gómez JM. González-Megías A. Long-term effects of ungulates on phytophagous insects. Ecological Entomology. 2007;32:229–234. [Google Scholar]
- *González-Megías A, Gómez JM. Sanchez-Pinero F. Effects of ungulates on epigeal arthropods in Sierra Nevada National Park (southeast Spain) Biodiversity and Conservation. 2004;13:733–752. [Google Scholar]
- Goulet H. Biodiversity of ground beetles (Coleoptera: Carabidae) in Canadian agricultural soils. Canadian Journal of Soil Science. 2003;83:259–264. [Google Scholar]
- *Grandchamp A-CC, Bergamini A, Stofer S, Niemelä J, Duelli P, Scheidegger C. Niemela J. The influence of grassland management on ground beetles (Carabidae, Coleoptera) in Swiss montane meadows. Agriculture, Ecosystems & Environment. 2005;110:307–317. [Google Scholar]
- Grime JP. Competitive exclusion in herbaceous vegetation. Nature. 1973;242:344–347. [Google Scholar]
- *Gudleifsson BE. Bjarnadottir B. Spider (Araneae) populations in hayfields and pastures in northern Iceland. Journal of Applied Entomology. 2004;128:284–291. [Google Scholar]
- Haddad NM, Crutsinger GM, Gross K, Haarstad J, Knops JMH. Tilman D. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecology Letters. 2009;12:1029–1039. doi: 10.1111/j.1461-0248.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- Haddad NM, Tilman D, Haarstad J, Ritchie M. Knops JM. Contrasting effects of plant richness and composition on insect communities: a field experiment. The American Naturalist. 2001;158:17–35. doi: 10.1086/320866. [DOI] [PubMed] [Google Scholar]
- *Hartley SE, Gardner SM. Mitchell RJ. Indirect effects of grazing and nutrient addition on the hemipteran community of heather moorlands. Journal of Applied Ecology. 2003;40:793–803. [Google Scholar]
- *Hatfield RG. LeBuhn G. Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biological Conservation. 2007;139:150–158. [Google Scholar]
- Hedges LV, Gurevitch J. Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Hobbs NT. Modification of ecosystems by ungulates. Journal of Wildlife Management. 1996;60:695–713. [Google Scholar]
- Hobbs NT. Large herbivores as sources of disturbance in ecosystems. In: Danell K, Bergstrom R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge: Cambridge University Press; 2006. pp. 261–288. [Google Scholar]
- Hobbs RJ. Huenneke LF. Disturbance, diversity, and invasion: implications for conservation. Conservation Biology. 1992;6:324–337. [Google Scholar]
- Hobbs NT. Swift DM. Grazing in herds – when are nutritional benefits realized. American Naturalist. 1988;131:760–764. [Google Scholar]
- Hofmann TA. Mason CFF. Importance of management on the distribution and abundance of Staphylinidae (Insecta: Coleoptera) on coastal grazing marshes. Agriculture, Ecosystems & Environment. 2006;114:397–406. [Google Scholar]
- *Hoffmann BD. James CD. Using ants to manage sustainable grazing: dynamics of ant faunas along sheep grazing gradients conform to four global patterns. Austral Ecology. 2011;36:698–708. [Google Scholar]
- *Holmes PR, Boyce DC. Reed DK. The ground beetle (Coleoptera, Carabidae) fauna of Welsh peatland biotopes – Factors influencing the distribution of ground beetles and conservation implications. Biological Conservation. 1993;63:153–161. [Google Scholar]
- *Holmes ND, Smith DS. Johnston A. Effect of grazing by cattle on the abundance of grasshoppers on fescue grassland. Journal of Range Management. 1979;32:310–311. [Google Scholar]
- *Holmquist JG, Schmidt-Gengenbach J. Haultain SA. Effects of a long-term disturbance on arthropods and vegetation in subalpine wetlands: manifestations of pack stock grazing in early versus mid-season. PLoS One. 2013;8:e54109. doi: 10.1371/journal.pone.0054109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth R, Magura T, Szinetar C. Tothmeresz B. Spiders are not less diverse in small and isolated grasslands, but less diverse in overgrazed grasslands: a field study (East Hungary, Nyirseg) Agriculture, Ecosystems & Environment. 2009;130:16–22. [Google Scholar]
- Humbert J-Y, Ghazoul J. Walter T. Meadow harvesting techniques and their impacts on field fauna. Agriculture, Ecosystems & Environment. 2009;130:1–8. [Google Scholar]
- Hutchinson KJ. King KL. The effects of sheep stocking level on invertebrate abundance, biomass and energy-utilization in a temperate, sown grassland. Journal of Applied Ecology. 1980;17:369–387. [Google Scholar]
- *Hutton SA. Giller PS. The effects of the intensification of agriculture on northern temperate dung beetle communities. Journal of Applied Ecology. 2003;40:994–1007. [Google Scholar]
- Jankielsohn A, Scholtz CH. Louw SV. Effect of habitat transformation on dung beetle assemblages: a comparison between a South African nature reserve and neighboring farms. Environmental Entomology. 2001;30:474–483. [Google Scholar]
- *Jansen R, Makaka L, Little IT. Dippenaar-Schoeman A. Response of ground-dwelling spider assemblages (Arachnida, Araneae) to Montane Grassland management practices in South Africa. Insect Conservation and Diversity. 2013;6:572–589. [Google Scholar]
- Jarman PJ. The social organisation of antelope in relation to their ecology. Behaviour. 1974;48:215–267. [Google Scholar]
- *Jáuregui BM, Rosa-García R, Garcia U, Wallisdevries MF, Osoro K, Celaya R. Rosagarcia R. Effects of stocking density and breed of goats on vegetation and grasshopper occurrence in heathlands. Agriculture, Ecosystems & Environment. 2008;123:219–224. [Google Scholar]
- Jay-Robert P, Niogret J, Errouissi F, Labarussias M, Paoletti E, Luis MV. Lumaret J-P. Relative efficiency of extensive grazing vs. wild ungulates management for dung beetle conservation in a heterogeneous landscape from Southern Europe (Scarabaeinae, Aphodiinae, Geotrupinae) Biological Conservation. 2008;141:2879–2887. [Google Scholar]
- *Jepson-Innes K. Bock CE. Response of grasshoppers (Orthoptera: Acrididae) to livestock grazing in southeastern Arizona: differences between seasons and subfamilies. Oecologia. 1989;78:430–431. doi: 10.1007/BF00379121. [DOI] [PubMed] [Google Scholar]
- *Joern A. Variation in grasshopper (Acrididae) densities in response to fire frequency and bison grazing in tallgrass prairie. Environmental Entomology. 2004;33:1617–1625. [Google Scholar]
- Joern A. Disturbance by fire frequency and bison grazing modulate grasshopper assemblages in tallgrass prairie. Ecology. 2005;86:861–873. [Google Scholar]
- Joern A. Lawlor LR. Guild structure in grasshopper assemblages based on food and microhabitat resources. Oikos. 1981;37:93–104. [Google Scholar]
- Joern A. Laws AN. Ecological mechanisms underlying arthropod diversity in grasslands. Annual Review of Entomology. 2013;58:19–36. doi: 10.1146/annurev-ento-120811-153540. [DOI] [PubMed] [Google Scholar]
- *Jonas JL. Joern A. Grasshopper (Orthoptera: Acrididae) communities respond to fire, bison grazing and weather in North American tallgrass prairie: a long-term study. Oecologia. 2007;153:699–711. doi: 10.1007/s00442-007-0761-8. [DOI] [PubMed] [Google Scholar]
- *Kaltsas D, Trichas A, Kougioumoutzis K. Chatzaki M. Ground beetles respond to grazing at assemblage level, rather than species-specifically: the case of Cretan shrublands. Journal of Insect Conservation. 2013;17:681–697. [Google Scholar]
- Kaplan I. Denno RF. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecology Letters. 2007;10:977–994. doi: 10.1111/j.1461-0248.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- *Kati V, Zografou K, Tzirkalli E, Chitos T. Willemse L. Butterfly and grasshopper diversity patterns in humid Mediterranean grasslands: the roles of disturbance and environmental factors. Journal of Insect Conservation. 2012;16:807–818. [Google Scholar]
- *Kearns CA. Oliveras DM. Environmental factors affecting bee diversity in urban and remote grassland plots in Boulder, Colorado. Journal of Insect Conservation. 2009;13:655–665. [Google Scholar]
- Keesing F, Allan BF, Young TP. Ostfeld RS. Effects of wildlife and cattle on tick abundance in central Kenya. Ecological Applications. 2013;23:1410–1418. doi: 10.1890/12-1607.1. [DOI] [PubMed] [Google Scholar]
- *Kleintjes-Neff PK, Fettig SM. Vanoverbeke DR. Variable response of butterflies and vegetation to elk herbivory: an exclosure experiment in ponderosa pine and aspen mixed conifer forests. Southwestern Naturalist. 2007;52:1–14. [Google Scholar]
- *Kőrösi Á, Batáry P, Orosz A, Rédei D. Báldi A. Effects of grazing, vegetation structure and landscape complexity on grassland leafhoppers (Hemiptera: Auchenorrhyncha) and true bugs (Hemiptera: Heteroptera) in Hungary. Insect Conservation and Diversity. 2013;5:57–66. [Google Scholar]
- Kruess A. Tscharntke T. Habitat fragmentation, species loss, and biological control. Science. 1994;264:1581–1584. doi: 10.1126/science.264.5165.1581. [DOI] [PubMed] [Google Scholar]
- Kruess A. Tscharntke T. Contrasting responses of plant and insect diversity to variation in grazing intensity. Biological Conservation. 2002a;106:293–302. [Google Scholar]
- Kruess A. Tscharntke T. Grazing intensity and the diversity of grasshoppers, butterflies, and trap-nesting bees and wasps. Conservation Biology. 2002b;16:1570–1580. [Google Scholar]
- Langellotto GA. Denno RF. Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia. 2004;139:1–10. doi: 10.1007/s00442-004-1497-3. [DOI] [PubMed] [Google Scholar]
- Larsen K, Purrington F, Brewer S. Taylor D. Influence of sewage sludge and fertilizer on the ground beetle (Coleoptera: Carabidae) fauna of an old-field community. Environmental Entomology. 1996;25:452–459. [Google Scholar]
- Lawton JH. Plant architecture and the diversity of phytophagous insects. Annual Review of Entomology. 1983;28:23–39. [Google Scholar]
- Lawton JH. Hassell MP. Asymmetrical competition in insects. Nature. 1981;289:793–795. [Google Scholar]
- Lawton JH. Schroder D. Effects of plant type, size of geographical range and taxonomic isolation on number of insect species associated with British plants. Nature. 1977;265:137–140. [Google Scholar]
- Lenoir L. Lennartsson T. Effects of timing of grazing on arthropod communities in semi-natural grasslands. Journal of Insect Science. 2010;10:60. doi: 10.1673/031.010.6001. article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezama F, Baeza S, Altesor A, Cesa A, Chaneton EJ. Paruelo JM. Variation of grazing-induced vegetation changes across a large-scale productivity gradient. Journal of Vegetation Science. 2014;25:8–21. [Google Scholar]
- Liddle M. Recreation Ecology: The Ecological Impact of Outdoor Recreation and Ecotourism. London: Chapman & Hall; 1997. [Google Scholar]
- Littlewood NA. Grazing impacts on moth diversity and abundance on a Scottish upland estate. Insect Conservation and Diversity. 2008;1:151–160. [Google Scholar]
- *Littlewood NA, Pakeman RJ. Pozsgai G. Grazing impacts on Auchenorrhyncha diversity and abundance on a Scottish upland estate. Insect Conservation and Diversity. 2013a;5:67–74. [Google Scholar]
- Littlewood NA, Stewart AJA. Woodcock BA. Science into practice – how can fundamental science contribute to better management of grasslands for invertebrates? Insect Conservation and Diversity. 2013b;5:1–8. [Google Scholar]
- Lorenzen ED, Nogues-Bravo D, Orlando L, Weinstock J, Binladen J, Marske KA, Ugan A, Borregaard MK, Gilbert MTP, Nielsen R, Ho SYW, Goebel T, Graf KE, Byers D, Stenderup JT, et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479:359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff ML. Abundance and diversity of beetle fauna of grass tussocks. Journal of Animal Ecology. 1966;35:189–208. [Google Scholar]
- Lumaret JP, Kadiri N. Bertrand M. Changes in resources: consequences for the dynamics of dung beetle communities. Journal of Applied Ecology. 1992;29:349–356. [Google Scholar]
- *Macagno ALM. Palestrini C. The maintenance of extensively exploited pastures within the Alpine mountain belt: implications for dung beetle conservation (Coleoptera: Scarabaeoidea) Biodiversity and Conservation. 2009;18:3309–3323. [Google Scholar]
- Madsen M, Nielsen BO, Holter P, Pedersen OC, Jespersen JB, Jensen K-MV, Nansen P. Gronvold J. Treating cattle with Ivermectin: effects on the fauna and decompsition of dung pats. Journal of Applied Ecology. 1990;27:1–15. [Google Scholar]
- Mbano BNN, Malpas RC, Maige MKS, Symonds PAK. Thompson DM. The Serengeti regional conservation strategy. In: Sinclair ARE, Arcese P, editors; Serengeti II Dynamics, Management, and Conservation of an Ecosystem. Chicago and London: University of Chicago Press; 1995. pp. 605–616. [Google Scholar]
- McCauley DJ, Keesing F, Young T. Dittmar K. Effects of the removal of large herbivores on fleas of small mammals. Journal of Vector Ecology. 2008;33:263–268. doi: 10.3376/1081-1710-33.2.263. [DOI] [PubMed] [Google Scholar]
- McNaughton SJ. Serengeti migratory wildebeest: facilitation of energy flow by grazing. Science. 1976;191:92–94. doi: 10.1126/science.191.4222.92. [DOI] [PubMed] [Google Scholar]
- McNaughton SJ. Grazing lawns – Animals in herds, plant form, and coevolution. American Naturalist. 1984;124:863–886. [Google Scholar]
- McNaughton SJ. On plants and herbivores. American Naturalist. 1986;128:765–770. [Google Scholar]
- *Melis C, Buset A, Aarrestad PA, Hanssen O, Meisingset EL, Andersen R, Moksnes A, Roskaft E. Røskaft E. Impact of red deer Cervus elaphus grazing on bilberry Vaccinium myrtillus and composition of ground beetle (Coleoptera, Carabidae) assemblage. Biodiversity and Conservation. 2006;15:2049–2059. [Google Scholar]
- *Melis C, Sundby M, Andersen R, Moksnes A, Pedersen B. Røskaft E. The role of moose Alces alces L. in boreal forest – the effect on ground beetles (Coleoptera, Carabidae) abundance and diversity. Biodiversity and Conservation. 2007;16:1321–1335. [Google Scholar]
- Melis C, Teurlings I, Linnell JC, Andersen R. Bordoni A. Influence of a deer carcass on Coleopteran diversity in a Scandinavian boreal forest: a preliminary study. European Journal of Wildlife Research. 2004;50:146–149. [Google Scholar]
- *Meyer H, Fock H, Haase A, Reinke HD. Tulowitzki I. Structure of the invertebrate fauna in salt marshes of the Wadden Sea coast of Schleswig-Holstein influenced by sheep-grazing. Helgolander Meeresuntersuchungen. 1995;49:563–589. [Google Scholar]
- Milchunas DG. Lauenroth WK. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecological Monographs. 1993;63:327–366. [Google Scholar]
- Milchunas DG, Sala OE. Lauenroth WK. A generalized model of the effects of grazing by large herbivores on grassland community structure. The American Naturalist. 1988;132:87–106. [Google Scholar]
- *Miller RH. Onsager JA. Grasshopper (Orthoptera, Acrididae) and plant relationships under different grazing intensities. Environmental Entomology. 1991;20:807–814. [Google Scholar]
- *Moran J, Gormally M. Skeffington MS. Turlough ground beetle communities: the influence of hydrology and grazing in a complex ecological matrix. Journal of Insect Conservation. 2012;16:51–69. [Google Scholar]
- *Moranz RA, Debinski DM, McGranahan DA, Engle DM. Miller JR. Untangling the effects of fire, grazing, and land-use legacies on grassland butterfly communities. Biodiversity and Conservation. 2012;21:2719–2746. [Google Scholar]
- Morris MG. Differences between invertebrate faunas of grazed and ungrazed chalk grassland. 1. Responses of some phytophagous insects to cessation of grazing. Journal of Applied Ecology. 1967;4:459–474. [Google Scholar]
- *Morris MG. Differences between invertebrate faunas of grazed and ungrazed chalk grasslands. 3. Heteropterous fauna. Journal of Applied Ecology. 1969;6:475–487. [Google Scholar]
- *Morris MG. Differences between invertebrate faunas of grazed and ungrazed chalk grassland. 4. Abundance and diversity of Homoptera-Auchenorhyncha. Journal of Applied Ecology. 1971a;8:37–52. [Google Scholar]
- Morris MG. The management of grassland for the conservation of invertebrate animals. In: Duffey E, Watt AS, editors. The Scientific Management of Animal and Plant Communities for Conservation. Oxford, London, Edinburgh: Blackwell Scientific Publications; 1971b. pp. 527–552. [Google Scholar]
- *Morris MG. Effects of seasonal grazing on Heteroptera and Auchenorrhyncha (Hemiptera) of chalk grasslands. Journal of Applied Ecology. 1973;10:761–780. [Google Scholar]
- Morris MG. The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biological Conservation. 2000;95:129–142. [Google Scholar]
- Morris MG, Clarke RT. Rispin WE. The success of a rotational grazing system in conserving the diversity of chalk grassland Auchenorrhyncha. Journal of Insect Conservation. 2005;9:363–374. [Google Scholar]
- *Mortimer SR, Hollier JA. Brown VK. Interactions between plant and insect diversity in the restauration of lowland calcareous grasslands in Southern Britain. Applied Vegetation Science. 1998;1:101–114. [Google Scholar]
- Murdoch WW, Peterson CH. Evans FC. Diversity and pattern in plants and insects. Ecology. 1972;53:819–829. [Google Scholar]
- *Mysterud A, Aaserud R, Hansen LO, Åkra K, Olberg S. Austrheim G. Large herbivore grazing and invertebrates in an alpine ecosystem. Basic and Applied Ecology. 2010;11:320–328. [Google Scholar]
- Nakagawa S. Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4:133–142. [Google Scholar]
- *Nash MS, Bradford DF, Franson SE, Neale AC, Whitford WG. Heggem DT. Livestock grazing effects on ant communities in the eastern Mojave Desert, USA. Ecological Indicators. 2004;4:199–213. [Google Scholar]
- Negro M, La Rocca C, Ronzani S, Rolando A. Palestrini C. Management tradeoff between endangered species and biodiversity conservation: the case of Carabus olympiae (Coleoptera: Carabidae) and carabid diversity in north-western Italian Alps. Biological Conservation. 2013;157:255–265. [Google Scholar]
- Negro M, Rolando A. Palestrini C. The impact of overgrazing on dung beetle diversity in the Italian Maritime Alps. Environmental Entomology. 2011;40:1081–1092. doi: 10.1603/EN11105. [DOI] [PubMed] [Google Scholar]
- *Ni Bhriain B, Skeffington MS. Gormally M. Conservation implications of land use practices on the plant and carabid beetle communities of two turloughs in Co. Galway, Ireland. Biological Conservation. 2002;105:81–92. [Google Scholar]
- Nickel H. Hildebrandt J. Auchenorrhyncha communities as indicators of disturbance in grasslands (Insecta, Hemiptera) – a case study from the Elbe flood plains (northern Germany) Agriculture, Ecosystems & Environment. 2003;98:183–199. [Google Scholar]
- *Noel NM. Finch O-D. Effects of the abandonment of alpine summer farms on spider assemblages (Araneae) Journal of Insect Conservation. 2010;14:427–438. [Google Scholar]
- Numa C, Verdu JR, Rueda C. Galante E. Comparing dung beetle species assemblages between protected areas and adjacent pasturelands in a Mediterranean savanna landscape. Rangeland Ecology & Management. 2012;65:137–143. [Google Scholar]
- Nykanen H. Koricheva J. Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos. 2004;104:247–268. [Google Scholar]
- *Öckinger E, Eriksson AK. Smith HG. Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biological Conservation. 2006;133:291–300. [Google Scholar]
- Oertli S, Muller A, Steiner D, Breitenstein A. Dorn S. Cross-taxon congruence of species diversity and community similarity among three insect taxa in a mosaic landscape. Biological Conservation. 2005;126:195–205. [Google Scholar]
- Olff H. Ritchie ME. Effects of herbivores on grassland plant diversity. Trends in Ecology & Evolution. 1998;13:261–265. doi: 10.1016/s0169-5347(98)01364-0. [DOI] [PubMed] [Google Scholar]
- Olff H, Ritchie ME. Prins HHT. Global environmental controls of diversity in large herbivores. Nature. 2002;415:901–904. doi: 10.1038/415901a. [DOI] [PubMed] [Google Scholar]
- Oliver I, Garden D, Greenslade PJ, Haller B, Rodgers D, Seeman O. Johnston B. Effects of fertiliser and grazing on the arthropod communities of a native grassland in south-eastern Australia. Agriculture, Ecosystems & Environment. 2005;109:323–334. [Google Scholar]
- *O'Neill KM, Olson BE, Rolston MG, Wallander R, Larson DP, Seibert CE. O'Neill KM. Effects of livestock grazing on rangeland grasshopper (Orthoptera: Acrididae) abundance. Agriculture, Ecosystems & Environment. 2003;97:51–64. [Google Scholar]
- *O'Neill KM, Olson BE, Wallander R, Rolston MG. Seibert CE. Effects of livestock grazing on grasshopper abundance on a native rangeland in Montana. Environmental Entomology. 2010;39:775–786. doi: 10.1603/EN09173. [DOI] [PubMed] [Google Scholar]
- Onsager JA. Suppression of grasshoppers in the Great Plains through grazing management. Journal of Range Management. 2000;53:592–602. [Google Scholar]
- Ostermann OP. The need for management of nature conservation sites designated under Natura 2000. Journal of Applied Ecology. 1998;35:968–973. [Google Scholar]
- Owensmith N. Megafaunal extinctions – the conservation message from 11,000 years bp. Conservation Biology. 1989;3:405–412. doi: 10.1111/j.1523-1739.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- *Paschetta M, La Morgia V, Masante D, Negro M, Rolando A. Isaia M. Grazing history influences biodiversity: a case study on ground-dwelling arachnids (Arachnida: Araneae, Opiliones) in the Natural Park of Alpi Marittime (NW Italy) Journal of Insect Conservation. 2012;17:339–356. [Google Scholar]
- Pétillon J, Georges A, Canard A, Lefeuvre JC, Bakker JP. Ysnel F. Influence of abiotic factors on spider and ground beetle communities in different salt-marsh systems. Basic and Applied Ecology. 2008;9:743–751. [Google Scholar]
- *Pétillon J, Georges A, Canard A. Ysnel F. Impact of cutting and sheep grazing on ground-active spiders and carabids in intertidal salt marshes (Western France) Animal Biodiversity and Conservation. 2007;30:201–209. [Google Scholar]
- *Pihlgren A, Lenoir L. Dahms H. Ant and plant species richness in relation to grazing, fertilisation and topography. Journal for Nature Conservation. 2010;18:118–125. [Google Scholar]
- Poelman EH, van Dam NM, van Loon JJA, Vet LEM. Dicke M. Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores. Ecology. 2009;90:1863–1877. doi: 10.1890/08-0977.1. [DOI] [PubMed] [Google Scholar]
- Potts SG, Woodcock BA, Roberts SPM, Tscheulin T, Pilgrim ES, Brown VK. Tallowin JR. Enhancing pollinator biodiversity in intensive grasslands. Journal of Applied Ecology. 2009;46:369–379. [Google Scholar]
- Pöyry J, Lindgren S, Salminen J. Kuussaari M. Restoration of butterfly and moth communities in semi-natural grasslands by cattle grazing. Ecological Applications. 2004;14:1656–1670. [Google Scholar]
- *Pöyry J, Lindgren S, Salminen J. Kuussaari M. Responses of butterfly and moth species to restored cattle grazing in semi-natural grasslands. Biological Conservation. 2005;122:465–478. [Google Scholar]
- Pöyry J, Luoto M, Paukkunen J, Pykälä J, Raatikainen K. Kuussaari M. Different responses of plants and herbivore insects to a gradient of vegetation height: an indicator of the vertebrate grazing intensity and successional age. Oikos. 2006;115:401–412. [Google Scholar]
- Prather CM, Pelini SL, Laws A, Rivest E, Woltz M, Bloch CP, Toro ID, Ho C-K, Kominoski J, Scott Newbold TA, Parsons S. Joern A. Invertebrates, ecosystem services and climate change. Biological Reviews. 2013;88:327–348. doi: 10.1111/brv.12002. [DOI] [PubMed] [Google Scholar]
- *Prendini L, Theron L-J, Van der Merwe K. Owensmith N. Abundance and guild structure of grasshoppers (Orthoptera: Acridoidea) in communally grazed and protected savanna. South African Journal of Zoology. 1996;31:120–130. [Google Scholar]
- Proulx M. Mazumder A. Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient rich ecosystems. Ecology. 1998;79:2581–2592. [Google Scholar]
- Pullin AS. Stewart GB. Guidelines for systematic review in conservation and environmental management. Conservation Biology. 2006;20:1647–1656. doi: 10.1111/j.1523-1739.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- *Purvis G. Curry JP. The influence of swardmanagement on foliage arthropod communities in a ley grassland. Journal of Applied Ecology. 1981;18:711–723. [Google Scholar]
- *Pykälä J. Effects of restoration with cattle grazing on plant species composition and richness of semi-natural grasslands. Biodiversity and Conservation. 2003;12:2211–2226. [Google Scholar]
- *Quinn MA. Walgenbach DD. Influence of grazing history on the community structure of grasshoppers of a mixed-grass prairie. Environmental Entomology. 1990;19:1756–1766. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Version 3.0.1. Vienna: R Foundation for Statistical Computing; 2013. Available at http://www.R-project.org. [Google Scholar]
- Reitz SR. Trumble JT. Competitive displacement among insects and arachnids. Annual Review of Entomology. 2002;47:435–465. doi: 10.1146/annurev.ento.47.091201.145227. [DOI] [PubMed] [Google Scholar]
- Rickert C, Fichtner A, van Klink R. Bakker JP. Alpha- and beta-diversity in moth communities in salt marshes is driven by grazing management. Biological Conservation. 2012;146:24–31. [Google Scholar]
- Ritchie ME. Nitrogen limitation and trophic vs. abiotic influence on insect herbivores in a temperate grassland. Ecology. 2000;81:1601–1612. [Google Scholar]
- *Roininen H, Price PW. Bryant JP. Response of galling insects to natural browsing by mammals in Alaska. Oikos. 1997;80:481–486. [Google Scholar]
- *Rosa-García R, Jáuregui BM, García U, Osoro K. Celaya R. Effects of livestock breed and grazing pressure on ground-dwelling arthropods in Cantabrian heathlands. Ecological Entomology. 2009a;34:466–475. [Google Scholar]
- *Rosa-García R, Jáuregui BM, García U, Osoro K. Celaya R. Responses of arthropod fauna assemblages to goat grazing management in northern Spanish heathlands. Environmental Entomology. 2009b;38:985–995. doi: 10.1603/022.038.0405. [DOI] [PubMed] [Google Scholar]
- Rothenwöhrer C, Scherber C. Tscharntke T. Grassland management for stem-boring insects: abandoning small patches is better than reducing overall intensity. Agriculture, Ecosystems & Environment. 2013;167:38–42. [Google Scholar]
- Roy DB. Thomas JA. Seasonal variation in the niche, habitat availability and population fluctuations of a bivoltine thermophilous insect near its range margin. Oecologia. 2003;134:439–444. doi: 10.1007/s00442-002-1121-3. [DOI] [PubMed] [Google Scholar]
- Rule S, Brook BW, Haberle SG, Turney CSM, Kershaw AP. Johnson CN. The aftermath of megafaunal extinction: ecosystem transformation in Pleistocene Australia. Science. 2012;335:1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- Rzanny M. Voigt W. Complexity of multitrophic interactions in a grassland ecosystem depends on plant species diversity. Journal of Animal Ecology. 2012;81:614–627. doi: 10.1111/j.1365-2656.2012.01951.x. [DOI] [PubMed] [Google Scholar]
- *Saarinen K. A comparison of butterfly communities along field margins under traditional and intensive management in SE Finland. Agriculture, Ecosystems & Environment. 2002;90:59–65. [Google Scholar]
- *Saarinen K. Jantunen J. Grassland butterfly fauna under traditional animal husbandry: contrasts in diversity in mown meadows and grazed pastures. Biodiversity and Conservation. 2005;14:3201–3213. [Google Scholar]
- *Samways MJ. Kreuzinger K. Vegetation, ungulate and grasshopper interactions inside vs. outside an African savanna game park. Biodiversity and Conservation. 2001;10:1963–1981. [Google Scholar]
- Sanders D. Platner C. Intraguild interactions between spiders and ants and top-down control in a grassland food web. Oecologia. 2007;150:611–624. doi: 10.1007/s00442-006-0538-5. [DOI] [PubMed] [Google Scholar]
- Sanderson EW, Redford KH, Weber B, Aune K, Baldes D, Berger J, et al. The ecological future of the North American bison: conceiving long-term, large-scale conservation of wildlife. Conservation Biology. 2008;22:252–266. doi: 10.1111/j.1523-1739.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, Schulze E-D, Roscher C, Weigelt A, Alan E, Bessler H, Bonkowski M, Buchmann N, Buscot F, Clement LW, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- *Schmidt AC, Fraser LH, Carlyle CN. Bassett ERL. Does cattle grazing affect ant abundance and diversity in temperate grasslands? Rangeland Ecology & Management. 2012;65:292–298. [Google Scholar]
- Schmitz OJ. Resolving Ecosystem Complexity, Monographs in Population Biology 47. Princeton: Princeton University Press; 2011. [Google Scholar]
- Scholl PJ. Biology and control of cattle grubs. Annual Review of Entomology. 1993;38:53–70. doi: 10.1146/annurev.en.38.010193.000413. [DOI] [PubMed] [Google Scholar]
- Scohier A, Ouin A, Farruggia A. Dumont B. Is there a benefit of excluding sheep from pastures at flowering peak on flower-visiting insect diversity? Journal of Insect Conservation. 2013;17:287–294. [Google Scholar]
- Seastedt TR. Crossley DA. The influence of arthropods on ecosystems. Bioscience. 1984;34:157–161. [Google Scholar]
- Shaw MR. Habitat considerations for parasitic wasps (Hymenoptera) Journal of Insect Conservation. 2006;10:117–127. [Google Scholar]
- Shaw MR. Hochberg ME. The neglect of parasitic Hymenoptera in insect conservation strategies: the British fauna as a prime example. Journal of Insect Conservation. 2001;5:253–263. [Google Scholar]
- Siegfried WR. Tail length and biting insects of ungulates. Journal of Mammalogy. 1990;71:75–78. [Google Scholar]
- Siemann E, Tilman D, Haarstad J. Ritchie M. Experimental tests of the dependence of arthropod diversity on plant diversity. American Naturalist. 1998;152:738–750. doi: 10.1086/286204. [DOI] [PubMed] [Google Scholar]
- *Sjödin NE. Pollinator behavioural responses to grazing intensity. Biodiversity and Conservation. 2007;16:2103–2121. [Google Scholar]
- *Sjödin NE, Bengtsson J. Ekbom B. The influence of grazing intensity and landscape composition on the diversity and abundance of flower-visiting insects. Journal of Applied Ecology. 2008;45:763–772. [Google Scholar]
- Smith CC. The effect of overgrazing and erosion upon the biota of the mixed-grass prairie of Oklahoma. Ecology. 1940;21:381–397. [Google Scholar]
- *Söderström B, Svensson B, Vessby K. Glimskar A. Plants, insects and birds in semi-natural pastures in relation to local habitat and landscape factors. Biodiversity and Conservation. 2001;10:1839–1863. [Google Scholar]
- *Spalinger LC, Haynes AG, Schütz M. Risch AC. Impact of wild ungulate grazing on Orthoptera abundance and diversity in subalpine grasslands. Insect Conservation and Diversity. 2012;5:444–452. [Google Scholar]
- *Sterling PH, Gibson CWD. Brown VK. Leaf miner assemblies – effects of plant succession and grazing management. Ecological Entomology. 1992;17:167–178. [Google Scholar]
- *Stoner KJL. Joern A. Landscape vs. local habitat scale influences to insect communities from tallgrass prairie remnants. Ecological Applications. 2004;14:1306–1320. [Google Scholar]
- Strong DR, Lawton JH. Southwood TRE. Insects on Plants – Community Patterns and Mechanisms. Oxford: Blackwell Scientific Publications; 1984. [Google Scholar]
- Suominen O. Danell K. Effects of large herbivores on other fauna. In: Danell K, Bergstrom R, Duncan P, Pastor J, editors; Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge: Cambridge University Press; 2006. pp. 383–412. [Google Scholar]
- *Suominen O, Danell K. Bergström R. Moose, trees, and ground-living invertebrates: indirect interactions in Swedish pine forests. Oikos. 1999;84:215–226. [Google Scholar]
- *Suominen O, Niemela J, Martikainen P, Niemela P. Kojola I. Impact of reindeer grazing on ground-dwelling Carabidae and Curculionidae assemblages in Lapland. Ecography. 2003;26:503–513. [Google Scholar]
- *Swengel AB. Effects of management on butterfly abundance in tallgrass prairie and pine barrens. Biological Conservation. 1998;83:77–89. [Google Scholar]
- *Szinetár C. Samu F. Intensive grazing opens spider assemblage to invasion by disturbance-tolerant species. Journal of Arachnology. 2012;40:59–70. [Google Scholar]
- Thomas JA, Thomas CD, Simcox DJ. Clarke RT. Ecology and declining status of the Silver-Spotted Skipper butterfly (Hesperia comma) in Britain. Journal of Applied Ecology. 1986;23:365–380. [Google Scholar]
- Tilman D. A consumer-resource approach to community structure. American Zoologist. 1986;26:5–22. [Google Scholar]
- Trimble SW. Mendel AC. The cow as a geomorphic agent – a critical-review. Geomorphology. 1995;13:233–253. [Google Scholar]
- Tscharntke T. Vertebrate effects on plant-invertebrate food webs. In: Gange K, Brown VK, editors. Multitrophic Interactions in Terrestrial Ecosystems. Oxford: Blackwell Science Ltd; 1997. pp. 277–297. [Google Scholar]
- Tscharntke T, Tylianakis JM, Rand TA, Didham RK, Fahrig L, Batáry P, Bengtsson J, Clough Y, Christ TO, Dormann CF, Ewers RM, Fründ J, Holt RD, Holzschuh A, Klein AM, et al. Landscape moderation of biodiversity patterns and processes – eight hypotheses. Biological Reviews. 2012;87:661–685. doi: 10.1111/j.1469-185X.2011.00216.x. [DOI] [PubMed] [Google Scholar]
- *Underwood EC. Christian CE. Consequences of prescribed fire and grazing on grassland ant communities. Environmental Entomology. 2009;38:325–332. doi: 10.1603/022.038.0204. [DOI] [PubMed] [Google Scholar]
- Vanbergen AJ, Hails RS, Watt AD. Jones TH. Consequences for host-parasitoid interactions of grazing-dependent habitat heterogeneity. Journal of Animal Ecology. 2006;75:789–801. doi: 10.1111/j.1365-2656.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- Van Klink R, Rickert C, Vermeulen R, Vorst O, WallisDeVries MF. Bakker JP. Grazed vegetation mosaics do not maximize arthropod diversity: evidence from salt marshes. Biological Conservation. 2013;164:150–157. [Google Scholar]
- Van Noordwijk CGE, Boer P, Mabelis AA, Verberk WCEP. Siepel H. Life-history strategies as a tool to identify conservation constraints: a case-study on ants in chalk grasslands. Ecological Indicators. 2012a;13:303–313. [Google Scholar]
- Van Noordwijk CGE, Flierman DE, Remke E, WallisDeVries MF. Berg MP. Impact of grazing management on hibernating caterpillars of the butterfly Melitaea cinxia in calcareous grasslands. Journal of Insect Conservation. 2012b;16:909–920. [Google Scholar]
- Van Straalen NM. Verhoef HA. The development of a bioindicator system for soil acidity based on arthropod pH preferences. Journal of Applied Ecology. 1997;34:217–232. [Google Scholar]
- Van Swaay C, Cuttelod A, Collins S, Maes D, López Munguira M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M. Wynhof I. European Red List of Butterflies. Luxembourg: 2010. Publications Office of the European Union. [Google Scholar]
- *Vazquez DP. Simberloff D. Ecological specialization and susceptibility to disturbance: conjectures and refutations. American Naturalist. 2002;159:606–623. doi: 10.1086/339991. [DOI] [PubMed] [Google Scholar]
- *Vazquez DP. Simberloff D. Changes in interaction biodiversity induced by an introduced ungulate. Ecology Letters. 2003;6:1077–1083. [Google Scholar]
- Verberk WCEP, van Noordwijk CGE. Hildrew AG. Delivering on a promise: integrating species traits to transform descriptive community ecology into a predictive science. Freshwater Science. 2013;32:531–547. [Google Scholar]
- Verdu JR, Moreno CE, Sanchez-Rojas G, Numa C, Galante E. Halffter G. Grazing promotes dung beetle diversity in the xeric landscape of a Mexican Biosphere Reserve. Biological Conservation. 2007;140:308–317. [Google Scholar]
- Vessby K, Söderström B, Glimskar A. Svensson B. Species-richness correlations of six different taxa in Swedish seminatural grasslands. Conservation Biology. 2002;16:430–439. [Google Scholar]
- Vicari M. Bazely DR. Do grasses fight back? The case for antiherbivore defences. Trends in Ecology & Evolution. 1993;8:137–141. doi: 10.1016/0169-5347(93)90026-L. [DOI] [PubMed] [Google Scholar]
- *Vogel JA, Debinski DM, Koford RR. Miller JR. Butterfly, responses to prairie restoration through fire and grazing. Biological Conservation. 2007;140:78–90. [Google Scholar]
- Völkl W, Zwölfer H, Romstöck-Völkl M. Schmelzer C. Habitat management in calcareous grasslands: effects on the insect community developing in flower heads of Cynarea. Journal of Applied Ecology. 1993;30:307–315. [Google Scholar]
- Vreysen MJB, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck VA, Msangi AR, Mkonyi PA. Feldmann HU. Glossina austeni (Diptera: Glossinidae) eradicated on the Island of Unguja, Zanzibar, using the sterile insect technique. Journal of Economic Entomology. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- Vulliamy B, Potts SG. Willmer PG. The effects of cattle grazing on plant-pollinator communities in a fragmented Mediterranean landscape. Oikos. 2006;114:529–543. [Google Scholar]
- Wall R. Strong L. Environmental consequences of treating cattle with the antiparasitic drug Ivermectin. Nature. 1987;327:418–421. doi: 10.1038/327418a0. [DOI] [PubMed] [Google Scholar]
- WallisDeVries MF. Large herbivores as key factors for nature conservation. In: WallisDeVries MF, Bakker JP, Van Wieren SE, editors. Grazing and Conservation Management. Dordrecht: Kluwer; 1998. pp. 1–20. [Google Scholar]
- WallisDeVries MF, Laca EA. Demment MW. The importance of scale of patchiness for selectivity in grazing herbivores. Oecologia. 1999;121:355–363. doi: 10.1007/s004420050939. [DOI] [PubMed] [Google Scholar]
- *WallisDeVries MF, Parkinson AE, Dulphy JP, Saye M. Diana E. Effects of livestock breed and grazing intensity on biodiversity and production in grazing systems. 4. Effects on animal diversity. Grass and Forage Science. 2007;62:185–197. [Google Scholar]
- *WallisDeVries MF. Raemakers I. Does extensive grazing benefit butterflies in coastal dunes? Restoration Ecology. 2001;9:179–188. [Google Scholar]
- Wardle DA, Barker GM, Yeates GW, Bonner KI. Ghani A. Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecological Monographs. 2001;71:587–614. [Google Scholar]
- *Warui CM, Villet MRH, Young TP. Jocque R. Influence of grazing by large mammals on the spider community of a Kenyan savanna biome. Journal of Arachnology. 2005;33:269–279. [Google Scholar]
- *Weiss N, Zucchi H. Hochkirch A. The effects of grassland management and aspect on Orthoptera diversity and abundance: site conditions are as important as management. Biodiversity and Conservation. 2013;22:2167–2178. [Google Scholar]
- *Wettstein W. Schmid B. Conservation of arthropod diversity in montane wetlands: effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. Journal of Applied Ecology. 1999;36:363–373. [Google Scholar]
- White TCR. The Inadequate Environment. Nitrogen and the Abundance of Animals. Berlin: Springer-Verlag; 1993. [Google Scholar]
- *Whitford WG, Van Zee J, Nash MS, Smith WE. Herrick JE. Ants as indicators of exposure to environmental stressors in North American desert grasslands. Environmental Monitoring and Assessment. 1999;54:143–171. [Google Scholar]
- Williams NM, Crone EE, Roulston TH, Minckley RL, Packer L. Potts SG. Ecological and life-history traits predict bee species responses to environmental disturbances. Biological Conservation. 2010;143:2280–2291. [Google Scholar]
- Wilson GWC. Control of warble fly in Great-Britain and the European-Community. Veterinary Record. 1986;118:653–656. doi: 10.1136/vr.118.24.653. [DOI] [PubMed] [Google Scholar]
- Wilson JB, Peet RK, Dengler J. Pärtel M. Plant species richness: the world records. Journal of Vegetation Science. 2012;23:796–802. [Google Scholar]
- Woodcock BA, Bullock JM, Mortimer SR. Pywell RF. Limiting factors in the restoration of UK grassland beetle assemblages. Biological Conservation. 2012;146:136–143. [Google Scholar]
- *Woodcock BA, Lawson CSS, Mann DJJ. McDonald AWW. Effects of grazing management on beetle and plant assemblages during the re-creation of a flood-plain meadow. Agriculture, Ecosystems & Environment. 2006;116:225–234. [Google Scholar]
- Woodcock BA, Potts SG, Westbury DB, Ramsay AJ, Lambert M, Harris SJ. Brown VK. The importance of sward architectural complexity in structuring predatory and phytophagous invertebrate assemblages. Ecological Entomology. 2007;32:302–311. [Google Scholar]
- Woodcock BA. Pywell RF. Effects of vegetation structure and floristic diversity on detritivore, herbivore and predatory invertebrates within calcareous grasslands. Biodiversity and Conservation. 2009;19:81–95. [Google Scholar]
- *Woodcock BA, Pywell RFF, Roy DBB, Rose RJJ. Bell D. Grazing management of calcareous grasslands and its implications for the conservation of beetle communities. Biological Conservation. 2005;125:193–202. [Google Scholar]
- Woodcock BA, Vogiatzakis IN, Westbury DB, Lawson CS, Edwards AR, Brook AJ, Harris SJ, Lock KA, Maczey N, Masters G, Brown VK. Mortimer SR. The role of management and landscape context in the restoration of grassland phytophagous beetles. Journal of Applied Ecology. 2010;47:366–376. [Google Scholar]
- Xie Z, Williams PH. Tang Y. The effect of grazing on bumblebees in the high rangelands of the Eastern Tibetan Plateau of Sichuan. Journal of Insect Conservation. 2008;12:695–703. [Google Scholar]
- Ydenberg RC. Prins HHT. Spring grazing and the manipulation of food quality by Barnacle geese. Journal of Applied Ecology. 1981;18:443–453. [Google Scholar]
- Yoshihara Y, Chimeddorj B, Buuveibaatar B, Lhaquasuren B. Takatsuki S. Effects of livestock grazing on pollination on a steppe in eastern Mongolia. Biological Conservation. 2008;141:2376–2386. [Google Scholar]
- Zaslavski V. Insect Development: Photoperiodic and Temperature Control. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Zeder MA. Domestication and early agriculture in the Mediterranean Basin: origins, diffusion, and impact. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang D, Wang L, Bai Y, Fang J. Liu J. The effects of large herbivore grazing on meadow steppe plant and insect diversity. Journal of Applied Ecology. 2012;49:1075–1083. [Google Scholar]
- *Zulka KP, Milasowszky N. Lethmayer C. Spider biodiversity potential of an ungrazed and a grazed inland salt meadow in the National Park “Neusiedler See-Seewinkel” (Austria): implications for management (Arachnida: Araneae) Biodiversity and Conservation. 1997;6:75–88. [Google Scholar]
- *Zürbrügg C. Frank T. Factors influencing bug diversity (Insecta: Heteroptera) in semi-natural habitats. Biodiversity and Conservation. 2006;15:275–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Geographic location, ecosystem, arthropod taxa, experimental setup and duration of the studies used for Figs 1 and 2.
Table S2. Studies on effects of large herbivores on arthropod diversity in wood- and scrublands.
Table S3. Definitions of taxonomic groups.


