Abstract
Objective
To explore asthma pathogenesis using data from upper and lower airways.
Data Source
English-language papers on human asthma and nasal polyp subjects from 1990 onwards.
Study Selection
High-quality studies in established journals.
Results
The recognition of its inflammatory nature led to a quantum leap in the understanding and treatment of asthma, with lives saved by inhaled corticosteroids. Further work at genetic, molecular, histological and clinical levels has shown that asthma is polymorphic and rarely involves isolated Th2 bronchial inflammation.
Viral infections may act as an initiating event in children and adults, showing synergy with atopy. Chronic staphylococcal colonization of the mucosa may act as a promoter, as in atopic dermatitis. These two observations may be linked, with viruses providing an entry for bacteria into the mucosal epithelium.
Conclusions
Most asthma begins in the nose and involves allergy and infection: both viral and bacterial. The combination of atopy and infection suggests new possibilities for therapy.
Keywords: airway epithelium, asthma, asthma mechanisms, bacterial infection, viral infection
The relationship between allergy and asthma has long been the subject of debate. It is obvious that allergen exposure in Immunoglobulin E (IgE)-sensitized asthmatic subjects can provoke wheezing, shortness of breath and falls in forced expiratory volume in 1 second (1). However, allergen avoidance and allergen-specific immunotherapy have not proven consistently effective in asthma (2, 3), which involves mechanisms other than Th2 inflammation, such as Th1 inflammation and remodeling (4, 5). Anti-IgE therapy has reopened the issue: it is undoubtedly effective in severe asthma (6), but it appears that eosinophils, rather than IgE, may be a better biomarker for those likely to benefit, since non-atopic severe asthmatics also respond (7), as do nasal polyps (8). Local IgE formation, without evidence of sensitization at systemic level, i.e. negative skin prick and blood IgE tests, may provide an answer (9–12), since the immunopathology of allergic and intrinsic asthma is similar (13).
An interaction between infection and allergy has been noted: children with allergic rhinitis suffer more and for longer with viral colds (14). Most acute asthma exacerbations start in the nose with a viral cold (15, 16). In allergic asthmatic children who are exposed to the relevant allergen and who then catch a cold, the odds ratio for hospital admission for asthma is 19 (17).
In fact, asthma itself usually begins with nasal disease. The European Community Respiratory Health Survey data show that both allergic and non-allergic rhinitis are risk factors for subsequent asthma development (18). The combination of recurrent viral colds and allergic rhinitis in children carries a high odds ratio for progression to asthma (19). The systemic link demonstrated between nose and chest by Braunstahl et al. – showing that nasal allergen challenge provokes inflammation in both the upper airway and the bronchi, and vice versa – provides one possible mechanism for the spread of inflammation from nose to bronchi (20).
The pathogenesis of one asthma phenotype – non-atopic (intrinsic) asthma associated with aspirin sensitivity and nasal polyposis, known as aspirin exacerbated respiratory disease (AERD) – is becoming unraveled. The European Network on Aspirin-Induced Asthma provided information from over 500 sufferers that the problem starts at age 29 ± 12 years with persistent rhinitis, and with subsequent development of nasal polyps, asthma and aspirin intolerance, in variable order. The disease tends to occur earlier in females and atopics (21).
The late Andrew Szczeklik suggested an initiating viral upper respiratory tract infection, based on the history given by many sufferers of being well until the sudden onset of a severe cold, which never went away, and upon the demonstration of virus-like particles in the bronchial mucosa (22). Interferon gamma hyperproduction, which differentiates AERD from other forms of eosinophilic asthma (23), is in accord with a chronic viral presence. Microorganisms other than viruses may also be involved: Bachert and his group have noted that nasal colonization with Staphylococcus aureus is particularly high in AERD patients at 88%, compared with around 30% in control subjects, and that most have formed IgE to staphylococcal enterotoxins (24). These appear to be disease drivers since they act as superantigens: activating over 20% of T lymphocytes to elaborate cytokines such as IL5 and IL4, which cause local polyclonal IgE production and predominantly eosinophilic inflammation (12). The systemic link is again apparent with elevated blood eosinophils which migrate out of the bone marrow and localize in both the upper and lower airways (25). Staphylococci are located within the mucosa and even within cells in biopsy studies (26).
The presence of IgE to staphylococcal enterotoxins in blood confers a risk factor of 7.25 (2.7–19.1) for asthma and 11.09 (4.1–29.6) for severe asthma, where 59.6% of sufferers were positive compared with 13% of controls (27).
These observations:– origin in the nose with atopy, systemic or local, as a factor; possible viral co-initiation and staphylococcal promotion – have been linked by recent observations that herpes simplex virus can penetrate the nasal epithelium and allow Staphylococci to enter the mucosa (28, 29). Rhinoviruses, the most frequent cause of the common cold, probably behave similarly. There is, thus, a multi-hit initiation of disease (Fig. 1), with epithelial genes and those for bacterial and viral resistance probably relevant, as well as those involved in atopy and remodeling (30, 31). Repeated observations of sphingolipid abnormalities in asthma may be relevant: sphingolipids are thought to protect the cell surface against harmful environmental factors by forming a mechanically stable and chemically resistant outer leaflet of the plasma membrane lipid bilayer. Polymorphisms controlling orosomucoid-like 3 (ORMDL3) expression in the asthma susceptibility locus 17q21 are associated with childhood asthma, but not with atopy (32, 33), suggesting that ORMDL3 affects asthma susceptibility independent of atopic or immunoglobulin E-mediated pathways (33).
Figure 1.
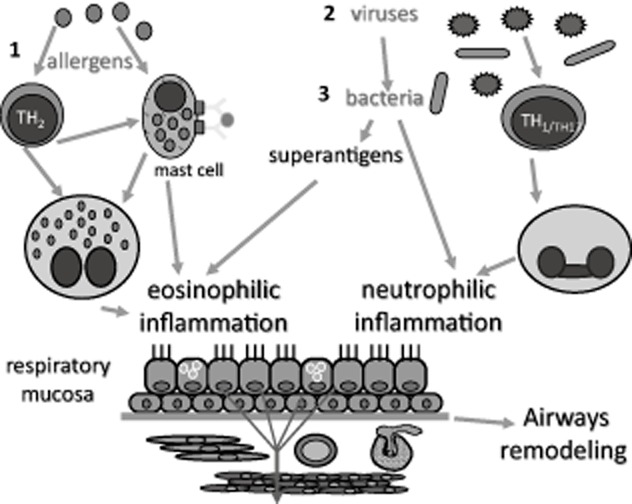
Allergic-type asthma could result from a multi-hit phenomenon: the primary event in the allergic airway is Th2 inflammation, usually arising in the upper airway (as rhinitis) before progressing to the lower, where it is initially intermittent. At this stage, the disease is amenable to treatments such as allergen avoidance and immunotherapy. However, infections such as rhinoviruses are handled less effectively by the inflamed mucosa, possibly because of defects in interferons and/or sphingolipids. Epithelial permeability is compromised, and persistence of microorganisms such as Staphylococci is permitted. This further perturbs local immunity with nonspecific activation of T lymphocytes via V beta receptors and chronic local Th1, Th17 and Th2 inflammation which is no longer as responsive to anti-allergic treatment. Since the bacteria are intra-epithelial, antibiotics cannot eliminate them. Topical corticosteroids are useful as anti-inflammatories at all stages, but doses needed rise as inflammation becomes more complex and persistent. There is a continued attempt at tissue repair, evident as airways remodeling. Non-allergic asthma could begin at stage 2.
There may be further microorganisms, including other bacteria, mycoplasma and fungi, that can initiate or maintain asthma in a similar fashion in other phenotypes: allergic bronchopulmonary aspergillosis is one example. The microbiome of induced sputum is different in asthma compared with controls: patients with mild asthma have an altered microbial composition in the respiratory tract that is similar to that observed in patients with more severe asthma (34). An IgE response to staphylococcal enterotoxins has also been demonstrated in adenoid tissues from atopic children (35). Interestingly in teenagers, although Staphylococci are implicated, some other microorganisms appear protective (36).
This has important implications. The first is that there is possibly a window of opportunity for prevention of asthma in allergic rhinitis. Grass pollen-specific immunotherapy by the subcutaneous route probably reduces asthma development (37); the sublingual route is under investigation (38). If proven effective, the threshold for its use should be reconsidered and the preventative effects of immunotherapy for other allergens, such as house dust mite, evaluated. Furthermore, pharmacotherapy for rhinitis used regularly with good control of minimal persistent upper respiratory tract inflammation (39) might also reduce progression to asthma, or reduce exacerbations in those already suffering from associated asthma. This needs exploration.
The second is that prevention of colds is vitally important and the search for a cure needs to continue. Whether amelioration of symptoms and inflammation during a viral upper respiratory tract infection (URTI) is helpful is unknown. Meanwhile, simple public health measures, such as hand washing, use of disposable tissues, etc., should be taught. Vitamin D may be relevant in protection against URTI, with low levels predisposing to infection frequency and/or severity (40).
Finally, if the pro-inflammatory stimulus is infective but intra-mucosal, then perhaps asthma therapy might benefit from a rethink. Inhaled corticosteroids are obviously needed, but instead of additional chronic anti-inflammatory therapy with increasingly expensive or toxic molecules the idea of stimulus removal could be mooted. Systemic antibiotics have shown some effectiveness in asthma and in nasal polyposis associated with asthma (41, 42), but intra-mucosal or surface biofilms are resistant to them and planktonic form reemerge once the course is completed. Local use of anti-microbials needs exploration. Bronchial thermoplasty, which destroys the respiratory epithelium, might act via bacterial killing, in a fashion analogous to the treatment of syphilis by heat (43).
The pathogenesis of asthma is unraveling further and provides us with an opportunity by careful research not only to improve the way in which patients are treated, but possibly also to prevent some of them from developing this lifelong, troubling and expensive disease in the first place.
References
- 1.Grainge C, Howarth P. Repeated high-dose inhalation allergen challenge in asthma. Clin Respir J. 2011;5:150–155. doi: 10.1111/j.1752-699X.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 2.Custovic A, Simpson A, Chapman MD, et al. Allergen avoidance in the treatment of asthma and atopic disorders. Thorax. 1998;53:63–72. doi: 10.1136/thx.53.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma [review] The Cochrane Database of Systematic Reviews. Cochrane Libr. 2004;4:1–89. [Google Scholar]
- 4.Chetta A, Foresi A, Del Donno M, et al. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest. 1997;111:852–857. doi: 10.1378/chest.111.4.852. [DOI] [PubMed] [Google Scholar]
- 5.Brightling CE, Gupta S, Gonem S, et al. Lung damage and airway remodelling in severe asthma. Clin Exp Allergy. 2012;42:638–649. doi: 10.1111/j.1365-2222.2011.03917.x. [DOI] [PubMed] [Google Scholar]
- 6.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 7.Menzella F, Piro R, Facciolongo N, et al. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol. 2011;7:9. doi: 10.1186/1710-1492-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gevaert P, Calus L, Van Zele T. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–161. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Takhar P, Smurthwaite L, Coker HA, et al. Allergen in the nose drives class switching. J Immunol. 2005;174:5024–5032. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 10.Powe DG, Jagger C, Kleinjan A, et al. ‘Entopy’: localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003;33:1374–1379. doi: 10.1046/j.1365-2222.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- 11.Rondón C, Campo P, Togias A, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012;129:1460–1467. doi: 10.1016/j.jaci.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Van Zele T, Gevaert P, Holtappels G, et al. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–1847. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 13.Humbert M, Menz G, Ying S, et al. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20:528–533. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 14.Fireman P. Virus-provoked rhinitis in patients who have allergies. Allergy Asthma Proc. 2002;23:99–102. Top of Form. [PubMed] [Google Scholar]
- 15.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 17.Custovic A, Murray CS, Simpson A. Allergy and infection: understanding their relationship. Allergy. 2005;60(Suppl. 79):10–13. doi: 10.1111/j.1398-9995.2005.00851.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–1057. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 19.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braunstahl GJ, Overbeek SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–476. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 21.Szczeklik A, Nizankowska E, Duplaga M, on behalf of the AIANE Investigators Natural history of aspirin-induced asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 22.Szczeklik A. Aspirin-induced asthma as a viral disease. Clin Exp Allergy. 1988;18:15–20. doi: 10.1111/j.1365-2222.1988.tb02838.x. [DOI] [PubMed] [Google Scholar]
- 23.Steinke J, Liu L, Huyett P, et al. Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013;132(4):856–865. doi: 10.1016/j.jaci.2013.05.008. e1–3. doi: 10.1016/j.jaci.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–983. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Bachert C, Zhang N, Patou J, et al. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–38. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 26.Corriveau MN, Zhang N, Holtappels G. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23:461–465. doi: 10.2500/ajra.2009.23.3367. [DOI] [PubMed] [Google Scholar]
- 27.Bachert C, van Steen K, Zhang N, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130:376–381. doi: 10.1016/j.jaci.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Glorieux S, Bachert C, Favoreel HW, et al. Herpes simplex virus type 1 penetrates the basement membrane in human nasal respiratory mucosa. PLoS ONE. 2011;6:e22160. doi: 10.1371/journal.pone.0022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhang N, Glorieux S, et al. Herpes simplex virus type 1 infection facilitates invasion of Staphylococcus aureus into the nasal mucosa and nasal polyp tissue. PLoS ONE. 2012;7:e39875. doi: 10.1371/journal.pone.0039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones G. Susceptibility to asthma and eczema from mucosal and epidermal expression of distinctive genes. Curr Allergy Asthma Rep. 2007;7:11–17. doi: 10.1007/s11882-007-0025-z. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson D, Andiappan AK, Halldén C, et al. Toll-like receptor gene polymorphisms are associated with allergic rhinitis: a case control study. BMC Med Genet. 2012;13:66. doi: 10.1186/1471-2350-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 33.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marri PR, Stern DA, Wright AL, et al. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–352. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin S, Choi G, Lee K, et al. IgE response to staphylococcal enterotoxins in adenoid tissues from atopic children. Laryngoscope. 2009;119:171–175. doi: 10.1002/lary.20046. [DOI] [PubMed] [Google Scholar]
- 36.Hollams EM, Hales BJ, Bachert C, et al. Th2-associated immunity to bacteria in teenagers and susceptibility to asthma. Eur Respir J. 2010;36:509–516. doi: 10.1183/09031936.00184109. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen L, Niggemann B, Dreborg S, et al. The PAT investigator group. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;628:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 38.Valovirta E. Effect of AIT in children including potential to prevent the development of asthma. Allergy. 2011;66(Suppl. 95):53–54. doi: 10.1111/j.1398-9995.2011.02640.x. [DOI] [PubMed] [Google Scholar]
- 39.de Groot EP, Nijkamp A, Duiverman EJ, et al. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–587. doi: 10.1136/thoraxjnl-2011-201168. [DOI] [PubMed] [Google Scholar]
- 40.Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax. 2012;67:1018–1020. doi: 10.1136/thoraxjnl-2012-202139. [DOI] [PubMed] [Google Scholar]
- 41.Cameron EJ, McSharry C, Chaudhuri R, et al. Long-term macrolide treatment of chronic inflammatory airway diseases: risks, benefits and future developments. Clin Exp Allergy. 2012;42:1302–1312. doi: 10.1111/j.1365-2222.2012.03979.x. [DOI] [PubMed] [Google Scholar]
- 42.Ragab S, Scadding GK, Lund VJ, et al. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J. 2006;28:68–74. doi: 10.1183/09031936.06.00043305. [DOI] [PubMed] [Google Scholar]
- 43.Cayetano KS, Chan A, Albertson TE, et al. Bronchial thermoplasty: a new treatment paradigm for severe persistent asthma. Clin Rev Allergy Immunol. 2012;43:184–193. doi: 10.1007/s12016-011-8295-6. [DOI] [PubMed] [Google Scholar]


