Abstract
Human plasma butyrylcholinesterase (BChE) contributes to cocaine metabolism and has been considered for use in treating cocaine addiction and cocaine overdose. TV-1380 is a recombinant protein composed of the mature form of human serum albumin fused at its amino terminus to the carboxy-terminus of a truncated and mutated BChE. In preclinical studies, TV-1380 has been shown to rapidly eliminate cocaine in the plasma thus forestalling entry of cocaine into the brain and heart. Two randomized, blinded phase I studies were conducted to evaluate the safety, pharmacokinetics, and pharmacodynamics of TV-1380, following single and multiple administration in healthy subjects. TV-1380 was found to be safe and well tolerated with a long half-life (43–77 hours) and showed a dose-proportional increase in systemic exposure. Consistent with preclinical results, the ex vivo cocaine hydrolysis, TV-1380 activity clearly increased upon treatment in a dose-dependent manner. In addition, there was a direct relationship between ex vivo cocaine hydrolysis (kel) and TV-1380 serum concentrations. There was no evidence that TV-1380 affected heart rate, the uncorrected QT interval, or the heart-rate-corrected QTcF interval. TV-1380, therefore, offers a safe once-weekly therapy to increase cocaine hydrolysis.
Keywords: TV-1380, butyrylcholinesterase, cocaine, pharmacokinetics, safety, tolerability, healthy volunteers
Drug addiction is a chronically relapsing disorder that is characterized by compulsive drug-seeking behavior, inability to limit intake of the drug, and emergence of a negative emotional state when access to the drug is prevented.1 Cocaine addiction is a significant public health problem worldwide, with serious consequences including public health risks such as infectious diseases,2 cardiovascular diseases,3–5 and increased crime and violence.6 Cocaine is thought to cause addiction in humans by activating the mesocorticolimbic system through increasing dopamine release in the nucleus accumbens.7–10 The subjective rewarding experience (“high”) caused by cocaine has been shown in preclinical studies to be mediated by increases in synaptic levels of both dopamine and noradrenaline.11–13 Cocaine also influences other neurotransmitter systems, including glutamate, GABA, endocannabinoid, and corticotrophin-releasing hormone.14–17 These neurotransmitter systems interact with each other in the modulation of the reward, motivation, and memory systems in the brain.18,19 Currently, behavioral therapy is the only option for treatment of cocaine dependence and there is no approved pharmacological treatment for patients seeking abstinence.20,21
A new strategy for treatment of cocaine addiction is to facilitate degradation of cocaine in the peripheral circulation. Plasma butyrylcholinesterase (BChE) contributes to cocaine metabolism and has been considered for use in treating cocaine toxicity. However, wild-type human BChE (hBChE) has poor catalytic efficiency.22 To overcome this limitation, TV-1380 was developed. The wild-type plasma hBChE was modified through successive rational mutations to increase its efficiency as a cocaine hydrolase. To express the mutated hBChE using mammalian expression system as a monomer, the C-terminus tetramerization domain was removed; to increase its stability and residence time in vivo, the modified BChE was fused to recombinant human serum albumin (rHSA) to express the recombinant protein as a single, continuous polypeptide.23,24 The increased selectivity and stability of the resulting fusion protein, TV-1380, enhances its potential as a protein-based treatment for cocaine dependence and toxicity.25
TV-1380 has been shown to have a potential therapeutic benefit in cocaine abstinence and in preventing relapse in a series of animal pharmacology experiments.25,26 Nonclinical studies have shown that TV-1380 can rapidly eliminate cocaine from plasma and, by that, prevent the entry of cocaine to the brain, heart, and periphery. Additionally, pretreatment with TV-1380 provided a marked, dose-dependent protection against cocaine toxicity and overdose, rescued rats from fatal cocaine overdose, stopped convulsions, allowed for resumption of upright posture, and limited further signs of cocaine-induced arousal.27 TV-1380 also selectively blocked cocaine-induced reinstatement of drug-seeking behavior in rats that had previously self-administered cocaine.23,26
The objectives of these 2 phase I studies were to evaluate the safety, tolerability, and pharmacokinetics (PK) of single and multiple doses of TV-1380 in healthy male and female subjects. In addition, ex vivo cocaine hydrolysis activity (TV-1380 activity assay) was determined as a pharmacodynamic (PD) assessment. The effect of TV-1380 on cardiac parameters was investigated in a dedicated analysis in the multiple dose study.
Methods
Study Approvals and Informed Consent
The studies were conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice (ICH) guidelines and in compliance with the European Union Clinical Trial Directive. An Independent Ethics Committee (Stichting Beoordeling Ethiek Bio-Medisch Onderzoek, Assen, The Netherlands) reviewed and approved the study protocols and the subject information. Written informed consent was obtained for each subject prior to enrolment in the studies.
Study Population
Two phase I double-blind, placebo controlled studies of single ascending dose (SAD) and multiple ascending dose (MAD) administration were conducted. Healthy male (SAD and MAD) and female (MAD) subjects were recruited by a single center in The Netherlands (PRA Health Sciences, Groningen). Participants underwent health status and urine drug screening within 3 weeks before dosing and had to meet the following main inclusion criteria: age within 18 and 45 years; body mass index within 18.0–30.0 kg/m2; and weight of at least 50 kg; nonsmokers. Main exclusion criteria included any clinically relevant medical disorder, blood pressure outside the ranges of 90–140 mm Hg systolic or 45–90 mm Hg diastolic, or heart rate <40 beats/min. Male subjects had to use medically acceptable contraceptive precautions during the study conduct. Female subjects had to be postmenopausal or surgically sterilized, or if of childbearing potential, they had to have a negative serum β-HCG test and needed to use a medically acceptable double-barrier form of birth control. With the exception of acetaminophen and ibuprofen, no concomitant medications were allowed unless needed to treat adverse events. Subjects exposed to pesticides or other organophosphates (eg, agricultural workers) were also to be excluded. No alcoholic or caffeinated drinks, grapefruit juice, smoking, and food containing poppy seeds were allowed.
Study Designs
Subjects were admitted to the clinical center on Day −1, the day before dosing and baseline measures of vital signs, ECG, urine drug screen, blood tests, laboratory, and neurological assessments were conducted. In the SAD study (Figure 1a), subjects were randomly assigned to 1 of 7 dose groups (0.5, 1.5, 5, 15, 50, 150, and 300 mg) and received TV-1380 (n = 6 per dose) or placebo (n = 2 per dose) by intramuscular (IM) injection. Subjects received single IM injections in volumes ranging from 0.5 to 5 mL for TV-1380 doses 0.5–150 mg and respective placebo groups; and received 2 IM injections, each a volume of 5 mL for the TV-1380 300 mg dose and respective placebo. The IM injections of TV-1380 and placebo occurred in the buttock (maximus gluteus muscle; for 1 injection into the left aspect and for 2 injections in the left and right aspects of the buttocks). A starting dose of 0.5 mg was selected on the basis of the minimal pharmacologically active dose (PAD) in Cynomolgus monkeys (0.2 mg/kg). The human equivalent dose (HED) of the PAD is 4.5 mg in a 70 kg individual. The proposed starting dose, therefore, was about 10 times lower than the HED of the PAD. A starting dose of 0.5 mg was also about 200 times lower than the NOEL in rats (9 mg/kg in rats) and 600 times lower than the NOEL in Cynomolgus monkeys (15 mg/kg), on an HED basis. There was an interval of at least 7 days between any 2 consecutive dose levels and doses were escalated only after a favorable safety review of the previous dose. Subjects remained in the clinic until the morning of Day 4 (72 hours postdosing) and thereafter returned for follow-up visits up to Day 35 (816 hours postdosing).
Figure 1.
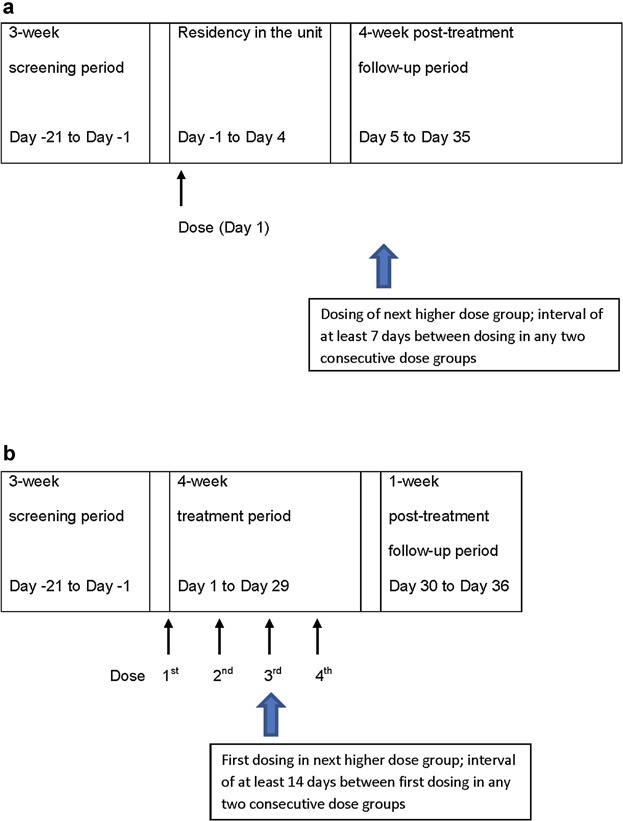
(a) The SAD study consisted of 7 dose groups (single doses of 0.5, 1.5, 5, 15, 50, 150, and 300 mg TV-1380 or placebo). (b) The MAD study consisted of 3 dose groups (multiple once-weekly doses of 50, 150, and 300 mg TV-1380 or placebo).
In the MAD study (Figure 1b), subjects were randomly assigned to 1 of 3 dose groups (50, 150, or 300 mg) and received a total of 4 IM injections of TV-1380 (n = 8 per dose) or placebo (n = 3 per dose) administered once-weekly. Subjects received a dose of TV-1380 (50, 100, or 300 mg) or placebo (formulation buffer) in a volume of 3 mL via a single IM injection into the left aspect of the maximus gluteus muscle. These doses were selected based on the results of the SAD study and the expected clinically effective dose levels. There was an interval of at least 14 days between any 2 consecutive dose groups, and doses were escalated only after a favorable safety review of the previous dose.
TV-1380 was produced and supplied by Teva Pharmaceutical Industries Ltd (Netanya, Israel) in sterile, single-use vials that could deliver TV-1380 at a concentration of 30 mg/mL for the SAD study or 100 mg/mL for the MAD study. The 2 formulations contained the same excipients and active substance but in different concentrations. The placebo contained the same excipients as the formulation buffer.
Safety and Tolerability Assessments
Safety and tolerability were assessed in both studies as adverse event (AE) monitoring, vital signs (supine blood pressure, heart rate, and body temperature), gross neurological examination (including pupil size in the SAD), and 12-lead electrocardiograms (ECGs). Standard clinical hematologic, blood chemistry, and urinalysis tests were performed.
Plasma BChE and red blood cells acetyl cholinesterase (AChE) activity levels were determined using a colorimetric method based on the Ellman assay28 at multiple time points throughout the study period in the SAD and MAD studies. The activity of both enzymes was monitored to detect an early signal for antidrug antibodies with crossreactivity to endogenous enzymes.
Salivary flow (to evaluate the possible AE of dry mouth caused by dysfunction of AChE) was determined in the SAD study at multiple time points throughout the study. Salivary flow was measured by placing preweighed dental cotton rolls in the oral cavity (1 in each buccal pouch and 1 on either side of the tongue) for 1 minute. The cotton rolls were then removed and reweighed. Salivary secretion was calculated from the weight gain of the rolls and expressed as gram saliva per minute.
Local tolerability at the injection site (evaluated through tenderness, erythema, and induration at the injection site) and pain at the injection site (evaluated using a visual analog scale) were evaluated at predetermined time points up to 24 hours postdose.
Immunogenicity
Titers for antibodies against HSA, (h)BChE, and TV-1380 were determined at predetermined time points during the study. Antidrug antibody (ADA) analysis was performed by Catalent Pharma Solutions (Morrisville, North Carolina) in the SAD study and by Teva Biopharmaceuticals (Rockville, Maryland) in the MAD study. Using a multitier approach, serum samples were first screened and confirmed for the presence of ADA using validated ELISA methods. Any samples tested above the screening and confirmatory cut points were deemed ADA-positive. Confirmed ADA-positive samples were then further characterized to evaluate the titer of the ADA using validated titration ELISA. Subjects with positive immunogenicity results on Day 35 were requested to return for another visit 3–5 months postdose.
Extensive ECG Analysis
In addition to standard ECGs taken for safety monitoring, during the MAD study extensive ECG analysis was also added to assess the effect of TV-1380 on cardiac repolarization, conduction, rhythm, and morphology in a dedicated analysis. ECGs were collected digitally in triplicates and read by a core ECG laboratory (eRT, Peterborough, UK). ECGs were captured at 3 time points prior to dosing on Day 1 and on Day 22, at multiple time points on Day 22 postdose, and on Days 23, 24, 26, 29, and 36. At each time point, the mean of the triplicate ECG was compared to baseline measurements. Concentration-effect (QTcF) analysis was also conducted.
Pharmacokinetic Assessments
Blood samples for the analysis of TV-1380 concentrations in plasma were collected at predose and 20, 40 minutes and 1, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, 72, 120, 168, 240, and 336 hours postdose (SAD) and at predose, 1, 2, 4, 6, 8, 12, 18, 24, 36, 48, 96, 168 (presecond dose), 180, 192, 264, 336 (prethird dose), 348, 360, 432, 504 (prefourth dose), 505, 506, 508, 510, 512, 516, 522, 528, 540, 552 (Day 24), 600 (Day 26), 672, 744, and 840 (Day 36) hours postfirst dose (MAD).
Drug concentration measurements of TV-1380 in subject serum were performed by Catalent Pharma Solutions (Morrisville, North Carolina; SAD study) and by Teva Biopharmaceuticals (Rockville, Maryland; MAD study) using validated ELISA methods. In brief, subject serum was incubated in a 96-well microplate coated with antihuman BChE mouse monoclonal antibody. After incubation, the captured TV-1380 on the microplate was detected by the addition of Horseradish peroxidase (HRP)-conjugated antihuman albumin (HSA) goat polycoclonal antibody (SAD) or HRP-conjugated antihuman (HSA) mouse monoclonal antibody (MAD), and subsequently color development of the TMB substrate. The absorbance is measured at 450 nm using 570 nm as a reference filter. Each plate contains ELISA standards, controls, and up to 30 diluted test serum samples in duplicate. The standard curve is generated by analysis software for each plate using the 4-PL model (SAD) or 5-PL model (MAD). The serum TV-1380 concentrations are interpolated from the standard curve, adjusted by dilution factor, and averaged for each sample (mean adjusted results). The assay has a dynamic range from 900 ng/mL to 156 ng/mL (SAD) and 12,000 ng/mL to 134 ng/mL (MAD). The minimum required dilution for the samples was 1:13.3 (SAD) or 1:40 (MAD).
Minor modifications to enhance assay performance of the assay were made by Teva, and the new assay was validated prior to use in the multiple dose. The modifications included an increase in the minimum required dilution and a switch from a polyclonal antibody to a monoclonal antibody in the secondary detector.
Pharmacodynamic Assessments
Blood samples for ex vivo cocaine hydrolysis analysis were obtained from the 50 and 150 mg dose groups in the SAD study at multiple time points from predose and up to Day 15 postdose. The measurements were performed by Tandem Labs (West Trenton, New Jersey). Serum samples were diluted in phosphate-buffered saline (PBS), spiked with cocaine to a final concentration of 200 ng/mL, and incubated at 37 °C. Samples for cocaine concentrations were taken at 8 time points over 90 minutes. Cocaine concentrations were determined by validated LC MS/MS assay and the elimination rate constant for ex vivo cocaine hydrolysis (kel) and t1/2 was calculated.
Pharmacokinetic and Statistical Analysis
For these 2 exploratory studies, no formal sample size calculation was performed. At least 6 (SAD) and 8 (MAD) subjects at each dose level were considered compatible with a reasonable clinical interpretation and descriptive statistics.
AEs were tabulated and summarized according to the Medical Dictionary for Regulatory Activities (MedDRA, version 13.0 for the SAD and version 14.1 for the MAD study). BChE and AChE activity, salivary flow (only in the single dose study), blood pressure, heart rate, ECG parameters, and clinical laboratory data were summarized using descriptive statistics.
The following pharmacokinetic parameters for TV-1380 were derived by noncompartmental analysis (NCA) from the individual plasma concentration-time profiles: peak serum concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration-time curve (AUC) from time 0 to last quantifiable concentration (AUC0–t) calculated using linear trapezoidal summation, AUC extrapolated to infinity (AUC0–inf), apparent volume of distribution (Vd/F where F represents bioavailability), apparent clearance (CL/F), terminal half-life (t1/2). In the MAD study, additional parameters were calculated: average concentration at steady state (Cavg), AUC from 0 hour to time tau (AUC0–tau) where tau is the dosing interval (168 hours), apparent total body clearance (steady state; CLss/F), and accumulation factor (Ra) calculated as the ratio of AUCtau at fourth dose compared to the first dose AUCtau. Pharmacokinetic parameters were calculated with WinNonlin® Professional Version 5.0.1 or higher (Pharsight Corp., Mountain View, California) and/or SAS® Version 9.1.3. Actual sampling times were used for the pharmacokinetic analysis. In addition, dose-normalized Cmax and AUC values were plotted against body weight to visualize the effect of subject weight on the PK parameters. The effect of sex was also evaluated in the multiple dose study. Dose proportionality was evaluated using the following power model: [log(PK) = slope*log(dose) + intercept + error].29 The estimate and 95% confidence intervals (CI) of the slope were determined. Time dependence in the MAD study was assessed by comparing AUC0–inf (first dose) with AUC0–tau (after fourth dose).
Parameters for cocaine hydrolytic activity (kel and t1/2) were estimated using NCA (model: the intravascular bolus injection) with WinNonlin® Professional Version 5.0.1 and SAS® Version 9.1.3.
ECG statistical analysis in the MAD study was designed to assess all ECG parameters with the corrected QT (QTc) interval as the key cardiac safety parameter of regulatory interest.30 The QT interval was corrected for heart rate (HR) using the Fridericia correction, QTcF = QT*RR1/3.31 For each time point, ECG intervals, including HR, RR, QT, PR, and QRS, were averaged from the triplicate traces. QTc values were derived from the average RR and QT values for each time point. The mean of the 9 individual predose observations on Day 1 was the baseline value for the study. All 9 placebo subjects were pooled to a single placebo group for summary and comparison purposes.
Summary statistics for all ECG parameters were derived for each time point and for each treatment dose. Formal comparisons of individual dose levels with placebo were made using an analysis of covariance, with factors for dose (including placebo) and sex, using the baseline value as a covariate. In addition outlier analyses of individual QTc values and, separately, ECG morphology and overall interpretation were performed.
The concentration-QTc analysis was performed using a linear modeling approach to quantify the relationship between the plasma concentration of TV-1380 and the change in the (baseline-adjusted) QTcF interval.
Results
Subject Disposition
Fifty-six and 33 subjects were dosed in the SAD and MAD studies, respectively. In the SAD study, 42 subjects received TV-1380 and 14 placebo. There were no differences in their demographic characteristics (Table1). In the MAD study, 24 subjects received TV-1380 and 9 placebo (Table2). One subject withdrew consent during the study and received 2 of the 4 planned administrations of 300 mg TV-1380. At baseline, there were no significant differences in demographics between the treatment groups although body weight and BMI were somewhat higher in the 150 mg TV-1380 dose group. A total of 16 female subjects (48%) and 17 male subjects (52%) participated in the study, and the number of subjects of each sex was approximately equal among groups.
Table 1.
Demographic Characteristics of Subjects in the Single Dose Administration Study
| Characteristic | Placebo (n = 14) | TV1380 Dose |
Overall(N = 56) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| .5 mg (n = 6) | 1.5 mg (n = 6) | 5.0 mg (n = 6) | 15 mg (n = 6) | 50 mg (n = 6) | 150 mg (n = 6) | 300 mg (n = 6) | |||
| Age, years, mean (SD) | 26 (8) | 25 (9) | 29 (8) | 25 (2) | 30 (9) | 30 (10) | 35 (6) | 25 (6) | 28 (8) |
| Weight, kg, mean (SD) | 74.3 (5.9) | 74.0 (7.7) | 78.3 (9.0) | 74.7 (5.5) | 80.9 (11.9) | 78.9 (18.9) | 82.4 (12.4) | 76.2 (11.4) | 77.0 (10.2) |
| Height, cm, mean (SD) | 181 (5) | 178 (6) | 187 (8) | 184 (8) | 184 (8) | 187 (13) | 179 (10) | 184 (4) | 183 (8) |
| BMI (kg/m2), mean (SD) | 22.8 (1.7) | 23.4 (1.6) | 22.5 (2.4) | 22.0 (1.3) | 23.9 (3.0) | 22.2 (3.0) | 25.6 (2.9) | 22.6 (3.3) | 23.1 (2.5) |
| Race, no. (%) | |||||||||
| Asian | 1 (7%) | – | – | 1 (17%) | – | – | 1 (17%) | – | 3 (5%) |
| Asian + black | – | – | – | – | – | – | – | 1 (17%) | 1 (2%) |
| Black | 1 (7%) | 1 (17%) | 1 (17%) | – | 1 (17%) | 1 (17%) | – | – | 5 (9%) |
| White | 10 (71%) | 5 (83%) | 5 (83%) | 5 (83%) | 4 (67%) | 4 (67%) | 5 (83%) | 5 (83%) | 43 (77%) |
| White + Asian | 1 (7%) | – | – | – | 1 (7%) | 1 (7%) | – | – | 3 (5%) |
| White + black | 1 (7%) | – | – | – | – | – | – | – | 1 (2%) |
BMI = body mass index.
Table 2.
Demographic Characteristics for Subjects in the Multiple Dose Administration Study
| Characteristic | Placebo (n = 9) | TV1380 Dose |
Overall (n = 33) | ||
|---|---|---|---|---|---|
| 50 mg (n = 8) | 150 mg (n = 8) | 300 mg (n = 8) | |||
| Age, years, mean (SD) | |||||
| Female | 29 (9) | 24 (2) | 27 (8) | 29 (10) | 28 (8) |
| Male | 30 (10) | 34 (10) | 32 (8) | 24 (1) | 30 (9) |
| Weight, kg, mean (SD) | |||||
| Female | 66.4 (13.2) | 66.2 (5.9) | 76.7 (9.1) | 60.3 (6.5) | 67.0 (10.4) |
| Male | 73.4 (10.0) | 76.7 (13.8) | 84.6 (10.6) | 73.0 (8.9) | 76.9 (11.2) |
| Height, cm, mean (SD) | |||||
| Female | 165 (8) | 179 (3) | 177 (4) | 165 (7) | 171 (8) |
| Male | 180 (3) | 179 (7) | 180 (9) | 175 (6) | 179 (6) |
| BMI (kg/m2), mean (SD) | |||||
| Female | 24.2 (3.7) | 20.7 (1.2) | 24.5 3. (7) | 22.2 (1.7) | 23.0 (2.9) |
| Male | 22.7 (3.7) | 23.9 (4.2) | 26.0 1. (9) | 23.9 (3.1) | 24.0 (3.4) |
| Sex, no. (%) | |||||
| Female | 4 (44%) | 3 (38%) | 4 (50%) | 5 (63%) | 16 (48%) |
| Male | 5 (56%) | 5 (63%) | 4 (50%) | 3 (38%) | 17 (52%) |
| Race, no. (%) | |||||
| American Indian or Alaska native | 1 (11%) | – | – | – | 1 (3%) |
| Black | – | – | 1 (12%) | – | 1 (3%) |
| White | 8 (89%) | 8 (100%) | 7 (88%) | 8 (100%) | 31 (94%) |
BMI = body mass index.
Safety
TV-1380 0.5–300 mg was well tolerated in both dosing schedules. For the single dose, a total of 41 treatment-emergent AEs (TEAEs) were reported by 25 subjects (45% of total number of subjects dosed). The percentage of subjects reporting TEAEs was 36% for placebo-treated subjects and varied between TV1380 dose groups (0.5, 1.5, 5.0, 15, 50, 150, and 300 mg) as 67%, 17%, 33%, 83%, 33%, 33%, 67%, and 45%, respectively, with no clear dose relationship.
For the multiple dose, a total of 108 TEAEs were reported by 25 subjects (76% of total number of subjects dosed). The percentage of subjects reporting TEAEs was 67% for placebo-treated subjects and 75%, 88%, and 75% for the 50 mg, 150 mg, and 300 mg TV-1380 dose groups, respectively. The number of TEAEs was approximately twice as high in the 150 and 300 mg TV-1380 dose groups as in the placebo and 50 mg TV-1380 dose groups.
Overall in the SAD study the most frequently reported TEAEs were injection site reactions and headaches, whereas in the MAD study the most frequent TEAEs were headaches, dizziness, injection site reactions, and gastrointestinal disorders (diarrhea, dyspepsia, and nausea) seen mainly in the 300 mg TV-1380 group. There were no deaths or serious AEs in both studies. No differences in injection site reactions were observed across the doses and no meaningful differences between placebo and TV-1380 were observed.
There were no findings of clinical relevance in clinical laboratory, vital signs, physical examination, gross neurological examination or pupil size and salivary flow.
There was no indication that the activities of endogenous BChE and AChE were changed in the TV-1380-treated subjects. No immunogenicity for TV-1380 was observed in the SAD study, whereas 3 subjects in the MAD study were identified as positive for anti-TV-1380 antibodies. One of these subjects had an anti-TV-1380 immune response from Day 22 up to 5 months after the last dose, but it was not accompanied by changes in AChE or BChE activity. One additional subject was identified as positive for anti-HSA antibodies at one time point but no changes in AChE or BChE activity were seen.
ECG
No evidence for any tendency of TV-1380 to affect the heart rate, the uncorrected QT interval, or the heart-rate-corrected QTcF interval was seen. The PR and QRS intervals also showed no strong evidence of an effect of TV-1380, although a small increase in the QRS interval was observed with the 300 mg dose group at the Day 26 and 29 time points, which may be related to a lower baseline value in this group. The fitted slope for the concentration-QTcF analysis was overall shallow (0.00070 msec/ng/mL) and not statistically significantly different from zero (Supplemental Figure 1). At the highest mean exposure of 2830 ng/mL (Cmax of the 300 mg dose level), the predicted mean QTcF prolongation was 1.23 milliseconds, with an upper confidence limit of 5.64 milliseconds, which is below the 10 millisecond threshold of regulatory concern.30 There was no evidence for an effect of TV-1380 on ECG morphology, conduction, rhythm, or overall ECG interpretation.
Pharmacokinetics
Mean plasma concentration time profiles following single dose administration are presented in Figure 2a and PK parameters in Table3. TV-1380 serum concentrations were below the lower limit of quantification (BLQ) for all or most samples treated with 0.5, 1.5, 5, and 15 mg. Median tmax ranged between 12 and 18 hours postdose and t1/2 ranged between 43 and 63 hours. Cmax and AUC increased in a dose-proportional manner (using the power model) over the 50–300 mg dose range. AUC0–t and AUC0–inf were calculated for all subjects at doses 50, 150, and 300 mg with AUC0–t being >80 in all cases, thus only AUC0–inf is reported in Table3.
Figure 2.
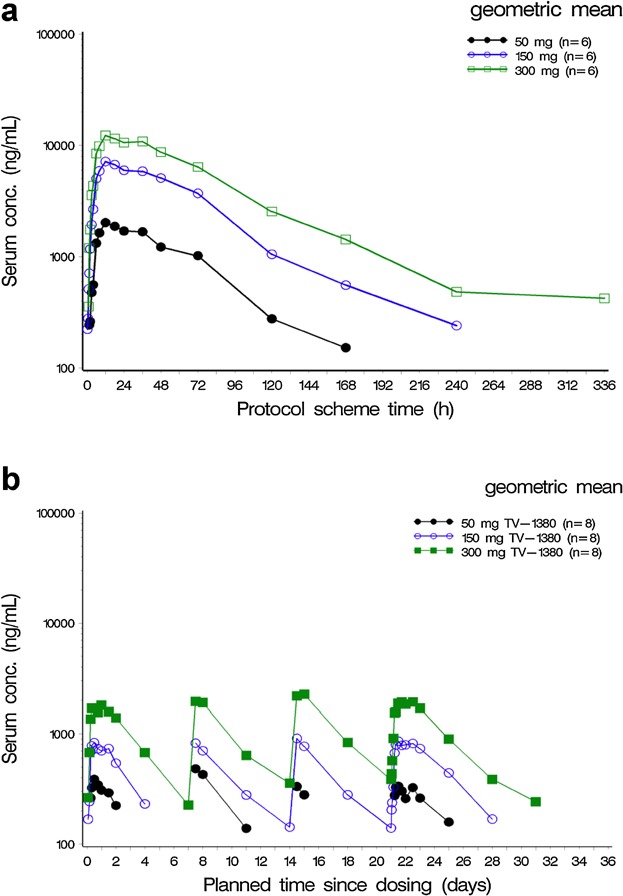
Geometric means of TV 1380 serum concentrations (semilogarithmic) following single administration (Panel a) and multiple doses (Panel b). N = number of subjects exposed per dose level.
Table 3.
Summary Statistics of TV-1380 Serum PK Parameters Following Single Dose Administered by IM Injection
| Single Dose Administration | |||
|---|---|---|---|
| Parameter | 50 mg TV-1380 | 150 mg TV-1380 | 300 mg TV-1380 |
| Cmax (ng/mL) | 2,030 (749–5591) | 8,020 (4002–15101) | 13,507 (7552–37343) |
| tmax (hours) | 12.0 (12.0–36.0) | 15.0 (12.0–24.0) | 18.0 (12.0–36.0) |
| AUC0–inf (ng · hr/mL) | 157,000 (68,900–376,000) | 580,000 (348,000–929,000) | 1,126,000 (689,000–1,626,000) |
| t1/2 (hours) | 42.7 (32.0–80.0) | 43.1 (30.7–73.8) | 62.8 (53.7–78.2) |
| CL/F (L/h) | 0.3 (0.1–0.7) | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) |
| Vd/F (L) | 19.6 (6.1–75.7) | 16.1 (9.2–33.5) | 24.1 (14.6–33.7) |
The pk parameters summarized included peak serum concentration (Cmax), time to Cmax (Tmax), AUC extrapolated to infinity (AUC0–inf), terminal half-life (t1/2), apparent clearance (CL/F), and apparent volume of distribution (Vd/F where F represents bioavailability). At lower doses of TV-1380, the PK results were below the LLoQ for all or most of the samples. N = 6 for each dose level. For all parameters except tmax the geometric mean (range) is presented; for tmax the median (range) is presented.
Mean serum drug concentration-time profiles for multiple-dose administration are presented in Figure 2b with the corresponding pharmacokinetic parameters displayed in Table4. Median tmax ranged between 12 and 36 hours postdose and was independent of dose. The t1/2 ranged between 50 and 77 hours (Day 1 and Day 22) and was independent of dose. In spite of slightly higher t1/2 values on Day 22 compared to Day 1, there were no indications that the t1/2 changed during multiple dosing as CL/F was also similar on both days.
Table 4.
Summary Statistics of TV-1380 Serum PK Parameters Following Multiple Doses Administered by IM Injection
| Multiple Dose Administration | ||||
|---|---|---|---|---|
| Parameter | Day | 50 mg TV-1380 | 150 mg TV-1380 | 300 mg TV-1380 |
| Cmax (ng/mL) | 1 | 533.9 (261.5–786.9)a | 900.7 (241.6–1,698.3) | 1,964.3 (592.5–2,715.3) |
| 22 | 468.8 (234.4–847.2)a | 952.0 (393.8–1,745.4) | 2,127.6 (927.1–3,738.7)a | |
| tmax (hours) | 1 | 12.0 (12.0–36.0)a | 12.0 (8.0–24.0) | 18.0 (12.0–36.0) |
| 22 | 36.0 (6.0–36.0)a | 12.0 (8–48.0) | 12.0 (6.0–48.0)a | |
| Cavg (ng/mL) | 22 | NC (195.8–420.2)e | 647.5 (507.5–936.7)b | 1,129.6 (562.2–1,848.0)a |
| AUC0–tau (ng · hr/mL) | 1 | 46,270 (33,189–59,554)d | 80,118 (48,002–126,523)b | 176,348 (110,707–244,735)a |
| 22 | NC (32,895–70,598)e | 108,784 (85,253–157,361)b | 189,765 (94,448–310,470)a | |
| AUC0–t (ng · hr/mL) | 1 | 22,597 (8,589–59,541)a | 50,858 (8,165–126,502) | 143,050 (40,492–244,701) |
| 22 | 28,312 (5,605–70,601)a | 74,866 (14,142–177,129) | 221,584 (109,128–377,037)a | |
| AUC0–inf (ng · hr/mL) | 1 | NC (36,660–79,453)e | 100,851 (70,616–148,047)c | 199,065 (122,982–272,938)a |
| t1/2 (hours) | 1 | 55.1 (40.3–82.6)d | 52.1 (46.9–61.6)b | 50.3 (45.2–56.4)a |
| 22 | NC (58.4–74.9)e | 64.27 (45.6–95.7)b | 77.4 (61.3–93.9)a | |
| CL/F (L/h) | 1 | NC (0.6–1.4)e | 1.49 (1.0–2.1)c | 1.5 (1.1–2.4)a |
| CLss/F (L/h) | 22 | NC (0.7–1.5)e | 1.38 (0.9–1.7)b | 1.6 (0.9–3.2)a |
| Vd/F (L) | 1 | NC (67.3–92.2)e | 112.0 (79.6–167.1)c | 109.4 (74.0–177.3)a |
| 22 | NC (65.6–128.0)e | 127.9 (93.4–242.9)b | 176.6 (101.9–383.9)a | |
| Ra | 22 | NC (1.2–1.2)f | 1.4 (1.1–2.2)b | 1.2 (0.8–1.5)b |
| Time dependence | 22 | NC (0.9–1.0)f | 1.2 (1.0–1.9)b | 1.0 (0.7–1.3)b |
The pk parameters summarized included peak serum concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration–time curve (AUC0–t) from time 0 to last quantifiable concentration, AUC extrapolated to infinity (AUC0–inf), terminal half-life (t1/2), apparent clearance (CL/F), and apparent volume of distribution (Vd/F where F represents bioavailability). In the MAD study, additional parameters were calculated: average concentration at steady state (Cavg), area under serum concentration-time curve from 0 hours to time tau (AUC0–tau) where tau is the dosing interval (168 hours), apparent total body clearance (steady state; CLss/F), and accumulation factor (Ra) calculated as the ratio of AUCtau at fourth dose compared to the first dose AUCtau. For all parameters except tmax the geometric mean (range) is presented; for tmax the median (range) is presented. NC = not calculated (due to insufficient data). N = 8, except for the following:
N = 7.
N = 6.
N = 5.
N = 4.
N = 3.
N = 2.
Dose-normalized Cmax and AUCtau at 50 mg (Figure 3 panels a and b) appeared to be slightly higher than for 150 and 300 mg, possibly due to the associated lower TV-1380 concentrations and the resulting missing data at this dose level. It was, however, concluded that exposure to TV-1380 increased in a dose-proportional manner both after single and multiple dosing.
Figure 3.
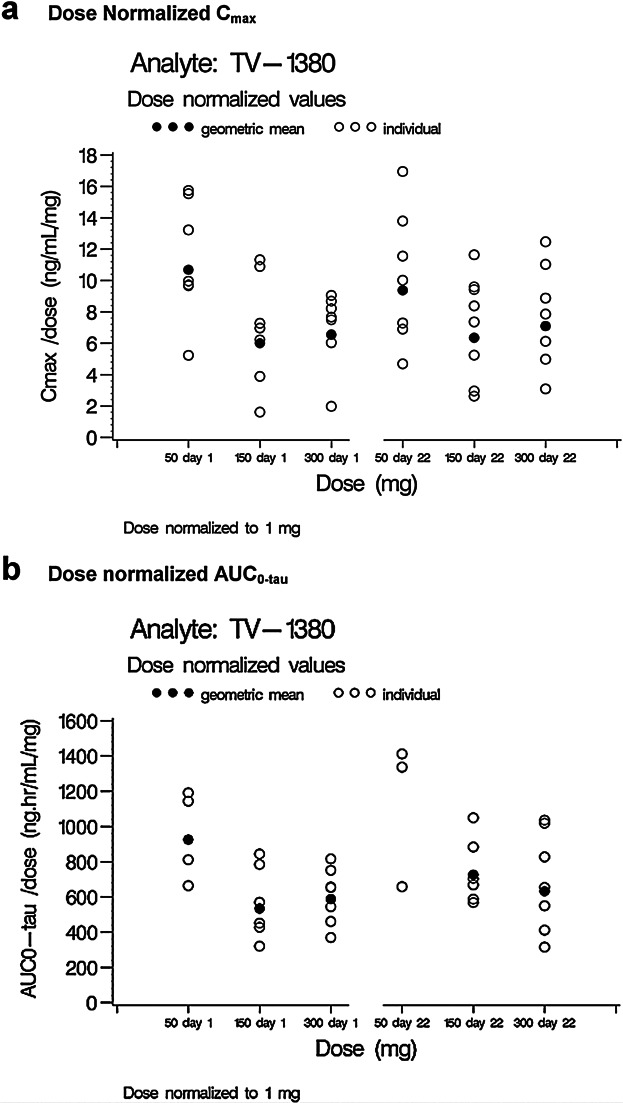
Individual and geometric mean dose-normalized TV-1380 Cmax and AUC0–tau on Days 1 and 22.
Dose-normalized exposure to TV-1380 was higher for male subjects than for female subjects. In the SAD study and in male subjects of the MAD study, TV-1380 exposure (expressed as Cmax, Cavg, Cmin, AUC0–tau, and AUC0–inf) decreased with increasing body weight. For the female subjects, a similar relationship was observed for Cmax only.
The AUC ratio AUC0–inf/AUC0–tau as a measure of time dependence could be calculated only for the 150 and 300 mg dose levels. These values were 1.19 and 1.02, respectively, indicating time-independent PK of TV-1380.
Very low residual levels of TV-1380 were detected in plasma 7 days after dosing indicating low ability for TV-1380 to accumulate in blood. This is further supported by accumulation ratios (Ra) of 1.36 and 1.16 for the 150 and 300 mg dose levels.
Pharmacodynamics
Ex vivo cocaine hydrolytic activity (kel) was determined for the 50 and 150 mg single dose levels and was directly correlated with TV-1380 plasma concentration in the sample (Figure 4). The median time after dosing when highest kel was seen was 12 hours postdose for both dose levels. Mean Emax (maximum observed hydrolytic activity) was approximately 2.8-fold higher for the 150 mg (kel = 0.0761/min) than for the 50 mg (kel = 0.0271/min) consistent with a dose proportional exposure and concentration-dependent Kel (Table5).
Figure 4.
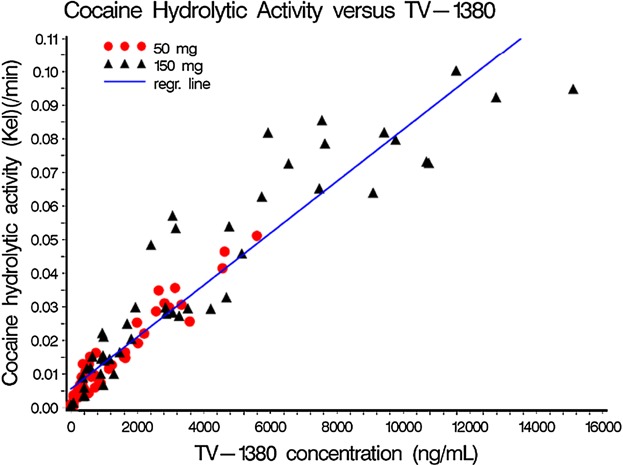
Ex vivo cocaine hydrolytic activity (kel) vs. TV-1380 serum concentrations per treatment.
Table 5.
Summary Statistics of Ex Vivo Cocaine Hydrolysis PD Parameters Following Single Doses Administered by IM Injection
| Parameter | 50 mg TV-1380 | 150 mg TV-1380 |
|---|---|---|
| Emax (/min) | 0.027 (0.01–0.05) | 0.076 (0.03–0.10) |
| tmax (hours) | 12.00 (12.00–24.00) | 12.00 (12.00–48.00) |
N = 6 for both dose levels. For Emax, maximum observed hydrolytic activity in kel, the mean (range) is presented; for tmax the time to Emax, the median (range) is presented.
Discussion
The studies presented herein evaluated the safety, tolerability, PK, and PD profiles of TV-1380, a novel-mutated BChE, after single and multiple intramuscular doses in healthy volunteers. Single and multiple doses of TV-1380 up to 300 mg were safe and well-tolerated with no apparent differences in injection site reactions across doses and compared to placebo. The most frequently reported TEAEs were injection site reactions and headaches in the SAD and headaches, dizziness, injection site reactions, and gastrointestinal disorders (diarrhea, dyspepsia, and nausea) in the MAD study.
TV-1380 is a large fusion protein designed to have high cocaine hydrolyse activity in blood, and is, therefore, unlikely to interact with the human Ether-à-go-go-Related Gene (hERG) potassium channel and lead to QT prolongation, which is a safety aspect that has to be addressed in development of new drugs.32 For this reason, regulators do not generally request the ICH-E14 mandated thorough QT (TQT) study for large therapeutic proteins such as monoclonal antibodies and fusion proteins.33 However, large therapeutic proteins are still expected to demonstrate a favorable cardiac safety profile (ie, no effect on the QT/QTc interval) in early phase clinical trials. The extensive ECG analysis and concentration-effect analysis of TV-1380 show no evidence that TV-1380 affects the heart rate, QT, or the QTcF intervals at the dose regimens tested.
The geometric mean values for t1/2 in the MAD study were between 50 and 77 hours (Day 1 and Day 22, respectively). This increase is most likely due to a more accurate estimation of the t1/2 after the last dose, due to the longer period during which PK samples were taken and during which serum concentrations were above LOQ.
Exposure to TV-1380 after single and multiple dosing was dose proportional at the 50–300 mg dose range. Multiple once-weekly doses of TV-1380 resulted in very limited accumulation of TV-1380, as expected considering the dosing interval of 7 days and the terminal elimination half live (t1/2) of approximately 2–3 days. The time dependence calculated was close to 1.0, indicating that the PK of TV-1380 was time independent.
In general, the shapes of the TV-1380 serum concentration-time profiles obtained in the multiple dose study were comparable to the profiles obtained in the single dose. However, when comparing exposure after single dose injection between the 2 studies, it is apparent that the overall exposure in the MAD study was 5–10-fold lower than in the SAD study. Geometric mean values for CL/F and Vz/F were approximately 5 times higher in the MAD study compared to SAD. In the MAD study, a different formulation for TV-1380 was used. One possible explanation for the observed difference could be a significantly lower bioavailability of TV-1380 in the formulation used in the MAD study. Another contributing factor to this difference could be the inclusion of female subjects in the multiple dose trial. Comparison of dose-normalized PK parameters by body weight for male and female subjects showed that at a given body weight, TV-1380 exposure for female subjects appeared to be lower than for male subjects. As a result, geometric mean values for TV-1380 serum concentrations could be expected to be lower in a study including approximately 50% female subjects. However, this sex effect on PK cannot be the only explanation for the observed difference. When considering male subjects only in the plots with the dose-normalized PK parameters vs. body weight, a considerable difference between the 2 studies was still evident. In addition, slightly different bioanalytical methods to quantitate TV-1380 were used. The range of detection of the 2 assays was almost identical; however 1 reagent was changed from a polyclonal detector to a monoclonal detector, and the minimum required dilution was increased from 1:13.3 to 1:40 to reduce matrix interference. Further evaluations are warranted.
Results from immunogenicity testing indicate a weak and transient immune response. Overall, based on the Ellman assays, the changes observed in BChE and AChE activities throughout the study are compatible with normal inter- and intrasubject variability and, therefore, there is no sign of development of antidrug antibodies with crossreactivity to endogenous enzymes.34,35
Ex vivo cocaine hydrolytic activity (kel) had a similar time course as TV-1380 serum concentrations and the effect increased with increasing TV-1380 serum concentrations. Further studies should be done in order to understand the therapeutic effect of the greater cocaine elimination rate in the presence of TV-1380.
In conclusion, TV-1380 is well tolerated and safe after multiple intramuscular injections and has a predictable pharmacokinetic profile. The cocaine hydrolase activity is directly correlated with blood levels. TV-1380, therefore, might offer a safe once-weekly pharmacological treatment for treating cocaine dependence.
Acknowledgments
These studies were financially supported by Teva Pharmaceuticals (Netanya Israel). The authors thank Teresa Nunes, MD, MSc (PRA Health Sciences, Zuidlaren, The Netherlands) and Pippa Loupe, PhD (Research and Scientific Affairs, Teva Pharmaceuticals, Kansas City, Missouri, USA) for assistance in manuscript development.
Declaration of Conflicting Interests
O. Cohen-Barak, PhD, A. Gross, M. Bassan, PhD, Y. Gilgun-Sherki, PhD, and O. Spiegelstein, PhD are employees of Teva Pharmaceuticals in Netanya Israel and S. Clark is an employee of Teva Biopharmaceuticals Rockville Maryland, USA. J. van de Wetering, MD, PhD, P. Schuilenga-Hut, PhD, Jacqueline Wildeman, MSc, and Judith Hettinga, PhD are employees of PRA Health Sciences (The Netherlands), and B. Mendzelevski, MD is an employee of Cardiac Safety Consultants Ltd (UK) and were contracted to perform these studies for Teva Pharmaceuticals.
Funding
This study, the manuscript preparation, and its publication were financially supported by Teva Pharmaceuticals, Inc., Netanya, Israel. Trial Registration numbers were EudraCT No. 2010-021561-76 and EudraCT No. 2011-004856-20.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. 4th ed. Arlington VA: American Psychiatric Publishing; 2000. p. 198. [Google Scholar]
- 2.Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 2006;47:330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor D, Parish D, Thompson L, Cavaliere M. Cocaine-induced prolongation of the QT interval. Emerg Med J. 2004;21:252–253. doi: 10.1136/emj.2002.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloner R, Rezkalla S. Cocaine and the heart. N Engl J Med. 2003;348:487–488. doi: 10.1056/NEJMp020174. [DOI] [PubMed] [Google Scholar]
- 5.Maceira M, Ripoll C, Cosin-Sales J. Long term effects of cocaine on the heart assessed by cardiovascular magnetic resonance at 3T. J Cardiov Magn Reson. 2014;16:26. doi: 10.1186/1532-429X-16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addict. Behav. 2003;28:1533–1554. doi: 10.1016/j.addbeh.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Cami J, Farre M. Drug addiction. N Engl J Med. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Le Moal M. Drug addiction, dysregulation of reward and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 10.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 11.Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57:685–687. [PubMed] [Google Scholar]
- 12.Wee S. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend. 2006;82:151–157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ungless M, Whistler J, Malenka C, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 14.Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81:396–406. doi: 10.1016/j.pbb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacol. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 16.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Role of the hypothalamic-pituitary-adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacol. 2003;168:177–183. doi: 10.1007/s00213-003-1391-4. [DOI] [PubMed] [Google Scholar]
- 17.Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA receptor positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacol. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Lingford-Hughes A, Nutt D. Neurobiology of addiction and implications for treatment. Br J Psychiat. 2003;182:97–100. doi: 10.1192/bjp.182.2.97. [DOI] [PubMed] [Google Scholar]
- 20.Shorter D, Kosten T. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2001;9:119. doi: 10.1186/1741-7015-9-119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Lawrence AJ. Drugs currently in Phase II clinical trial for cocaine addiction. Exp Opin Invest Drugs. 2014;23(8):1105–1122. doi: 10.1517/13543784.2014.915312. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Gao D, Yang W. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA. 2005;102(46):16656–16661. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brimijoin S, Gao Y, Anker JJ. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacol. 2008;33:2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact. 2008;175:83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindler CW, Justinova Z, Lafleur D. Modification of pharmacokinetic and abuse-related effects of cocaine by human-derived cocaine hydrolase in monkeys. Addict Biol. 2013;18(1):30–39. doi: 10.1111/j.1369-1600.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll ME, Gao Y, Brimijoin S, Anker JJ. Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacol. 2011;213:817–829. doi: 10.1007/s00213-010-2040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godin Shemesh-Darvish CSL, Sklair-Tavron L, Rosenstock M. TV-1380 (Albumin-Fused Mutated bche) Attenuates the Cardiovascular and Respiratory Effects Induced by Cocaine in Cynomolgus Monkeys. Gaithersburg, MD: AVANZA Laboratories; [Google Scholar]
- 28.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 29.Smith BP, Vandenhende FR, DeSante KA. Confidence interval criteria for assessment of dose proportionality. Pharmaceut Res. 2000;17:10. doi: 10.1023/a:1026451721686. [DOI] [PubMed] [Google Scholar]
- 30.Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drug. U.S. Department of Health and Human Services; 2005.
- 31.Frederica LS. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bein Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- 32.Vargas HM, Amouzadeh HR, Engwall MJ. Nonclinical strategy considerations for safety pharmacology: evaluation of biopharmaceuticals. Exp Opin Drug Saf. 2013;12(1):91–102. doi: 10.1517/14740338.2013.745851. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez I, Erdman A, Padhi D. Electrocardiographic assessment for therapeutic proteins-scientific discussion. Am Heart J. 2010;160:627–634. doi: 10.1016/j.ahj.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Sidell FR, Kaminski A. Temporal intrapersonal physiological variability of cholinesterase activity in human plasma and erythrocytes. Clin Chem. 1975;21:1961–1963. [PubMed] [Google Scholar]
- 35.Brock A. Inter- and intraindividual variations in plasma cholinesterase activity and substance concentration in employees of an organophosphorus insecticide factory. Br J Ind Med. 1991;48:562–567. doi: 10.1136/oem.48.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


