Abstract
Background
After surgical resection of pancreatic adenocarcinoma, most patients will develop recurrence within 2 years. Intense follow-up is often recommended; however, its impact on survival is unknown. Patient and clinician attitudes towards follow-up were qualitatively assessed along with the perceived benefits and challenges.
Methods
A semi-structured interview guide was developed. Purposive sampling identified patients who were in active surveillance or had developed recurrence. Clinicians involved in patient care were also interviewed. Interviews were conducted until saturation was reached and themes were derived using standard qualitative methods.
Results
A total of 15 patients and seven clinicians were interviewed. Patient themes included a limited understanding of disease prognosis, a desire for reassurance, a desire to know if and when recurrence occurred and minimal difficulties with follow-up. Clinician themes included expectation that patients are aware of the recurrence risk, a desire to provide reassurance, support for intense follow-up and perceived patient challenges in follow-up. Overall, the dominant theme was one of disconnect between patients and clinicians in the understanding of the disease and its prognosis.
Discussion
Patients have an intense need for reassurance and obtain this through follow-up appointments with their oncologists. Consequently, they express few difficulties with the process. Clinicians recognize this desire for reassurance. Patients' understanding and expectations contrast starkly with clinicians' perspectives regarding prognosis.
Introduction
Pancreatic ductal adenocarcinoma is the 12th most common cancer in Canada and the United States; however, it is the 4th leading cause of cancer death.1,2 While surgery remains the only potentially curative treatment, after resection the 5-year survival rates remain low at 5–27%.3–6 Unfortunately 80% of patients with pancreatic cancer will develop a recurrence within the first 2 years after a resection, for which there are no curative treatment options.7–9 Palliative chemotherapy improves survival in those with a good performance status; however, the median survival after recurrence only ranges from 6 to 12 months.10 Clinical trials are therefore strongly recommended and best supportive care remains a valid option10 for those who are not fit for chemotherapy. There is no compelling evidence to suggest that early detection of recurrence or early initiation of treatment impacts survival.
Some clinicians advocate for intensive surveillance after a resection of pancreatic cancer owing to the high risk of recurrence.10–12 The National Comprehensive Cancer Network (NCCN) recommends a history and physical examination, laboratory investigations (including Ca19-9) and computed tomography (CT) every 3 to 6 months for the first 2 years and then annually.10 No randomized controlled trials have evaluated the benefit of intensive follow-up and data from observational studies have been conflicting.11,13 A recent analysis suggests that a less intensive protocol with no routine imaging is the most cost-effective surveillance protocol.14 It should be noted that none of these studies evaluated the benefit of follow-up in the setting of clinical trials, where there may be other potential benefits to an intensive follow-up plan. However, given the lack of strong supporting evidence towards a follow-up protocol, current guidelines are poorly followed.15
Intensive surveillance carries the potential for harm. Patients undergoing follow-up testing exhibit significant anxiety and fear of cancer recurrence, with a substantial impact on quality of life.16–18 False-positive test results followed by additional invasive testing may perpetuate harm to the patients undergoing intensive surveillance.19 Beyond individual patient harm, there are resource consequences to intensive follow-up and surveillance.19–22
In spite of the high risk of recurrence, the intense follow-up strategies, and the potential consequences of such regimens, no qualitative study has previously investigated patient understanding and the impact of surveillance on patients with pancreatic cancer. This study sought to assess patient and clinician experiences, expectations and attitudes towards surveillance after resection of pancreatic and peri-ampullary cancer.
Patients and methods
Participants
Patients were recruited from a specialized Hepato-pancreatico-biliary clinic at the Odette Cancer Centre (OCC), a tertiary cancer centre at Sunnybrook Health Sciences Centre (SHSC), from November 2012 to March 2013. Consecutive eligible patients who had undergone surgical resection of pancreatic or peri-ampullary adenocarcinoma with curative intent and were undergoing surveillance or had developed recurrence were identified for inclusion in the study. No patient was enrolled into a clinical trial as none was being conducted during the study period. No standardized patient education material, support group or follow-up protocol was available during this period. In keeping with the qualitative research design, the sample size was expanded as necessary until redundancy on core issues, known as saturation, was observed.23–26 Informed consent was obtained from each participant prior to the interview. No additional educational materials were given to patients beyond that required to obtain informed consent. This study was approved by the SHSC Research Ethics Board.
Data collection
Each patient completed a questionnaire to supply demographic and general follow-up information. Pathological information was obtained through retrospective chart review.
Qualitative methodology and Content Analysis (CA) directed the generation of the interview guide, data collection and data analysis. Data collection was accomplished through private semi-structured interviews to encourage honest opinions and allow for discussion of sensitive issues.23 Patients were encouraged to bring a support person, such as a family member; however, the family member was not interviewed. A single trained researcher (E.C.), who was not involved in the clinical care of these patients, conducted all the interviews using a semi-structured interview guide. The interview guide was piloted during the first three interviews.23 The interview guide was adjusted to ensure all areas of interest were addressed. Interviews were conducted once per participant.
All clinicians in radiation oncology, medical oncology and surgical oncology involved in treating pancreatic adenocarcinoma, except for those directly involved in this study, were invited to participate. Semi-structured interviews were conducted with the clinicians by a single interviewer (E.C.) using a different interview guide.
The interviewer made notes concerning important interactions, mood or tone of responses, and any other non-verbal behaviour for both sets of interviews.23
Statistical analysis
Interviews were transcribed verbatim and CA was used to analyse the data. This is an iterative approach which involves multiple readings of the transcripts; simultaneous data collection and analysis generates a coding schema reflecting unique ideas.25–28 Constant comparative analysis of the schema allowed similar concepts to be grouped together into larger themes driving the research towards an overarching theory or theme construction.24–28 Interviews were coded independently by three investigators (E.C., R.D. and F.C.W.), findings were discussed with the entire research team and consensus of interpretation was achieved. One dominant theme or overarching theory was identified. Descriptive statistical analysis of participant demographics and clinical characteristics was performed.
Results
Patient characteristics
Seventeen patients were invited to participate in the study; however, one patient was excluded after the interview owing to a different histological presentation (intraductal papillary mucinous neoplasm) and one patient declined to participate as a result of physical discomfort. Fifteen patients were included in the analysis. The median time from pancreatic cancer resection to the interview was 247 days (range 41 to 1140). Patient demographic and follow-up data are included in Table 1. Three patients had been diagnosed with recurrence whereas the remaining 12 had no evidence of disease. Patient pathology and treatment data are included in Table 2.
Table 1.
Patient data
| Patients N = 15 (n) | |
|---|---|
| Age [median (range)] | |
| 64 (43–84) | |
| Gender | |
| M | 8 |
| F | 7 |
| Ethnicity | |
| Caucasian | 13 |
| Korean | 1 |
| West Indian | 1 |
| Partner status | |
| Married | 11 |
| Separated | 1 |
| Widowed | 2 |
| Never married | 1 |
| Occupation | |
| Retired | 13 |
| Working | 2 |
| Highest educational level | |
| High School | 7 |
| Undergraduate/College | 6 |
| Graduate | 2 |
| Distance travelled to cancer centre (km) | |
| <25 | 8 |
| 26–100 | 5 |
| >100 + | 2 |
| Accompaniment to appointments | |
| Yes (family or friend) | 13 |
| No | 2 |
| Frequency of seeing oncologist(s) | |
| Every month | 7 |
| Every 3 months | 6 |
| Every 6 months | 2 |
| CT scan frequency | |
| Every month | 3 |
| Every 3 months | 5 |
| Every 6 months | 4 |
| Annually | 3 |
CT, computed tomography.
Table 2.
Patient pathological and treatment data
| Patients N = 15 (n) | |
|---|---|
| Site of primary | |
| Pancreatic ductal | 10 |
| Peri-ampullary | 5 |
| TNM stage | |
| T1 N0 | 1 |
| T2 N1 | 2 |
| T3 N0 | 2 |
| T3 N1 | 9 |
| T4 N1 | 1 |
| Recurrence | |
| Yes | 3 |
| No | 12 |
| Chemotherapy received | |
| Yes | 14 |
| No | 1 |
| Radiation received | |
| Yes | 10 |
| No | 5 |
Patient themes (Table 3)
Table 3.
Patient themes and quotes
| Theme | Quote |
|---|---|
| 1 Limited understanding of prognosis | ‘They want to make sure there is no recurrence and that everything is working the way it should be […] and making sure I'm around for another ten, twenty years!’ (15) |
| ‘Hopefully the chemo and radiation will look after that [positive margin & lymph nodes] and get healthy for another 30 years.’ (11) | |
| 2 Reassurance through follow-up | ‘It's that comfortable level of just seeing them all and having each one in their own way tell me that everything's fine’ (6) |
| 3 Desire to know if or when recurrence occurred | ‘We're very educated individuals to be able to understand the data so it's better to know than not to know. So any information that may be available should be available to us.’ (6) |
| 4 Minimal difficulties with follow-up protocol | ‘Because it was like a walk in the park like, okay, I come in and everything's fine and I get to go away for three months.’ (14) |
Median interview times for patients were 28 min (range 14 min to 47 min). A family member was present for ten interviews. Saturation was reached after twelve interviews.
Subtheme 1: limited understanding of disease prognosis
Most patients had a vague understanding of the disease and its prognosis. Some patients understood that it was an ‘aggressive’ cancer. Patients viewed themselves as being cancer free after resection and most patients with advanced disease did not recognize their poor prognosis. ‘Hopefully the chemo and radiation will look after that [positive margin and lymph nodes] and get healthy for another 30 years’. The effectiveness of treatments was also overestimated. ‘I would have to do chemo because they see lesions on my liver and they want to get rid of them before they become tumors’. Patients with recurrence in particular lacked understanding, often expecting to survive 10 or more years without symptoms. ‘I did go to the hospice and I will end up there some year, maybe ten years’.
Subtheme 2: reassurance through follow-up
Patients experienced reassurance through attending follow-up appointments and hearing from their oncologists that they did not have recurrence, including the only two patients who acknowledged that follow-up was stressful. Many patients expressed the importance of timely access to clinicians or nurses at the cancer centre in order to have questions or concerns addressed between follow-up appointments. Patients did not convey anything specific that was reassuring, but generally conveyed ‘that comfortable level of just seeing them’ was what was desired. When ‘he says in three months time, I'm going to see you again … it's giving you hope that everything is going to be okay’.
Subtheme 3: desire to know if or when recurrence occurred
Patients stated that they would want to know if or when recurrence occurred. Some patients felt that early detection of recurrence through follow-up would allow for earlier treatment, which they felt would improve their survival. ‘If something's going to show up, get it detected earlier, get it treated’. Others still wished to know if a recurrence developed in spite of not wanting to receive further treatment. Despite this desire, few patients reported knowing or having discussed with their oncologists what the treatment options would be if a recurrence were detected.
Subtheme 4: minimal difficulties with follow-up protocol
The majority of patients had a positive follow-up experience. This was expressed through comments such as: ‘It's been great’, ‘It's nice to see them’ and ‘extremely positive’. ‘It's the people, honest! … Going there is healing’. The frequency of follow-up and travel were not major challenges for most patients as most were retired or could easily take time off work. Minor challenges identified included wait times at the clinic and parking costs. Anxiety as a result of the overall follow-up process was not a major difficulty reported by patients. However, patients did not note experiencing anxiety with CT scans, a few patients identified that CT scans were the most difficult part of the follow-up process. In some cases this was clearly because of physical discomfort, not anxiety, for example, ‘I really hate them [CT scan] and it's because I have great difficulty drinking’. Only one patient questioned the utility of a CT scan detecting a recurrence if she was asymptomatic.
Clinician themes (Table 4)
Table 4.
Clinician themes and quotes
| Theme | Quote |
|---|---|
| 1 Think patients are aware of recurrence risk | ‘Patients are very intelligent … they know. They will say, “Doctor, you know, I want you to make sure that I don't get it again. And if I get it, I want you to do something and so forth.” So they are very much aware.’ (S2) |
| 2 Intense follow-up despite lack of supporting evidence | ‘Usually after chemo, we initially plan to scan them once every 6 months and after 1–2 years, we usually scan them once a year, even if there's no evidence supporting that.’ (M2) |
| 3 Desire to provide reassurance to patients | ‘They love to come back … unless unfortunately … in pancreatic cancer, there's a high, high rate of recurrence, and when that happens, it's a sad visit. But other than that, however many times they come back, and you give them good news that there's no recurrence, they're quite happy.’ (S2) |
| 4 Secondary goals for surveillance | ‘I'd rather them know and know at a time where they're functional rather than symptomatic so that while they're functional, they have a better opportunity to see who they need to see, close their affairs, get things in order, or go on that cruise that they needed to do.’ (S1) |
| 5 Challenges in follow-up | ‘There's a huge anxiety that many patients tell me that they don't sleep for a week or two before a test […] so then they have the test and […] it's a great relief after that, but they've built up all this anxiety around the test and at the end of the day, the test doesn't really change the day that they're going to die.’ (M1) |
Seven of the nine eligible clinicians were interviewed including three medical oncologists, two surgical oncologists and two radiation oncologists. The median interview times for clinicians were 19 min (range 11–32). Saturation was reached with seven interviews.
Subtheme 1: think patients are aware of recurrence risk
All the clinicians interviewed stated that they discuss the risk of recurrence with patients before or after resection so that patients can understand their treatment options and prognosis. ‘We know that almost all of them will recur … It'd be wrong to have patients going on thinking “I'm cured. There's never going to be a problem again” ’. Some clinicians felt that patients implicitly knew that they were looking for recurrence with the follow-up protocol, and consequently did not re-discuss the risk of recurrence at follow-up visits. ‘They are well aware of their recurrence risk … most of them are already very aware that it's pretty grave disease’. Discussion of possible treatment options for recurrent disease happens rarely during the follow-up period prior to the detection of recurrence.
Subtheme 2: intense follow-up in spite of lack of supporting evidence
Almost all of the clinicians conducted intense follow-up including clinical appointments every 3 to 6 months and CT scans and blood tests every 6 months for the first 2 years. ‘Usually after chemo, we initially plan to scan them once every 6 months and after 1–2 years, we usually scan them once a year, even if there's no evidence supporting that’. Most acknowledged the lack of evidence to support this approach with statements like ‘we don't know if surveillance makes a difference’. In spite of this, many clinicians believe there are benefits with early palliative therapy before functional decline when a patient would not be able to receive treatment. Others described their follow-up protocol as a routine, ‘it's just what I've been doing in practice’ or ‘It's what we do. I'm not sure how else I would do it [follow-up] otherwise’. Only one clinician reported following patients clinically and tried to ‘avoid doing blood work or scans unless clinically necessary’.
Subtheme 3: desire to provide reassurance to patients
Most clinicians felt that patients want to have follow-up for reassurance. One described it as ‘that constant reassurance that somebody's with them and somebody's making a plan for them’. The clinicians recognized that follow-up could bring ‘peace of mind’ and provide psychosocial benefits. Some clinicians expected that patients would be resistant to the proposal of decreased follow-up.
Subtheme 4: secondary goals for surveillance
Clinicians believe that follow-up can provide additional benefits for patients beyond early detection and treatment of recurrence. Post-treatment symptoms can be identified and managed. For patients who develop recurrence, it provides an opportunity for end-of-life planning prior to functional decline. It can ‘allow the patient to know as early as possible what their fate will be’. Other clinicians desire to maintain the ‘doctor–patient relationship’ through the follow-up process.
Subtheme 5: challenges in follow-up
All clinicians noted significant challenges for patients caused by the follow-up process. The majority of clinicians identified that their patients get anxious surrounding follow-up, especially with CT scans. ‘There's a lot of anxiety surrounding the whole issue of follow-up and waiting’. ‘Many patients tell me that they don't sleep for a week or two before a test’. Some clinicians also identified the potential harms of CT scans. Others noted logistical problems for patients due to travel and time away from work as well as system challenges due to resource allocation. ‘If you do a lot of follow-up scans, maybe the next patient who was jaundice … you can't get time on the CT scan’.
Dominant theme or overarching theory: ‘disconnect between patients and clinicians in the understanding of the disease and its prognosis’
The dominant theme identified in this study is disconnect between patients and clinicians in their understanding of the disease, its prognosis and the patient's experience with follow-up. Clinicians feel patients understand their risk of recurrence and prognosis, while patients demonstrate unrealistic expectations of therapy and survival in spite of poor pathological staging or even recurrence of disease. Patients have an intense desire for reassurance. In response, clinicians offer intensive surveillance in spite of limited evidence for its benefit and the acknowledgement that follow-up is anxiety inducing and difficult for patients. Despite these concerns, patients report minimal difficulties with the follow-up process and a desire to continue their follow-up with oncologists. (See Fig. 1).
Figure 1.
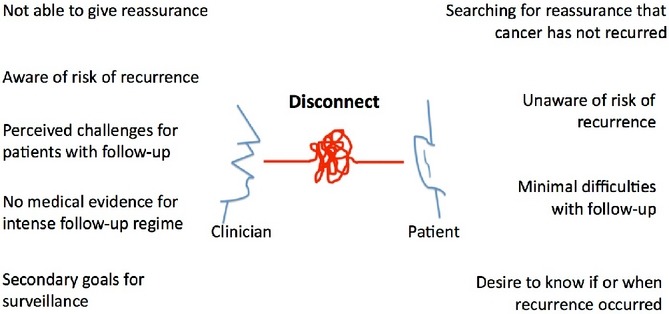
Overarching theory for patient and clinician views of follow-up after pancreatic cancer surgery
Discussion
This is, to our knowledge, the first qualitative study investigating patient and clinician experiences with surveillance after resection of pancreatic and peri-ampullary cancer. These malignancies are unique in their high propensity for recurrence, overall poor survival and non-curative treatment when recurrence develops. The recommended follow-up regimen for these patients remains one of the most intense of all malignancies. In spite of the demands of surveillance, the patients in our study noted few difficulties with the follow-up process. Patients express an intense desire for reassurance and derive this from their follow-up, despite additional anxiety related to the investigations. This is consistent with the literature from other cancer sites.29–35 The clinicians interviewed in our study recognized this need for reassurance in their patients and provide intensive follow-up in response; however, they do acknowledge the limited evidence to support the benefits of surveillance in this patient population and the potential harms of surveillance. The clinicians also suggest follow-up has secondary benefits including maintaining the doctor–patient relationship.
Patients and clinicians in our study exhibited a major disconnect in their respective understanding of the risk of recurrence and prognosis after resection for pancreatic cancer. Both groups recognized that discussions surrounding risk of recurrence had occurred; however, patients, especially those with poor prognostic factors, had unrealistic expectations of the benefits of treatment. For example, one patient with recurrent disease expected to live another ten years and another patient with a positive margin and nodal involvement expected chemotherapy and radiation to ‘look after that’ and be ‘healthy for another thirty years’. Our study was unable to determine if there exists a difference in expectations based on different stages of disease; however, it did appear that patients with a poorer prognosis, including recurrent disease, had a worse understanding of their prognosis. While surprising, this is in keeping with other studies that have shown poor patient understanding of cancer prognosis.36–39 Other studies have revealed that patients note improved satisfaction and less depression or anxiety when their desire for prognostic information is met and when their interactions with clinicians are perceived as open and honest.36,38,40 Notably, no studies suggest patient outcomes are worse after being informed of a poor prognosis.36,38,40 The clinicians in our study either felt patients implicitly knew the aggressive biology of pancreatic cancer or had discussed prognosis but avoided focusing on the recurrence rate. Studies have noted that physicians struggle with individualizing the information for each patient and ensuring patients understand their prognosis while still providing hope.36,41
Our study is limited in that it was confined to one cancer centre; it is possible that patients at other cancer centres understand their prognosis better and have more realistic expectations. However, our centre includes four hepatopancreaticobiliary surgeons, five gastrointestinal medical oncologists and four gastrointestinal radiation oncologists, all of whom treat patients with pancreatic cancer. Thus, patients were treated by a heterogeneous group of experienced clinicians who reported remarkably similar misunderstandings and unrealistic expectations. It is possible that patients on clinical trials may have a different understanding. The results of this study are likely transferable to other centres with similar patient populations; however, studies from other centres, especially those with patients of different cultural and socioeconomic backgrounds as well as those on clinical trials, would be valuable.
A second limitation was the difficulty in recruiting patients with recurrent disease due to physical discomfort, functional decline and missed clinic appointments. Thus, the majority of our findings were identified by patients undergoing active surveillance yet without evidence of recurrence. It is possible that perspectives on follow-up would change after recurrence, although we did not observe a difference in the limited number of patients who we interviewed with recurrence.
Interviews were not designed to identify barriers to effective patient–clinician communication or reasons for unrealistic expectations. It is possible that clinicians included in this study avoided discussing prognosis with the patients or presented unclear information, although the heterogeneity and experience of the clinicians included makes this unlikely. Clinician hopefulness or a desire to provide reassurance may have affected the patients' understanding of their prognosis. It is also possible that patients seek reassurance so strongly that they consciously or subconsciously perceived the information presented differently. However, as this study did not directly observe the patient–clinician interactions, it is not possible to determine the cause for this disconnect. It is known that patients receive an overwhelming burden of information during the early phases of their cancer journey and do not retain all of it.42–44 Further research is needed to identify patient information needs and optimal techniques of presenting information, such as repetitive education methods, particularly surrounding prognosis. Studies should also explore barriers and facilitators to effective patient–clinician communication at this vulnerable time in a patient's cancer journey.
Patients desire active surveillance after resection of pancreatic cancer and clinicians generally support intense follow-up. However, patients and clinicians express discordant goals motivating the follow-up process. Patients seek follow-up to obtain reassurance that the cancer has not returned, whereas clinicians provide surveillance to maintain the doctor–patient relationship as well as facilitate further treatments, palliative care and end-of-life planning. There exists a conflict between the patient's desire for reassurance and clinicians knowledge of prognosis. In many cases, patients exhibit unrealistic expectations of risk of recurrence and treatment benefits. Efforts to improve patient–physician communication at this particularly vulnerable point in the cancer journey would be valuable. Future studies should identify what information patients wish to know and how they wish to receive this information, which we are currently investigating, as well as clarify whether the addition of imaging to the follow-up regimen, as is currently recommended, is beneficial.
Conflicts of interest
None declared.
References
- Canadian Cancer Society's Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2012. Toronto, ON: Canadian Cancer Society; 2012. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- Katz MHG, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;298:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Winter JM, Brennan MF, Tang LH, D'Angelica MI, DeMatteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck A, Sergeant G, Ectors E, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kleef J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245:566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JM, Morton CA, Al-Saadi S, Villadolid D, Cooper J, Bowers C, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg. 2010;76:480–485. [PubMed] [Google Scholar]
- Tempero MA, Arnoletti JP, Behrman S, Ben-Josef E, Benson AB, III, Berlin JD, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng CWD, Fleming JB, Lee JE, Wang X, Pisters PWT, Vauthey JN, et al. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB. 2012;14:365–372. doi: 10.1111/j.1477-2574.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascinu S, Falconi M, Valentini V, Jelic S. Pancreatic cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):55–58. doi: 10.1093/annonc/mdq165. [DOI] [PubMed] [Google Scholar]
- Witkowski ER, Smith JK, Ragulin-Coyne E, Ng SC, Shah SA, Tseng JF. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a national study. J Gastrointest Surg. 2012;16:121–128. doi: 10.1007/s11605-011-1699-z. [DOI] [PubMed] [Google Scholar]
- Tzeng CWD, Abbott DE, Cantor SB, Fleming JB, Lee JE, Pisters PWT, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol. 2013;20:2197–2203. doi: 10.1245/s10434-013-2889-6. [DOI] [PubMed] [Google Scholar]
- Sheffield KM, Crowell KT, Lin YL, Djukom C, Goodwin JS, Riall TS. Surveillance of pancreatic cancer patients after surgical resection. Ann Surg Oncol. 2012;19:1670–1677. doi: 10.1245/s10434-011-2152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Charlson ME, Schenkein E, Wells MT, Furman RR, Elstrom R, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21:2262–2266. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology. 2006;15:306–320. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- Petzel MQB, Parker NH, Valentine AD, Simard S, Nogueras-Gonzales GM, Lee JE, et al. Fear of cancer recurrence after curative pancreatectomy: a cross-sectional study in survivors of pancreatic and periampullary tumors. Ann Surg Oncol. 2012;19:4078–4084. doi: 10.1245/s10434-012-2566-1. [DOI] [PubMed] [Google Scholar]
- Edelman MJ, Meyers FJ, Siegel D. The utility of follow-up testing after curative cancer therapy: a critical review and economic analysis. J Gen Intern Med. 1997;12:318–331. doi: 10.1046/j.1525-1497.1997.012005318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364:2060–2065. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper LE, Smith TJ, Raghavan D, Blayney DW, Ganz PA, Mulvey TM, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1716–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- Guy JGP, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriquez JL, et al. Economic burden of cancer survivorship among adults in United States. J Clin Oncol. 2013;31:3749–3757. doi: 10.1200/JCO.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Miller FA. An introduction to qualitative research. In: Harvey BJ, Lang ES, Frank JR, editors. The Research Guide: A Primer for Residents, Other Health Care Trainees, and Practitioners. Ottawa, ON: Royal College of Physicians and Surgeons of Canada; 2011. pp. 119–130. Chapter 16. [Google Scholar]
- McCann TV, Clark E. Grounded theory in nursing research: part 1 – methodology. Nurse Res. 2003;11:7–18. doi: 10.7748/nr2004.01.11.2.7.c5918. [DOI] [PubMed] [Google Scholar]
- Lingard L, Albert M, Levinson W. Grounded theory, mixed methods, and action research. BMJ. 2008;337:a567. doi: 10.1136/bmj.39602.690162.47. . doi: 10.1136/bmj.39602.690162.47. [DOI] [PubMed] [Google Scholar]
- Cho JY, Lee EH. Reducing confusion about grounded theory and qualitative content analysis: similarities and differences. Qual Rep. 2014;19:1–20. [Google Scholar]
- Charmaz K. Constructing Grounded Theory. Thousand Oaks, CA: SAGE Publications Ltd; 2009. [Google Scholar]
- Charmaz K. Grounded theory in the 21st century. In: Denzin NK, Lincoln YS, editors. Handbook of Qualitative Research. Thousand Oaks, CA: SAGE Publications Ltd; 2005. pp. 507–536. [Google Scholar]
- Kantsiper M, McDonald EL, Geller G, Shockney L, Snyder C, Wolff AC. Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med. 2009;24:459–466. doi: 10.1007/s11606-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muss HB, Tell GS, Case LD, Robertson P, Atwell BM. Perceptions of follow-up care in women with breast cancer. Am J Clin Oncol (CCT) 1991;14:55–59. doi: 10.1097/00000421-199102000-00013. [DOI] [PubMed] [Google Scholar]
- Kelly L, Caldwell K, Henshaw L. Involving users in service planning: a focus group approach. Eur J Oncol Nurs. 2006;10:283–293. doi: 10.1016/j.ejon.2005.12.008. [DOI] [PubMed] [Google Scholar]
- O'Brien R, Rose PW, Campbell C, Weller D, Neal RD, Wilkinson C, et al. Experiences of follow-up after treatment in patients with prostate cancer: a qualitative study. BJU Int. 2009;106:998–1003. doi: 10.1111/j.1464-410X.2010.09292.x. [DOI] [PubMed] [Google Scholar]
- Lydon A, Beaver K, Newbury C, Wray J. Routine follow-up after treatment for ovarian cancer in the United Kingdom (UK); Patient and health professional views. Eur J Oncol Nurs. 2009;13:336–343. doi: 10.1016/j.ejon.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Blom RL, Nieuwkerk PT, van Heijl M, Bindels P, Kinklenbijl JH, Sprangers MA, et al. Patient preferences in screening for recurrent disease after potentially curative esophagectomy. Dig Surg. 2012;29:206–212. doi: 10.1159/000338256. [DOI] [PubMed] [Google Scholar]
- Papagrigoriadis S, Heyman B. Patients' views on follow up of colorectal cancer: implications for risk communication and decision making. Postgrad Med J. 2003;79:403–407. doi: 10.1136/pmj.79.933.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerty RG, Butow PN, Ellis PM, Dimitry S, Tattersall MHN. Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol. 2005;16:1005–1053. doi: 10.1093/annonc/mdi211. [DOI] [PubMed] [Google Scholar]
- Mitera G, Zhang L, Sahgal A, Barnes E, Tsao M, Danjoux C, et al. A survey of expectations and understanding of palliative radiotherapy from patients with advanced cancer. Clin Oncol. 2012;24:134–138. doi: 10.1016/j.clon.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Dow LA, Virago EA, Khatcheressian J, Matsuyama R, Lyckholm LJ. A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer. J Support Oncol. 2011;9:79–86. doi: 10.1016/j.suponc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Hope and Prognostic Disclosure. J Clin Oncol. 2007;25:5636–5642. doi: 10.1200/JCO.2007.12.6110. [DOI] [PubMed] [Google Scholar]
- Elit L, Charles C, Gafni A, Ranford J, Gold ST, Gold I. Walking a tightrope: oncologists' perspective on providing information to women with recurrent ovarian cancer during the medical encounter. Support Care Cancer. 2012;20:2327–2333. doi: 10.1007/s00520-011-1344-0. [DOI] [PubMed] [Google Scholar]
- Gabrijel S, Grize L, Helfenstein E, Brutsche M, Grossman P, Tamm M, et al. Receiving the diagnosis of lung cancer: patient recall of information and satisfaction with physician communication. J Clin Oncol. 2008;26:297–302. doi: 10.1200/JCO.2007.13.0609. [DOI] [PubMed] [Google Scholar]
- Kumaravel V, Heald B, Lopez R, Hasson H, Schneider K, Burke C. Patients do not recall important details about polyps required for colorectal cancer prevention. Clin Gastroenterol Hepatol. 2013;11:543–547. doi: 10.1016/j.cgh.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Scheer AS, O'Connor AM, Chan BPK, Moloo H, Poulin EC, Mamazza J, et al. The myth of informed consent in rectal cancer surgery: what do patients retain? Dis Colon Rectum. 2012;55:970–975. doi: 10.1097/DCR.0b013e31825f2479. [DOI] [PubMed] [Google Scholar]


