Abstract
Background
Studies of pancreaticoduodenectomy (PD) frequently overlook diagnosis as a variable when evaluating postoperative outcomes or generically group patients according to whether they have ‘benign’ or ‘malignant’ disease. Large multicentre studies comparing postoperative outcomes in PD stratified by diagnosis are lacking. The present study was conducted to verify the hypothesis that postoperative morbidity and length of stay (LoS) following PD vary by diagnosis and that patients may be grouped into low- and high-risk categories.
Methods
The database of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) was reviewed for all PDs performed during 2005–2011. Diagnoses were identified using ICD-9 codes and grouped based on the incidence of major morbidity. Univariate and multivariate analyses were utilized to assess the impact of diagnosis on PD outcomes.
Results
Of 5537 patients, those with pancreas cancer (n = 3173) and chronic pancreatitis (n = 485) experienced similar incidences of major morbidity (P = 0.95) and were grouped as having low-risk diagnoses. Patients with bile duct and ampullary (n = 1181), duodenal (n = 558) and neuroendocrine (n = 140) disease experienced similar levels of major morbidity (P = 0.78) and were grouped as having high-risk diagnoses. A high-risk diagnosis was identified as an independent risk factor for a prolonged LoS [odds ratio (OR) 1.67], organ space infection (OR 2.57), sepsis or septic shock (OR 1.83), and major morbidity (OR 1.70). Diagnosis did not predict readmission.
Conclusions
The high-risk diagnosis is independently associated with postoperative morbidity and prolonged LoS. Patients with PD should be stratified by diagnosis to more accurately reflect their risk for postoperative complications and the complexity of care they will require.
Introduction
Although significant advancements in the perioperative care of patients undergoing pancreaticoduodenectomy (PD) have resulted in a reduction in postoperative mortality, overall postoperative morbidity remains high and relatively unchanged.1–6 Several studies have established soft gland texture and small duct size as consistent predictors of complications after PD.7–12 Importantly, both of these parameters are associated with considerable subjectivity as a result of variability in their definitions and measurement. However, the underlying diagnosis prompting surgical intervention is a non-modifiable, definitive parameter that appears to have direct association with gland texture and duct size. Chronic pancreatitis and pancreatic cancer are often characterized by firmer glands and pancreatic ductal dilation caused by fibrosis, oedema and ductal obstruction, by contrast with other diagnoses, such as duodenal, biliary and pancreatic cystic lesions. Classification by diagnosis is therefore intriguing as a potential indicator of risk for the occurrence of postoperative complications. Currently, studies of postoperative outcomes following PD, including those using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, frequently overlook diagnosis or group patients into categories according to whether they have ‘benign’ or ‘malignant’ disease, rather than performing stratification and analyses by individual diagnoses.13–15 Grouping patients into ‘benign’ or ‘malignant’ categories may be overly simple and may lead to the under- or overestimation of the true morbidity associated with a given diagnosis within each category. The averaging effect within each of the ‘benign’ and ‘malignant’ disease groups may encourage the false impression that no significant differences in morbidity exist between the two categories. This is because the categories of ‘benign’ and ‘malignant’ both include diagnoses classically associated with gland textures and duct sizes at each end of the spectrum. The present study was conducted with the aim of developing a more clinically relevant approach towards the identification of groups at ‘high’ and ‘low’ risk, respectively, for postoperative complications based on underlying diagnosis. Currently, several small studies have reported a relationship between diagnosis and outcomes following PD.7,16–21 However, no large multicentre study has yet reported on 30-day postoperative outcomes following PD by diagnosis.
Because of the associations between pathological diagnosis and pancreatic texture and duct size, and therefore risk for complications, it can be inferred that patients undergoing PD can be easily stratified into high- and low-risk groups based on diagnosis alone without further assessment or calculation. This type of stratification is clinically useful as it can be applied at any time in the clinical course of the patient. Therefore, the present study was based on the hypotheses that postoperative morbidity and length of stay (LoS) following PD vary by diagnosis and that patients may be grouped into low- and high-risk categories.
Materials and methods
Data source
The ACS-NSQIP database is a prospectively maintained, risk-adjusted outcomes database of 135 variables including 30-day morbidity and mortality in patients undergoing surgical procedures. Specific details regarding data collection, outcome variable definitions, quality control and personnel training are available on the NSQIP website.22
Design
The 2005–2011 ACS-NSQIP Participant Use Data File was reviewed for all PDs performed between 2005 and 2011. Patients were identified by Current Procedural Terminology (CPT) codes 48150 and 48153.23 Diagnoses were identified and stratified using International Classification of Disease, 9th Revision (ICD-9) codes for: pancreas cancer (157.0, 157.1); chronic pancreatitis (577.1, 577.8); duodenal neoplasm (152, 152.0, 211.2, 230.7, 235.2); neuroendocrine tumour (157.4, 209.3, 209.30, 209.69, 211.7), and bile duct and ampullary neoplasm (156.1, 156.2, 156.8, 156.9, 211.5, 230.8, 235.3).24
Exclusions
In an attempt to achieve mutually exclusive groups and minimize any ambiguity of diagnosis, data for patients who were identified using the aforementioned CPT codes but whose disease did not fit these ICD-9 codes were excluded. There are no ICD-9 codes that specifically identify intraductal papillary mucinous neoplasm (IPMN), serous cystic neoplasm or mucinous cystic neoplasm. Therefore, no diagnosis group exclusive to IPMN, serous cystic neoplasm and mucinous cystic neoplasm could be established. Patients with acute pancreatitis, PD without pancreaticojejunostomy, or PD with autologous islet cell transplant were excluded. Patients with any of the following preoperative conditions were also excluded: emergent surgery; American Society of Anesthesiologists (ASA) class 5 status; ventilator dependence; severe sepsis or septic shock; pneumonia; an open wound; wound infection; acute renal failure; coma; receipt of >4 units of red blood cells in the prior 72 h; renal failure; dialysis, and disseminated cancer. In an attempt to reduce confounding and to create a sample population that best represents the true risks associated with PD, patients undergoing concurrent operations not typically performed with this procedure were also excluded. Excluded concurrent operations included hepatectomy, colectomy, hysterectomy, splenectomy, hernia repair, vena cava resection, nephrectomy, etc. Patients were not excluded for common concomitant operations, such as feeding tube insertion, vein resection or reconstruction, vein harvest, central line placement and laparoscopy.
Demographic characteristics
Patient demographic characteristics identified included: gender; race/ethnicity; age; year of operation; discharge destination; body mass index (BMI); diabetes; current smoking within 1 year; dyspnoea; functional status; history of severe chronic obstructive pulmonary disease (COPD); congestive heart failure; history of myocardial infarction within the 6 months prior to operation; previous percutaneous coronary intervention; previous cardiac surgery; history of angina within the 1 month prior to surgery; hypertension requiring medication; steroid use for any chronic condition; weight loss of >10% of body weight in the previous 6 months; chemotherapy within the 30 days prior to operation; radiotherapy for malignancy within the 90 days prior to operation; preoperative creatinine, bilirubin, albumin, alkaline phosphatase, haematocrit and international normalized ratio (INR) values; wound classification, and ASA class.
Outcome variables
Patient outcome variables studied included: total operative time; 30-day mortality; hospital LoS; superficial surgical site infection (SSI); deep incisional SSI; organ space SSI; any SSI; wound disruption; pneumonia; unplanned intubation; pulmonary embolism; ventilator requirement for >48 h; progressive renal insufficiency; acute renal failure; urinary tract infection (UTI); stroke or cerebral vascular accident (CVA) with neurological deficit; cardiac arrest requiring cardiopulmonary resuscitation (CPR); myocardial infarction; deep vein thrombosis (DVT); sepsis or septic shock; reoperation; unplanned readmission; number of complications; overall morbidity, and total relative value units (RVUs). For the purpose of this study, a prolonged LoS was indicated by discharge on or after postoperative day (PoD) 14, as previously defined.25 Transfusion was not investigated as the outcome variable reported was not consistently collected across all of the years of the study. Readmission was only available as a qualitative variable for 2011. Relative value units were reviewed on a yearly basis and not totalled across all years because there are year-to-year variations in the assigned RVUs.
Complications and morbidity were then stratified as ‘any’, ‘major’ and ‘minor’. Complications defined as ‘major’ included: cardiac arrest requiring CPR; myocardial infarction; stroke or CVA with neurological deficit; wound disruption; deep incisional SSI; organ space SSI; sepsis or septic shock; unplanned intubation; ventilator dependency for >48 h; pneumonia; acute renal failure; progressive renal insufficiency; DVT or thrombophlebitis; pulmonary embolism, and return to the operating room. Complications defined as ‘minor’ included superficial SSIs and UTIs.
Statistical analysis
Rates of major morbidity were compared among diagnoses using chi-squared tests. Diagnoses were then categorized as being of ‘high’ or ‘low’ risk based on the incidence of major morbidity. Chi-squared tests, Fisher's exact tests and rank sum tests were then performed to compare demographic characteristics and postoperative outcomes in patients in the two risk groups. Multivariate logistic regression was performed to assess the impact of diagnosis on outcomes. In an attempt to control for both clinically and statistically significant risk factors, all multivariate models were adjusted for: age; race; BMI; diabetes status; smoking status; presence of dyspnoea; functional status; history of COPD; history of myocardial infarction; weight loss of >10% of body weight in the 6 months prior to operation; chemotherapy within 30 days; radiotherapy within 90 days; preoperative serum albumin; preoperative total bilirubin; preoperative alkaline phosphatase; preoperative INR; ASA class, and total operative time quartile. Univariate and multivariate secondary data analyses were also performed to assess the impacts of other clinically and statistically significant variables on outcomes. All statistical analyses were performed using sas Version 9.3 (SAS Institute, Inc., Cary, NC, USA). A P-value of <0.05 was considered to indicate statistical significance.
Results
Descriptive statistics: defining high- and low-risk diagnosis categories
Within the NSQIP database, 5537 patients submitted to PD during 2005–2011 were identified and included for analysis. These included 3173 (57.3%) patients with pancreas cancer, 485 (8.8%) with chronic pancreatitis, 1181 (21.3%) with bile duct or ampullary neoplasms, 558 (10.1%) with duodenal neoplasms, and 140 (2.5%) with neuroendocrine neoplasms.
Major morbidity was greatest in patients with diagnoses of bile duct and ampullary neoplasms (33.1%), duodenal neoplasms (34.6%) and neuroendocrine neoplasms (32.1%). The incidence of major morbidity did not differ statistically among these three diagnoses (P = 0.78). Therefore, these three diagnoses were categorized as being of ‘high’ risk. Incidences of major morbidity were lowest among patients with pancreas cancer (23.4%) and chronic pancreatitis (23.3%), and did not differ statistically (P = 0.95). Therefore, pancreas cancer and chronic pancreatitis were categorized as being of ‘low’ risk (Fig. 1).
Figure 1.
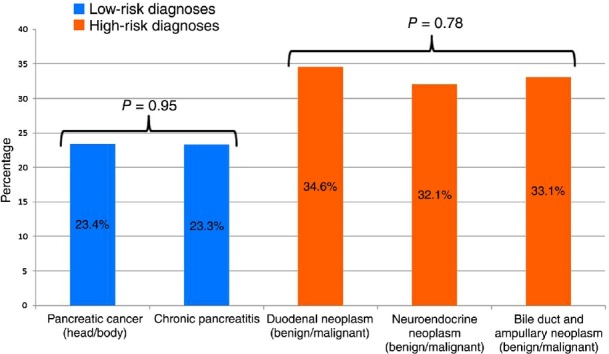
Categorizations of high- and low-risk diagnoses based on incidences of major morbidity (any major complication: P < 0.001)
Demographic characteristics: high- versus low-risk diagnoses
A comparison between the high- and low-risk diagnosis groups showed no differences with respect to gender, race, functional status, cardiac history, hypertension, steroid use, preoperative haematocrit or preoperative INR (all P > 0.05). High-risk patients were slightly older, by a median of 1 year, and more likely to have elevated creatinine or be overweight (BMI 25.0–29.9 kg/m2) or obese (BMI ≥30 kg/m2). High-risk patients were less likely to have traditional risk factors for postoperative complications. They were less likely to have diabetes, to smoke, to have COPD, to have lost > 10% of body weight, to have received neoadjuvant chemotherapy or radiotherapy, to have preoperative hyperbilirubinaemia, to have elevated preoperative alkaline phosphatase, to have preoperative hypo-albuminaemia, and to have ASA class 3 or 4 status (Table 1).
Table 1.
Univariate comparison of patient demographics and risk factors by diagnosis risk group
| Characteristic/factor | All patients (n = 5537) | Patients with low-risk diagnoses (n = 3658) | Patients with high-risk diagnoses (n = 1879) | P-value |
|---|---|---|---|---|
| CPT code, n (%) | 0.084 | |||
| 48150 | 3194 (57.7%) | 2080 (56.9%) | 1114 (59.3%) | |
| 48153 | 2343 (42.3%) | 1578 (43.1%) | 765 (40.7%) | |
| Gender, n (%) | ||||
| Male | 2882 (52.1%) | 1893 (51.8%) | 989 (52.7%) | 0.520 |
| Female | 2648 (47.9%) | 1761 (48.2%) | 887 (47.3%) | |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 4293 (77.5%) | 2871 (78.5%) | 1422 (75.7%) | 0.060 |
| Black or African-American | 432 (7.8%) | 273 (7.5%) | 159 (8.5%) | |
| Other or unknown | 812 (14.7%) | 514 (14.1%) | 298 (15.9%) | |
| Age, years, median (IQR) | 66 (57–74) | 65 (57–73) | 66 (57–75) | <0.001 |
| Age category, n (%) | ||||
| <50 years | 629 (11.4%) | 417 (11.4%) | 212 (11.3%) | <0.001 |
| 50–64 years | 1955 (35.3%) | 1340 (36.6%) | 615 (32.7%) | |
| 65–74 years | 1687 (30.5%) | 1139 (31.1%) | 548 (29.2%) | |
| ≥75 years | 1266 (22.9%) | 762 (20.8%) | 504 (26.8%) | |
| Discharge destination (from 2011), n (%) | ||||
| Home/home facility | 1227 (85.7%) | 836 (86.8%) | 391 (83.5%) | 0.097 |
| Other | 204 (14.3%) | 127 (13.2%) | 77 (16.5%) | |
| BMI (WHO classification), n (%) | ||||
| <18.5 kg/m2 | 188 (3.4%) | 145 (4.0%) | 43 (2.3%) | <0.001 |
| 18.5–24.9 kg/m2 | 2073 (37.8%) | 1470 (40.6%) | 603 (32.5%) | |
| 25.0–29.9 kg/m2 | 1965 (35.9%) | 1240 (34.2%) | 725 (39.1%) | |
| 30.0–34.9 kg/m2 | 791 (14.4%) | 498 (13.7%) | 293 (15.8%) | |
| 35.0–39.9 kg/m2 | 303 (5.5%) | 173 (4.8%) | 130 (7.0%) | |
| ≥40 kg/m2 | 158 (2.9%) | 98 (2.7%) | 60 (3.2%) | |
| Diabetes mellitus, n (%) | ||||
| None | 4261 (77.0%) | 2719 (74.3%) | 1542 (82.1%) | <0.001 |
| Insulin or non-insulin/oral | 1276 (23.0%) | 939 (25.7%) | 337 (17.9%) | |
| Current smoker within 1 year, n (%) | 1210 (21.9%) | 899 (24.6%) | 311 (16.6%) | <0.001 |
| Dyspnoea at rest/moderate exertion, n (%) | 433 (7.8%) | 265 (7.2%) | 168 (8.9%) | 0.026 |
| Functional status partially/totally dependent, n (%) | 116 (2.1%) | 75 (2.1%) | 41 (2.2%) | 0.745 |
| History of severe COPD, n (%) | 243 (4.4%) | 175 (4.8%) | 68 (3.6%) | 0.045 |
| Congestive heart failure in 30 days prior to surgery, n (%) | 15 (0.3%) | 8 (0.2%) | 7 (0.4%) | 0.297 |
| Myocardial infarction in 6 months prior to surgery, n (%) | 14 (0.3%) | 8 (0.3%) | 6 (0.4%) | 0.575 |
| Previous PCI, n (%) | 317 (6.7%) | 204 (6.6%) | 113 (7.0%) | 0.591 |
| Previous cardiac surgery, n (%) | 257 (5.4%) | 163 (5.2%) | 94 (5.8%) | 0.422 |
| Angina in 30 days prior to surgery, n (%) | 21 (0.4%) | 13 (0.4%) | 8 (0.5%) | 0.711 |
| Hypertension requiring medication, n (%) | 2926 (52.8%) | 1914 (52.3%) | 1012 (53.9%) | 0.279 |
| Steroid use for chronic condition, n (%) | 99 (1.8%) | 68 (1.9%) | 31 (1.6%) | 0.578 |
| Loss of > 10% of body weight in 6 months prior to surgery, n (%) | 1047 (18.9%) | 789 (21.6%) | 258 (13.7%) | <0.001 |
| Chemotherapy for malignancy in 30 days prior to surgery, n (%) | 125 (2.6%) | 117 (3.8%) | 8 (0.5%) | <0.001 |
| Radiotherapy for malignancy in 90 days prior to surgery, n (%) | 135 (2.9%) | 126 (4.1%) | 9 (0.6%) | <0.001 |
| Preoperative serum creatinine, n (%) | ||||
| ≤1.0 mg/dl | 4326 (79.9%) | 2927 (81.6%) | 1399 (76.6%) | <0.001 |
| >1.0 mg/dl to < 1.5 mg/dl | 898 (16.6%) | 543 (15.1%) | 355 (19.4%) | |
| ≥1.5 mg/dl | 191 (3.5%) | 118 (3.3%) | 73 (4.0%) | |
| Preoperative serum albumin, n (%) | ||||
| <3.5 g/dl | 1531 (30.4%) | 1060 (31.5%) | 471 (28.1%) | 0.015 |
| ≥3.5 g/dl | 3509 (69.6%) | 2306 (68.5%) | 1203 (71.9%) | |
| Preoperative total bilirubin, n (%) | ||||
| ≤1.0 mg/dl | 2829 (57.0%) | 1760 (53.8%) | 1069 (63.3%) | <0.001 |
| >1.0 mg/dl to < 2.0 mg/dl | 728 (14.7%) | 481 (14.7%) | 247 (14.6%) | |
| ≥2.0 mg/dl | 1403 (28.3%) | 1030 (31.5%) | 373 (22.1%) | |
| Preoperative alkaline phosphatase, n (%) | ||||
| <120 IU/l | 2184 (43.3%) | 1323 (39.6%) | 861 (50.6%) | <0.001 |
| ≥120 IU/l | 2860 (56.7%) | 2019 (60.4%) | 841 (49.4%) | |
| Preoperative haematocrit, n (%) | ||||
| ≤24% | 27 (0.5%) | 16 (0.4%) | 11 (0.6%) | 0.675 |
| >24% to 35% | 1582 (29.2%) | 1054 (29.4%) | 528 (28.8%) | |
| >35% | 3811 (70.3%) | 2514 (70.1%) | 1297 (70.6%) | |
| Preoperative INR, n (%) | ||||
| <1.5 | 4798 (98.6%) | 3184 (98.4%) | 1614 (99.0%) | 0.079 |
| ≥1.5 | 68 (1.4%) | 52 (1.6%) | 16 (1.0%) | |
| ASA class, n (%) | ||||
| 1–2 (No or mild disturbance) | 1653 (29.9%) | 1033 (28.3%) | 620 (33.0%) | <0.001 |
| 3–4 (Severe disturbance to life-threatening) | 3880 (70.1%) | 2623 (71.7%) | 1257 (67.0%) |
ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; INR, international normalized ratio; IQR, interquartile range; PCI, percutaneous coronary intervention; WHO, World Health Organization.
Univariate outcome variables: high- versus low-risk diagnoses
Patients with high-risk diagnoses experienced slightly shorter operative times. There was no significant difference in postoperative 30-day mortality.
High-risk diagnosis patients were more likely to experience: a prolonged LoS (≥14 days); superficial SSI; deep SSI; organ space SSI; any SSI; wound disruption; progressive renal insufficiency; acute renal failure; UTI; sepsis or septic shock; return to the operating room; overall morbidity (one or more complications), and major morbidity. There was no significant difference in readmission rate (Table 2 and Fig. 2).
Table 2.
Univariate comparison of patient outcomes by diagnosis risk group
| Characteristic/factor | All patients (n = 5537) | Patients with low-risk diagnoses (n = 3658) | Patients with high-risk diagnoses (n = 1879) | P-value |
|---|---|---|---|---|
| Total operation time, hours, median (IQR) | 5.87 (4.72–7.22) | 5.93 (4.77–7.33) | 5.72 (4.65–7.05) | <0.001 |
| Total operation time quartile, n (%) | ||||
| Q1: < 4.75 h | 1411 (25.6%) | 896 (24.6%) | 515 (27.5%) | 0.003 |
| Q2: 4.75 h to < 6 h | 1495 (27.1%) | 969 (26.6%) | 526 (28.1%) | |
| Q3: 6 h to < 7.25 h | 1247 (22.6%) | 827 (22.7%) | 420 (22.4%) | |
| Q4: ≥7.25h | 1368 (24.8%) | 955 (26.2%) | 413 (22.0%) | |
| Total work RVU (primary, concurrent and other procedures), median (IQR) | ||||
| 2005 | 47.93 (47.93–54.22) | 47.93 (47.93–59.83) | 47.93 (47.93–49.13) | 0.076 |
| 2006 | 52.91 (47.93–63.28) | 53.32 (47.93–64.03) | 50.44 (47.93–61.49) | 0.160 |
| 2007 | 55.55 (52.63–69.98) | 56.83 (52.63–70.99) | 55.24 (52.63–69.98) | 0.117 |
| 2008 | 57.31 (52.63–71.45) | 57.51 (52.63–72.58) | 55.25 (52.63–70.22) | 0.280 |
| 2009 | 57.72 (52.63–72.60) | 58.35 (52.63–72.60) | 57.51 (52.63–72.60) | 0.778 |
| 2010 | 57.93 (52.84–72.94) | 59.38 (52.84–75.41) | 55.46 (52.84–71.39) | 0.018 |
| 2011 | 59.38 (52.84–72.99) | 59.38 (52.84–72.99) | 59.38 (52.84–73.92) | 0.861 |
| Death within 30 days, n (%) | 142 (2.6%) | 84 (2.3%) | 58 (3.1%) | 0.078 |
| Days from operation to discharge | ||||
| Mean ± SD | 12.03 ± 9.20 | 11.35 ± 7.95 | 13.36 ± 11.13 | <0.001 |
| Median (IQR) | 9 (7–14) | 9 (7–13) | 10 (7–15) | |
| Days from operation to discharge, n (%) | ||||
| <14 | 4089 (74.0%) | 2811 (76.9%) | 1278 (68.2%) | <0.001 |
| ≥14 | 1440 (26.0%) | 845 (23.1%) | 595 (31.8%) | |
| Superficial SSI, n (%) | 580 (10.5%) | 344 (9.4%) | 236 (12.6%) | <0.001 |
| Deep incisional SSI, n (%) | 137 (2.5%) | 76 (2.1%) | 61 (3.2%) | 0.008 |
| Organ space SSI, n (%) | 530 (9.6%) | 249 (6.8%) | 281 (15.0%) | <0.001 |
| Any SSI, n (%) | 1173 (21.2%) | 641 (17.5%) | 532 (28.3%) | <0.001 |
| Wound disruption, n (%) | 84 (1.5%) | 45 (1.2%) | 39 (2.1%) | 0.015 |
| Pneumonia, n (%) | 265 (4.8%) | 163 (4.5%) | 102 (5.4%) | 0.109 |
| Unplanned intubation, n (%) | 249 (4.5%) | 151 (4.1%) | 98 (5.2%) | 0.064 |
| Pulmonary embolism, n (%) | 50 (0.9%) | 29 (0.8%) | 21 (1.1%) | 0.226 |
| On ventilator for > 48 h, n (%) | 252 (4.6%) | 157 (4.3%) | 95 (5.1%) | 0.197 |
| Progressive renal insufficiency, n (%) | 36 (0.7%) | 15 (0.4%) | 21 (1.1%) | 0.002 |
| Acute renal failure, n (%) | 63 (1.1%) | 33 (0.9%) | 30 (1.6%) | 0.021 |
| Urinary tract infection, n (%) | 287 (5.2%) | 166 (4.5%) | 121 (6.4%) | 0.003 |
| Stroke/CVA with neurological deficit, n (%) | 17 (0.3%) | 15 (0.4%) | 2 (0.1%) | 0.053 |
| Cardiac arrest requiring CPR, n (%) | 63 (1.1%) | 38 (1.0%) | 25 (1.3%) | 0.333 |
| Myocardial infarction, n (%) | 37 (0.7%) | 26 (0.7%) | 11 (0.6%) | 0.588 |
| DVT/thrombophlebitis, n (%) | 116 (2.1%) | 69 (1.9%) | 47 (2.5%) | 0.130 |
| Sepsis or septic shock, n (%) | 759 (13.7%) | 401 (11.0%) | 358 (19.1%) | <0.001 |
| Return to operating room, n (%) | 330 (6.0%) | 197 (5.4%) | 133 (7.1%) | 0.012 |
| Readmission (from 2011), n (%) | 220 (16.2%) | 140 (15.4%) | 80 (18.1%) | 0.199 |
| Any complications, n (%) | 1972 (35.6%) | 1166 (31.9%) | 806 (42.9%) | <0.001 |
| Any major complications, n (%) | 1485 (26.8%) | 856 (23.4%) | 629 (33.5%) | <0.001 |
CPR, cardiopulmonary resuscitation; CVA, cerebral vascular accident; DVT, deep vein thrombosis; IQR, interquartile range; RVU, relative value unit; SD, standard deviation; SSI, surgical site infection.
Figure 2.
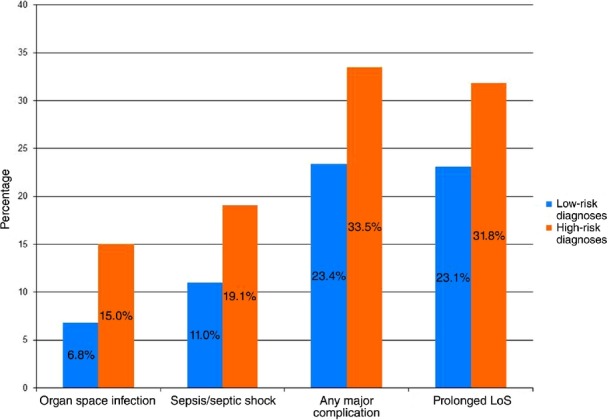
Outcomes by diagnosis risk group (all: P < 0.001). LoS, length of stay
Relative value unit: high- versus low-risk diagnoses
The median RVU was observed to increase slightly over time (Table 2). Median RVU ranged from 47.93 in 2005 to 59.38 in 2011. There was no significant difference in RVU between the high- and low-risk cohorts except in 2010, for which a higher median RVU was observed in the low-risk cohort (Table 2).
Major complications: high- versus low-risk diagnoses
Univariate analysis demonstrated that although overall major morbidity was experienced by 26.8% of all patients studied, those with a high-risk diagnosis experienced a significantly greater incidence of major morbidity than did patients with low-risk diagnoses (33.5% versus 23.4%; P < 0.001). Multivariate analysis revealed the high-risk diagnosis category to be an independent risk factor for major complications [odds ratio (OR) 1.70, 95% confidence interval (CI) 1.45–2.00].
Multivariate analysis also revealed other preoperative independent risk factors for the occurrence of major complications, including: age ≥75 years versus 50–64 years (OR 1.47, 95% CI 1.19–1.82); BMI of ≥40 kg/m2 versus 18.5–24.9 kg/m2 (OR 1.93, 95% CI 1.25–2.98); dyspnoea at rest or in moderate exertion (OR 1.46, 95% CI 1.11–1.93); history of myocardial infarction in the 6 months prior to surgery (OR 4.06, 95% CI 1.31–12.53); ASA class III/IV (OR 1.23, 95% CI 1.02–1.47); and preoperative serum albumin of < 3.5 g/dl (OR 1.43, 95% CI 1.19–1.72). Fourth-quartile total operative time (≥7.25 h) in comparison with operative time of < 4.75 h was also an independent risk factor (OR 1.37, 95% CI 1.09–1.71).
Any SSI: high- versus low-risk diagnoses
On univariate analysis, SSIs were observed in 21.2% of patients. High-risk diagnosis patients experienced significantly more SSIs than patients with low-risk diagnoses (28.3% versus 17.5%; P < 0.001). Multivariate analysis revealed the high-risk category to be an independent risk factor for any SSI (OR 1.78, 95% CI 1.50–2.11).
Multivariate analysis also revealed other preoperative independent risk factors for any SSI including: a BMI of ≥40 kg/m2 versus a BMI of 18.5–24.9 kg/m2 (OR 2.50, 95% CI 1.61–3.87), and preoperative serum albumin of < 3.5 g/dl (OR 1.37, 95% CI 1.13–1.66). Fourth-quartile total operative time (≥7.25 h) in comparison with operative time of < 4.75 h was also an independent risk factor (OR 1.42, 95% CI 1.13–1.80).
Organ space SSI: high- versus low-risk diagnoses
Organ space infection was observed in 9.6% of all patients studied. High-risk diagnoses were associated with significantly more organ space infections than low-risk diagnoses (15.0% versus 6.8%; P < 0.001). Multivariate analysis revealed a high-risk diagnosis to be an independent risk factor for organ space SSIs (OR 2.57, 95% CI 2.02–3.28).
Multivariate analysis also revealed other preoperative independent risk factors for organ space SSIs, including: overweight and obesity at a BMI of 25.0–29.9 kg/m2 (OR 1.38, 95% CI 1.03–1.84), a BMI of 30.0–34.9 kg/m2 (OR 1.54, 95% CI 1.07–2.22), a BMI of 35.0–39.9 kg/m2 (OR 1.81, 95% CI 1.10–2.97), and a BMI of ≥ 40 kg/m2 (OR 2.51, 95% CI 1.38–4.56), all in comparison with a BMI of 18.5–24.9 kg/m2. Additionally, preoperative serum albumin of < 3.5 g/dl (OR 1.41, 95% CI 1.06–1.86) and fourth-quartile total operative time (≥7.25 h) in comparison with operative time of < 4.75 h (OR 1.68, 95% CI 1.19–2.38) were also independent risk factors on multivariate analysis.
Sepsis and septic shock: high- versus low-risk diagnoses
A total of 13.7% of all PD patients experienced sepsis or septic shock. A high-risk diagnosis was associated with a significantly greater incidence of sepsis and septic shock than a low-risk diagnosis (19.1% versus 11.0%; P < 0.001). Multivariate analysis revealed a high-risk diagnosis to be an independent risk factor for sepsis and septic shock (OR 1.83, 95% CI 1.49–2.25).
Other preoperative independent risk factors identified in multivariate analysis included: a BMI of ≥40 kg/m2 versus a BMI of 18.5–24.9 kg/m2 (OR 2.05, 95% CI 1.24–3.39); preoperative serum albumin of < 3.5 g/dl (OR 1.39, 95% CI 1.10–1.75), and dyspnoea (OR 1.49, 95% CI 1.06–2.08).
Prolonged LoS: high- versus low-risk diagnoses
High-risk patients experienced significantly longer hospital stays. A total of 90.0% of low-risk patients were discharged on or before PoD 19, whereas 90.0% of high-risk patients were discharged by PoD 23. Of all PD patients, 26.0% were discharged at or after PoD 14. Additionally, high-risk patients had an increased incidence of prolonged LoS compared with low-risk patients (31.8% versus 23.1%; P < 0.001). Multivariate analysis revealed a high-risk diagnosis to be an independent risk factor for a prolonged LoS (OR 1.67, 95% CI 1.41–1.97) (Table 2, Figs 2 and 3).
Figure 3.
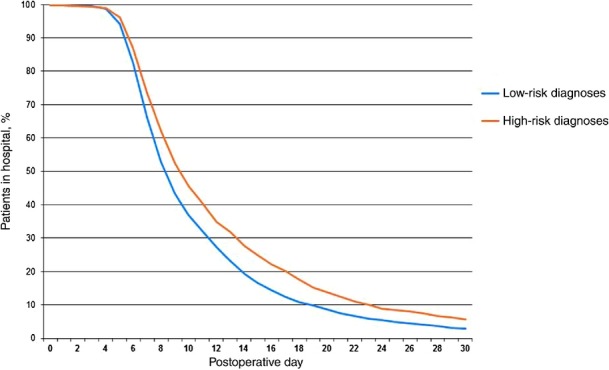
Observed rate of hospital discharge by diagnosis risk group (P < 0.001)
Other preoperative independent risk factors for a prolonged LoS identified in multivariate analysis included: age ≥75 years versus age of 50–64 years (OR 1.58, 95% CI 1.27–1.96), dyspnoea (OR 1.38, 95% CI 1.04–1.83), and preoperative serum albumin of < 3.5 g/dl (OR 1.78, 95% CI 1.48–2.14). Fourth-quartile total operative time (≥ 7.25 h) in comparison with operative time of < 4.75 h was also an independent risk factor (OR 1.68, 95% CI 1.34–2.10).
Other patient outcomes: high- versus low-risk diagnoses
Multivariate analysis identified no differences between the high- and low-risk diagnosis groups with respect to the occurrence of UTIs, reoperation, 30-day mortality or readmission.
Discussion
In this analysis of multicentre data, outcomes in 5537 patients after PD were associated with these patients' underlying diagnoses. Although the patients in the low-risk diagnosis group had an increased incidence of traditional risk factors for postoperative complications, patients in the high-risk group were identified as having increased risk for complications including a prolonged LoS, organ space infection, sepsis or septic shock and major morbidity. Additionally, this study demonstrated that despite the significant increase in the rates of adverse events associated with diagnosis, there was no corresponding difference in RVU assignment between the high- and low-risk diagnosis groups. Therefore, this study has considerable implications because diagnosis is a non-modifiable, independent risk factor for the occurrence of complications following PD and thus facilitates the calculation of added requirements for complex care and resource utilization in high-risk patients.
Recently, there has been increasing interest in risk calculators for surgery, including PD.12,13,15,26,27 Existing risk calculators for PD vary based on the parameters suggested as inputs. Some are designed to include only preoperative variables, whereas others are meant to use an intraoperative assessment. These calculators are very useful for aiding pre- and intraoperative decision making, as well as for providing insight into the relative relationships among different risk factors. They are, however, subject to several limitations. Both forms require time and access to the information required. Preoperative risk calculators require a number of inputs, many of which may not be known. They also, by their very nature, are unable to accurately account for critically important variables such as gland texture and duct size. Diagnoses are either not considered or are generically grouped as ‘benign’ or ‘malignant’. Intraoperative risk calculators include important, albeit subjective, parameters (i.e. gland texture, estimated blood loss), yet these are limited by inter-observer variability. Furthermore, external independent validation of such risk calculators is currently lacking. Existing calculators may also be too complex to allow for simple integration into risk stratification systems and the modification of value assignments. Although risk calculators may prove to be more sensitive or specific for predicting postoperative complications, their use is certainly more complex than the method of stratifying by diagnosis alone. As there is a plausible underlying pathological basis for the inherent risk for complications after PD, risk calculators should certainly incorporate the influence of diagnosis. Further, in an effort to improve the relative value assignment, it may be more prudent to assign additional value to a patient undergoing PD with a ‘high-risk’ underlying diagnosis than to base it on the sum of a more subjective risk calculator.
Currently, in the USA, RVUs are assigned by the Relative Value Update Committee (RUC) to individual operations on the basis of physician work effort. The Centers for Medicare and Medicaid Services (www.CMS.gov) provide a physician fee schedule search tool which allows the RVU for a given CPT to be queried by year.28 For CPT 48150 work, the RVUs assigned varied from 47.93 in 2005 to 52.84 in 2011; likewise, for CPT 48153 work, the RVUs assigned varied from 47.82 in 2005 to 52.79 in 2011.28 The NSQIP dataset contains total (index and concurrent operations) work RVU data, but these have not been utilized to evaluate PD outcomes. A historical comparison of RVUs assigned for PD as provided by CMS.gov with the total work RVUs reported by the NSQIP shows that the RVU assignments from neither source have increased greatly over time, but that the NSQIP-reported total RVU has increased at a slightly greater rate than that reported by CMS.gov for PD. The increase in total RVU reported in the NSQIP over that seen in the CMS.gov physician fee schedule may potentially be explained by an increase in concurrent operations. For the purposes of this study, patients with concurrent operations that are not typically performed with PD were excluded. However, concurrent operations more typically performed, such as vascular resection, were included and may potentially explain this difference although this study did not specifically investigate the incidence of concurrent operations. Its analysis of total work RVU by diagnosis did demonstrate there to be no relationship between RVU assignment and diagnosis, complications experienced and, therefore, resource utilization. These results were anticipated because there is currently no modification of RVU assignment for operations, including PD, based on diagnosis, risk or actual complications encountered. Diagnosis, as a non-modifiable independent risk factor for patient outcomes and resource utilization after PD, may facilitate a more appropriate assignment of relative value.
Current demands for quality and outcomes improvement require not only a multidisciplinary team-based approach, but also proper patient selection and stratification. Patient stratification involves the identification of both modifiable and non-modifiable risk factors, and is essential to developing proper individualized patient-centred approaches and ensuring high-quality outcomes. Proper patient stratification is also essential to a more accurate reflection of outcomes and takes into account case mix variation. Institutional quality metrics are increasingly used to assess the care provided. Such quality metrics should therefore be adjusted for institutional case mix variation. For example, a case mix consisting primarily of high-risk patients would be expected to have considerable differences in outcomes, resource utilization and complexity of care compared with a case mix of primarily low-risk patients. Furthermore, externally reported performance measures, payer value assignment and performance-related compensation should also take into account these non-modifiable risk strata to more accurately account for variation in case mix and complexity of care.
Limitations
This study has several potential limitations. Firstly, this is a retrospective review of a prospective database. Although the ACS has put in place stringent quality control measures to maintain the integrity of the data, such datasets are subject to inherent limitations. Additionally, the database does not contain information on PD-specific variables such as pancreatic fistula, delayed gastric emptying, pancreas texture and duct size. There are also no hospital- or surgeon-specific variables to enable comparisons by volume or experience. This study relies on ICD-9 diagnosis codes for patient identification and thus only the codes for diagnoses that are clearly related to the diagnosis categories created can be studied. The ACS-NSQIP captures only the primary diagnosis and thus only patients with a primary diagnosis representative of a diagnosis of interest could be identified. Further, the ICD-9 diagnosis information in the ACS-NSQIP is limited to one postoperative diagnosis code per patient. The ACS-NSQIP database does not contain preoperative diagnoses and as a result no correlation between pre- and postoperative diagnoses can be made. However, modern preoperative diagnostic modalities make it increasingly common to have a correct diagnosis preoperatively.
Several ICD-9 codes were not included because they either did not translate directly into a defined diagnostic category commonly referenced or were not exclusive to one particular diagnosis. For example, IPMN, serous cystic neoplasm and mucinous cystic neoplasm do not have specific ICD-9 codes and therefore were not evaluated in this study. In addition, although the present authors assigned codes to the created categories to the best of their ability, there is no objective way to evaluate the extent to which the categories are mutually exclusive. Notwithstanding these limitations, however, this is the first large multicentre analysis to evaluate the impact of diagnosis on postoperative outcomes following PD.
Conclusions
This study demonstrates that patients undergoing PD can be easily stratified into high- and low-risk groups based on diagnosis alone. This non-modifiable parameter allows a clinically useful stratification that can be applied at any time in the clinical course of the patient.
The present findings suggest that future outcomes studies, performance measures, value assignment, and the obtaining of patient-informed consent for PD should include diagnosis, or diagnosis risk group, as a predictive factor. The reporting of outcomes of PD without such stratification may represent a missed opportunity to improve risk prediction and anticipate resource needs.
Conflicts of interest
None declared.
References
- Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg. 1999;23:913–919. doi: 10.1007/s002689900599. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. ; discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcom JH, 4th, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzoli S, Liessi G, Pasquali C, Ragazzi R, Berselli M, Sperti C. Postoperative pancreatic fistulas: preventing severe complications and reducing reoperation and mortality rate. Ann Surg. 2009;249:97–104. doi: 10.1097/SLA.0b013e31819274fe. [DOI] [PubMed] [Google Scholar]
- Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207:490–498. doi: 10.1016/j.jamcollsurg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM., Jr Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Zhang B, Zhang Y, Jiang X, Yi B, Luo X, et al. Predictive factors for complications after pancreaticoduodenectomy. J Surg Res. 2007;139:22–29. doi: 10.1016/j.jss.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Choe YM, Lee KY, Oh CA, Lee JB, Choi SK, Hur YS, et al. Risk factors affecting pancreatic fistulas after pancreaticoduodenectomy. World J Gastroenterol. 2008;14:6970–6974. doi: 10.3748/wjg.14.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Callery MP, Vollmer CM., Jr Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–2135. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Turaga KK, Miller WJ, Loggie BW, Foster JM. Determinants of outcomes in pancreatic surgery and use of hospital resources. J Surg Oncol. 2011;104:634–640. doi: 10.1002/jso.21923. [DOI] [PubMed] [Google Scholar]
- Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–497. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117–1123. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- Veillette G, Dominguez I, Ferrone C, Thayer SP, McGrath D, Warshaw AL, et al. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: the Massachusetts General Hospital experience. Arch Surg. 2008;143:476–481. doi: 10.1001/archsurg.143.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoi S, Takai S, Matsui Y, Terakawa N, Iwaki R, Fukui J, et al. Less morbidity after pancreaticoduodenectomy of patients with pancreatic cancer. Pancreas. 2006;33:45–52. doi: 10.1097/01.mpa.0000234645.64483.5c. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588. doi: 10.1097/00000658-199510000-00014. ; discussion 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanjian KK, Hines OJ, Eibl G, Reber HA. Management of pancreatic fistulas after pancreaticoduodenectomy: results in 437 consecutive patients. Arch Surg. 2005;140:849–854. doi: 10.1001/archsurg.140.9.849. ; discussion 854–856. [DOI] [PubMed] [Google Scholar]
- Liang TB, Bai XL, Zheng SS. Pancreatic fistula after pancreaticoduodenectomy: diagnosed according to International Study Group Pancreatic Fistula (ISGPF) definition. Pancreatology. 2007;7:325–331. doi: 10.1159/000105498. [DOI] [PubMed] [Google Scholar]
- American College of Surgeons. 2014. National Quality Improvement Program. Participant Use Data File. Available at http://site.acsnsqip.org/participant-use-data-file/ (last accessed 30 November 2014)
- American Medical Association. 2013. Current Procedural Terminology: CPT 2013 Professional Edition. Chicago, IL.
- American Medical Association. 2013. 2013 ICD-9-CM for Hospitals. Chicago, IL.
- Schneider EB, Hyder O, Wolfgang CL, Dodson RM, Haider AH, Herman JM, et al. Provider versus patient factors impacting hospital length of stay after pancreaticoduodenectomy. Surgery. 2013;154:152–161. doi: 10.1016/j.surg.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Are C, Afuh C, Ravipati L, Sasson A, Ullrich F, Smith L. Preoperative nomogram to predict risk of perioperative mortality following pancreatic resections for malignancy. J Gastrointest Surg. 2009;13:2152–2162. doi: 10.1007/s11605-009-1051-z. [DOI] [PubMed] [Google Scholar]
- Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF, et al. A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol. 2010;17:1802–1807. doi: 10.1245/s10434-010-0947-x. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. 2014. Physician fee schedule search. Available at http://www.cms.gov/apps/physician-fee-schedule/ (last accessed 2 December 2014) [PubMed]


