Abstract
Guanine-adenine-thymine-adenine 2 (GATA2) mutated disorders include the recently described MonoMAC syndrome (Monocytopenia and Mycobacterium avium complex infections), DCML (dendritic cell, monocyte, and lymphocyte deficiency), familial MDS/AML (myelodysplastic syndrome/acute myeloid leukemia) (myeloid neoplasms), congenital neutropenia, congenital lymphedema (Emberger's syndrome), sensorineural deafness, viral warts, and a spectrum of aggressive infections seen across all age groups. While considerable efforts have been made to identify the mutations that characterize this disorder, pathogenesis remains a work in progress with less than 100 patients described in current literature. Varying clinical presentations offer diagnostic challenges. Allogeneic stem cell transplant remains the treatment of choice. Morbidity, mortality, and social costs due to the familial nature of the disease are considerable. We describe our experience with the disorder in three affected families and a comprehensive review of current literature.
Keywords: GATA2, leukemia, lymphedema, MonoMAC, viral warts
Introduction
The guanine-adenine-thymine-adenine (GATA) family is comprised of six zinc-finger transcription factors that recognize approximately 7 million GATA motifs in the human genome 1,2. GATA1 is instrumental in development of erythrocytes, mast cells, eosinophils, and megakaryocytes 3–8 and is implicated in Down syndrome-related acute megakaryocytic leukemia and transient myeloproliferative disorder 9,10. GATA1 is also associated with X-linked thrombocytopenia and dyserythropoeitic anemia (Diamond–Blackfan anemia) 75–77. GATA2, located on 3q21 11 is pivotal in proliferation of hematopoietic stem cells (HSC) and mutations were first described in aplastic anemia 12–15. Pedigree studies have initially recognized two mutations in GATA2 in familial AML, p.T354M, and p.T355del, both in the second zinc finger (ZF-2) of GATA2 16–18, while two other mutations; p.R308P and p.A350-N351ins8 are associated with de novo AML 78. During erythropoiesis; GATA switching results in displacement of GATA2 by GATA1 from chromatin causing inhibition of GATA2, promoting downstream erythroid differentiation 5,19. In contrast, GATA2 overexpression induces megakaryocytic differentiation in cell lines 20. GATA2 exerts an inhibiting influence on the PU.1 gene which is essential for monocytic, granulocytic, and lymphoid differentiation 21. In contrast to RUNX1, which is essential for generation of HSC, GATA2 appears to be essential for HSC generation and subsequent survival 22. Other GATA genes perform a diverse array of functions. GATA3 promotes T-cell lymphopoiesis 23–28 but deficiency has been associated only with hypoparathyroidism, deafness, and renal disease. GATA4 has recently been implicated in childhood onset diabetes 29. GATA 5 CpG island hypermethylation in renal carcinoma appear to identify aggressive phenotypes with poor outcomes 30. In animal models, GATA6 has been shown to orchestrate cardiac muscle hypertrophy in response to pressure stress and increase hepcidin expression in inflammatory states 31,32. In summary, GATA factors 1–3 appear to be involved in hematopoiesis, while GATA 4–6 appear to be more important for cardiac development and function 33 although expression has been demonstrated in other endodermal and mesodermal organs such as lung, liver and gonads and gut 34.
The study of germline mutations such as GATA2 provides profound insights into leukemogenesis, immune dysfunction and cross-talk of seemingly diverse genetic pathways such as CEBPA, PU.1 35–38, and RUNX1 39–43. The clinical phenotype of germline GATA2 mutations include, but is not limited to, spectrum of immune deficits such as MonoMAC syndrome 44–46, dendritic cell, monocyte and lymphoid deficiency (DCML) 47, familial MDS (myelodysplastic syndrome)/AML (acute myeloid leukemia), and Emberger's syndrome 48. Of note, sporadic mutations in GATA2 are described and may have no familial implications as described below. Our focus in this article is the haploinsufficiency induced by spontaneous germline mutations in GATA2 resulting in an autosomal dominant inheritance of diverse phenotypes 44,46,49. The differential diagnosis of GATA-2 deficiency includes other related disorders with overlapping features and are summarized in Table1.
Table 1.
Mutations/disorders in differential diagnosis of GATA2 deficiency
| Familial MDS/AML 67 | Warts/HPV infections 51 | Mycobacterial infections 68–70 | Congenital lymphedema 71–73,75,76 | Pulmonary alveolar proteinosis 77 |
|---|---|---|---|---|
|
TERT/TERC CEBPA RUNX1 |
DOCK 8 CXCR4 HIV/CD4↓ TMC6/8 SPINK5/LEKT1 STK4/MST1 |
IFNGR1 IFNGR2 IL12RB1 STAT1 (loss of function; AR and AD) IRF8 CYBB (macrophage-specific mutation) TYK2 ISG15 IKKG (NEMO) |
FLT4 GJC2 FOXC2 SOX18 CCBE1 PTPN14 |
Anti-GM-CSF Ab CSF2RB |
MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; HPV, human papillomavirus.
Case Series
Family 1
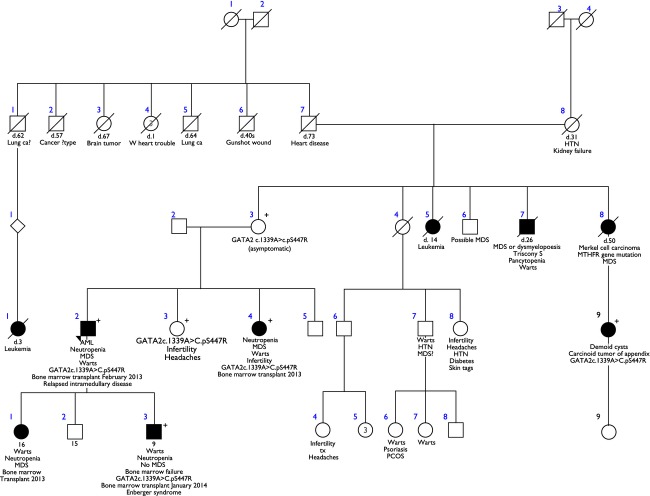
The proband is a 38-year-old Caucasian male, who presented with progressive dyspnea and fatigue of 3 months duration and was found to have pancytopenia. A bone marrow biopsy revealed hypocellular marrow but demonstrated acute myeloid leukemia (AML) with the following cytogenetic abnormalities: t (1; 21) (q10; q10) [9]/+8[4]/46XY [7]. He received standard induction chemotherapy with idarubicin and cytarabine, and his course was complicated by an orbital fungal infection with Absidia lithemia, medically and surgically managed, following which he underwent a reduced-intensity conditioning-matched unrelated donor allogeneic hematopoietic stem cell transplant (MUD-Allo-HCT). Posttransplant course was complicated by severe refractory immune-mediated thrombocytopenia requiring a splenectomy and an orbital relapse of AML. Due to history of multiple family members being affected (Fig.2) by AML and extra genital warts (sister, son, and daughter), congenital lymphedema (son), and cytopenias (sister) a work-up for familial bone marrow failure syndromes was carried out. GATA2 mutation analysis performed at the National Institutes of Health (NIH) confirmed the presence of a missense mutation (1339A>C, p S447R) in the patient, a female sibling, a son, and a daughter. The female sibling with MDS and viral warts also underwent MUD-Allo-HSCT (hematopoietic stem cell transplant) and remains symptom-free 14 months posttransplant. The probands son and daughter also underwent MUD allogeneic HSCT and are doing very well more than 12 months posttransplant. Patient characteristics and outcomes are shown in Table2.
Figure 2.
Family Tree of Proband 1 with the GATA2 c.1339A>C.pS447R mutation.
Table 2.
Clinical characteristics and outcomes of patients with GATA2 mutations that underwent allogeneic stem cell transplantation
| No. | Diagnosis Age/Sex | Cytogenetics/GATA2 mutation | Associated features | Prior Rx | HCT-CI | CMV | Regimen | ABO/HLA | GVHD Prop | aGVHD Status | Complications | Day 30 Chimerism /Marrow | Day 100 Chimerism /Marrow | Last follow up (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AML 38/M |
46,XY, t(1;21) [9] +8[4]46,XY [7] S447R |
NK/B-cell def Viral warts Hydrocele |
Idarubicin Cytarabine | 0 | D+ R+ |
Fludarabine TBI (200) | ABO− mismatch HLA 9/10 | Tacrolimus MTX |
GI Grade 1 |
E.Faecalis CMV Anti-platelet antibodies/Thrombocytopenia |
100% Donor 30% Hypo- Cellular |
100% Donor 30% Hypo- Cellular |
356 (extra medullary relapse day 307) |
| 2 | MDS 35/F |
S447R | NK/B-cell def Viral warts Infertility |
None | 0 | D− R+ |
Busulfan Cytoxan |
ABO− match HLA 10/10 |
Tacrolimus MTX |
Skin Grade 1 |
CMV | 100% Donor 30% Hypocellular |
100% Donor 60% Hypocellular |
208 |
| 3 | MDS 10/F |
S447R | NK/B-cell def Viral warts |
None | 0 | D− R+ |
Cytoxan, TBI Alemtuzumab |
ABO− match HLA 9/10 |
Tacrolimus MTX |
Skin Grade 1 |
C.difficile | 100% Donor 30% Hypo- Cellular |
100% Donor 20% Hypo- Cellular |
247 |
| 4 | Chronic Neutropenia 7/M |
S447R | NK/B cell def Viral warts Emberger's syndrome Pulmonary stenosis |
None | 0 | D− R+ |
Cytoxan, TBI Alemtuzumab |
ABO− match HLA 10/10 |
Tacrolimus | – | – | Pending 30% Hypo cellular |
NA | 31 |
MDS, myelodysplastic syndrome; AML, acute myeloid leukemia.
Family 2
Family 2 was discovered by the birth of a newborn with dysmorphic features (head size larger than stomach) resulting in a cytogenetic examination in infancy with identification of deletion 3q13.2-q21.3, which includes the GATA2 gene. The child exhibited monocytopenia without lymphopenia or neutropenia. Dendritic cell activity was not assessed for. The parents were tested and did not have the same gene defect. She is being followed with monthly blood tests and has not demonstrated any systemic infections or signs of MDS/AML although cognitive development appears to be delayed.
Family 3
In Family 3, the proband is a Caucasian female who presented at age 17 years with abdominal pain, hemoptysis, and mild pancytopenia. A CT scan revealed mild diffuse thoracic and abdominal lymphadenopathy. A detailed evaluation found acute Epstein–Barr virus (EBV) infection. A bone marrow biopsy was mildly hypocellular with mild erythroid hypoplasia and megakaryocytic hyperplasia with atypia. The cytogenetics were 46, XX 20. She had a history of recurrent episodes of hidradenitis suppurativa, skin abscesses, folliculitis, otitis media, and throat infections. Several years later, she was initiated on therapy with pegylated G-CSF. In spite of this, otitis media and abscesses continued. Two years later, she presented with a hypercatabolic state, with progressive hepato-splenomegaly and constitutional features. A bone marrow biopsy demonstrated progressive megakaryocytic atypia. While cytogenetics were once again normal, however, a MDS-fluorescence in situ hybridization (FISH) panel identified a deletion of -3q21 in 99% of analyzed nuclei. A phytohemagglutinin-stimulated karyotyping of peripheral blood lymphocytes also demonstrated the -3q21 (RPN1 deletion) in 99% of analyzed nuclei. GATA2 is located within this region, Expression studies confirmed GATA2 haploinsufficiency. She is awaiting a donor for a MUD-HSCT.
Discussion
MonoMAC syndrome and DCML deficiency
The terms MonoMAC and DCML are synonymous, in terms of the genetic etiology, and refer to a primary immunodeficiency with predisposition to MDS/AML. MonoMAC refers to a recently described syndrome of MONOcytopenia and Mycobacterium Avium Complex infections characterized by germline GATA2 mutations 44,46. DCML, also caused by germline GATA2 mutations refers specifically to the cytopenias frequently seen in most patients—DCML deficiency (both B and NK cell) 50. Two independent groups studied 24 individuals with these syndromes and reported similar mutations noted above in familial syndromes (T354M and T355 del). The scope of immune deficiency in this group is vast and not limited to mycobacterial infections. Opportunistic viral (disseminated human papillomavirus [HPV] and HPV-associated squamous cell carcinoma) 51, parasitic and fungal infections, as well as pulmonary alveolar proteinosis (GATA2 is known to influence the phagocytic activity of pulmonary alveolar macrophages) can be seen 52. A majority of patients with GATA2 mutations eventually show deficiency of B lymphocytes, NK cells, CD4 lymphocytes, and monocytes 53.
Emberger's syndrome
Emberger's Syndrome is primary lymphedema with cutaneous warts, deafness, and a propensity to develop MDS/AML. Intact GATA2 function is required for proper lymphatic vascular development during embryogenesis in mice 54. Ostergaard and colleagues identified eight mutations in GATA2 in three patients with this syndrome by whole-exome sequencing identifying this mutation as the only common denominator between the group 48. At least 1 other patient with propensity to varicella zoster and salmonella infections has been reported 48. Complications secondary to prolonged lymphedema such as secondary cellulitis and deep vein thrombosis (DVT) are frequent 53. Null mutations in GATA2 appear to be associated with severe viral infections and lymphedema 52.
Familial MDS/AML
GATA2 overexpression has been documented in one-third to one half of nonfamilial AML and correlates with a poor prognosis with shorter overall and event-free survival when treated with standard chemotherapy 55,56. Of the original four families with GATA2 mutations, described by Hahn et al. with MDS/AML, three had the T354M mutation, and one had deletion T355. Both mutations occurred in the second zinc finger (ZF) of GATA2 (Fig 1). In the T354 mutation families, all members had the mutation but not all had developed hematological disease at least by the time of reporting 16. Bone marrow biopsies are typically hypocellular in contrast to the common MDS marrow picture, with abundant atypical megakaryocytes in >90% patients 53. Some patients have also fulfilled the diagnostic criteria for CMML (chronic myelomonocytic leukemia) and LGL (large granular lymphocytic leukemia) suggesting overlap syndromes 53. Other acquired mutations such as ASXL1 may herald the development of AML 57. Increased levels of FLT3 ligand have also been reported to be associated with clinical progression 58.
Figure 1.
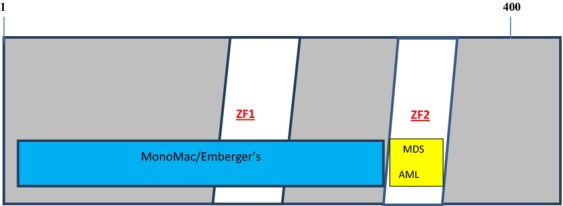
Genotype/phenotype relations of GATA2 mutations.
Chronic myeloid leukemia
A novel GATA2 mutation L359V has been found in nearly 10% of patients with accelerated or blast phase CML, but not CLL or ALL 59,60. This is thought to be mediated through PU.1 inhibition. It is interesting to note that GATA2 overexpression or the L359V gain-of-function mutation have been associated with AML and CML, respectively; whereas loss-of-function mutation of GATA2 such as T354M have been linked to MDS. L359 and T354 located in the same region on the second zinc finger of GATA2 thus highlighting the vital role GATA2 plays in hemostasis of myeloid precursors.
Aplastic anemia
Expression of GATA-2 mRNA in purified CD34-positive cells was significantly decreased in aplastic anemia compared with normal subjects when examined by immunocytochemical analysis 61. The changes extend further to stromal cells, with lower expression of GATA2 in patients with aplastic anemia when compared to controls by RT-PCR-ELISA 62. GATA-2 is instrumental in both hematopoiesis and adipogenesis. Overexpression of peroxisome proliferator-activated receptor-gamma (PPAR-γ, an adipogenic factor) and underexpression of GATA2 by mesenchymal stem cells may explain fatty marrow replacement in AA patients 63.
Pediatric neutropenia
A high frequency of GATA2 mutations has been reported in pediatric patients with mild chronic neutropenia 64. Analysis of French Neutropenia registry data revealed chronic familial neutropenia in seven families predisposing to MDS/AML associated with GATA2 mutations that included a complete deletion of GATA2 locus as well as additional mutations (p.R396Q, R204X, R330X, E224X, A372T, and M388V) 64.
Pulmonary disease
Ventilation-diffusion defects can be demonstrated in about two-thirds of GATA2-deficient patients while pulmonary hypertension (PAH) and pulmonary alveolar proteinosis (PAP) are some of the rare manifestations occurring in <20% in one series 53. PAP in GATA2 deficiency is not due to GM-CSF-(Granulocyte Monocyte- Colony Stimulating Factor) autoantibodies and is refractory to GM-CSF inhalational and subcutaneous therapy 53.
Treatment
Immune deficiency
Allogeneic HSCT remains the main therapy for GATA2-deficient patients with immunodeficiency. Timing of HSCT for immune deficiency alone is less well defined as compared to MDS/AML and should focus on risk-benefit ratios for the individual and the family. The incidence of HPV, mycobacterial, and fungal infections decreases considerably after successful allogeneic HSCT 53,65. Notably, it may take more than 3.5 years for reversal of phenotype and full immune reconstitution of B, NK, and monocyte populations 66. This may be especially problematic with delayed engraftment typical of umbilical cord grafts. Both PAP and PAH also respond well to HSCT and repeated lung infections or declining lung function should be considered an indication in clinical context 53. Earlier transplantation, before organ dysfunction ensues, results in less morbidity and mortality.
MDS/AML
Allogeneic HCT remains the only treatment with favorable responses in GATA2-mutated MDS/AML (Fig.3). In the NIH experience, 21 patients were transplanted for either hematological (MDS/AML) or immunological indications (age 15–49 years) with good responses (Fig.1). Of note, half the patients who were not transplanted passed away by age 40 51. A similar NIH experience further outlined use of conditioning regimens for nonmyeloablative allogeneic HCT 66. Donors included fully matched related and unrelated donors (conditioning-fludarabine + total body radiation 200 cGy) and alternative sources such as umbilical cord blood and haploidentical bone marrow (fludarabine + cyclophosphamide and total body irradiation 200 cGy, with posttransplant cyclophosphamide for T-cell replete grafts). Busulfan was later added for a more robust eradication of the GATA2 clone. Azithromycin was started before and continued for 1 year posttransplant due to increased propensity to nontuberculous mycobacterial (NTM) infections, in addition to standard prophylaxis. No NTM infections during or after transplant were reported using prophylaxis. Overall survival was 57% at 36 months. Our patient characteristics and outcomes are shown in Table2. Tacrolimus was used for graft versus host disease prophylaxis. All four patients have engrafted with 100% donor chimerisms (CD3 and CD33 fractions). One patient had CMV-Cytomegalovirus reactivation and refractory thrombocytopenia which failed to improve despite splenectomy. Three developed acute GVHD and one had chronic GVHD involving esophagus with dysphagia and strictures that improved with steroids.
Figure 3.
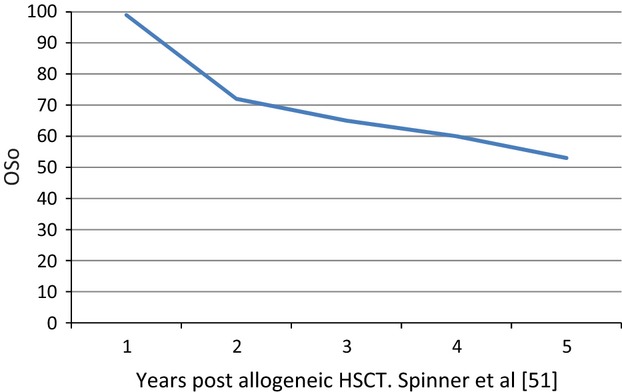
Years post allogeneic HSCT for GATA2 mutation Spinner et al. [53]HSCT, 5 hematopoietic stem cell transplant.
Two clinical trials are currently recruiting patients for myeloablative and reduced-intensity conditioning allogeneic HSCT for GATA2 mutations and enrollment is encouraged whenever feasible (NCT01861106 and NCT00923364 at www.clinicaltrails.gov).
Genetic counseling
Early genetic diagnosis and screening is paramount 53. Patients and families should be seen in conjunction with a geneticist. Suggested screening categories are listed in Table3. Variable phenotypes resulting from different GATA2 mutations are listed in Table4.
Table 3.
Suggested screening categories for GATA2 mutation
| Pediatric neutropenia |
| Monocytopenia |
| B-cell or NK cell cytopenia |
| Dendritic cell deficiency |
| MDS with hypocellular bone marrow |
| Familial MDS/AML |
| PAP in absence of anti-GM-CSF autoantibodies |
| Recurrent extra genital HPV warts or severe refractory genital HPV |
| Severe viral infection (HSV, EBV) |
| Lymphedema, often later onset |
| Sensorineural deafness with immunodeficiency |
| Disseminated nontuberculous mycobacteria (NTM) |
| Disseminated or severe fungal infections |
| Pulmonary NTM without bronchiectasis |
Modified from Spinner et al. 53. MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; HPV, human papillomavirus; PAP, pulmonary alveolar proteinosis; EBV, Epstein–Barr virus.
Table 4.
| Mutation type | AA location | Phenotype |
|---|---|---|
| Nonsense | 337(ZF1) | Emberger syndrome |
| Missense | 254 | MonoMAC/DCML |
| 354(ZF2) | Familial MDS/AML, MonoMAC/DCML | |
| 361(ZF2) | MonoMAC/DCML | |
| 371(ZF2) | MonoMAC/DCML | |
| 373(ZF2) | Emberger syndrome | |
| 396(ZF2) | MonoMAC/DCML | |
| 398(ZF2) | MonoMAC/DCML | |
| Frameshift | 1 | MonoMAC/DCML |
| 78 | Emberger syndrome | |
| 81 | MonoMAC/DCML | |
| 105 | Emberger syndrome | |
| 194 | Emberger syndrome | |
| 200 | MonoMAC/DCML | |
| 259 | MonoMAC/DCML | |
| 317(ZF1) | MonoMAC/DCML | |
| 341(ZF1) | Emberger syndrome | |
| In-frame insertion or deletion | 355(ZF2) | Familial MDS/AML |
| 361(ZF2) | MonoMAC/DCML, Emberger syndrome | |
| Large deletion | 340–381(ZF1 & 2) | MonoMAC/DCML |
MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; DCML, dendritic cell, monocyte, and lymphocyte.
Conflicts of Interest
None declared.
References
- Simon MC. Gotta have GATA. Nat. Genet. 1995;11:9–11. doi: 10.1038/ng0995-9. [DOI] [PubMed] [Google Scholar]
- Bresnick EH, Katsumura KR, Lee HY, Johnson KD. Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–5831. doi: 10.1093/nar/gks281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Simon MC, Pevny L, Wiles MV, Keller G, Costantini F. Orkin SH. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat. Genet. 1992;1:92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Weiss MJ. Orkin SH. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl. Acad. Sci. USA. 1995;92:9623–9627. doi: 10.1073/pnas.92.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Browne CP, Cunniff K, Goff SC. Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Shivdasani RA, Fujiwara Y. McDevitt MA. Transcription factor GATA-1 in megakaryocyte development. Stem Cells. 1998;16(Suppl. 2):79–83. doi: 10.1002/stem.5530160710. [DOI] [PubMed] [Google Scholar]
- Caldwell JT, Edwards H, Dombkowski AA, Buck SA, Matherly LH, Ge Y. Overexpression of GATA1 confers resistance to chemotherapy in acute megakaryocytic leukemia. PLoS One. 2013;8:e68601. doi: 10.1371/journal.pone.0068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC, Patel HV, Chen Q, van Remmen H, Matherly LH, Ge Y. Mutational spectrum at GATA1 provides insights into mutagenesis and leukemogenesis in Down syndrome. Blood. 2009;114:2753–2763. doi: 10.1182/blood-2008-11-190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente C, Conchillo A, Garcia-Sanchez MA. Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol. Hematol. 2012;82:1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Harigae H. GATA transcription factors and hematological diseases. Tohoku J. Exp. Med. 2006;210:1–9. doi: 10.1620/tjem.210.1. [DOI] [PubMed] [Google Scholar]
- Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsai FY. Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119:1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor C, Renneville A, Smith M, Charazac A, Iqbal S, Etancelin P. Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival. Haematologica. 2012;97:890–894. doi: 10.3324/haematol.2011.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick EH, Lee HY, Fujiwara T, Johnson KD. Keles S. GATA switches as developmental drivers. J. Biol. Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Crossley M, Hill J, Orkin SH. Adams JM. The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol. Cell. Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou ST, Khandros E, Bailey LC, Nichols KE, Vakoc CR, Yao Y. Graded repression of PU.1/Sfpi1 gene transcription by GATA factors regulates hematopoietic cell fate. Blood. 2009;114:983–994. doi: 10.1182/blood-2009-03-207944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pater E, Kaimakis P, Vink CS, Yokomizo T, Yamada-Inagawa T, van der Linden R. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013;210:2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH. Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- Ting CN, Olson MC, Barton KP. Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Pai SY, Brigl M, Besra GS, Gumperz J. Ho IC. GATA-3 regulates the development and function of invariant NKT cells. J. Immunol. 2006;177:6650–6659. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- Pai SY, Kang BY, Sabadini AM, Parisini E, Truitt ML. Ho IC. Distinct structural requirements of GATA-3 for the regulation of thymocyte and Th2 cell differentiation. J. Immunol. 2008;180:1050–1059. doi: 10.4049/jimmunol.180.2.1050. [DOI] [PubMed] [Google Scholar]
- Pai SY, Truitt ML. Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl. Acad. Sci. USA. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai TS, Pai SY. Ho IC. GATA-3 regulates the homeostasis and activation of CD8+ T cells. J. Immunol. 2013;190:428–437. doi: 10.4049/jimmunol.1201361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Smith C, De Franco E, Allen HL, Batlle M, Flanagan SE, Borowiec M. GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes. 2014;63:2888–2894. doi: 10.2337/db14-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters I, Dubrowinskaja N, Kogosov M, Abbas M, Hennenlotter J, von Klot C. Decreased GATA5 mRNA expression associates with CpG island methylation and shortened recurrence-free survival in clear cell renal cell carcinoma. BMC Cancer. 2014;14:101. doi: 10.1186/1471-2407-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagu ET, Layoun A, Calve A. Santos MM. Friend of GATA and GATA-6 modulate the transcriptional up-regulation of hepcidin in hepatocytes during inflammation. Biometals. 2013;26:1051–1065. doi: 10.1007/s10534-013-9683-6. [DOI] [PubMed] [Google Scholar]
- van Berlo JH, Aronow BJ. Molkentin JD. Parsing the roles of the transcription factors GATA-4 and GATA-6 in the adult cardiac hypertrophic response. PLoS One. 2013;8:e84591. doi: 10.1371/journal.pone.0084591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforest B. Nemer M. GATA5 interacts with GATA4 and GATA6 in outflow tract development. Dev. Biol. 2011;358:368–378. doi: 10.1016/j.ydbio.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- Grigorakaki C, Morceau F, Chateauvieux S, Dicato M. Diederich M. Tumor necrosis factor alpha-mediated inhibition of erythropoiesis involves GATA-1/GATA-2 balance impairment and PU.1 over-expression. Biochem. Pharmacol. 2011;82:156–166. doi: 10.1016/j.bcp.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Tanaka M, Zheng J, Yen H, Sato A, Sugiyama D. Redirecting differentiation of hematopoietic progenitors by a transcription factor, GATA-2. Blood. 2006;107:1857–1863. doi: 10.1182/blood-2005-06-2527. [DOI] [PubMed] [Google Scholar]
- Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Radomska HS. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. USA. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann V, Haferlach C, Nadarajah N, Fasan A, Weissmann S, Roller A. CEBPA double-mutated acute myeloid leukaemia harbours concomitant molecular mutations in 76.8% of cases with TET2 and GATA2 alterations impacting prognosis. Br. J. Haematol. 2013;161:649–658. doi: 10.1111/bjh.12297. [DOI] [PubMed] [Google Scholar]
- Mandoli A, Singh AA, Jansen PW, Wierenga AT, Riahi H, Franci G. CBFB-MYH11/RUNX1 together with a compendium of hematopoietic regulators, chromatin modifiers and basal transcription factors occupies self-renewal genes in inv(16) acute myeloid leukemia. Leukemia. 2013;28:770–778. doi: 10.1038/leu.2013.257. [DOI] [PubMed] [Google Scholar]
- Nottingham WT, Jarratt A, Burgess M, Speck CL, Cheng JF, Prabhakar S. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007;110:4188–4197. doi: 10.1182/blood-2007-07-100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijssen MR, Cvejic A, Joshi A, Hannah RL, Ferreira R, Forrai A. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev. Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NK, Timms RT, Kinston SJ, Cheng YH, Oram SH, Landry JR. Gfi1 expression is controlled by five distinct regulatory regions spread over 100 kilobases, with Scl/Tal1, Gata2, PU.1, Erg, Meis1, and Runx1 acting as upstream regulators in early hematopoietic cells. Mol. Cell. Biol. 2010;30:3853–3863. doi: 10.1128/MCB.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo JF, Lobo SA, Hsu AP, Zerbe CS, Wormser GP. Holland SM. MonoMAC syndrome in a patient with a GATA2 mutation: case report and review of the literature. Clin. Infect. Dis. 2013;57:697–699. doi: 10.1093/cid/cit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Imai K, Honma K, Tamura S, Imamura T, Ito M. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. Eur. J. Pediatr. 2012;171:1273–1276. doi: 10.1007/s00431-012-1715-7. [DOI] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat. Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- Hsu AP, Johnson KD, Falcone EL, Sanalkumar R, Sanchez L, Hickstein DD. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121:3830–3837. doi: 10.1182/blood-2012-08-452763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiding JW. Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J. Allergy Clin. Immunol. 2012;130:1030–1048. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasbury ME, Tang X, Durant PJ. Lee CH. Effect of transcription factor GATA-2 on phagocytic activity of alveolar macrophages from Pneumocystis carinii-infected hosts. Infect. Immun. 2003;71:4943–4952. doi: 10.1128/IAI.71.9.4943-4952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics and immunity. Blood. 2013;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J. Clin. Invest. 2012;122:3705–3717. doi: 10.1172/JCI61619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente C, Vazquez I, Conchillo A, Garcia-Sanchez MA, Marcotegui N, Fuster O. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2012;26:550–554. doi: 10.1038/leu.2011.235. [DOI] [PubMed] [Google Scholar]
- Ayala RM, Martinez-Lopez J, Albizua E, Diez A. Gilsanz F. Clinical significance of Gata-1, Gata-2, EKLF, and c-MPL expression in acute myeloid leukemia. Am. J. Hematol. 2009;84:79–86. doi: 10.1002/ajh.21332. [DOI] [PubMed] [Google Scholar]
- West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J. Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2013;99:276–281. doi: 10.3324/haematol.2013.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N. The evolution of cellular deficiency in GATA2 mutation. Blood. 2013;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Ma LY, Huang QH, Li G, Gu BW, Gao XD. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:2076–2081. doi: 10.1073/pnas.0711824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Shi JY. Li JY. GATA-2 L359 V mutation is exclusively associated with CML progression but not other hematological malignancies and GATA-2 P250A is a novel single nucleotide polymorphism. Leuk. Res. 2009;33:1141–1143. doi: 10.1016/j.leukres.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Fujimaki S, Harigae H, Sugawara T, Takasawa N, Sasaki T. Kaku M. Decreased expression of transcription factor GATA-2 in haematopoietic stem cells in patients with aplastic anaemia. Br. J. Haematol. 2001;113:52–57. doi: 10.1046/j.1365-2141.2001.02736.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Zhu K, Wang Z, Chen S. Yang L. GATA-1, -2 and -3 genes expression in bone marrow microenviroment with chronic aplastic anemia. Hematology. 2007;12:331–335. doi: 10.1080/10245330701255288. [DOI] [PubMed] [Google Scholar]
- Xu Y, Takahashi Y, Wang Y, Hama A, Nishio N, Muramatsu H. Downregulation of GATA-2 and overexpression of adipogenic gene-PPARgamma in mesenchymal stem cells from patients with aplastic anemia. Exp. Hematol. 2009;37:1393–1399. doi: 10.1016/j.exphem.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Pasquet M, Bellanne-Chantelot C, Tavitian S, Prade N, Beaupain B, Larochelle O. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T. Nonmyeloablative allogeneic hematopoietic stem-cell transplantation for GATA2 deficiency. Biol. Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.08.004. , et al. S1083–8791(14):00503–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme H, Hossain U, Kirwan M, Walne A, Vulliamy T. Dokal I. Marked genetic heterogeneity in familial myelodysplasia/acute myeloid leukaemia. Br. J. Haematol. 2012;158:242–248. doi: 10.1111/j.1365-2141.2012.09136.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Sanchez F, Cobos-Carrascosa E, Sanchez-Forte M, Martinez-Lirola M, Lopez-Ruzafa E, Galera-Martinez R. Different penetrance of disseminated infections caused by non-tuberculous mycobacteria in mendelian susceptibility to mycobacterial disease associated with a novel mutation. Pediatr. Infect. Dis. J. 2013;33:328–330. doi: 10.1097/INF.0000000000000099. [DOI] [PubMed] [Google Scholar]
- Machaczka M, Lorenz F, Kleinotiene G, Bulanda A, Markuszewska-Kuczynska A, Raistenskis J. Recurrent pulmonary aspergillosis and mycobacterial infection in an unsplenectomized patient with type 1 Gaucher disease. Ups. J. Med. Sci. 2013;119:44–49. doi: 10.3109/03009734.2013.857373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quispel WT, Stegehuis-Kamp JA, Santos SJ, van Wengen A, Dompeling E, Egeler RM. Intact IFN-gammaR1 expression and function distinguishes Langerhans cell histiocytosis from mendelian susceptibility to mycobacterial disease. J. Clin. Immunol. 2013;34:600. doi: 10.1007/s10875-013-9959-1. [DOI] [PubMed] [Google Scholar]
- Alders M, Mendola A, Ades L, Al Gazali L, Bellini C, Dallapiccola B. Evaluation of clinical manifestations in patients with severe lymphedema with and without CCBE1 mutations. Mol. Syndromol. 2013;4:107–113. doi: 10.1159/000342486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeer Karaca N, Boisson-Dupuis S, Aksu G, Bustamante J, Kandiloglu G, Ozsan N. Granulomatous skin lesions, severe scrotal and lower limb edema due to mycobacterial infections in a child with complete IFN-gamma receptor-1 deficiency. Immunotherapy. 2012;4:1121–1127. doi: 10.2217/imt.12.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K, Schulte D, Brice G, Simpson MA, Roukens MG, van Impel A. Mutation in vascular endothelial growth factor-C, a ligand for vascular endothelial growth factor receptor-3, is associated with autosomal dominant milroy-like primary lymphedema. Circ. Res. 2013;112:956–960. doi: 10.1161/CIRCRESAHA.113.300350. [DOI] [PubMed] [Google Scholar]
- Itoh M. Nakagawa H. A novel complex insertion-deletion mutation in the FOXC2 gene in a Japanese patient with Lymphedema-Distichiasis Syndrome. Eur. J. Dermatol. 2013;23:411–413. doi: 10.1684/ejd.2013.2022. [DOI] [PubMed] [Google Scholar]
- Mendola A, Schlogel MJ, Ghalamkarpour A, Irrthum A, Nguyen HL, Fastre E. Mutations in the VEGFR3 signaling pathway explain 36% of familial lymphedema. Mol. Syndromol. 2013;4:257–266. doi: 10.1159/000354097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Conlin LK, Gomez L, Aagenaes O, Eiklid K, Knisely AS. CCBE1 mutation in two siblings, one manifesting lymphedema-cholestasis syndrome, and the other, fetal hydrops. PLoS One. 2013;8:e75770. doi: 10.1371/journal.pone.0075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Motoi N, Tsuchihashi Y, Tazawa R, Kaneko C, Nei T. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J. Med. Genet. 2011;48:205–209. doi: 10.1136/jmg.2010.082586. [DOI] [PubMed] [Google Scholar]
- Hyde RK. Liu PP. GATA2 mutations lead to MDS and AML. Nat. Genet. 2011;43:926–927. doi: 10.1038/ng.949. [DOI] [PubMed] [Google Scholar]