Abstract
Cervical cancer is more common in the Somali immigrant population than the general population in the United States (US). There are low rates of cervical cancer screening among Somali women. This study compares cervical cancer screening test completion rates for a home human papilloma virus (HPV) test and standard clinic Pap test. Sixty-three Somali immigrant women aged 30–70 years who had not undergone cervical cancer screening within the past 3 years were randomly assigned to a home HPV test group (intervention) or a clinic Pap test group (control). Test completion rates were measured at 3 months. Univariate and multivariate logistic regression models were used to explore factors associated with test completion (intention-to-treat analysis). Participants in the HPV test group were 14 times more likely to complete the test compared to those in the Pap test group (P = 0.0002). Women who reported having friends/family members to talk about cancer screening were approximately three times more likely to complete any screening test than those who did not (P = 0.127) and participants who reported residing in the US longer were more likely to complete a screening test (P = 0.011). Future research should explore the potential of using the home-based HPV test kits as an initial approach to cervical cancer screening. Impact: The use of a self-sampling HPV kit has the potential to increase cervical cancer screening in under-served communities in the US.
Keywords: Cancer screening HPV self-collection test, Pap test, Somali women
Introduction
Significant gains have been made in cervical cancer screening over the past three decades in the United States (US); however, cervical cancer continues to cause morbidity and mortality among immigrant women 1,2. The current literature shows that some racial and ethnic groups in the US have disproportionately higher cervical cancer incidence and mortality rates that is associated with low use of screening services 3,4. One group is US immigrants whose cancer screening rates are far below the national goals 5,6. Studies have shown that more recent immigrants (<10 years) are less likely to screen for cervical cancer compared to women born in the US 7–9.
Cervical cancer is the second most frequent cancer among Somali women between 15 and 44 years of age 10. Harcourt and colleagues showed that among African immigrant women in Minnesota, Somali women were least likely to undergo screening for cervical cancer 11. Other studies by Morrison et al. showed that cervical cancer screening prevalence and adherence among Somali women was below the state and national goals 12,13. To date, several well-established barriers to cervical cancer have been documented in the Somali community and many communities worldwide 14–18.
Human papilloma virus (HPV)–DNA testing in cervical cancer prevention programs has been a topic at the forefront of cervical cancer policy discussions in recent years in developing countries 19,20. HPV–DNA testing provides a novel and alternative pathway to increasing cervical cancer screening among women who may not readily access a clinic Pap test 21. It is important to note that that a self-collected sample is equivalent in sensitivity to a direct collected specimen 22–25. With this new development, there is a growing need to examine preference, acceptability and reach among women who are less likely to access the clinic-based Pap test. Currently in the US, HPV–DNA testing is used in conjunction (cotesting) with the traditional clinic-based Pap test; however, there is growing evidence that use of HPV–DNA testing alone as a primary cervical cancer screening test may be an alternative in resource-limited regions 19,21,26,27, where significant barriers to access clinic-based Pap tests exists and substantial loss to follow up which cripples the effectiveness of cervical cancer screening programs 28–31. Furthermore, the HPV home test kit is growing in use in developing countries and has shown tremendous progress in identifying women at risk of cervical cancer and thus reducing mortality 27,32. There is growing evidence as to the test's sensitivity and efficiency rendering it as an alternative to the traditional Pap test. These findings suggest that primary HPV testing merits consideration as another alternative for cervical cancer screening 33,34.
Given that more than half of cervical cancer deaths in the US are among immigrants, and the incidence and mortality from cervical cancer are increasing among foreign-born women living in the US, 6 understanding the factors that influence women's cervical cancer screening practices and examining which screening options are acceptable in different populations may be an effective way to reduce the existing cervical cancer screening disparities. The objective of this pilot study was to examine the difference in successful test completion rates between home-based HPV tests and clinic-based Pap tests among a sample of Somali immigrant women residing in the Minneapolis/St. Paul area, to see if this innovative testing method might improve cervical cancer screening rates in this particular underserved population.
Methods
This work was a result of a partnership between a community-based Somali community organization; Somali Health Solutions and the University of Minnesota. Prior to the initiation of the study, community and university research partners met to develop culturally acceptable study informational materials. Using a community-based participatory approach similar to one used by Belinson and colleagues 35; Somali community health workers (CHWs) were trained by University researchers on the study protocol. The protocol training specifically included information on cervical cancer screening guidelines, randomization and a step-by-step instructional guide on use of the home-based HPV kit (“Just For Me,” Preventive Oncology International, Cleveland Heights, Ohio). Study procedures were approved and monitored by the University of Minnesota Institutional Review Board.
Study design
We planned a two-group randomized controlled pilot study of 64 Somali adult women designed to assess the completion rates of a home-based HPV kit versus standard of care clinic-based Pap test within a 3-month follow-up period. We hypothesized that women offered the HPV—DNA test kit would have a higher test completion rate compared to those in the clinic-based Pap test group.
Recruitment and randomization
Recruitment started in November 2013 and was completed in February 2014. Screening for study eligibility was conducted by Somali CHWs who were able to speak both Somali and English. The Somali Health Solutions team recruited participants by word of mouth and flyers. Prior to assessing eligibility and obtaining informed consent, we held an hour long informational meetings in an informal setting such as at one of the Somali women's homes or at a community center identified by the Somali Health Solutions staff. Ten to 20 women attended each informational session. These sessions focused on providing information about the home HPV test kit and the clinic Pap test. All participants were provided with written printed materials during the informational sessions. Following the training, all participants at the training completed a survey to determine study eligibility. Participants who were eligible and wanted to participate in the study provided informed consent and medical release forms for their primary care clinics to obtain potential Pap test data. Participants were then randomly assigned in a 1:1 ratio to the clinic based Pap test versus the home-based HPV test using permuted block randomization with varying block sizes. The study statistician provided the CHWs with randomization assignments in sealed envelopes. Each envelope was opened only after the participant was eligible and had provided a signed consent. Participants were compensated with a $25 gift card for their time.
Participants
This study recruited women of Somali origin, aged 25–70 years, who lived in the US for 10 years or less and who reported not having had a Pap test in the last 3 years. Women with a self- reported past history of a total hysterectomy, cervical cancer, and/or active history of cervical dysplasia were excluded.
Study procedures
All participants received education materials on HPV and Pap screening tests. The information provided was similar to that currently used by the Minnesota Sage Program and adapted in the study for Somali women. Following certain eligibility criteria, the Sage program provides free or subsidized office visits for breast and cervical exams, as well as mammogram and Pap tests 36.
Home-based HPV kit group
Participants randomized to the home-based HPV kit group were given a kit to perform their own vaginal HPV sample collection with detailed written instructions. Text and illustrations were translated and tailored for the Somali community. Participants were asked to complete specimen collection and return the sample to the CHWs within 3 months of study enrollment. Participants were provided with a study phone number in case of any questions pertaining to the test. Three months after the study, women in this group were contacted and provided with information by the CHWs to follow up with a Pap test, regardless of whether they completed home HPV testing. Participants were provided with a letter explaining their participation in the study and a request to the clinic to follow up with a clinic-based Pap test as it is the current standard of care. The self-collected samples were placed on specimen cards contained in the kits that were in turn sent to BGI Clinical Laboratories (BGI Shenzhen, Shenzhen, China), certified by National Health and Family Planning Commission of the People's Republic of China for analysis. They were analyzed using SEQHPV, a new validated high-risk HPV genotyping assay based on next-generation genomic sequencing 25.
Standard clinic Pap group
Participants randomized to clinic pap group (standard of care) were asked to follow up with their established clinic for a Pap test within 3 months of enrollment. Women without insurance or those lacking an established care clinic were given a list of Minnesota-based clinics which have a cancer screening Sage program close to their place of residence.
Measures
The primary outcome was the successful completion of the assigned screening test within a period of 3 months after the enrollment date. In the clinic Pap test group, completion was defined by documentation of a Pap test result by their primary care clinic during the study time frame. For the home-based HPV collection group, completion was defined as return of the HPV DNA self-sampling kit by the patient to the CHWs within 3 months of study enrollment.
Surveys
Baseline and other outcome study data were collected using paper surveys administered by the CHWs.
Baseline survey
Socio-demographic data collected included age, sex, marital status (single/married/widowed/divorced), need for an interpreter, use of emergency room services, health care provider preference, history of pregnancies, total annual household income (<$25,000, $25,000–99,999, or $100,000 and more), educational level (no formal education to greater than high school), and preference of receiving and sending medical test results.
HPV home test acceptability survey
An acceptability survey was conducted among participants who returned the home vaginal-based kit. This survey was conducted on receipt of a completed test from the participants. The test assessed participant's ease to do the test, difficulties encountered to do the test and any concerns they had while collecting the sample.
Data analysis
Demographic characteristics were summarized as means and standard deviations (SD) for continuous variables and percent in each category for discrete variables. Screening and baseline characteristics were compared by randomization group to check balance due to the small sample size. The primary analysis used an intention-to-treat approach and compared the proportions of successful completion of cervical cancer screening within 3 months of study entry between the clinic-based Pap group and home-based HPV group using a Pearson chi-square test. In addition, univariate and multivariate logistic regression models were conducted to explore factors associated with screening completion. Factors were included in the multivariate model as potential confounders if they were associated with randomization group and/or screening completion (P < 0.10). Analyses were conducted using SAS version 9.3 (Cary, NC) and P < 0.05 were considered statistically significant.
Results
Of the 242 women screened for the study, 75 (31%) were eligible and 11 declined to participate (Fig.1). Most women who attended the screening sessions were ineligible based on several reasons; a recent Pap smear (within 3 years), unknown Pap history, and history of hysterectomy. A total of 64 women were enrolled and randomized; 32 to the clinic-based Pap group and 32 to the home-based HPV group. One participant in the clinic-based Pap group was removed from analysis due to a protocol violation.
Figure 1.
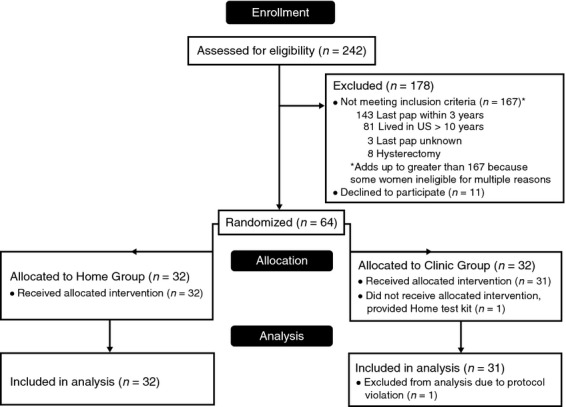
Participant flow chart. *One participant in the clinic group was assigned to clinic but given kit and returned it. This person was removed from the analysis. **There are two additional kits from the Home group that were returned after the 3-month time frame; their outcomes are not included in this analysis.
The average age of the 63 participants included in the analysis was 55.1 years (SD = 13.4), the majority were married, had no formal education, primarily spoke Somali, and had an annual household income of less than $25,000 (Table1). There were no significant differences in the demographic characteristics of participants between both groups.
Table 1.
Baseline characteristics (n = 63)
| Characteristic | Clinic-based Pap group | Home-based HPV kit group | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age mean (SD) | 31 | 54.2 (11.4) | 32 | 56.0 (15.3) | 0.585 |
| Years in US, mean (SD) | 31 | 11.2 (5.7) | 32 | 11.8 (5.5) | 0.683 |
| Years in MN mean (SD) | 30 | 9.9 (5.6) | 31 | 9.7 (5.6) | 0.907 |
| Marital status | |||||
| Single/never married | 2 | 6.3 | 4 | 12.9 | 0.119 |
| Married | 13 | 40.6 | 9 | 29.0 | |
| Separated | 4 | 12.5 | 3 | 9.7 | |
| Divorced | 2 | 6.3 | 9 | 29.0 | |
| Widowed | 11 | 34.4 | 6 | 19.4 | |
| Education | |||||
| No formal education | 11 | 36.7 | 14 | 45.2 | 0.189 |
| Less than fifth grade | 7 | 23.3 | 1 | 3.2 | |
| 5th–8th grade | 6 | 20.0 | 8 | 25.8 | |
| 8th–10th grade | 2 | 6.7 | 1 | 3.2 | |
| 10th–12th grade | 1 | 3.3 | 3 | 9.7 | |
| Completed high school | 0 | 0.0 | 2 | 6.5 | |
| More than high school | 3 | 10.0 | 2 | 6.5 | |
| Income | |||||
| <$25,000 | 24 | 80.0 | 25 | 80.7 | 1.00 |
| $25,000–49,999 | 4 | 13.3 | 5 | 16.1 | |
| $50,000–74,999 | 1 | 3.3 | 0 | 0.0 | |
| $75,000–99,999 | 1 | 3.3 | 0 | 0.0 | |
| Prefer not to answer | 0 | 0.0 | 1 | 3.2 | |
| Primary language | |||||
| Somali | 31 | 100.0 | 31 | 100.0 | – |
| Need an interpreter | |||||
| No | 6 | 19.4 | 3 | 9.7 | 0.473 |
| Yes | 25 | 80.7 | 28 | 90.3 | |
Table2 shows the health-related characteristics of participants by treatment group. More than half of the participants never had a Pap test, although this differed by randomization group (n = 12, 38.7% in clinic group vs. n = 20, 62.5% in home group; P = 0.062). The majority of women reported having health insurance and a regular doctor and did not think they were at risk of cervical cancer. More than half of the women reported having more than four previous pregnancies. All but two (96.6%) of the women reported that their doctors had never discussed with them anything to do with HPV vaccine. Beyond timing of last Pap test, there was no evidence of differences between randomized groups on these characteristics.
Table 2.
Health-related characteristics (n = 63)
| Characteristic | Clinic-based Pap group | Home-based HPV kit group | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Last Pap test | |||||
| 3–5 years | 14 | 45.2 | 8 | 25.0 | 0.062 |
| 5–10 years | 2 | 6.5 | 4 | 12.5 | |
| >10 years | 3 | 9.7 | 0 | 0.0 | |
| Never | 12 | 38.7 | 20 | 62.5 | |
| Regular doctor | |||||
| No | 6 | 19.4 | 3 | 9.7 | 0.473 |
| Yes | 25 | 80.7 | 28 | 90.3 | |
| Health care coverage | |||||
| No | 4 | 12.9 | 3 | 9.7 | 1.00 |
| Yes | 27 | 87.1 | 28 | 90.3 | |
| Time since last general exam | |||||
| Within past 2 years | 25 | 80.7 | 25 | 80.7 | 0.776 |
| More than 2 years | 4 | 12.9 | 3 | 9.7 | |
| Never | 1 | 3.2 | 3 | 9.7 | |
| Don't Know | 1 | 3.2 | 0 | 0.0 | |
| Doctor visits last year | |||||
| None | 15 | 48.4 | 10 | 32.3 | 0.410 |
| 1–4 times | 10 | 32.3 | 12 | 38.7 | |
| 5 + times | 6 | 19.4 | 9 | 29.0 | |
| Difficult to get primary care clinic | |||||
| No | 29 | 96.7 | 28 | 90.3 | 0.612 |
| Yes | 1 | 3.3 | 3 | 9.7 | |
| Prefer female provider | |||||
| Strongly disagree | 1 | 3.3 | 2 | 6.5 | 0.514 |
| Somewhat disagree | 2 | 6.7 | 4 | 12.9 | |
| Somewhat agree | 1 | 3.3 | 3 | 9.7 | |
| Strongly agree | 26 | 86.7 | 22 | 71.0 | |
| Difficult to get interpreter for health care | |||||
| Strongly disagree | 25 | 83.3 | 25 | 80.7 | 1.00 |
| Somewhat disagree | 1 | 3.3 | 1 | 3.2 | |
| Somewhat agree | 0 | 0.0 | 0 | 0.0 | |
| Strongly agree | 4 | 13.3 | 5 | 16.1 | |
| Want to know chance of getting cancer | |||||
| Strongly disagree | 11 | 36.7 | 17 | 54.8 | 0.286 |
| Somewhat disagree | 2 | 6.7 | 2 | 6.5 | |
| Somewhat agree | 0 | 0.0 | 1 | 3.2 | |
| Strongly agree | 17 | 56.7 | 11 | 35.5 | |
| Think you are at risk for cervical cancer | |||||
| Strong disagree | 21 | 70.0 | 28 | 90.3 | 0.130 |
| Somewhat disagree | 6 | 20.0 | 2 | 6.5 | |
| Somewhat agree | 2 | 6.7 | 0 | 0.0 | |
| Strongly agree | 1 | 3.3 | 1 | 3.2 | |
| See benefit in cancer screening | |||||
| Strong disagree | 1 | 3.3 | 2 | 6.5 | 1.00 |
| Somewhat disagree | 0 | 0.0 | 0 | 0.0 | |
| Somewhat agree | 0 | 0.0 | 1 | 3.2 | |
| Strongly agree | 29 | 96.7 | 28 | 90.3 | |
| History of C-section | |||||
| No | 28 | 93.3 | 28 | 90.3 | 1.00 |
| Yes | 2 | 6.7 | 3 | 9.7 | |
| History of female circumcision | |||||
| No | 2 | 6.7 | 4 | 12.9 | 0.671 |
| Yes | 28 | 93.3 | 27 | 87.1 | |
| Number of previous pregnancies | |||||
| None | 4 | 13.3 | 3 | 9.7 | 0.767 |
| 1–2 | 3 | 10.0 | 3 | 9.7 | |
| 3–4 | 3 | 10.0 | 6 | 19.4 | |
| More than 4 | 20 | 66.7 | 19 | 61.3 | |
| Friends/family members to talk about health | |||||
| No | 11 | 36.7 | 10 | 32.3 | 0.717 |
| Yes | 19 | 63.3 | 21 | 67.7 | |
| Friends/family members to talk about cancer screening | |||||
| No | 21 | 70.0 | 16 | 51.6 | 0.142 |
| Yes | 9 | 30.0 | 15 | 48.4 | |
| Anyone in family ever had cancer | |||||
| No | 26 | 86.7 | 24 | 77.4 | 0.508 |
| Yes | 4 | 13.3 | 7 | 22.6 | |
| Ever told by doctor to have mammogram | |||||
| No | 11 | 36.7 | 10 | 32.3 | 0.717 |
| Yes | 19 | 63.3 | 21 | 67.7 | |
| Doctor ever discussed HPV vaccine with you? | |||||
| No | 29 | 100.0 | 27 | 93.1 | 0.491 |
| Yes | 0 | 0.0 | 2 | 6.9 | |
| Feel comfortable sending testing materials in mail | |||||
| No | 13 | 43.3 | 12 | 38.7 | 0.714 |
| Yes | 17 | 56.7 | 19 | 61.3 | |
| Prefer to receive medical test results | |||||
| In-person | 7 | 23.3 | 3 | 9.7 | 0.262 |
| 20 | 66.7 | 22 | 71.0 | ||
| Phone | 3 | 10.0 | 6 | 19.4 | |
Analysis of the primary outcome indicated that participants randomized to the home test group were more likely to complete the test (21/32, 65.6%) than those randomized to the clinic group (6/31, 19.4%); P = 0.0002). Univariate logistic regression analyses indicated that those randomized to the home group, those who reported having family/friends to talk about cancer screening, and those who have been in the US longer were more likely to have completed screening (Table3). After adjusting for time since last Pap test, whether friends/family members talk about cancer screening and number of years in the US, multivariate logistic regression analyses indicated that women randomized to the home-based HPV group were about 14 times more likely to complete screening than those assigned to the clinic-based Pap group (OR: 14.18 [95% CI: 2.73–73.51]). While this was highly statistically significant (P = 0.002), this odds ratio should be cautiously interpreted given the sample size and large confidence interval.
Table 3.
Factors associated with test completion (n = 63)
| Variable | Test completed | Univariate analysis | Multivariate analysis1 | |||
|---|---|---|---|---|---|---|
| N | % | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Randomization group | ||||||
| Clinic | 6 | 19.4 | 1.00 | 0.0004 | 1.00 | 0.002 |
| Home | 21 | 65.6 | 7.95 (2.52–25.16) | 14.18 (2.73–73.51) | ||
| Last Pap test | ||||||
| 3–5 years | 10 | 45.5 | 1.00 | 0.949 | 1.00 | 0.973 |
| 5–10 years | 3 | 50.0 | 1.20 (0.20–7.31) | 1.43 (0.05–44.80) | ||
| >10 years | 1 | 33.3 | 0.60 (0.05–7.63) | 1.64 (0.11–25.30) | ||
| Never | 13 | 40.6 | 0.82 (0.27–2.46) | 0.86 (0.16–4.73) | ||
| Marital status | ||||||
| Single/never | 2 | 33.3 | 0.44 (0.06–3.11) | 0.706 | ||
| Married | 8 | 36.4 | 0.51 (0.14–1.84) | |||
| Widowed | 8 | 47.1 | 0.79 (0.21–3.04) | |||
| Divorced/separated | 9 | 52.9 | 1.00 | |||
| Education | ||||||
| No formal education | 9 | 36.0 | 1.00 | 0.525 | ||
| Less than high school | 12 | 48.0 | 1.64 (0.53–5.09) | |||
| Completed high school or more | 6 | 54.6 | 2.13 (0.51–9.01) | |||
| Regular doctor | ||||||
| Yes | 21 | 39.6 | 0.33 (0.07–1.46) | 0.143 | ||
| No | 6 | 66.7 | 1.00 | |||
| Friends/family members to talk about cancer screening | ||||||
| Yes | 15 | 62.5 | 3.47 (1.18–10.18) | 0.023 | 3.14 (0.72–13.67) | 0.127 |
| No | 12 | 32.4 | 1.00 | 1.00 | ||
| Want to know chance of getting cancer | ||||||
| Strongly agree/somewhat agree | 17 | 53.1 | 0.46 (0.17–1.31) | 0.146 | ||
| Strongly disagree/somewhat disagree | 10 | 34.5 | 1.00 | |||
| Anyone in family ever had cancer | ||||||
| Yes | 7 | 63.6 | 2.63 (0.68–10.15) | 0.162 | ||
| No | 20 | 40.0 | 1.00 | |||
| Age (per 5 year increase) | 27 | 0.98 (0.81–1.18) | 0.804 | |||
| Years in United States (per 1 year increase) | 27 | 1.16 (1.04–1.28) | 0.007 | 1.23 (1.05–1.44) | 0.011 | |
Adjusted for randomization group, time since last Pap test, whether friends/family members to talk about cancer screening and number of years in the United States.
Women who reported having friends/family members to talk about cancer screening were approximately three times more likely to complete screening than those who do not, although this was not statistically significant after multivariate adjustment (OR: 3.14; [95% CI: 0.72–13.67], P = 0.127). Finally, women who have lived in the US longer were more likely to complete screening remained statistically significant after adjustment (OR per 1 year longer: 1.23; [95% CI: 1.05–1.44], P = 0.011).
The results from the acceptability survey completed by women in the HPV test group showed that 83% of these women indicated that if they had to make a choice between using the self- collection and the regular Pap test, they would choose the self-collection method. All participants indicated that the instructions were easy to follow and had no difficulties in collecting the sample. In addition, 30% of women agreed to DNA sample storage.
HPV genotyping results showed that 3/21 (14.2%) of women who completed and returned the home-based HPV test kit had a positive result; two participants were positive for HPV 16 while one participant was positive for HPV 68; both HPV types are considered high risk for cervical cancer. Test results for all the 21 women will be delivered and they will be strongly encouraged to obtain a clinic-based Pap test as this is the current standard of care in the US. Results from the clinic-based Pap test obtained from six participants were all negative for intraepithelial lesion or malignancy.
Discussion
This pilot study compares HPV home-based cervical cancer screening rates versus clinic-based Pap test among Somali immigrant women in the US. We found a screening completion rate of 65.6% for the home-based HPV kit versus 19.4% for the clinic-based Pap test. The screening completion rates in our study are comparable to findings from a systemic review and meta-analysis that showed that the overall relative compliance of HPV self-collected testing was significantly greater compared to Pap testing 37. Several other studies have shown a high acceptability rate for the home-based self-sampling over Pap test 38–42. However, some studies have assessed the barriers to acceptance of the self-sampling HPV test and showed that some women preferred Pap testing by a health care professional because they were accustomed to pelvic examinations, the test was more convenient or they trusted the results 15,43–45.
Furthermore, the Pap test completion rates for Somali women in our study were low at 19.4% compared to those reported in a previous study done by Morrison and colleagues in Minnesota, where the Pap test completion rates for Somali immigrant women were at 48.8% 13. This discrepancy could be a result of two very different recruitment strategies: Morrison et al. used a clinic data base for his study, while our study recruited participants who may or may not have been enrolled in a clinic, an approach which could have created the potential for such a difference. A larger size study is needed to validate these findings. Our findings further confirm that foreign-born women are less likely to receive a Pap test compared to US born women 6.
In addition, our study showed that women who reported having a social network of friends and family to talk about cancer screening and had more than 10 years of residency in the US were more likely to complete the screening test. This information further justifies the need for a community-based model that involves friends and family members in programs designed to increase screening utilization in immigrant communities. This implies that an intervention targeting the individual and their support system may enhance cervical cancer screening among under screened women. Our findings are similar to those from studies finding that one of the strong factors associated with cervical cancer screening among immigrants is suggestion from friends, followed by longer residency 9,46–48.
A large number of our study participants were already engaged in the health care system, with more than 80% having had a general physical exam within the past 2 years. The majority reported visiting a regular health care provider and had no difficulty in getting to their primary care clinic. Despite their encounters in the health care environment, more than 50% reported never receiving a Pap test. These patient clinic encounters should be used as opportunities to expand alternative methods of cervical cancer screening within the current clinic setting.
Lack of insurance coverage is considered a significant barrier to cancer screening 49; however, in our study, the majority of women reported having health insurance. More than 50% of the women reported that they were not at risk for cervical cancer and nearly 50% did not want to know their chances of getting cancer. This supports other studies highlighting the significance of increasing awareness as component to any cervical cancer prevention initiative among immigrant communities 14.
Studies have documented several sociocultural factors that influence cervical cancer screening among immigrants and ethnic minorities. Commonly held beliefs cut across several cultural groups 50–52. One unique finding in our study is that more than 80% of our study participants strongly believed that they were not at any risk of getting cervical cancer, which is in agreement with the HPV positivity test results we obtained. Our findings are similar to the perceptions and barriers that have been well documented showing that many Somali women held fatalistic attitudes and lacked an understanding of the risk factors for cervical cancer 14,51. The strong belief that one is not at risk of a getting a particular disease has been documented as a deterrent for an individual to make an effort to undergo screening. The health belief model framework shows that individuals who believe they are at no risk are less likely to screen for a disease 53.
This study has some limitations. First, it was conducted in a metropolitan area in the upper Midwest of the US and there may be differences between cities, which limits the external validity. However, this generalizability concern is somewhat mitigated by the fact that Minneapolis has the largest Somali immigrant population in the US. In addition, this was a pilot study and therefore the sample size used here was small. Due to the small sample size and random chance, the randomization did not completely balance the two groups for all potential confounders. In this case, there was a difference in the proportion who had never had a pap test before between the groups (n = 20 (63%) in the home group versus n = 12 (39%) in the pap group). We attempted to account for this difference in the analysis but this could potentially be avoided in future studies by either enrolling a larger sample and/or by stratifying the randomization on this variable. Finally, we solely relied on self-report of Pap test history; this may have created bias if some of the participants were not aware if they had the screening test within the past 3 years.
This study adds to the literature by providing evidence that despite the existing barriers to cancer screening in the Somali immigrant community, women are willing and able to use an alternative screening test as an initial approach to cervical cancer screening.
Conclusion
The use of a self-sampling HPV kit has the potential to increase screening in this community; it addresses some major sociocultural barriers such as lack of privacy created by the need for an interpreter during a clinic-based Pap test. Future research should explore using the home-based HPV test kits as an initial approach to encourage use of cervical cancer screening in the Somali community. In addition, we need to develop culturally and linguistically relevant screening education programs that address the sociocultural factors that continue to fuel the growing cervical cancer screening disparities in immigrants and minority ethnic populations in the US.
Acknowledgments
This work was supported by the grants from the National Cancer Institute: Minnesota Community Networks Center for Eliminating Cancer Disparities (U54CA153603), Cancer-related Health Disparities Education and Career Development Program (1R25CA163184), and P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award number UL1TR000114. Special thanks to the Somali immigrant women for volunteering to participate in this study. We also thank the management and staff of the Somali Health Solutions Services team who aided in the recruitment and moderating the interviews.
Conflict of Interest
None declared.
References
- Collins Y, Holcomb K, Chapman-Davis E, Khabele D. Farley JH. Gynecologic cancer disparities: a report from the health disparities taskforce of the society of gynecologic oncology. Gynecol. Oncol. 2014;133:353–361. doi: 10.1016/j.ygyno.2013.12.039. , and. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeff LC. McKenna MT. Cervical cancer mortality among foreign-born women living in the United States, 1985 to 1996. Cancer Detect. Prev. 2003;27:203–208. doi: 10.1016/s0361-090x(03)00062-x. [DOI] [PubMed] [Google Scholar]
- (CDC), C. f. D. C. a. P., & Control, D. o. C. P. a. 2013. Cervical cancer rates by race and ethnicity; 1999-2010. Available at http://www.cdc.gov/cancer/cervical/statistics/race.htm (accessed 20 July 2014)
- Rositch AF, Nowak RG. Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032–2038. doi: 10.1002/cncr.28548. , and. doi: 10.1002/cncr.28548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel MS, Wee CC, McCarthy EP, Davis RB, Ngo-Metzger Q. Phillips RS. Racial and ethnic disparities in cancer screening: the importance of foreign birth as a barrier to care. J. Gen. Intern. Med. 2003;18:1028–1035. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Saraiya M, Thompson T, Dey A. Richardson L. Cervical cancer screening among foreign-born women by birthplace and duration in the United States. J. Womens Health (Larchmt) 2007;16:1447–1457. doi: 10.1089/jwh.2006.0279. , and. doi: 10.1089/jwh.2006.0279. [DOI] [PubMed] [Google Scholar]
- Carrasquillo O. Pati S. The role of health insurance on Pap smear and mammography utilization by immigrants living in the United States. Prev. Med. 2004;39:943–950. doi: 10.1016/j.ypmed.2004.03.033. , and. doi: 10.1016/j.ypmed.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Swan J, Breen N, Coates RJ, Rimer BK. Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. doi: 10.1002/cncr.11208. , and. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- Swan J, Breen N, Graubard BI, McNeel TS, Blackman D, Tangka FK, et al. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–4881. doi: 10.1002/cncr.25215. . doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni LBRL, Serrano B. Brotons M, Cosano R, Muñoz J, Bosch FX, deSanjosé S, et al. 2014. Human papillomavirus and related diseases in Somalia Available at http://www.hpvcentre.net/statistics/reports/SOM.pdf (accessed 22 July 2014)
- Harcourt N, Ghebre RG, Whembolua GL, Zhang Y, Warfa Osman S. Okuyemi KS. Factors Associated with breast and cervical cancer screening behavior among African immigrant women in Minnesota. J. Immigr. Minor. Health. 2013 doi: 10.1007/s10903-012-9766-4. , and. doi: 10.1007/s10903-012-9766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TB, Flynn PM, Weaver AL. Wieland ML. Cervical cancer screening adherence among Somali immigrants and refugees to the United States. Health Care Women Int. 2013;34:980–988. doi: 10.1080/07399332.2013.770002. , and. doi: 10.1080/07399332.2013.770002. [DOI] [PubMed] [Google Scholar]
- Morrison TB, Wieland ML, Cha SS, Rahman AS. Chaudhry R. Disparities in preventive health services among Somali immigrants and refugees. J. Immigr. Minor. Health. 2012;14:968–974. doi: 10.1007/s10903-012-9632-4. , and. doi: 10.1007/s10903-012-9632-4. [DOI] [PubMed] [Google Scholar]
- Abdullahi A, Copping J, Kessel A, Luck M. Bonell C. Cervical screening: perceptions and barriers to uptake among Somali women in Camden. Public Health. 2009;123:680–685. doi: 10.1016/j.puhe.2009.09.011. , and. doi: 10.1016/j.puhe.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Howard M, Lytwyn A, Lohfeld L, Redwood-Campbell L, Fowler N. Karwalajtys T. Barriers to acceptance of self-sampling for human papillomavirus across ethnolinguistic groups of women. Can. J. Public Health. 2009;100:365–369. doi: 10.1007/BF03405272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano-Ponce EC, Castro R, Allen B, Najera P, Alonso de Ruiz PA. Hernandez-Avila M. Barriers to early detection of cervical-uterine cancer in Mexico. J. Womens Health. 1999;8:399–408. doi: 10.1089/jwh.1999.8.399. [DOI] [PubMed] [Google Scholar]
- Ndukwe EG, Williams KP. Sheppard V. Knowledge and perspectives of breast and cervical cancer screening among female African immigrants in the Washington D.C. metropolitan area. J. Cancer Educ. 2013;28:748–754. doi: 10.1007/s13187-013-0521-x. , and. doi: 10.1007/s13187-013-0521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood-Campbell L, Fowler N, Laryea S, Howard M. Kaczorowski J. ‘Before you teach me, I cannot know’: immigrant women's barriers and enablers with regard to cervical cancer screening among different ethnolinguistic groups in Canada. Can. J. Public Health. 2011;102:230–234. doi: 10.1007/BF03404903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. . doi: 10.1016/s1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan A, Efrusy M, Lindeque G, Dreyer G. Santas C. Cost effectiveness of high-risk HPV DNA testing for cervical cancer screening in South Africa. Gynecol. Oncol. 2009;112:377–383. doi: 10.1016/j.ygyno.2008.08.030. , and. doi: 10.1016/j.ygyno.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Castle PE. Cremer M. Human papillomavirus testing in cervical cancer screening. Obstet. Gynecol. Clin. North Am. 2013;40:377–390. doi: 10.1016/j.ogc.2013.03.002. , and. doi: 10.1016/j.ogc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Belinson JL, Du H, Yang B, Wu R, Belinson SE, Qu X, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int. J. Cancer. 2012;130:1855–1860. doi: 10.1002/ijc.26202. . doi: 10.1002/ijc.26202. [DOI] [PubMed] [Google Scholar]
- Belinson JL, Hu S, Niyazi M, Pretorius RG, Wang H, Wen C, et al. Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: implications for vaginal self-collection. Int. J. Cancer. 2010;127:1151–1157. doi: 10.1002/ijc.25144. . doi: 10.1002/ijc.25144. [DOI] [PubMed] [Google Scholar]
- Gravitt PE, Belinson JL, Salmeron J. Shah KV. Looking ahead: a case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int. J. Cancer. 2011;129:517–527. doi: 10.1002/ijc.25974. , and. doi: 10.1002/ijc.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Zou J, Xu J, Liu T, Hua S, Xi F, et al. Development and validation of a new HPV genotyping assay based on next-generation sequencing. Am. J. Clin. Pathol. 2014;141:796–804. doi: 10.1309/AJCP9P2KJSXEKCJB. . doi: 10.1309/ajcp9p2kjsxekcjb. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl. 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. . doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Gage JC, Ajenifuja KO, Wentzensen N, Adepiti AC, Stoler M, Eder PS, et al. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. Int. J. Cancer. 2012;131:2903–2909. doi: 10.1002/ijc.27563. . doi: 10.1002/ijc.27563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravitt PE, Coutlee F, Iftner T, Sellors JW, Quint WG. Wheeler CM. New technologies in cervical cancer screening. Vaccine. 2008;26(Suppl. 10):K42–K52. doi: 10.1016/j.vaccine.2008.05.002. , and. doi: 10.1016/j.vaccine.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Budukh AM. Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull. World Health Organ. 2001;79:954–962. [PMC free article] [PubMed] [Google Scholar]
- Swaminathan R, Lucas E. Sankaranarayanan R. Cancer survival in Africa, Asia, the Caribbean and Central America: database and attributes. IARC Sci. Publ. 2011;162:23–31. [PubMed] [Google Scholar]
- Wong LP, Wong YL, Low WY, Khoo EM. Shuib R. Cervical cancer screening attitudes and beliefs of Malaysian women who have never had a pap smear: a qualitative study. Int. J. Behav. Med. 2008;15:289–292. doi: 10.1080/10705500802365490. , and. doi: 10.1080/10705500802365490. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N. Engl. J. Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. . doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- Delere Y, Schuster M, Vartazarowa E, Hansel T, Hagemann I, Borchardt S, et al. Cervicovaginal self-sampling is a reliable method for determination of prevalence of human papillomavirus genotypes in women aged 20 to 30 years. J. Clin. Microbiol. 2011;49:3519–3522. doi: 10.1128/JCM.01026-11. . doi: 10.1128/jcm.01026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N. Engl. J. Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. . doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- Belinson JL, Wang G, Qu X, Du H, Shen J, Xu J, et al. The development and evaluation of a community based model for cervical cancer screening based on self-sampling. Gynecol. Oncol. 2014;132:636–642. doi: 10.1016/j.ygyno.2014.01.006. . doi: 10.1016/j.ygyno.2014.01.006. [DOI] [PubMed] [Google Scholar]
- (MDH), M. D. o. H. 2014. Breast and Cervical Cancer Screening Sage Program. Available at http://www.health.state.mn.us/divs/hpcd/ccs/screening/sage/ (accessed 17 July 2014)
- Racey CS, Withrow DR. Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta- analysis. Can. J. Public Health. 2013;104:e159–e166. doi: 10.1007/BF03405681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhang R, Nelson JA, Telerant R, Chiasson MA. Wright TC., Jr Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J. Womens Health (Larchmt) 2005;14:721–728. doi: 10.1089/jwh.2005.14.721. , and. doi: 10.1089/jwh.2005.14.721. [DOI] [PubMed] [Google Scholar]
- Dzuba IG, Diaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, et al. The acceptability of self-collected samples for HPV testing vs. the pap test as alternatives in cervical cancer screening. J. Womens Health Gend. Based Med. 2002;11:265–275. doi: 10.1089/152460902753668466. . doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- Levinson KL, Abuelo C, Salmeron J, Chyung E, Zou J, Belinson SE, et al. The Peru Cervical Cancer Prevention Study (PERCAPS): the technology to make screening accessible. Gynecol. Oncol. 2013;129:318–323. doi: 10.1016/j.ygyno.2013.01.026. . doi: 10.1016/j.ygyno.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Garnier H, Tamalet C, Halfon P, Leandri FX, Le Retraite L, Djoufelkit K, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int. J. Cancer. 2013;133:2681–2687. doi: 10.1002/ijc.28283. . doi: 10.1002/ijc.28283. [DOI] [PubMed] [Google Scholar]
- Tamalet C, Le Retraite L, Leandri FX, Heid P, Sancho Garnier H. Piana L. Vaginal self-sampling is an adequate means of screening HR-HPV types in women not participating in regular cervical cancer screening. Clin. Microbiol. Infect. 2013;19:E44–E50. doi: 10.1111/1469-0691.12063. , and. doi: 10.1111/1469-0691.12063. [DOI] [PubMed] [Google Scholar]
- Arriba LN, Enerson CL, Belinson S, Novick L. Belinson J. Mexican Cervical Cancer Screening Study II: acceptability of human papillomavirus self- sampler. Int. J. Gynecol. Cancer. 2010;20:1415–1423. doi: 10.1111/IGC.0b013e3181f58678. , and. doi: 10.1111/IGC.0b013e3181f58678. [DOI] [PubMed] [Google Scholar]
- Bansil P, Wittet S, Lim JL, Winkler JL, Paul P. Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health. 2014;14:596. doi: 10.1186/1471-2458-14-596. , and. doi: 10.1186/1471-2458-14-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisci S, Shen YH, Fife D, Huang J, Goycoolea J, Ma CP, et al. Patient acceptance of self-sampling for human papillomavirus in rural China. J. Low Genit. Tract. Dis. 2003;7:107–116. doi: 10.1097/00128360-200304000-00007. [DOI] [PubMed] [Google Scholar]
- Ma GX, Toubbeh JI, Wang MQ, Shive SE, Cooper L. Pham A. Factors associated with cervical cancer screening compliance and noncompliance among Chinese, Korean, Vietnamese, and Cambodian women. J. Natl Med. Assoc. 2009;101:541–551. doi: 10.1016/s0027-9684(15)30939-1. [DOI] [PubMed] [Google Scholar]
- Nguyen-Truong CK, Lee-Lin F, Leo MC, Gedaly-Duff V, Nail LM, Wang PR, et al. A community-based participatory research approach to understanding pap testing adherence among Vietnamese American immigrants. J. Obstet. Gynecol. Neonatal. Nurs. 2012;41:E26–E40. doi: 10.1111/j.1552-6909.2012.01414.x. . doi: 10.1111/j.1552-6909.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- Schleicher E. Immigrant women and cervical cancer prevention in the United States. Baltimore, MD: Women's and Children's Health Policy Center, Johns Hopkins Bloomberg School of Public Health; 2007. [Google Scholar]
- Carroll J, Epstein R, Fiscella K, Volpe E, Diaz K. Omar S. Knowledge and beliefs about health promotion and preventive health care among somali women in the United States. Health Care Women Int. 2007;28:360–380. doi: 10.1080/07399330601179935. , and. doi: 10.1080/07399330601179935. [DOI] [PubMed] [Google Scholar]
- Ackerson K. Gretebeck K. Factors influencing cancer screening practices of underserved women. J. Am. Acad. Nurse Pract. 2007;19:591–601. doi: 10.1111/j.1745-7599.2007.00268.x. , and. doi: 10.1111/j.1745-7599.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- Ghebre RG, Sewali B, Osman S, Adawe A, Nguyen HT, Okuyemi KS, et al. Cervical cancer: barriers to screening in the Somali community in Minnesota. J. Immigr. Minor. Health. 2014 doi: 10.1007/s10903-014-0080-1. (Epub ahead of print). doi: 10.1007/s10903-014-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CE, Mues KE, Mayne SL. Kiblawi AN. Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the Health Belief Model. J. Low Genit. Tract. Dis. 2008;12:232–241. doi: 10.1097/LGT.0b013e31815d8d88. , and. doi: 10.1097/LGT.0b013e31815d8d88. [DOI] [PubMed] [Google Scholar]
- Moore de Peralta A, Holaday B. McDonell JR. Factors affecting Hispanic women's participation in screening for cervical cancer. J. Immigr. Minor. Health. 2014 doi: 10.1007/s10903-014-9997-7. , and (Epub ahead of print). doi: 10.1007/s10903-014-9997-7. [DOI] [PubMed] [Google Scholar]


