Abstract
Background & Aims
There have been inconsistent reports of pre- and perinatal factors that affect risk for development of celiac disease. We assessed the association of fetal growth, birth weight, and mode of delivery with development of celiac disease within the Norwegian Mother and Child (MoBa) cohort study.
Methods
The MoBa cohort contains pregnancy information on 95,200 women and data on their 114,500 children, collected in Norway from 1999 through 2008; it is linked to the Medical Birth Registry. Women and children with celiac disease were identified from the National Patient Register and from women's responses to MoBa questionnaires. We calculated odds ratios (ORs) for celiac disease using a multivariable logistic regression model, adjusting for maternal celiac disease, sex of children, and children's age (model 1); in a second model, we adjusted for age of gluten introduction and duration of breastfeeding (model 2).
Results
We identified 650 children with celiac disease and 107,828 controls in the MoBa database. We found no association between birth weight or height with celiac disease (born small for gestational age was not associated). Celiac disease was not associated with mode of delivery (Cesarean section, model 1: OR=0.84; 95% confidence interval [CI], 0.65–1.09 and model 2: OR=0.83; 95% CI, 0.63−1.09). Maternal celiac disease, adjusted for age and sex of the children (OR=12.45; 95% CI, 8.29−18.71) and type 1 diabetes (model I: OR=2.58; 95% CI, 1.19−5.53 and model 2: OR=2.61; 95% CI, 1.14−5.98) were associated with development of celiac disease in children, whereas maternal type 2 diabetes and gestational diabetes were not.
Conclusion
Based on analysis of the Norwegian MoBa cohort, development of celiac disease in children is significantly associated with sex of the child, maternal celiac disease and type 1 diabetes, but not with intrauterine growth.
Keywords: coeliac, pregnancy outcome, prematurity, perinatal factors
Introduction
Celiac disease (CD) is an immune-mediated disease triggered by the ingestion of gluten. CD affects about 1% of the Western population1 and is diagnosed in 0.4% of Norwegian children aged 0-12 years2. The genetic impact of CD is well known but it is still unclear which additional environmental factors (outside gluten ingestion) affect the development of the disease3. Earlier studies have suggested that Cesarean section, particularly elective, is associated with an increased risk of CD4, 5, although other studies have suggested that Cesarean section is not associated with CD or might even be protective6. Being small for gestational age (SGA) has been associated with future CD4, 7. Also maternal smoking and parity have been reported to increase the risk of CD in offspring6 as well as low birth weight7.
As previous studies have indicated that reduced birth weight is a risk factor, we tested this hypothesis as well as pregnancy factors known to impair fetal growth such as maternal smoking, preeclampsia or hypertension. Also impact of maternal diabetes, which on the contrary is likely to increase birth weight of the child, was assessed. Furthermore we analyzed whether maternal education and parity had an impact on future CD since earlier studies has suggested that children of mothers with higher social class and parity are at an increased risk of future CD6. In the current study we also examined the impact of Cesarean section on future development of CD in the Norwegian Mother and Child (MoBa) cohort.
Methods
Data for this study was extracted from the prospectively collected Norwegian MoBa cohort8 conducted by the Norwegian Institute of Public Health. Pregnant women were recruited around 18 gestational weeks from all over Norway from 1999 to 2008. The participation rate was 40.6%. Mothers could participate with more than one pregnancy. In total approximately 95,200 mothers and 114,500 children were included in the cohort. Written informed consent was obtained from all participants. The current study used version VII of the quality assured data files which included all follow-up information available around June 2013 and included 114,285 children. The questionnaires used for this study were filled out around weeks 18 and 30 of pregnancy and at ages 6 and 18 months as well as 7 and 8 years of age. (All of the questionnaires are available at www.fhi.no/moba.)
The cohort database is also linked to the Medical Birth Registry of Norway (MBRN) and the National Patient Register (NPR) using unique personal identification numbers. The Norwegian Data Inspectorate has approved the ongoing data collection in MoBa. The Regional Committee for Medical Research Ethics in Southeastern Norway approved of the current study.
The National Patient Register
The NPR contains information on all diagnoses according to the International Classification of Diseases, 10th revision (ICD-10) codes, set in all government-owned Norwegian hospitals and outpatient clinics. The information is mandatory and virtually complete. Information in this registry has been available on an individual level since 2008, when the Norwegian 11-digit personal identification number was included. The ICD-10 code used to classify CD was K90.0.
Selection of patients
CD was defined as being reported in the questionnaires at 7 or 8 years of age (n=159) or at least two different registrations of CD diagnosis in NPR (in absence of being reported in the questionnaires, n=491, many participants have not reached the age of 7). All other participants were defined as controls / non-CD. We demanded two registrations in the NPR since the diagnosis sometimes is set only once as a working diagnosis, before knowing the result of biopsies. No further restrictions were made. Children participating in MoBa without data from MBRN available (n=5,689) or uncertain diagnosis of CD (e.g. recorded only once in the NPR, n=118) were excluded. A comprehensive flow chart of study participants in our different models is found in figure 1. In a validation sample, participants identified with CD through questionnaires or NPR completed a separate questionnaire to provide details about the diagnostic process. Of 468 respondents, 94.2% (277/294) of those identified with two or more entries in the NPR confirmed the diagnosis, and only 4% and 1.8% had a false positive or uncertain diagnosis, respectively. Of those who had reported the CD diagnosis in a previous questionnaire, 92.4% confirmed the diagnosis in the validation study. Diagnosis confirmed by biopsy was reported in 83.0%, 15.7% had been diagnosed through positive antibody tests and only 1.3% without serology or biopsy.
Figure I.
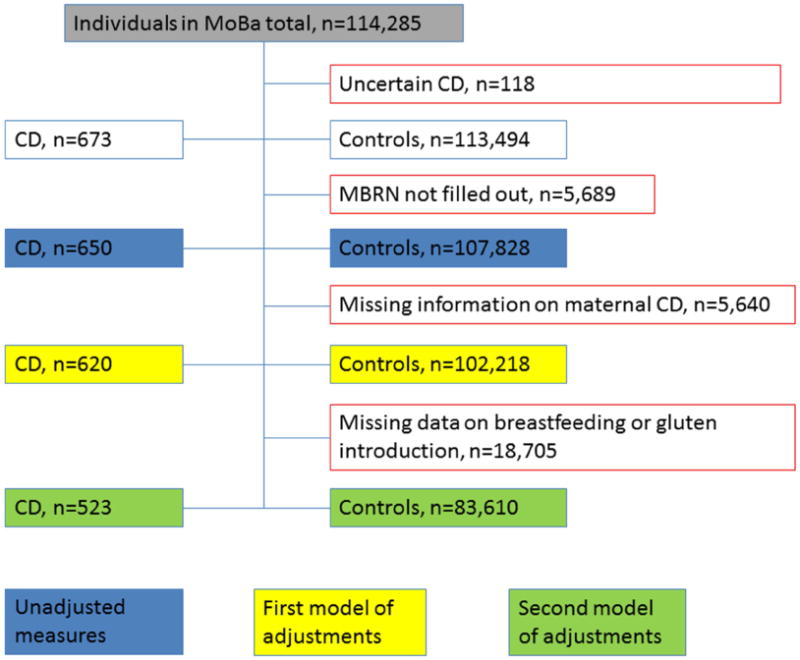
Flow chart of participants. Abbreviations: CD, Celiac disease; MBRN, medical birth register of Norway; MoBa, the Norwegian mother-child cohort.
Data collection
Exposures
Data on birth-weights and lengths, maternal diabetes and pregnancy factors; maternal age at delivery, gestational age, gestational hypertension and preeclampsia was gathered from the MBRN. Data on mode of delivery and indication for Cesarean section, parental smoking, maternal education, maternal CD and parity were collected from the MoBa follow-up questionnaires. Concerning maternal and paternal smoking the data came from the questionnaire filled out at 18 weeks of pregnancy (current smoking) and at 6 months after birth where the mothers answered if they themselves or their partner were smoking (partly or daily) in different time frames; last three months pre-partum, 0-3 post-partum and 4-6 months post-partum. Being SGA was defined as having a birth weight below the 10th percentile at a given gestational week of certain reference points based on data derived from a reference population9 and since we had such a big sample within MoBa we used internally standardized reference values according to gestational week for this assessment. We also calculated SGA for the lowest 2.5 percentile.
Adjustment variables
All analyses were adjusted for attained age, sex and maternal CD, this data was available for 620 individuals in the CD cohort and 102,218 individuals in the non-CD cohort. We also obtained data on gluten introduction and breastfeeding from the questionnaires. These variables were added into a second model, as adjustment variables, complete data on these variables were available for 523 / 83,610 individuals.
Statistical analysis
We used chi square tests to assess unadjusted statistical significance, e.g. for Table I. For all main exposures we used logistic regression adjusted for the child's attained age, maternal CD and sex (referred to as Model I) to assess odds ratios with 95% confidence intervals. We also performed analyses additionally adjusted for gluten introduction and breastfeeding (referred to as Model II). Statistical significance was defined as p-values below the 0.05 level. We also performed a sensitivity analysis restricted to singletons concerning birth weight and length (z scores) since multiplicity impacts these variables. We calculated the sex specific z-scores based on internal standardization. Standardization of height and weight measures at birth was conducted using STATA (using STATA version 12, Statacorp, Texas. All other analyses were performed using SPSS version 21.0 (SPSS, Inc, Chicago, IL).
Table I.
Characteristics of included individuals and mothers with and without CD.
| CD, % | Non-CD cohort, % | P-value | |
|---|---|---|---|
| Total number | 650 (100) | 107,828 (100) | |
| Female sex | 61.4 | 48.7 | <0.001 |
| Parity (mother) | 0.18 | ||
| 0 | 41.7 | 44.5 | |
| 1 | 40.3 | 35.7 | |
| 2 | 14.0 | 15.3 | |
| 3 | 3.2 | 3.4 | |
| >3 | 0.8 | 1.1 | |
| Maternal education | N=620 | N=102,218 | 0.78 |
| 0-12 years | 32.3 | 33.7 | |
| 13-16 years | 41.1 | 41.2 | |
| 17- years | 26.6 | 25.1 | |
| Maternal CD | N=620 | N=102,218 | <0.001 |
| 4.0 | 0.4 | ||
| Maternal diabetes total | 2.5 | 1.5 | 0.051 |
| Type 1 before pregnancy | 1.1 | 0.4 | <0.01 |
| Type 2 before pregnancy | 0.2 | 0.2 | 0.91 |
| Before pregnancy unspecified | 0.2 | <0.1 | 0.01 |
| Gestational diabetes | 0.9 | 0.9 | 0.86 |
| Registration of antidiabetic treatment during pregnancy without diagnosis | 0.2 | <0.1 | 0.48 |
| Delivery by Cesarean section | N=506 | N=80,909 | 0.18 |
| Elective Cesarean section | 12.8 4.8 | 15.0 6.8 | 0.17 |
| Maternal age | 0.65 | ||
| -19 | 0.9 | 1.1 | |
| 20-24 | 11.2 | 10.2 | |
| 25-29 | 33.7 | 32.5 | |
| 30-34 | 38.9 | 38.6 | |
| 35-39 | 13.4 | 15.6 | |
| 40- | 1.8 | 2.1 | |
| Birth weight (kg) | 0.07 | ||
| <1.5 | 0.3 | 0.5 | |
| 1.5-2.49 | 2.8 | 3.6 | |
| 2.5-3.49 | 43.4 | 38.1 | |
| 3.5-4.49 | 49.2 | 53.4 | |
| >4.5 | 4.3 | 4.4 | |
| Mean age at end of follow-up (range) | 7.93 (3.5-13.5) | 7.41 (3.5-13.5) | <0.001 |
Results
Unadjusted associations
Being female and older at follow up was significantly associated with the diagnosis of CD, whereas parity, maternal education, mode of delivery, maternal age and birth-weight were not associated with CD in unadjusted models. Maternal diabetes of any type was of borderline significance in the unadjusted model, specifically diabetes type I was significantly more common in mothers of CD children (Table I).
Adjusted associations of birth-weight and birth-length
No association of CD with birth weight and length, e.g. internal Z-scores was seen in this cohort (table II). Neither did we find any associations to anthropometric measures at birth or being born within any of the following birth-weight categories; very low, low, normal or high birth-weight (Table II). SGA status, neither the lowest 10th percentile nor the 2.5th percentile was significantly associated with the risk of future CD, Model I OR=0.96; 95%CI= (0.73-1.26) and Model II OR=0.94; 95%CI= (0.70-1.27).
Table II.
Anthropometric measures at birth; Model 1 adjusted for sex, attained age and maternal CD and Model 2 Additionally adjusted for breastfeeding and gluten introduction.
| Age | Model 1 N CD/N non-CD |
Model 1 OR (95% CI) |
Model 2 N CD/N non-CD, |
Model 2 OR (95%CI) |
|---|---|---|---|---|
| Birth weight (Z score) | 620/101,637 | 0.98 (0.91-1.06) | 523/83,441 | 0.99 (0.91-1.08) |
| Birth weight (Z score, only singletons) | 613/99,862 | 0.96 (0.89-1.04) | 517/82,028 | 0.98 (0.91-1.06) |
| Birth height (Z score) | 595/97,422 | 1.01 (0.93-1.09) | 504/80,067 | 1.02 (0.93-1.12) |
| Birth height (Z score, only singletons) | 588/95,911 | 1.00 (0.92-1.08) | 498 / 78,859 | 1.01 (0.93-1.09) |
| Small for gestational age; lowest 10 percentile (birth weight Z-score <-1.23 adjusted for gestational age) | 620/102,218 | 0.96 (0.73-1.26) | 523/83,610 | 0.94 (0.70-1.27) |
| Small for gestational age; lowest 2.5 percentile (birth weight Z-score <-1.86 adjusted for gestational age) | 620/102,218 | 1.12 (0.70-1-80) | 523/83,610 | 1.01 (0.58-1.75) |
| Birth weight | ||||
| Very low birth weight (<1500g) | 2/524 | 0.60 (0.15-2.43) | 1/333 | 0.45 (0.06-3.19) |
| Low birth weight (1500-2499g) | 14/3,647 | 0.63 (0.37-1.07) | 14/2,783 | 0.79 (0.46-1.35) |
| Normal birth weight (2500-4499g) | 579/93,054 | 1.36 (0.99-1.87) | 487/76,650 | 1.19 (0.85-1.68) |
| High birth weight (>4500g) | 25/4,412 | 0.97 (0.65-1.46) | 21/3,675 | 0.98 (0.63-1.51) |
Adjusted associations of pregnancy factors, parental smoking and maternal education
In this study we found no association of mode of delivery with CD. Cesarean section overall, independent of indication and electivity, was not associated with future CD, OR=0.85; 95%CI=0.65-1.09 (Table III). We did not find any association of CD with being born preterm (Table III). CD was less common in children of mothers who smoked at 18 weeks of pregnancy. Furthermore, maternal and paternal smoking during the first six months showed an inverse association to CD of borderline significance (Table III). However, the inverse association between maternal smoking during the first six months of the child's life and CD disappeared with additional adjustment for gluten introduction and breastfeeding. Higher maternal education was not independently associated with a higher risk of CD diagnosis in the children even though there was a tendency that the higher educational level the more probable CD diagnosis (Table III). Maternal diabetes type I was associated with future CD in the children whereas type II and gestational diabetes was not (Table III). Parity, gestational hypertension and preeclampsia were not significantly associated with future CD in the adjusted model (table III). Maternal CD (only adjusted for age and sex) was significantly associated with future CD in the children OR= 12.45; 95% CI= (8.29-18.71).
Table III.
Risk of CD by different pregnancy and parental factors.
| CD (n exposed/n total) |
Non-CD cohort (n exposed/n total) |
OR 95% CI | p-value | Additionally adjusted OR, (Model 2) 95%CI (breastfeeding and gluten introduction)* | |
|---|---|---|---|---|---|
| Cesarean section | |||||
| Cesarean section (overall) | 64 / 503 | 11,987 / 79,997 | 0.84 (0.65-1.09) | 0.20 | 0.83 (0.63-1.09) |
| Elective section | 24 / 503 | 5,427 / 80,004 | 0.69 (0.46-1.04) | 0.08 | 0.67 (0.44-1.03) |
| Child-growth indication | 5 / 503 | 713 / 79,998 | 1.10 (0.46-2.68) | 0.83 | 0.93 (0.34-2.49) |
| Maternal indication | 8 / 503 | 1,761 / 80,000 | 0.73 (0.36-1.46) | 0.37 | 0.78 (0.39-1.58) |
| Breech birth | 11 / 503 | 1,966 / 80,000 | 0.86 (0.47-1.57) | 0.63 | 0.75 (0.39-1.45) |
| Preterm birth (<37 weeks) | 34 / 620 | 6,812 / 102,218 | 0.82 (0.58-1.16) | 0.25 | 0.99 (0.69-1.43) |
| Maternal smoking | |||||
| At week 18 of pregnancy | 35/617 | 8,430/101,413 | 0.61 (0.44-0.87) | <0.01 | 0.66 (0.44-0.97) |
| Last three months of pregnancy | 25 / 530 | 5,553 / 83,287 | 0.80 (0.63-1.00) | 0.05 | 0.86 (0.68-1.08) |
| Month 0-3 after birth | 27 / 521 | 6,374 / 82,232 | 0.77 (0.60-0.97) | 0.03 | 0.83 (0.65-1.05) |
| Month 4-6 after birth | 53 / 523 | 11,151 / 82,529 | 0.80 (0.67-0-96) | 0.02 | 0.85 (0.71-1.03) |
| Paternal smoking (reported by mother at 6 months of age) | |||||
| Last three months of pregnancy | 88 / 523 | 17,181 / 82,279 | 0.86 (0.76-0.97) | 0.02 | 0.88 (0.77-1.00) |
| Month 0-3 after birth | 83 / 520 | 16,719 / 81,840 | 0.84 (0.74-0.96) | 0.01 | 0.87 (0.76-0.99) |
| Paternal smoking; month 4-6 after birth | 80 / 520 | 17,130 / 81,805 | 0.82 (0.72-0.94) | <0.01 | 0.84 (0.73-0.96) |
| Maternal education | |||||
| 13-16 years (compared to 0-12 years) | 189 / 620 | 32,267 / 102,228 | 1.08 (0.90-1.31) | 0.75 | 1.06 (0.86-1.31) |
| >17 years (compared to 0-12 years) | 165 / 620 | 25,679 / 102,228 | 1.20 (0.97-1.48) | 0.10 | 1.13 (0.89-1.43) |
| Gestational hypertension | 35/620 | 6,190 /102,218 | 0.94 (0.67-1.33) | 0.73 | 1.00 (0.70-1.44) |
| Preeclampsia | 19/620 | 4020/102,218 | 0.76 (0.48-1.20) | 0.24 | 0.82 (0.50-1.33) |
| Maternal diabetes (any type) | 15/620 | 1,547/102,218 | 1.56 (0.93-2.62) | 0.09 | 1.57 (0.88-2.80) |
| Maternal diabetes type 1 | 7/620 | 408/102,218 | 2.58 (1.19-5.53) | 0.02 | 2.61 (1.14-5.98) |
| Maternal diabetes type 2 | 1/620 | 187/102,218 | 0.86 (0.12-6.19) | 0.88 | Noobservation in the CD cohort |
| Gestational diabetes | 6/620 | 924/102,218 | 0.98 (0.41-2.37) | 0.96 | 1.25 (0.52-3.02) |
| Parity (mother) (compared to 0 para) | |||||
| 1 | 243/620 | 35,293/102,218 | 1.15 (0.97-1.37) | 0.11 | 1.11 (0.92-1.34) |
| 2 | 88/620 | 15,509/102,218 | 0.96 (0.75-1.22) | 0.71 | 0.98 (0.75-1.27) |
| 3 | 20/620 | 3,370/102,218 | 0.96 (0.61-1.52) | 0.87 | 1.10 (0.68-1.78) |
| >3 | 5/620 | 1,039/102,218 | 0.79 (0.33-1.92) | 0.60 | 0.81 (0.30-2.18) |
Based on 477 children in the CD cohort and 76,402 in the non CD cohort for Cesarean section and 523/83,610 respectively for all other exposures.
Discussion
(Comparison with earlier literature)
In this study we found that female sex and maternal CD was associated with increased risk of future CD in the children. These findings are consistent with earlier knowledge; that there is a strong genetic association in CD10, 11 and that CD is more common in females12, 13. The strong association with maternal CD is, in addition to the known marked heritability of CD, also likely increased by disease awareness in the family with a detection bias. However, this is unlikely to influence the perinatal factors in the present study. Naturally, since cumulative incidence of any chronic disease will increase with age of the individual, we also observed an association with attained age within the cohort.
Unlike some other studies4, 5 but in similarity to Roberts et al 6, we did not find any association with any type of Cesarean section (whether it was due to elective, breech, child-growth, maternal indication or overall). The earlier prospectively collected study reported a fairly small significant OR (1.15) for elective Cesarean section but showed no significant association to Cesarean section overall4. The Swedish registry-based study consisted of both children and adults being born from 1973 and onwards, with a larger study sample than the present study. In total Cesarean section was less common in the Swedish study, 11.1% of CD and 10.7% of controls, compared to 12.8% and 15% respectively in our study, of which elective section was 5.7%/5.1% compared to 4.8%/6.8% in our study. These differences are likely due to changing indication over time, rather than practice differences in between the two countries, and may dilute the earlier observed association with elective section. The age groups differ between the studies, we would expect a potential association with Cesarean section to be more likely in a young cohort like ours compared to the Swedish study also including adults. However, in similarity to our study, the Swedish study showed no association of Cesarean section to CD with onset before two years of age. Another possible explanation to the significant Swedish finding is that having an elective section might be associated with an increased health care seeking behavior or other unknown risk factors that may have inflated the estimate or chance of diagnosing CD more in a matched registry-based design than in our classic cohort design. The other study that suggested an association with Cesarean section was based on retrospective questionnaires from 1,950 children of whom 123 developed CD5. Therefore we believe that the result from this study adds knowledge that Cesarean section truly does not have a major impact on future childhood CD.
Some earlier studies have suggested an increased risk of CD in children being born SGA4, 7. In the present study we found no such association. Factors contributing to impaired fetal growth may be less prevalent in this cohort, e.g. parents accepting to take part in a prospectively collected questionnaire study may be healthier, more nutritionally well served and suffer from less comorbidities, compared to other entirely registry-based studies. In fact smokers are indeed underrepresented whereas individuals taking vitamin supplements are overrepresented in MoBa compared to the general population14, which may impact the results. We also assessed the possibility of a more linear association of z-score to CD, without finding any association.
We did not find associations to other adverse fetal outcomes such as prematurity, preeclampsia, low or very low birth weight, which was in accordance with previously published data 4, 6. In contrast to earlier studies6, 7 reporting an increased risk of CD in children of mothers who smoke, our study found instead a weak protective effect of exposure to maternal smoking. This might reveal some small detection bias in the cohort (detection/diagnosing of CD might be less common in parents who smoke even though true prevalence is not). However it is plausible that smoking is truly inversely related to development of autoimmune diseases such as CD since some earlier studies have suggested an inverse relation of maternal smoking with diabetes mellitus type I15, 16 and inflammatory bowel disease (both Mb Crohns and ulcerative colitis)17 and that nicotine has also been shown to reduce the incidence of type I diabetes in mice18. It is also possible that maternal smoking affects breastfeeding and gluten introduction practice in being significantly associated in model I but not in model II (additionally adjusted for those factors). Our study found an increased risk of CD in children born to mothers with diabetes mellitus type I, but not type II or gestational diabetes. We do not know of any earlier study that has specifically addressed this question. However in the study from Mårild4 et al any diabetes was reported in 0.7% of mothers to celiac children compared to 0.4% in the control cohort, this is markedly lower than the corresponding 2.5/1.5% found in our study, probably due to under-reporting, but ratio of the proportions are similar. Our finding that only maternal type I diabetes is associated to future CD in the offspring (in contrast to type II and gestational diabetes) suggests that it is rather the genetic predisposition than the fetal exposure of elevated fasting glucose that confers the increased risk. Shared genetics between CD and diabetes type I are well documented within the HLA loci19 but also other common genes are established20.
Misclassification
Some individuals that have not yet been diagnosed with CD are likely to be found in the non-CD cohort. This risk of misclassification is unlikely to affect the results substantially since CD is relatively rare and diagnosed only in 0.6% of the cohort, and even a prevalence of undiagnosed CD in the non-CD cohort of 1-2% would not impact the associations given that it is non-differential by the exposures of interest. Access to clinical, biochemical, and histologic information would likely add cases with single entries in the NPR to the celiac group. Additional screening for celiac disease in all the participants to find additional cases of celiac disease would similarly identify more cases, primarily with silent disease or low-grade symptoms 21. It is recommended that Norwegian children with CD are seen biannually by a pediatrician but naturally this is not 100% fulfilled.
All exposure data for this study was collected prospectively, and therefore there should be low risk of misclassification.
Strengths and limitations
Strengths of this study are the large setting and the prospectively collected data, reducing the risk of recall bias seen in case-control studies. Furthermore, the availability of detailed data that enabled us to adjust for factors like gluten introduction and breastfeeding (unlike earlier registry-based studies) improves the credibility of the findings. A limitation is that many of the children are still young and consequently their follow-up time is rather short. Many of them are still about to develop and detect CD in the future. Consequently, the observations may be less valid regarding the long-term risk for CD.
Conclusion
This study found no association of mode of delivery, intrauterine growth or factors pertaining to intrauterine fetal growth on future development of CD. CD was significantly associated to female sex, maternal CD and maternal type I diabetes mellitus.
Abbreviations used in this article
- CD
celiac disease
- CI
confidence interval
- MBRN
medical birth register of Norway
- MoBa
the Norwegian mother-child cohort
- NPR
Norwegian patient register
- OR
odds ratio
- SGA
small for gestational age
Footnotes
Competing interests: None declared
Data sharing: No additional data.
Details of ethics approval: Approved by the Regional Ethics Committee of Medical Research, Oslo, Norway no S-97045 and S-95113.
Conflicts of interest/Disclosure requirement: Ketil Stordal is funded by an unrestricted grant from Oak Foundation, Geneva, Switzerland.
Author involvement: LE: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
MM: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis.
KS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Stordal K, Bakken IJ, Suren P, Stene LC. Epidemiology of coeliac disease and comorbidity in Norwegian children. J Pediatr Gastroenterol Nutr. 2013;57:467–71. doi: 10.1097/MPG.0b013e3182a455dd. [DOI] [PubMed] [Google Scholar]
- 3.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 4.Marild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45. e3. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker E, Engelmann G, Findeisen A, Gerner P, Laass M, Ney D, Posovszky C, Hoy L, Hornef MW. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125:e1433–40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 6.Roberts SE, Williams JG, Meddings D, Davidson R, Goldacre MJ. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharmacol Ther. 2009;29:222–31. doi: 10.1111/j.1365-2036.2008.03871.x. [DOI] [PubMed] [Google Scholar]
- 7.Sandberg-Bennich S, Dahlquist G, Kallen B. Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Paediatr. 2002;91:30–3. doi: 10.1080/080352502753457905. [DOI] [PubMed] [Google Scholar]
- 8.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Merialdi M, Platt LD, Kramer MS. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;202:522–8. doi: 10.1016/j.ajog.2009.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio-Tapia A, Van Dyke CT, Lahr BD, Zinsmeister AR, El-Youssef M, Moore SB, Bowman M, Burgart LJ, Melton LJ, 3rd, Murray JA. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–7. doi: 10.1016/j.cgh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Green PH. Clinical management of coeliac disease. J Intern Med. 2011;269:560–71. doi: 10.1111/j.1365-2796.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 12.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 13.Ivarsson A, Persson LA, Nystrom L, Hernell O. The Swedish coeliac disease epidemic with a prevailing twofold higher risk in girls compared to boys may reflect gender specific risk factors. Eur J Epidemiol. 2003;18:677–84. doi: 10.1023/a:1024873630588. [DOI] [PubMed] [Google Scholar]
- 14.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Haynes A, Cooper MN, Bower C, Jones TW, Davis EA. Maternal smoking during pregnancy and the risk of childhood type 1 diabetes in Western Australia. Diabetologia. 2014;57:469–72. doi: 10.1007/s00125-013-3122-7. [DOI] [PubMed] [Google Scholar]
- 16.Rasouli B, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Smoking is associated with reduced risk of autoimmune diabetes in adults contrasting with increased risk in overweight men with type 2 diabetes: a 22-year follow-up of the HUNT study. Diabetes Care. 2013;36:604–10. doi: 10.2337/dc12-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspberg S, Dahlquist G, Kahan T, Kallen B. Fetal and perinatal risk factors for inflammatory bowel disease. Acta Paediatr. 2006;95:1001–4. doi: 10.1080/08035250600573151. [DOI] [PubMed] [Google Scholar]
- 18.Mabley JG, Pacher P, Southan GJ, Salzman AL, Szabo C. Nicotine reduces the incidence of type I diabetes in mice. J Pharmacol Exp Ther. 2002;300:876–81. doi: 10.1124/jpet.300.3.876. [DOI] [PubMed] [Google Scholar]
- 19.Sumnik Z, Cinek O, Bratanic N, Kordonouri O, Kulich M, Roszai B, Arato A, Lebl J, Soltesz G, Danne T, Battelino T, Schober E. Risk of celiac disease in children with type 1 diabetes is modified by positivity for HLA-DQB1*02-DQA1*05 and TNF -308A. Diabetes Care. 2006;29:858–63. doi: 10.2337/diacare.29.04.06.dc05-1923. [DOI] [PubMed] [Google Scholar]
- 20.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–77. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stordal K, Haugen M, Brantsaeter AL, Lundin KE, Stene LC. Association between maternal iron supplementation during pregnancy and risk of celiac disease in children. Clin Gastroenterol Hepatol. 2014;12:624–631. e2. doi: 10.1016/j.cgh.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


