Abstract
Schizophrenia is associated with abnormalities of hippocampal structure and function. Neuroimaging studies have shown that the hippocampus is hyperactive in schizophrenia. Here we explore GABAergic mechanisms of this hippocampal hyperactivity.
The initial evidence for GABAergic abnormalities of the hippocampus in schizophrenia came from post-mortem studies of interneuron number, protein expression, and gene expression. These studies revealed marked decreases in gene and protein expression of somatostatin-positive and parvalbumin-positive interneurons, and indicated reduced interneuron numbers. Animal studies of decreased parvalbumin and NMDA-receptor function have shown that selective abnormalities of hippocampal interneurons mimic some of the cognitive deficits and clinical features of schizophrenia.
The post-mortem and animal studies are consistent with the neuroimaging finding of increased hippocampal activity in schizophrenia, which can explain some of the psychotic symptoms and cognitive deficits. Taken together, these findings may guide the development of biomarkers and the development of new treatments for psychosis.
The hippocampus is abnormal in schizophrenia (Benes, 1999; Heckers and Konradi, 2010; Nelson et al., 1998; Tamminga et al., 2010; Wright et al., 2000). Two prominent features of schizophrenia, reality distortion (delusions and hallucinations) and cognitive deficits have been linked to hippocampal dysfunction and several neural models of schizophrenia posit abnormalities of the hippocampus (Heckers and Konradi, 2010; Tamminga et al., 2010).
In this review we will focus on the hypothesis that hippocampal hyperactivity is a core feature of schizophrenia. In particular, we will highlight GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. We will review the evolution of the hippocampal hyperactivity model of schizophrenia and clarify the term hippocampal hyperactivity. We will summarize the evidence generated so far in support of GABAergic mechanisms and propose a developmental model of GABAergic mechanisms, giving rise to hippocampal dysfunction in schizophrenia.
Evolution of the hippocampal hyperactivity model
Even before any studies of the hippocampus, several authors proposed models of hippocampal hyperactivity in schizophrenia (Krieckhaus et al., 1992; Venables, 1992). These authors hypothesized that deficits seen in patients with schizophrenia (e.g., latent inhibition and negative priming) are caused by hippocampal hyperactivity, but did not provide any experimental data. This changed with Peter Liddle’s 1992 study of patterns of cerebral blood flow in schizophrenia and Francine Benes’ 1998 study of a selective decrease in the number of hippocampal interneurons in schizophrenia.
Liddle et al. studied 30 patients with schizophrenia, who were classified into three syndromes based on a stable pattern of psychopathology: 1) psychomotor poverty (i.e., mainly negative symptoms), 2) disorganization and 3) reality distortion (i.e., mainly positive symptoms). The authors reported significant positive correlations between reality distortion and rCBF in the left temporal lobe, especially the parahippocampal region (Liddle et al., 1992).
A complementary analysis of the same data set (Friston et al, 1992) provided a more detailed discussion of temporal lobe hyperactivity in schizophrenia: “An obvious inference is that severe schizophrenia is associated with increased transynaptic activity (inhibitory or excitatory) mediating the transformation of neuronal firing patterns. In other words, a reduction in the efficiency of these transformations. The anatomical substrate of this positive physiological correlate may include atrophic changes, but the nature of the presumed dysplasia is such that it results in higher rCBF.” This introduces several components of current models of hippocampal hyperactivity in schizophrenia: synaptic dysfunction, abnormal neuronal firing pattern, efficiency of neurotransmission, and atrophy of the hippocampus.
Benes et al. reported that the density of pyramidal cells (glutamatergic neurons) is normal in schizophrenia, but that nonpyramidal (GABAergic) neurons are selectively reduced in sector CA2 (Benes et al., 1998). Benes and Berretta conjectured “that decreased GABAergic transmission in specific cortical areas could result in rearrangement, and possibly enlargement, of sensory, memory and ‘cognitive’ fields and thereby lead to overinclusive, disorganized thought processes.” (Benes and Berretta, 2001). This introduces several other aspects of current hippocampal hyperactivity models: decreased GABAergic neurotransmission, rearrangement of cognitive fields, and, as a result, abnormal thought processes.
In addition to these two important studies, several other observations support a hippocampal hyperactivity model of schizophrenia. First, schizophrenia is associated with impaired habituation, i.e. an inability to modulate responses after repeated presentations of sensory stimuli (Rankin et al., 2009). Freedman and colleagues have proposed that alpha-7 nicotinic receptors on hippocampal interneurons are the primary site of pathology underlying the impaired filtering of sensory information in schizophrenia (Freedman et al., 2000). Support for this model from neuroimaging data was recently reviewed by Tregellas (Tregellas, 2014). For example, patients with schizophrenia demonstrate significantly greater activation of the hippocampus while passively viewing facial expressions (Holt et al., 2006) and do not show the normal pattern of habituation to the repeated presentation of fearful faces (Holt et al., 2005). This failure of habituation is correlated with the degree of memory deficits in schizophrenia (Williams et al., 2013).
Second, specific memory deficits in schizophrenia have been linked to abnormal hippocampal function. The initial study showed an increased activity of the hippocampus and decreased recruitment during the performance of a memory retrieval task (Heckers et al., 1998). This finding was confirmed and extended by several studies, which revealed that hippocampus-dependent memory is more impaired than memory that does not rely on the hippocampus (Achim et al., 2007; Ongur et al., 2006; Weiss et al., 2003). More recently, Tregellas provided evidence that intrinsic hippocampal activity at rest is related to cognitive dysfunction in schizophrenia (Tregellas et al., 2014).
Third, the dopamine hypothesis of schizophrenia has been linked to hippocampal hyperactivity. Elevated regional cerebral blood flow in the hippocampus of schizophrenia patients is normalized by D2-antagonists (Medoff et al., 2001). Dopamine facilitates hippocampus-specific cognitive tasks at higher levels, but is detrimental at levels above the optimal range (Chowdhury et al., 2012; Rocchetti et al., 2014). One proposed mechanism is the inhibition of perforant path input to CA1 by dopamine, which is reversed with antipsychotic drugs (Lisman and Otmakhova, 2001#12809). In addition, a hyperactive dopamine system increases hippocampal activity and increased hippocampal activity triggers a hyperactive dopamine system (via reciprocal connections between hippocampal formation and midbrain dopamine neurons) (Lisman et al., 2008). For example, in the methylazoxymethanol acetate (MAM) rodent model of schizophrenia, which disrupts prenatal hippocampal development, a primary disturbance in the hippocampus increases the spontaneous firing rate of dopaminergic neurons in the ventral tegmental area (Lodge and Grace, 2007). In this model, antipsychotic drugs reduced the number of spontaneously active dopamine neurons (Valenti et al., 2011) and transplanted GABAergic neuronal precursors reversed the physiological and behavioral deficits (Perez and Lodge, 2013).
Taken together, there is now compelling evidence that hippocampal hyperactivity is a possible mechanism for psychosis. Several models have been proposed, which focus on hippocampal-cortical interactions (Heckers, 2001) and the distinct roles of subfields CA1 (Small et al., 2011), CA2/3 (Benes, 1999), and hilus/dentate gyrus (Tamminga et al., 2010).
Excitation-inhibition balance in the hippocampus
The hyperactivity model of schizophrenia states that the excitation-inhibition (E/I) balance is abnormal in psychosis (Uhlhaas, 2013). This has been proposed for several brain areas, including the hippocampus. Here we will briefly review the E/I balance in the human hippocampus.
Excitation in the hippocampus
The vast majority of hippocampal neurons (approximately 90%) are glutamatergic pyramidal cells (also referred to as principal cells), (Freund and Buzsaki, 1996; Olbrich and Braak, 1985). This is markedly different from the cerebral cortex, where the numbers of pyramidal and nonpyramidal cells are more balanced (Mouton, 2014). This E/I imbalance makes the hippocampus more vulnerable to focal excitation and seizures. Pyramidal cells project either within hippocampal subfields (intrinsic circuitry) or via long-range projections to several target areas (hippocampal efferents). These glutamatergic neurons have a high baseline firing rate and are synchronized into oscillatory patterns by a plethora of interneuron subtypes (Klausberger and Somogyi, 2008). Synaptic input to the soma, the axon and dendrites of pyramidal neurons is arranged according to the origin and characteristics of the presynaptic source. Distal dendrites receive excitatory input predominantly from distant brain areas, whereas basal and proximal dendrites receive excitatory input from local sources (Spruston, 2008). Inhibitory interneurons synapse in the perisomatic area, the axons and dendrites, with different interneuron subtypes targeting specific subcellular components (Freund and Buzsaki, 1996; McBain and Fisahn, 2001).
Inhibition in the hippocampus
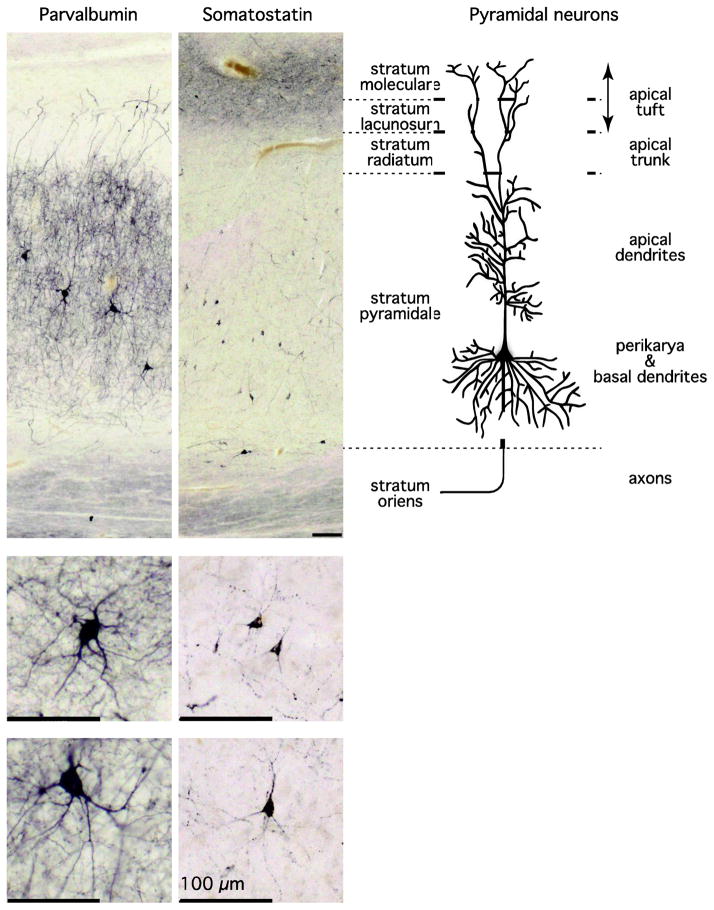
Only 10% of hippocampal neurons are GABAergic, non-pyramidal cells, which form intrinsic connections. Despite the small proportion, these interneurons exert ultimate control by synchronizing pyramidal cell populations and regulating information flow through the hippocampus, achieved via elaborate synaptic networks (Freund, 2003). Synchrony in neuronal networks is the foundation of higher brain function (Varela et al., 2001). Interneurons vary greatly in shape, dendritic arborization and axonal projections, giving rise to population subtypes characterized by morphological, neurochemical and electrophysiological properties (McBain and Fisahn, 2001). These population subtypes differ further in their functional impact on the principal cells (Freund and Buzsaki, 1996). Here we will focus on two of the largest population subtypes of hippocampal interneurons, characterized by the expression of the neuromodulator somatostatin, or the Ca2+ binding protein parvalbumin (Figure 1).
Figure 1. Parvalbumin-positive and Somatostatin-positive neurons in the human hippocampus.
The photomicrographs show the cell bodies, axon and dendrites of parvalbumin-positive (left column) and somatostatin-positive (middle column) neurons in subfield CA1 of the human hippocampus, next to a schematic depiction of the position of subcellular components of pyramidal cells (right column; modified from (Spruston, 2008)). Cell somata of both interneuron populations are dispersed throughout the pyramidal cell layer. Parvalbumin-positive neurons have large somata and a high density of neurites throughout the pyramidal cell layer. A lesser density of neurites is seen in the stratum radiatum, with increasing density toward the stratum lacunosum. In contrast, somatostatin-positive neurons have smaller somata. In CA1, a group of these neurons is aligned at the border to the stratum oriens. Faint somatostatin-positive projections are seen in the pyramidal cell layer, but the highest density of neurites is seen in stratum moleculare. All scale bars = 100 μm.
Somatostatin-positive interneurons constitute about a third of all hippocampal interneurons and regulate the efficacy and plasticity of excitatory inputs to hippocampal pyramidal cells (Freund and Buzsaki, 1996; Viollet et al., 2008). Somatostatin neurons play an important role in seizure control, and perturbations of the inhibitory role of somatostatin-positive neurons can lead to abnormal pyramidal cell firing (Tallent and Qiu, 2008). In the human hippocampus, somatostatin-positive neurons are dispersed throughout the pyramidal cell layer, while most of their axons terminate in the molecular cell layer on dendrites of pyramidal neurons (Figure 1), (Konradi et al., 2011a; Konradi et al., 2011b).
Parvalbumin-positive interneurons represent about 20% of all GABAergic neurons in the hippocampus (Freund and Buzsaki, 1996). They are crucial for organized temporal encoding and retrieval of information, by synchronizing the firing pattern of pyramidal cells in the 30–100 Hz range (i.e. gamma oscillations) (Bartos et al., 2007; Lewis et al., 2005). Gamma oscillations, mediated by parvalbumin-expressing interneurons, play a central role in higher brain function (Bartos et al., 2007; Varela et al., 2001).
There are several well-known examples of E/I imbalance in the human hippocampus. Most notably, seizure disorders that originate in the hippocampus are caused by an unregulated firing of principal cells (Fritschy, 2008). In support of a role of E/I imbalance in the hippocampus, individuals with temporal lobe epilepsy have a higher incidence of psychotic symptoms than patients with generalized epilepsy (Roberts et al., 1990; Stevens, 1988). Anticonvulsants strengthen inhibition (e.g., i.v. administration of benzodiazepines in status epilepticus) or weaken excitation. More recently, increased activity of the hippocampus has also been shown in animal models of Alzheimer’s Disease (AD) and in AD patients, and this hippocampal hyperactivity can explain some of the cognitive deficits and neuropsychiatric symptoms (Bakker et al., 2012; Verret et al., 2012; Wragg and Jeste, 1989).
Abnormal hippocampal activity in schizophrenia
Studies of regional cerebral glucose metabolic rates (rCMRglc), regional cerebral blood flow (rCBF) and blood oxygen level-dependent (BOLD) signal demonstrate abnormal hippocampal activity in schizophrenia (Buchsbaum et al., 1992; Nordahl et al., 1996; Tamminga et al., 1992) (Friston et al., 1992; Kawasaki et al., 1996; Kawasaki et al., 1992; Lahti et al., 2003; Liddle et al., 1992; Malaspina et al., 2004; Medoff et al., 2001). Changes of hippocampal metabolism and blood flow are associated with more severe psychopathology (Friston et al., 1992), especially positive symptoms (Gur et al., 1995; Liddle et al., 1992), such as auditory hallucinations (Dierks et al., 1999; Silbersweig et al., 1995). Intriguingly, the abnormally increased hippocampal blood flow in patients with schizophrenia is partially normalized by treatment with dopamine D2 antagonists (Lahti et al., 2003; Malaspina et al., 2004; Medoff et al., 2001).
These earlier studies of hippocampal glucose metabolism and cerebral blood flow in schizophrenia have been complemented by three recent studies of cerebral blood volume (CBV), (Schobel et al., 2013; Schobel et al., 2009; Talati et al., 2014). CBV measurements using fMRI provide sub-millimeter spatial resolution at the level of hippocampal subfields (Small et al., 2011). The two studies by Schobel et al. revealed a striking pattern in patients with prodromal and chronic schizophrenia: CBV was selectively increased in hippocampal sector CA1, which was associated with CBV increases in the orbitofrontal cortex and CBV decreases in the dorsolateral prefrontal cortex (Schobel et al., 2013; Schobel et al., 2009). The increased CBV in CA1 was recently confirmed in a cohort of chronic schizophrenia patients (Talati et al., 2014). These studies of hippocampal blood flow and blood volume support the hypothesis of abnormally elevated hippocampal activity in schizophrenia (Lisman et al., 2008; Small et al., 2011).
Hippocampal function in schizophrenia is abnormal during the performance of cognitive tasks (Hall et al., 2009; Heckers et al., 1999; Heckers et al., 1998; Jessen et al., 2003; Leavitt and Goldberg, 2009; Ongur et al., 2006; Ragland et al., 2001; Weiss et al., 2003; Weiss et al., 2004). During memory retrieval, patients with schizophrenia rely less on the recruitment of the hippocampus and show more widespread activation of the prefrontal cortex (Weiss et al., 2003). Schizophrenia patients are especially impaired on relational memory tasks that depend on the hippocampus (Ongur et al., 2006).
While cognitive task studies have revealed impaired hippocampal recruitment in the context of increased baseline activity, several studies have provided additional evidence pointing to increased hippocampal activity in schizophrenia. In normal control probands, the hippocampal BOLD signal decreases over time when passively viewing visual stimuli. This type of hippocampal habituation is not seen in schizophrenia (Holt et al., 2006; Holt et al., 2005) and is associated with impaired memory function (Williams et al., 2013). These studies of impaired hippocampal habituation in schizophrenia have been complemented by a study of intrinsic hippocampal connectivity at rest, which also explained some of the cognitive impairments seen in schizophrenia (Tregellas et al., 2014).
Several studies have used magnetic resonance spectroscopy to measure GABA and glutamate in the hippocampus of patients with schizophrenia. Of particular interest is a recent study that linked increased hippocampal glutamate to subsequent hippocampal volume deficits in schizophrenia (Kraguljac et al., 2013).
The imaging studies reviewed here cannot reveal the cellular and molecular substrate of hippocampal hyperactivity, but this can be addressed with post-mortem and animal studies.
Reduced number of interneuron populations
While the total number of hippocampal neurons is normal in schizophrenia (Heckers et al., 1991; Konradi et al., 2011a; Schmitt et al., 2009; Walker et al., 2002), several studies have revealed selective abnormalities of interneurons. Benes et al. provided evidence for a decreased density of non-pyramidal cells in the hippocampus in schizophrenia and psychotic bipolar disorder (Benes et al., 1998). A subsequent study in these patient populations revealed decreased mRNA levels of glutamic acid decarboxylase (GAD), a marker specific for GABA synthesizing neurons (Heckers et al., 2002).
More recently, several studies have provided compelling evidence for abnormalities in subpopulations of hippocampal interneurons. Interneurons, though small in number, govern hippocampal function: they exert a dynamic, spatio-temporal control of hippocampal cell firing and are crucial for normal cognition and behavior (Somogyi and Klausberger, 2005). In particular, fast-spiking parvalbumin-expressing interneurons generate gamma oscillations which are crucial for higher brain function (Bartos et al., 2007). Neural oscillations are essential for the establishment of precise temporal relationships between neuronal responses and abnormalities in GABAergic interneurons have been proposed as the anatomical correlate for abnormal neural oscillations in schizophrenia (Uhlhaas and Singer, 2010). An initial study reported a decreased density of parvalbumin-positive neurons in all hippocampal regions (Zhang and Reynolds, 2000). This finding was independently confirmed in another study which revealed a reduction in the total number of somatostatin-positive interneurons as well as in the level of somatostatin and parvalbumin mRNA (Konradi et al., 2011a).
While the decrease in specific interneuron populations observed in post-mortem studies fits well with the neuroimaging evidence of hippocampal hyperactivity, animal models can further examine the relationship between interneurons and hippocampal hyperactivity, and elucidate the functional basis of the readouts in imaging.
Animal models of hippocampal hyperactivity
Several animal studies linked an impairment of parvalbumin-positive interneurons to abnormal hippocampal activity and schizophrenia-like behavior. Inhibition of NMDA-receptors in the hippocampus (especially in sector CA 1) decreases activity of parvalbumin-positive interneurons, which in turn leads to a disinhibition of hippocampal pyramidal cells (Behrens et al., 2007; Bickel and Javitt, 2009; Greene, 2001; Kinney et al., 2006; Lisman et al., 2008). Furthermore, administration of methylazoxymethanol (MAM) disrupts DNA synthesis in dividing cells, and when administered on gestational day 17 targets parvalbumin-containing interneurons especially in the ventral hippocampus, leading to diminished oscillatory activity (Lodge et al., 2009; Lodge and Grace, 2009). Two recent studies tested the hypothesis that the hippocampal hyperactivity seen in CBV and BOLD signal studies of schizophrenia patients (see above) are caused by abnormalities in glutamatergic or GABAergic neurotransmission. Schobel et al showed that ketamine administration leads to increased hippocampal activity as measured by gadolinium-enhanced cerebral blood volume (CBV) mapping (Schobel et al., 2013). Ketamine, a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, induces psychosis. It preferentially blocks NMDA receptors on GABA interneurons, which leads to a disinhibition of glutamate neurons, glutamate release and increased neuronal activity (Stone et al., 2012).
Gilani et al showed that modulation of parvalbumin-positive interneurons lead to deficits in synaptic inhibition, increased in-vivo spike activity of projection neurons and increased in-vivo basal metabolic activity (as assessed with fMRI) (Gilani et al., 2014). Even more intriguing, transplantation of cells from the embryonic ganglionic eminence (the major origin of cerebral cortical interneurons) reversed this phenotype (Perez and Lodge, 2013). These recent studies raise the question when hippocampal hyperactivity develops in psychosis.
Hippocampal hyperactivity develops in stages
Schobel et al. pioneered the longitudinal study of hippocampal activity in schizophrenia (Schobel et al., 2013). They followed subjects at risk for schizophrenia for 2 years and measured changes in CBV and hippocampal structure. They reported that subjects with higher CA1 CBV values showed greater hippocampal volume reduction after 2 years (Schobel et al., 2013). We propose that hippocampal hyperactivity in schizophrenia develops in stages.
Stage 1: Baseline risk
Several static and dynamic risk factors make subjects more vulnerable to hippocampal hyperactivity later in life (e.g., hypoxia, stress, trauma). Early stress perturbs the development of GABA neurons, which migrate over extensive distances to reach their final location in the hippocampus. This makes them more vulnerable than interneurons in other cortical regions (Tricoire et al., 2011). Several genotypes and clinical phenotypes have been associated with a greater risk for psychosis, which include a hyperactive hippocampus in subjects at risk for psychosis.
Stage 2: Early stage of psychosis
There is growing evidence that hippocampal dysfunction (hyperactivity and smaller volume) is present in the early stage of psychosis. However, not all psychotic patients progress to a chronic psychotic disorder and some will convert to an affective disorder (Bromet et al., 2011; Salvatore et al., 2008). While it is unclear how hippocampal dysfunction can differentiate between these various trajectories, hippocampal hyperactivity is related to cognitive dysfunction (Tregellas et al., 2014; Williams et al., 2013) and reality distortion (Schobel et al., 2013).
Stage 3: Chronic psychosis
There is emerging evidence that the degree of hippocampal hyperactivity (Schobel et al., 2013) or the amount of glutamatergic dysfunction (Kraguljac et al., 2013) predict the severity of hippocampal volume loss over time in patients with schizophrenia. This provides the basis for a developmental model of hippocampal dysfunction and may offer the opportunity for a biomarker (Tregellas, 2014).
Accounting for heterogeneity
Brain changes, even robust findings such as smaller hippocampal volume, are not diagnostic for schizophrenia (Heckers et al., 2013). Several scenarios can account for this heterogeneity and need to be considered as we test the model of hippocampal hyperactivity in psychosis.
Patients who carry a significant burden of risk for the development of an abnormal hippocampus might show cognitive deficits and smaller hippocampal volume already at baseline and in the at-risk state. This includes patients with elevated genetic (e.g., DISC-1, VCFS) or environmental (hypoxia, stress) risks for a smaller hippocampus (Heckers et al., 2013). Patients with smaller hippocampal volumes in the at-risk state might show more pronounced cognitive impairment before the onset of psychosis.
Environmental risk factors may accelerate the progression of hippocampal pathology in psychotic disorders. In particular, the early and heavy use of cannabis alters hippocampal structure (Cousijn et al., 2012). Patients with such environmental risk factors might show more rapid changes in hippocampal activity and volume.
Finally, pharmacological as well as non-pharmacological treatment can alter the excitation-inhibition balance of the hippocampus and may lead to changes in hippocampal function and structure. There are compelling examples for such effects, including antipsychotic medication (Medoff et al., 2001; Schobel et al., 2013), exercise (Pajonk et al., 2010) and psychotherapy (Eack et al., 2010).
Implications for treatment
Treating the hyperactive hippocampus is a possible intervention strategy in the early stages of psychosis. This approach shows great promise in the early stages of Alzheimer’s Disease. In animals that overexpress human amyloid precursor protein, the hippocampus shows spontaneous epileptiform discharges, primarily during reduced gamma oscillatory activity, which was linked to parvalbumin cell dysfunction (Verret et al., 2012). The anticonvulsant levetiracetam suppressed the abnormal spiking activity of the hippocampus and improved memory performance (Sanchez et al., 2012). In human studies, levetiracetam was able to normalize hippocampal hyperactivity (measured via the BOLD signal) and improved cognition in subjects with mild cognitive impairment (Bakker et al., 2012). These translational and treatment studies provide compelling evidence that hippocampal hyperactivity can be a treatment target for schizophrenia as well.
Summary
We propose that the neuroimaging (BOLD, CBV, and rCBF) finding of hippocampal hyperactivity in schizophrenia is due to cellular and molecular changes of hippocampal interneurons, primarily somatostatin-positive and parvalbumin-positive interneurons. We do not dispute the important role of other mechanisms (e.g., NMDA-receptor hypofunction, alpha7 nicotinergic receptor dysfunction), but for the purpose of this review we have focused on GABAergic mechanisms. We suggest that hippocampal hyperactivity in schizophrenia predates a) the onset of cognitive deficits and psychosis and b) the full course of hippocampal volume reduction seen in chronic patients. This may guide the development of biomarkers in patient populations and the development of new treatments for psychosis.
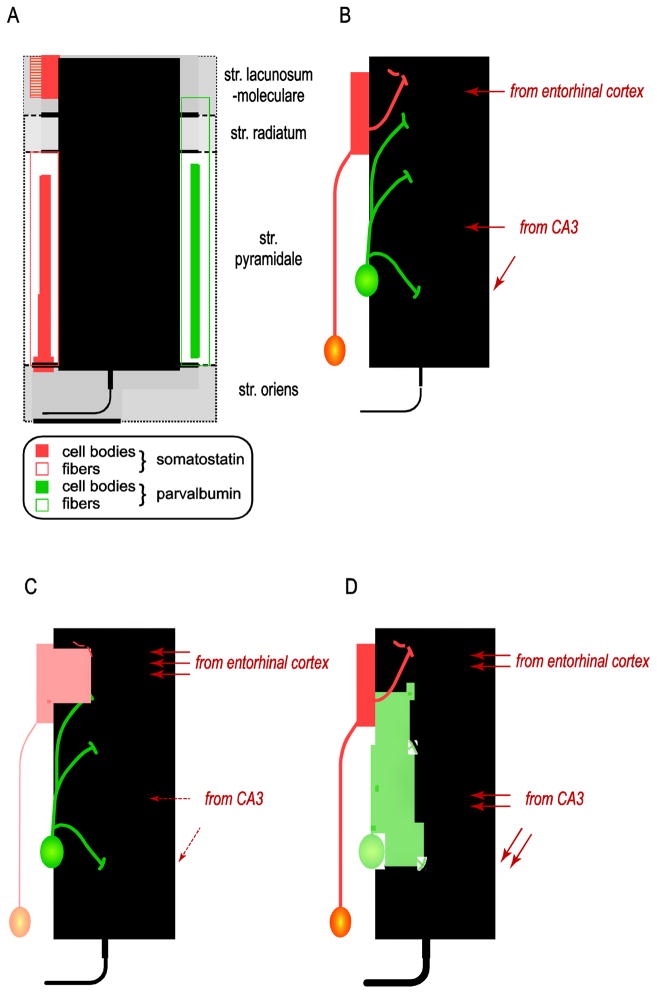
Figure 2. Altered excitation/Inhibition balance in the human hippocampus.
Panel A. The majority of neurons in the human hippocampus are large, excitatory (glutamatergic) pyramidal cells. Pyramidal cells are surrounded by local interneurons that can inhibit their firing pattern at various subcellular sites. Somatostatin-positive interneurons inhibit the distal dendrites in the stratum moleculare (apical tuft; orange column on the left). In contrast, parvalbumin-positive interneurons modulate pyramidal cells at more proximal dendrites and the cell body (panel A, green column on the right).
Panel B. Somatostatin-positive interneurons control information flow arriving from the entorhinal cortex. Proximal apical dendrites in CA1 receive input primarily from CA3. Thus, parvalbumin neurons control pyramidal cell activity originating from both extrahippocampal and intrahippocampal sources.
Panel C. A loss of somatostatin neuron activity will lead to a disinhibition of the input from the entorhinal cortex.
Panel D. A loss of parvalbumin neurons will lead to asynchronous firing of pyramidal cells and increased excitatory drive from the hippocampus.
Acknowledgments
SH is supported by National Institute of Mental Health (grant R01 MH70560).
Role of the Funding Source
This work was supported by the National Institute of Mental Health (grant R01 MH070560 to SH).
Footnotes
Contributors
SH and CK wrote, edited and approved the manuscript.
Conflict of Interest
Dr. Heckers has received funding from the National Institute of Mental Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Bertrand MC, Sutton H, Montoya A, Czechowska Y, Malla AK, Joober R, Pruessner JC, Lepage M. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64(9):999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews Neuroscience. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46(5):589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Gabaergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biological Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Bickel S, Javitt DC. Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav Brain Res. 2009;204(2):352–362. doi: 10.1016/j.bbr.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang SW. Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Haier RJ, Potkin SG, Nuechterlein K, Bracha HS, Katz M, Lohr J, Wu J, Lottenberg S, Jerabek PA, Trenary M, Tafalla R, Reynolds C, Bunney WE. Frontostriatal disorder of cerebral metabolism in never-medicated schizophrenics. Arch Gen Psychiatry. 1992;49(12):935–942. doi: 10.1001/archpsyc.1992.01820120023005. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Duzel E. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32(41):14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22(3):615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67(7):674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. Journal of chemical neuroanatomy. 2000;20(3–4):299–306. doi: 10.1016/s0891-0618(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26(9):489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain. 1992;115(2):367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Frontiers in molecular neuroscience. 2008;1:5. doi: 10.3389/neuro.02.005.2008. eCollection 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, Chuhma N, Glickstein S, Merker RJ, Xu Q, Small SA, Anderson SA, Ross ME, Moore H. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci U S A. 2014;111(20):7450–7455. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11(5):569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- Gur RE, Mozley PD, Resnick SM, Mozley LH, Shtasel DL, Gallacher F, Arnold SE, Karp JS, Alavi A, Reivich M, Gur RC. Resting cerebral glucose metabolism in first-episode and previously treated patients with schizophrenia relates to clinical features. Arch Gen Psychiatry. 1995;52(8):657–667. doi: 10.1001/archpsyc.1995.03950200047013. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Marwick K, McKirdy J, Sussmann J, Romaniuk L, Johnstone EC, Wan HI, McIntosh AM, Lawrie SM. Hippocampal function in schizophrenia and bipolar disorder. Psychol Med. 2009:1–10. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, Rauch SL. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Archives of General Psychiatry. 1999;56:1117–1123. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Archives of General Psychiatry. 1991;48(11):1002–1008. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. In: Swerdlow NR, editor. Current Topics in Behavioral Neurosciences: Behavioral Neurobiology of Schizophrenia and Its Treatment. Springer; New York: 2010. pp. 529–553. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Archives of General Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Heckers S, Woodward ND, Ongur D. Neuroimaging of psychotic disorders. In: Charney DS, Buxbaum J, Sklar P, Nestler EJ, editors. Neurobiology of mental illness. Oxford University Press; 2013. pp. 256–268. [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Research. 2006;82(2–3):153–162. doi: 10.1016/j.schres.2005.09.021. Epub 2005 Dec 2027. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, Ditman T, Welsh RC, Heckers S. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biological Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU, Maier W, Schild HH, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. The American journal of psychiatry. 2003;160(7):1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Maeda Y, Sakai N, Higashima M, Yamaguchi N, Koshino Y, Hisada K, Suzuki M, Matsuda H. Regional cerebral blood flow in patients with schizophrenia: relevance to symptom structures. Psychiatry Res. 1996;67(1):49–58. doi: 10.1016/0925-4927(96)02685-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Suzuki M, Takashima T. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. Eur Arch Psychiatry Clin Neurosci. 1992;241(4):195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26(5):1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia Research. 2011a;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011b;68(4):340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA psychiatry. 2013;70(12):1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieckhaus EE, Donahoe JW, Morgan MA. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biological Psychiatry. 1992;31(6):560–570. doi: 10.1016/0006-3223(92)90242-r. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53(7):601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19(3):312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Jones T, Hirsch SR, Frackowiak RSJ. Patterns of cerebral blood flow in schizophrenia. British Journal of Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nature reviews Neuroscience. 2001;2(1):11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Neurostereology: Unbiased stereology of neural systems. Wiley; Oxford, UK: 2014. [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O’Shora-Celaya L, Eberling J, Robertson L, Huesman RH, Jagust W, Budinger TF. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15(6):541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- Olbrich HG, Braak H. Ratio of pyramidal cells versus non-pyramidal cells in sector CA1 of the human Ammon’s horn. Anatomy and Embryology. 1985;173:105–110. doi: 10.1007/BF00707308. [DOI] [PubMed] [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Archives of General Psychiatry. 2006;63(4):1268–1277. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Molecular psychiatry. 2013;18(11):1193–1198. doi: 10.1038/mp.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. American Journal of Psychiatry. 2001;158(7):1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92(2):135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Done DJ, Bruton C, Crow TJ. A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry. 1990;28(2):127–143. doi: 10.1016/0006-3223(90)90630-k. [DOI] [PubMed] [Google Scholar]
- Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, Tronche F, Levesque D, Moquin L, Gratton A, Wong TP, Rubinstein M, Giros B. Presynaptic D Dopamine Receptors Control Long-Term Depression Expression and Memory Processes in the Temporal Hippocampus. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Salvatore P, Baldessarini RJ, Tohen M, Khalsa HM, Sanchez-Toledo JP, Zarate CA, Vieta E, Maggini C. McLean-Harvard international first-episode project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2008;70(4):458–466. doi: 10.4088/jcp.08m04227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. 2012;109(42):E2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathologica. 2009;117(4):395–407. doi: 10.1007/s00401-008-0430-y. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhry N, Khan U, Paniagua B, Styner M, Asllani I, Innbar BP, Corcoran CM, Lieberman JA, Moore M, Small SA. Imaging patients with psychosis and a mouse model establishes a spatiotemporal pattern of hippocampal dysfunction and implicates glutamate elevation as a pathogenic driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of General Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews Neuroscience. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nature reviews Neuroscience. 2008;9(3):206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stevens JR. Epilepsy, psychosis and schizophrenia. Schizophr Res. 1988;1(1):79–89. doi: 10.1016/0920-9964(88)90044-8. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Molecular psychiatry. 2012;17(7):664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, Heckers S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage: Clinical. 2014;5:359–364. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent MK, Qiu C. Somatostatin: an endogenous antiepileptic. Molecular and cellular endocrinology. 2008;286(1–2):96–103. doi: 10.1016/j.mce.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. American Journal of Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Archives of General Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry. 2014;76(2):111–119. doi: 10.1016/j.biopsych.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. The American journal of psychiatry. 2014;171(5):549–556. doi: 10.1176/appi.ajp.2013.13070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31(30):10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Current opinion in neurobiology. 2013;23(2):283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews Neuroscience. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31(34):12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature reviews Neuroscience. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Venables PH. Hippocampal function and schizophrenia. Experimental psychological evidence. Annals of the New York Academy of Sciences. 1992;658:111–127. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Molecular and cellular endocrinology. 2008;286(1–2):75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, Crow TJ. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. American Journal of Psychiatry. 2002;159:821–828. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biological Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biological Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Williams LE, Blackford JU, Luksik A, Gauthier I, Heckers S. Reduced habituation in patients with schizophrenia. Schizophr Res. 2013;151(1–3):124–132. doi: 10.1016/j.schres.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg RE, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. The American journal of psychiatry. 1989;146(5):577–587. doi: 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective deficit in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia Research. 2000;49(1–2 Suppl):65. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]