Abstract
Background
Exhaled nitric oxide (FeNO), a marker of airway inflammation, is often elevated in lung transplant recipients (LTxR) with acute rejection or infection. Isolated measurements in the setting of bronchiolitis obliterans syndrome have been variable. We sought to assess the utility of serial FeNO in predicting chronic allograft dysfunction or the presence of acute rejection or infection.
Methods
Eighty-six LTxR underwent 325 serial FeNO measurements at an expiratory flow rate of 50 ml/s. The change in FeNO (ΔFeNO) between two measurements obtained during a stable state (ΔFeNO-SS) was compared with ΔFeNO where the first measurement was during a stable state and the second taken during an unstable state (defined as a subsequent decline in FEV1 of > 10% over 3 months; ΔFeNO-SU) or an acute complication (acute rejection, lymphocytic bronchiolitis or acute infection; ΔFeNO-SAC). The median follow-up time after the baseline FeNO was 10 months (range 3 – 25).
Results
ΔFeNO-SS in 117 FeNO pairs was similar to ΔFeNO-SU in 26 pairs (2.1 ± 3 ppb vs. 2.3 ± 4 ppb; p = 0.2). ΔFeNO-SAC in 17 pairs was markedly increased (27 ± 20 ppb; p < 0.001 vs. ΔFeNO-SS). The area under the receiver operating characteristic curve for ΔFeNO in detecting an acute complication was 0.93 (p < 0.001). By applying a cut off > 10 ppb, the sensitivity and specificity was 82 and 100%, respectively, with positive and negative predictive values of 100 and 97.5%.
Conclusions
Changes in FeNO may serve as a useful adjunct in the detection of acute complications after lung transplantation. In this limited analysis, ΔFeNO was not predictive of a subsequent decline in allograft function.
Introduction
Lung transplantation (LTx) is an important therapeutic option for patients with endstage pulmonary disorders (1). However, chronic lung allograft dysfunction manifesting as bronchiolitis obliterans syndrome (BOS) continues to be highly prevalent and is the leading cause of long term mortality after LTx (2). Although the pathogenesis of BOS is not fully understood, airway injury is thought to induce an initial inflammatory process that eventually leads to fibrosis and small airway obliteration (3,4). Detection of airway inflammation that could precede the development of BOS offers an opportunity for early intervention, e.g. introduction of azithromycin or other potential treatment strategies (5,6). In addition, acute rejection (AR), lymphocytic bronchiolitis (LB) and respiratory infections have been implicated as risk factors for BOS (5,7–11). Early diagnosis and treatment of these acute events may reduce the incidence of BOS (8). Unfortunately, the current diagnostic modalities for early detection of both BOS and its risk factors have some limitations (12). Thus, a reliable, noninvasive diagnostic biomarker is needed.
Exhaled nitric oxide (the fractional expired concentration or FeNO) is a standardized and validated technique for assessing airway inflammation and the response to pharmacological treatment in patients with asthma (13–15). In LTx, previous reports suggest elevated FeNO in the setting of acute infections, LB and AR (16–18). FeNO appears to be highly variable in subjects with BOS (19). Most of these studies have been cross-sectional in design with a single measurement. Two longitudinal studies have assessed changes in FeNO and subsequent development of BOS (20,21). Van Muylem et al found limited diagnostic utility using a high expiratory flow rate for FeNO of 200 ml/s, compared with the ATS recommended rate of 50 ml/s (22). Neurohr and co-workers report excellent negative predictive value for the subsequent development of BOS, but limited positive predictive value for a single FeNO value of > 20 ppb. Since normal individuals demonstrate a fairly wide range of FeNO (23), the magnitude of increase may be more informative than an absolute threshold value. Moreover, studies of longitudinal FeNO changes among recipients developing acute complications have been limited. In this study, we hypothesized that the change in FeNO measured serially in lung transplant recipients would predict a subsequent decline in lung function and acute complications.
Materials and methods
Study subjects and FeNO Measurements
The study included 86 consecutive bilateral or combined heart-lung LTxR seen in the outpatient clinic of the Johns Hopkins transplant program during the period from June 2010 to March 2012. The study protocol was approved by the local institutional review board and all subjects gave written informed consent. Basilximab or dacluzimab were given for induction and immunosuppression was maintained with triple therapy consisting of tacrolimus or cyclosporine, mycophenalate or azathioprine, and corticosteroids.
FeNO was obtained during outpatient visits and/or before surveillance or clinically indicated bronchoscopies according to ATS guidelines using an Aerocrine ® NIOX Flex analyzer at an expiratory flow rate of 50 ml/s (22). Active smokers, those using inhaled steroids within 2 weeks of study, and patients with bronchial stenosis or symptoms of allergic rhinitis at the time of testing were not included (24–27). Sixty-six healthy subjects (non-smokers and without history of asthma) served as a normal control group, providing a single measurement.
Definitions and groups
The clinical status at each FeNO measurement was categorized into 5 different groups as modified from Neurohr et al (20):
Stable non-BOS: BOS stage 0/0-p (4) without any acute complications (AR, LB, or infection) within the previous month and < 10% decline in FEV1 during the subsequent 3 months following the FeNO measurement.
Unstable non-BOS: BOS stage 0/0-p without any acute complications within previous month but a decline of ≥ 10% in FEV1 during the following 3 months not attributable to an acute complication or other process.
Stable BOS: BOS stage ≥ 1 without any acute complications within previous month and < 10% decline in FEV1 during the subsequent 3 months following the FeNO measurment.
Unstable BOS: BOS stage ≥ 1 without any acute complications within the previous month but an otherwise unexplained decline of ≥ 10% in FEV1 during the the subsequent 3 months following the FeNO measurment.
Acute complications group were diagnosed with AR, LB or an acute respiratory infection within one month of the FeNO measurement. AR and LB diagnoses were based on transbronchial biopsy (TBB) findings. Respiratory infection was defined as signs and symptoms, e.g. new cough, sputum, radiographic abnormality or positive cultures and the addition of a new antimicrobial agent by the treating physician.
Serial FeNO
The change in FeNO between 2 consecutive measurements (ΔFeNO), where both were taken during a stable state (ΔFeNO-SS), was compared with ΔFeNO in which the first measurement was during a stable state and the second taken during an unstable state (ΔFeNO-SU). Similarly, ΔFeNO-SS was compared with FeNO pairs where the first value was during a steady state and the second taken during an acute complication (ΔFeNO-SAC). A receiver operating characteristic (ROC) curve was constructed to assess the diagnostic performance of ΔFeNO for the detection of an acute complication.
Statistical analysis
Data are expressed as mean ± SD unless otherwise indicated. Comparison between groups was made with unpaired or paired t test as appropriate. Non-normally distributed variables were analyzed by Mann-Whitney test. Differences between multiple groups were analyzed with the Kruskal-Wallis test. The coefficient of variation (CV) for FeNO was computed in 36 stable non- BOS subjects who had 3 FeNO measurements at least one month apart.
Results
Subjects characteristics
A total of 325 FeNO measurements were obtained from 86 patients. Subject characteristics are summarized in table 1. The mean follow up time after the baseline FeNO measurement was 10.6 ± 6.2 months (median 10, range 3 – 25 months). Ninety percent (291) of the measurements were eligible to be included under one of the study groups. Thirty-four (10%) met the exclusion criteria. Of these, 24 measurements were excluded because of bronchial stenosis (new/unstable), 6 were excluded because of inhaled steroids use 2 weeks prior to FeNO measurement and rest were excluded because of allergic rhinitis symptoms at the time of the measurement. At the beginning of the study, 11 patients were in stage BOS 1 or greater and 2 more recipients who were BOS free at baseline developed BOS by the end of the study. These patients had an overwhelmingly obstructive pattern of chronic allograft dysfunction with only one having an FEV1/FVC ratio of > 0.7 and all had ratios < 0.8 (28). The remaining patients (73) were BOS free during the entire follow up period.
Table 1.
Baseline characteristics
| Total # of LTxR (male/female) | 86 (34/52) |
| Race (black/white/other) | 20/62/4 |
| LTx type (Bilateral lung/Heat-Lung) | 80/6 |
| LTx reason (COPD/CF/IPF/other) | 25/21/9/31 |
| Age (Yrs) | 51.2 ± 13.1 |
| Post-Operative months (min/max) | 39.4 ± 46.9 (1/263) |
| Best post-operative FEV1 (L) | 2.0 ± 0.6 |
| Best post-operative FEV1 ( % predicted) | 73 ± 21.1 |
| # of measurements/patient | 3.8 ± 2.3 |
LTxR = lung transplant recipient; COPD = chronic obstructive lung disease; CF = cystic fibrosis; IPF = idiopathic pulmonary fibrosis; FEV1 = forced expiratory volume in 1 second.
Single FeNO Measurements in Recipients without an Acute Complication
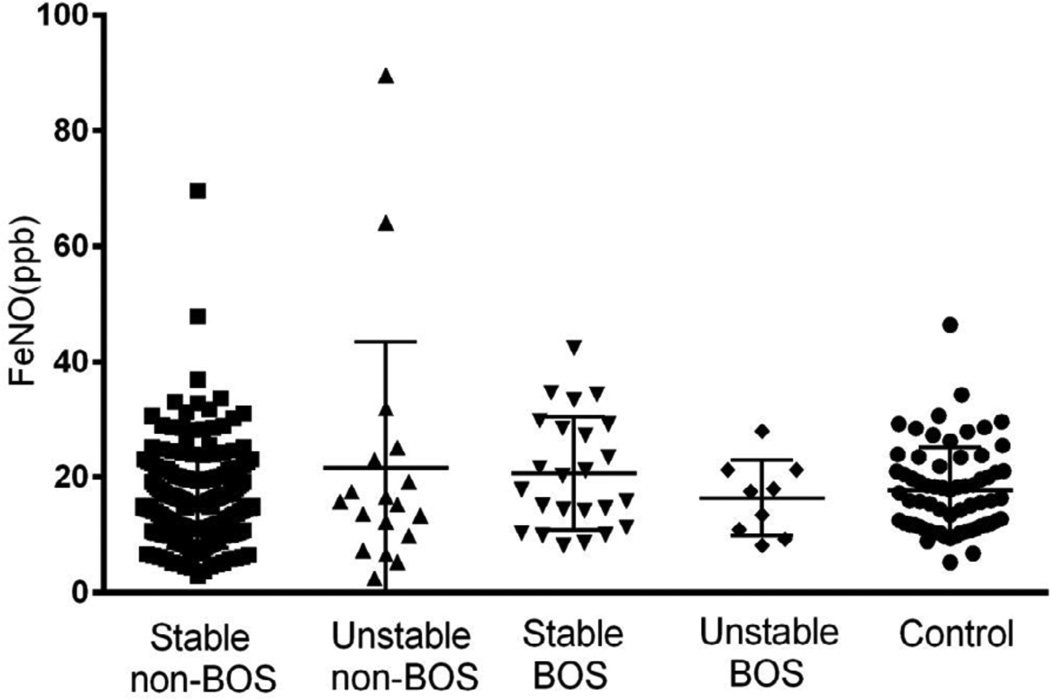
FeNO measurements in stable non-BOS subjects were comparable to healthy controls (16 ± 8.2 vs. 17.6 ± 7.5 ppb). No significant differences were found between BOS vs. non-BOS or stable vs. unstable LTxR without acute complications (Figure 1). Based on this data, we calculate that we had 78% power to detect a 10 ppb difference between stable and unstable subjects at an alpha level of 0.05. The CV of FeNO among stable non-BOS subjects was 25.5 ± 11.3 %.
FIGURE 1.
FeNO levels in lung transplant recipients and healthy control subjects. Stable non- BOS N = 198, unstable non-BOS N = 18, stable BOS N = 12, unstable BOS N = 9 and Control subjects N = 66. Mean values with SD. p > 0.05.
ΔFeNO in stable and unstable LTxR
In order to ascertain whether a change in FeNO could predict a subsequent decline in lung function, 117 ΔFeNO-SS pairs in 51 patients (46 stable non-BOS and 5 stable BOS) were compared with 26 ΔFeNO-SU pairs in 16 patients where the first measurement was during a steady state and the second an unstable state (12 unstable non-BOS and 4 unstable BOS). No difference was observed (2.1 ± 2.5 ppb vs. 2.3 ± 4.3 ppb; p = 0.2). Based on this data, we had > 99% power to detect a change in FeNO of 10 ppb in the unstable subjects and > 90% power to detect a change of 5 ppb. The mean fall in FEV1 in the unstable subjects was 16.6 ± 5%. Subsequent pulmonary function was available for review beyond the initial 3 month follow-up window in 14 patients. In11of these, FEV1 had returned to within 10% of the baseline value. For the 2 patients who developed BOS during the course of the study the ΔFeNO was + 2.9 and – 2.9 ppb, respectively.
FeNO in LTxR with acute complications
Forty-two FeNO measurements were taken from 27 LTxR with an acute complication (AR = 9, LB = 5, Acute infection = 28). FeNO was measured before any treatment in 21 of these episodes in 16 subjects. In all these cases, testing occurred within 2 days of the diagnosis. FeNO during untreated acute complications was significantly increased compared with the stable non-BOS group (40.9 ± 28.7 vs. 16 ± 8.2 ppb; p < 0.001). Those studied after therapy for an acute complication had comparable levels (16.7 ± 10.5 ppb; p = 0.8) to stable non-BOS.
ΔFeNO and Acute Complications
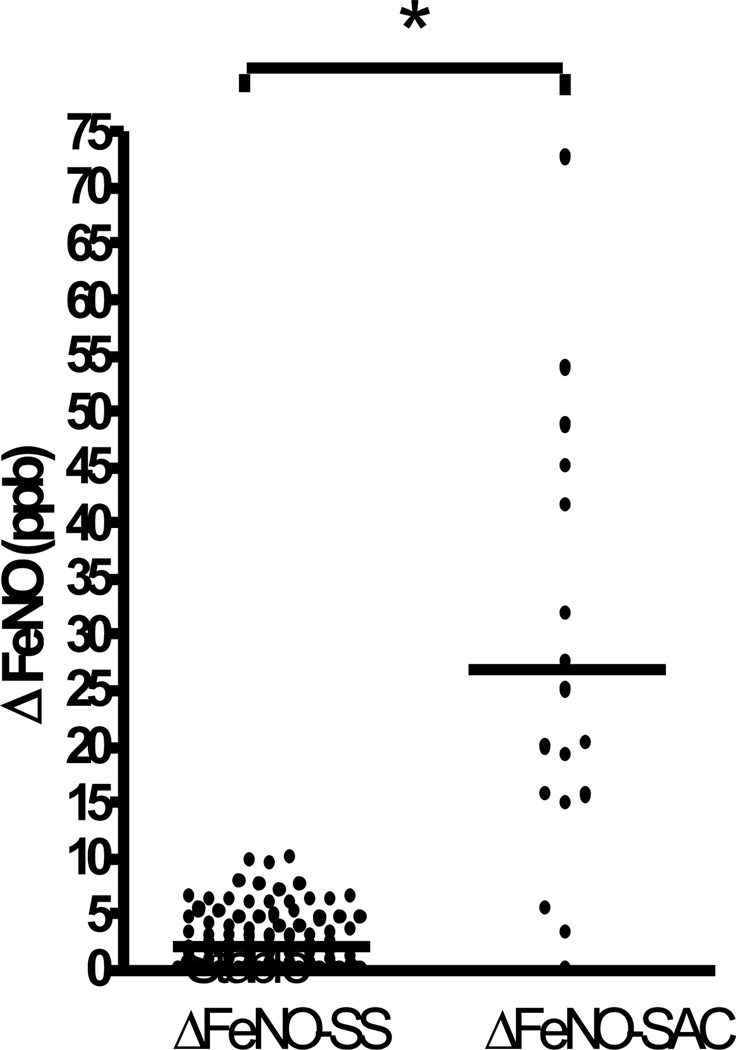
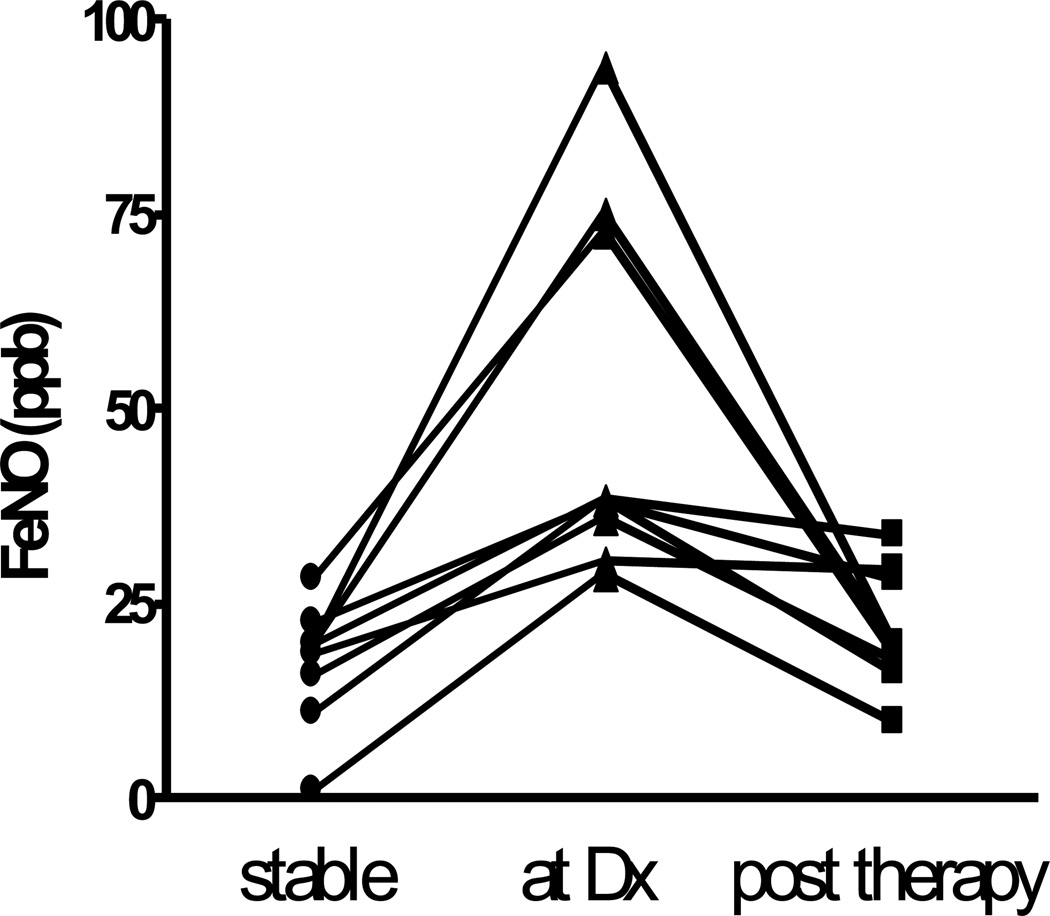
Seventeen FeNO pairs were obtained from 13 subjects, where the first measurement was during a stable state and the second during an untreated acute complication (AR = 2, LB = 3, acute infection = 12). As shown in figure 2, ΔFeNO-SAC (26.9 ± 19.6 ppb) was dramatically increased in this group compared with essentially no change observed in ΔFeNO-SS (p < 0.001). Details of these 17 acute complication episodes are presented in table 2. FeNO was repeated after therapy for 9 episodes. As demonstrated in figure 3, values fell considerably after treatment (50 ± 24 vs. 22 ± 7 ppb; p = 0.01). Accompanying the ΔFeNO reduction was an improvement in FEV1 after therapy of 0.2 ± 0.2 L (p = 0.02).
FIGURE 2.
ΔFeNO in stable LTxR and untreated acute complication. Dot plots show the change in FeNO between 2 consecutive measurements taken during a stable state (ΔFeNO-SS) and where first measure was during a stable state and second during an untreated acute complication (ΔFeNO-SAC). * p < 0.001.
Table 2.
Characteristics of Recipients with Serial FeNO during Acute Complications
| LTx R No. |
Reason for LTx |
POD (months) |
BOS stage |
Baseline FEV1 (%) |
FEV1 (%) at Dx |
Acute complication |
Radiological findings |
Biopsy | BAL culture |
Rx | ΔFeNO | ΔFe NO after Rx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A1AD | 9.6 | 0 | 4.4 (93.4) |
3.2 (84.2) |
AR | none | A2B0 | none | ST | 15.45 | −9.8 |
| 2 | CF | 14.7 | 0-p | 3.6 (76.7) |
2.9 (74.7) |
AR | none | A2Bx | none | ST | 19.00 | −5 |
| 3 | PH | 44.4 | 0 | 1.7 (58.8) |
1.5 (53.6) |
infection Abx treated |
none | N/A | N/A | Abx | 20.20 | −18.2 |
| 4 | COPD | 31.4 | 0-p | 2 (69.1) |
1 (42.5) |
infection Abx treated |
none | N/A | N/A | Abx | 27.55 | −22.6 |
| 5 | CF | 148.9 | 2 | 3.1 (10 5) |
1.6 (67) |
infection Abx treated |
none | N/A | N/A | Abx | 31.80 | −21.1 |
| 6 | CF | 10.6 | 0-p | 3.6 (77.7) |
2.9 (75.9) |
infection Abx treated |
new infiltrate |
A0B0 | PA | Abx | 53.70 | −55.8 |
| 7 | CF | 13.1 | 0-p | 3.6 (77.7) |
2.9 (75.9) |
infection Abx treated |
new infiltrate |
A0B0 | PA | Abx | 72.55 | −74.6 |
| 8 | CHD | 48.4 | 0 | 3.7 (84.8) |
2.9 (78.4) |
infection Abx treated |
none | N/A | N/A | Abx | 48.60 | N/A |
| 9 | PH | 72.2 | 0 | 3.5 (85.2) |
2.5 (75.5) |
infection Abx treated |
none | N/A | N/A | Abx | 3.15 | N/A |
| 10 | PH | 31.1 | 1 | 1.9 (72.6) |
1.3 (59) |
infection Abx treated |
none | A0Bx | none | Abx | 5.40 | N/A |
| 11 | IPF | 12.6 | 1 | 3.5 (103) |
1.9 (75.2) |
infection other Rx |
none | A0B0 | MPV | IVIG | 19.75 | −19 |
| 12 | CF | 143.8 | 0-p | 3 (103) |
1.6 (65.6) |
infection other Rx |
none | A0B0 | RSV | IVIG | 45.00 | −54.5 |
| 13 | PH | 96.1 | 0-p | 3.2 (94.9) |
2.1 (72.5) |
infection other Rx |
none | A0B0 | ASP | AF | 41.35 | N/A |
| 14 | IPF | 3.2 | 0 | 2.2 (69.5) |
1.8 (61) |
infection other Rx |
new infiltrate |
A0B0 | RSV | IVIG | 0.00 | N/A |
| 15 | COPD | 48.9 | 0 | 2.5 (79) |
1.2 (51.6) |
LB | none | LB | none | ST | 14.85 | N/A |
| 16 | CF | 15.1 | 0 | 3.3 (95.9) |
1.9 (65.6) |
LB | none | LB | none | ST | 24.90 | N/A |
| 17 | CF | 17.2 | 0 | 3.3 (95.9) |
1.9 (65.6) |
LB | none | LB | none | ST | 15.50 | N/A |
A1AD = alpha-1 antitrypsin deficiency; Abx = antibiotics; AF = antifungal; ASP = aspergillosis; BAL = bronchoalveolar lavage; CF = cystic fibrosis; CHD = congenital heart disease; COPD = chronic obstructive pulmonary disease; Dx = diagnosis; FEV1 = forced expiratory volum in 1 second (L/% predicted); IPF = idiopathic pulmonary fibrosis; IVIG = Intravenous immunoglobulin; MPV = metapneumovirus; N/A = not applicable; PA = pseudomonas aeruginosa; PH = pulmonary hypertension; RSV = respiratory syncytial virus; Rx = therapy; ST = steroids.
FIGURE 3.
Serial individual FeNO values in LTxR with an acute complication during stable state, at the time of diagnosis (Dx) and post therapy. p = 0.01.
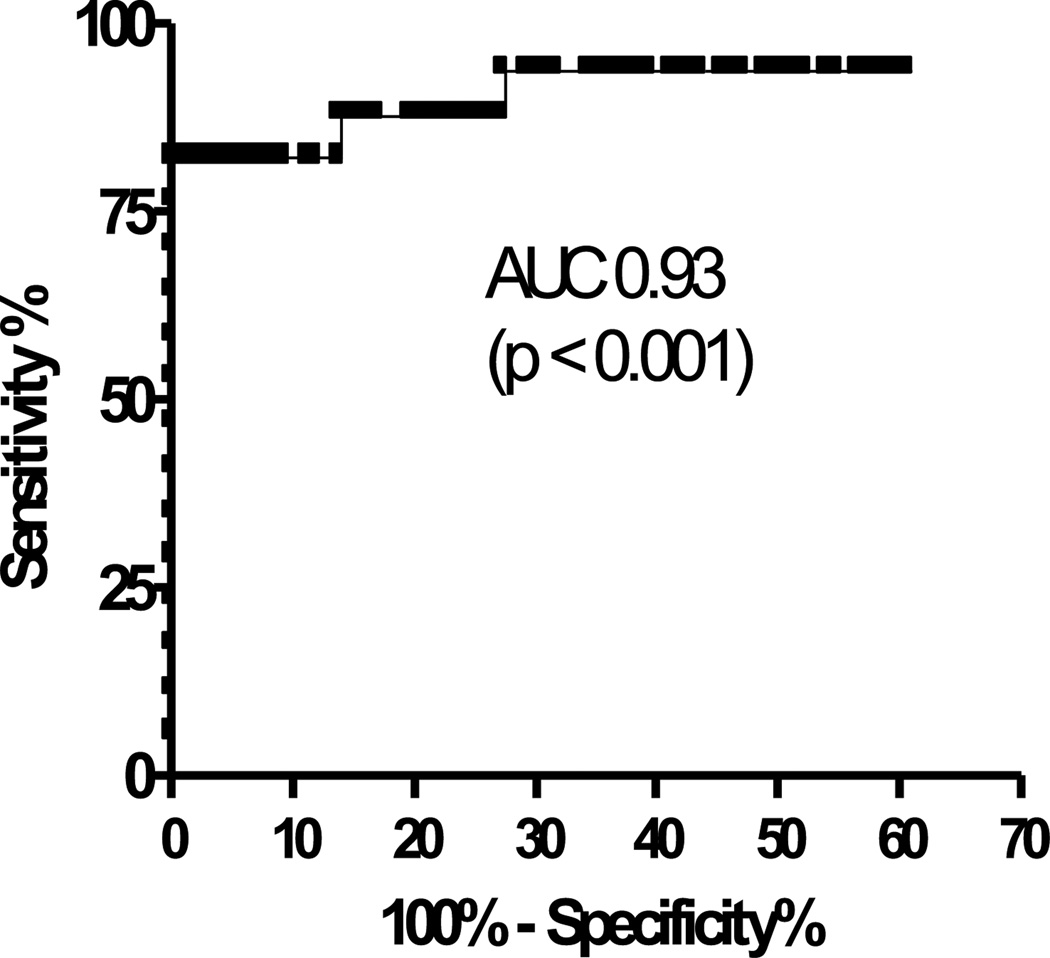
Diagnostic Utility of the Change in FeNO for the Detection of an Acute Complication
The area under the ROC curve (figure 4) for ΔFeNO in detecting an acute complication was quite high at 0.93 (p < 0.001). By applying the optimal cut off ≥ 10 ppb, we calculated a sensitivity of 82.3% and a specificity of 100%. The positive and negative predictive values were 100% and 97.5%, respectively.
FIGURE 4.
Receiver operating characteristic (ROC) curve for ΔFeNO to detect an acute complication.
Discussion
The main finding of this study is the robust rise in serial FeNO during acute complications (acute rejection, lymphocytic bronchiolitis and acute infection). While previous reports have shown elevated values during these episodes compared with stable LTxR, this is the first longitudinal study to describe the operating characteristics of a change in FeNO for detection of these episodes. Given that such events are established risk factors for BOS, exhaled nitric oxide may be a useful adjunct in the monitoring of LTxR.
Several previous cross-sectional studies have demonstrated increased FeNO in LTxR with lymphocytic bronchiolitis and acute infection, suggesting a role in identifying airway inflammation (17,18). A report by De Soyza et al showed an increase in FeNO (at an expiratory flow rate of 250 ml/sec) in fourteen LTxR who developed LB, and subsequent return to baseline after inhaled steroid treatment (24). FeNO values during acute rejection have been variable (17,18). We only had 2 cases of acute rejection and in one a B grade could not be ascertained. A longitudinal study by Antus et al showed increased levels of FeNO in LTxR during acute infection. However this study had a relatively small number of LTxR and included both upper and lower respiratory infection. FeNO has been correlated with BAL neutrophilia and epithelial inducible nitric oxide synthase (iNOS) expression in LTxR (29). Moreover, FeNO is an established marker of airway inflammation in asthma. Interestingly, the optimal cut-point for a change in FeNO to detect an acute complication in our study of ≥ 10 ppb is identical to the increase suggested as a significant change in asthma in a recent consensus statement (13).
If airway inflammation precedes BOS, FeNO could be a useful predictor. Previous studies showed higher FeNO in early (17) and unstable BOS (19), but not advanced stages of disease without an active decline in lung function, suggesting that occult airway inflammation precedes fibrous obliteration. Normal individuals have a wide range of FeNO (23). Hence, serial measurements are likely to provide more information compared with a single test. In a longitudinal study of 65 patients, Van Muyhem et al found a small rise in FeNO as LTxR progressed from BOS stage 0 to 0-p, but no change between stages 0 and 1 (21). Similarly, a study by Verleden et al demonstrated an increase in FeNO at early stages of BOS (30). However, both studies measured FeNO at an expiratory flow rate of 200 ml/s. FeNO falls exponentially with higher flow rates, potentially diluting changes induced by airway inflammation. We measured FeNO at the ATS recommended rate of 50 ml/s (22). Neurohr and colleagues (20), using a rate of 50 ml/s, demonstrated higher FeNO values among LTxR who subsequently developed new or worsening BOS (defined as unstable). Furthermore, a single FeNO measurement of ≥ 20 ppb had a positive and negative predictive value of 69% and 97%, respectively, for a subsequent unstable course. With serial testing, unstable patients had greater variability in FeNO compared with stable subjects. However, the diagnostic performance characteristics of a change in FeNO were not reported.
We did not detect a difference in single FeNO values between non-BOS and BOS consistent with previous data (31), although the number of BOS patients was small. We also could not detect a difference in FeNO between stable vs. unstable LTxR, defined as a subsequent 10% fall in FEV1. Thus, we were unable to duplicate the results of Neurohr et al (20). While our study had about half the number of subjects, and was somewhat underpowered to detect a difference in single FeNO values, others have had a similar experience (31). Moreover, we did not detect a change in serial measurements between stable and unstable clinical states and did have quite adequate power to detect such a change. It is possible that a large proportion of patients develop fibro-proliferative airways disease without a preceding inflammatory phase. Alternatively, inflammation may be transient and not detected during periodic FeNO testing. An important limitation to this observation is the subsequent recovery of lung function in many of the unstable patients.
While the number of acute complication episodes in this study is small, the ΔFeNO data suggests that this simple, non-invasive and inexpensive test could serve as a useful adjunct in the monitoring of lung transplant recipients. An increase in 10 ppb was both highly sensitive and specific for an acute complication. The high negative predictive value suggests that patients with a decline in lung function, but less than a 10 ppb rise in FeNO could be safely observed, whereas those with an increase of ≥ 10 ppb would require additional investigation, including bronchoscopy. Additional studies are required to confirm these findings and determine whether isolated acute vascular rejection is also associated with a rise in FeNO.
Small, portable nitric oxide analyzers are becoming increasingly utilized in the clinical outpatient setting (32). Hand held devices suitable for home use are in development making daily monitoring potentially feasible (33,34). Using such devices, future studies could ascertain if FeNO does indeed predict the development of BOS and its utility in detecting acute complications.
Acknowledgments
MAG contributed to the design of the study, analysis of data and preparation of the final manuscript. MAG also serves as the guarantor for this manuscript. Guarantor of the paper takes responsibility for the integrity of the work as a whole from inception to publication. CAM, MRP, JFM and JBO contributed to recruiting patients and writing/reviewing the manuscript. JAH assisted with the statistical analysis and writing/reviewing the manuscript. REG was responsible for the conception of the project, assisted in the writing and data analysis and final manuscript approval.
Sources of Support and Funding: Supported in part by NHLBI HL092831 (RG). MAG received scholarship fund from the Libyan Petroleum Training and Qualifying Institute (PTQI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest to declare.
Contributor Information
Mohamed A. Gashouta, Saint Luke’s Hospital, Internal Medicine Department, 222 S Woods Mill Rd. Suite 760N, Chesterfield MO 63017, mgashouta@gmail.com
Christian A. Merlo, Johns Hopkins University School of Medicine, Pulmonary and Critical Care Medicine, Baltimore, MD, cmerlo@jhmi.edu
Matthew R. Pipeling, University of Pittsburgh, Pulmonary, Allergy and Critical Care Medicine, Pittsburgh, PA, pipelingm@upmc.edu
John F. McDyer, University of Pittsburgh, Pulmonary, Allergy and Critical Care Medicine, Pittsburgh, PA, mcdyerjf@upmc.edu
J. W. Awori Hayanga, Spectrum Health/Michigan State University, Richard DeVos Heart and Lung Transplant Program, Grand Rapids, MI, Jw.Hayanga@spectrumhealth.org
Jonathan B. Orens, Johns Hopkins University School of Medicine, Pulmonary and Critical Care Medicine, Baltimore, MD, jorens@jhmi.edu
Reda E. Girgis, Spectrum Health/Michigan State University, Richard DeVos Heart and Lung Transplant Program, Grand Rapids, MI, Reda.girgis@spectrumhealth.org
Reference List
- 1.Reitz BA, Wallwork JL, Hunt SA, Pennock JL, Billingham ME, Oyer PE, Stinson EB, Shumway NE. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982;306:557–564. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 4.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 5.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–1263. [PubMed] [Google Scholar]
- 6.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26:681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, Ting A, Johnstone I, Miller J, Robbins RC, Reitz BA, Theodore J. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–1208. [PubMed] [Google Scholar]
- 8.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80:1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 9.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 10.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, Gould K, Fisher AJ. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85:771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 11.Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, Snell G, Westall G. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11:2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant. 2002;21:1062–1067. doi: 10.1016/s1053-2498(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 13.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montuschi P, Mondino C, Koch P, Ciabattoni G, Barnes PJ, Baviera G. Effects of montelukast treatment and withdrawal on fractional exhaled nitric oxide and lung function in children with asthma. Chest. 2007;132:1876–1881. doi: 10.1378/chest.07-1587. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 16.Antus B, Csiszer E, Czebe K, Horvath I. Pulmonary infections increase exhaled nitric oxide in lung transplant recipients: a longitudinal study. Clin Transplant. 2005;19:377–382. doi: 10.1111/j.1399-0012.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 17.Fisher AJ, Gabbay E, Small T, Doig S, Dark JH, Corris PA. Cross sectional study of exhaled nitric oxide levels following lung transplantation. Thorax. 1998;53:454–458. doi: 10.1136/thx.53.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silkoff PE, Caramori M, Tremblay L, McClean P, Chaparro C, Kesten S, Hutcheon M, Slutsky AS, Zamel N, Keshavjee S. Exhaled nitric oxide in human lung transplantation. A noninvasive marker of acute rejection. Am J Respir Crit Care Med. 1998;157:1822–1828. doi: 10.1164/ajrccm.157.6.9707159. [DOI] [PubMed] [Google Scholar]
- 19.Brugiere O, Thabut G, Mal H, Marceau A, Dauriat G, Marrash-Chahla R, Castier Y, Leseche G, Colombat M, Fournier M. Exhaled NO may predict the decline in lung function in bronchiolitis obliterans syndrome. Eur Respir J. 2005;25:813–819. doi: 10.1183/09031936.05.00057004. [DOI] [PubMed] [Google Scholar]
- 20.Neurohr C, Huppmann P, Leuschner S, von Wulffen W, Meis T, Leuchte H, Ihle F, Zimmermann G, Baezner C, Hatz R, Winter H, Frey L, Ueberfuhr P, Bittmann I, Behr J. Usefulness of exhaled nitric oxide to guide risk stratification for bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2011;11:129–137. doi: 10.1111/j.1600-6143.2010.03327.x. [DOI] [PubMed] [Google Scholar]
- 21.Van Muylem A, Knoop C, Estenne M. Early detection of chronic pulmonary allograft dysfunction by exhaled biomarkers. Am J Respir Crit Care Med. 2007;175:731–736. doi: 10.1164/rccm.200609-1301OC. [DOI] [PubMed] [Google Scholar]
- 22.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.See KC, Christiani DC. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: results from the National Health and Nutrition Examination Survey 2007–2010. Chest. 2013;143:107–116. doi: 10.1378/chest.12-0416. [DOI] [PubMed] [Google Scholar]
- 24.De Soyza A, Fisher AJ, Small T, Corris PA. Inhaled corticosteroids and the treatment of lymphocytic bronchiolitis following lung transplantation. Am J Respir Crit Care Med. 2001;164:1209–1212. doi: 10.1164/ajrccm.164.7.2011034. [DOI] [PubMed] [Google Scholar]
- 25.Kalpaklioglu AF, Kalkan IK. Comparison of orally exhaled nitric oxide in allergic versus nonallergic rhinitis. Am J Rhinol Allergy. 2012;26:e50–e54. doi: 10.2500/ajra.2012.26.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 27.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–457. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 28.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, Palmer SM. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabbay E, Haydn WE, Orsida B, Whitford H, Ward C, Kotsimbos TC, Snell GI, Williams TJ. In stable lung transplant recipients, exhaled nitric oxide levels positively correlate with airway neutrophilia and bronchial epithelial iNOS. Am J Respir Crit Care Med. 1999;160:2093–2099. doi: 10.1164/ajrccm.160.6.9902088. [DOI] [PubMed] [Google Scholar]
- 30.Verleden GM, Dupont LJ, Van Raemdonck DE, Vanhaecke J. Accuracy of exhaled nitric oxide measurements for the diagnosis of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;78:730–733. doi: 10.1097/01.tp.0000131814.44106.c0. [DOI] [PubMed] [Google Scholar]
- 31.Antus B, Barta I, Csiszer E. Exhaled nitric oxide in diagnosis of bronchiolitis obliterans syndrome in lung transplant recipients: possible limitations. Am J Transplant. 2011;11:2774–2775. doi: 10.1111/j.1600-6143.2011.03780.x. [DOI] [PubMed] [Google Scholar]
- 32.Menzies D, Nair A, Lipworth BJ. Portable exhaled nitric oxide measurement: Comparison with the "gold standard" technique. Chest. 2007;131:410–414. doi: 10.1378/chest.06-1335. [DOI] [PubMed] [Google Scholar]
- 33.Schneider A, Tilemann L, Schermer T, Gindner L, Laux G, Szecsenyi J, Meyer FJ. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement--results of a prospective diagnostic study: FENO< or = 16 ppb better than FENO< or =12 ppb to rule out mild and moderate to severe asthma [added] Respir Res. 2009;10:15. doi: 10.1186/1465-9921-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Laurentiis G, Maniscalco M, Cianciulli F, Stanziola A, Marsico S, Lundberg JO, Weitzberg E, Sofia M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharmacol Ther. 2008;21:689–693. doi: 10.1016/j.pupt.2008.04.006. [DOI] [PubMed] [Google Scholar]