Abstract
Purpose
We investigated whether the initial CT distribution of metastatic disease is predictive of overall survival in patients with stage IV colorectal cancer.
Materials and Methods
A retrospective study of 65 patients (37 male, 28 female, mean age 56, range 28-88 years) with stage IV colorectal cancer was derived from an institutional database. Inclusion criteria required KRAS mutation testing and pretreatment CT examinations to be available (65 abdomen/pelvis, 63 chest). Disease burden was jointly characterized by two radiologists in consensus. Median follow-up was 39 months (range 8 – 115 months). Survival was assessed using Cox proportional hazards models.
Results
Univariate analysis showed that stratified site(s) of measurable disease and counts of measurable lesions >= 1cm in the liver, peritoneum, and retroperitoneum were statistically significant risk factors for overall mortality (univariate HR 8.2 [CI 2.7 to 25.4] for isolated peritoneal disease, HR 1.11 per 5 lesions [CI1.05 to 1.17] for liver lesions, HR 1.15 per lesion [CI 1.05 to 1.26] for peritoneal lesions, and HR 1.11 [CI 1.03 to 1.19] for retroperitoneal lymph nodes >= 1cm in short axis). The stratified site(s) of disease and counts of measurable liver lesions remained significant in the multivariate model (p<0.0001 for isolated peritoneal disease and count of liver lesions). Thoracic metastases were not statistically significant predictors of overall mortality in this cohort.
Conclusions
This study identified site(s) of measurable metastasis and counts of measurable liver lesions as independent predictors of overall survival. These findings may have value for future prognostic assessments once validated in a larger, independent and potentially prospective cohort.
INTRODUCTION
Colorectal cancer is the second most common cause of cancer death in the United States [1]. Patients with colorectal cancer are risk-stratified into stages based on the local extent of the primary tumor, involvement of regional lymph nodes, and presence of metastatic disease using the American Joint Committee on Cancer’s TNM staging system [2]. Approximately 21% of patients have synchronous metastatic disease at presentation, and up to 50% of patients with colorectal cancers develop metastases, and the burden of disease at some sites of metastasis is a known prognostic factor in colorectal cancer [3,4]. While extensive research has been performed to refine and validate the local and regional staging for colon and rectal cancers [5], risk stratification within stage IV disease remains unclear to our knowledge, leading to calls for refined staging criteria for patients with advanced disease [6].
We hypothesize that some features of the initial radiographic distribution and volume of disease in patients presenting with stage IV colorectal cancer will be predictive of overall survival. We therefore performed a retrospective cohort study to test this hypothesis.
MATERIALS and METHODS
Study population
Our HIPAA-compliant, retrospective study was approved by our institutional review board and the requirement for informed consent was waived. A cohort of subjects with stage IV colorectal cancer was identified using a gastrointestinal oncology clinical trials database at our institution. This database included all of the subjects who enrolled in one of the indexed gastrointestinal oncology clinical trials from 2004 to 2010 at our institution, and all 65 subjects from the database that matched our criteria were included in our study. Inclusion criteria included biopsy-proven colorectal adenocarcinoma, stage IV disease at initial diagnosis, no prior chemotherapy at the time of baseline imaging, the presence of pre-chemotherapy CT of the abdomen and pelvis in our radiology system, and tumor KRAS mutation testing documented in our medical record. Subjects were initially diagnosed between March 2003 and August 2009, and median follow-up was 39 months (range 8 – 115 months). No subjects were lost to follow-up.
Assessment of Metastatic Disease
All available baseline CT studies were reviewed. All subjects had baseline pre-chemotherapy CT imaging of the abdomen and pelvis (n=65), and almost all had baseline CT of the chest (n=63). Among the 65 subjects, 13 had baseline imaging examinations performed at outside hospitals that were imported into our PACS, and 52 patients had baseline CT performed in our institution according to the protocol described as below. No significant difference in protocol or image quality was observed between the imported examinations and those performed at our institution. Imaging was performed within an average of 33 days of the start of chemotherapy.
CT scans were performed by using a 64-row MDCT scanner (Aquilion 64; Toshiba America Medical Systems, California). Our CT protocol is as follows: (1) 64-row MDCT scanner at 0.5 mm collimation, 120 kVp, tube current maximum of 500 mA using dose modulation with noise index of 12.5 HU, 0.5 seconds gantry rotation time, and a table speed of 26.5 mm per rotation. One hundred milliliters of iopromide (Ultravist 300®; Bayer HealthCare, San Francisco, CA) were injected intravenously at a rate of 2-3 mL/s, with a scan delay of 60 seconds. Oral contrast (Gastrografin®, Bracco Diagnostics, Princeton, NJ) was administrated prior to the CT scans. Axial images with 5 mm thickness and coronal images with 4 mm thickness were reconstructed using standard abdominal algorithms.
All baseline staging CT scans were evaluated in consensus by two radiologists (M.R. and K.W.K., with 7 and 8 years of postgraduate radiology experience) for the location and volume of metastatic disease using counts of individual lesions. Radiologists were blinded to outcomes and official reports at the time of the image review. Follow-up imaging was only reviewed to resolve indeterminate lesions, as detailed below. Soft tissue, lung, and bone windows were reviewed. To provide broad assessments of the burden of disease that would not be limited by the two lesions per organ standard of RECIST 1.1, we counted the number of discrete lesions that measured 1 cm or greater in longest axial dimension in each site of disease [7]. The 1 cm axial threshold was chosen for consistency with the accepted RECIST thresholds. We assessed for osseous metastases, but none were present in this cohort. For retroperitoneal and thoracic lymph nodes, we counted lesions with an axial short axis measurement greater than 1 cm to remain consistent with clinical practice.
If any lesion was felt to be indeterminate for metastasis on the baseline scan, then two radiologists reviewed all available follow-up imaging and clinical information and made an assignment decision in consensus. Disagreements in scoring and lesion confirmation were resolved by consensus with a third reviewer (N.R.).
Only lesions in the lungs, liver, peritoneum, or lymph nodes were included in the quantitative analyses due to the small numbers of subjects with lesions at other sites.
Medical Record Review
Information was retrieved for each subject using the previously described oncology database, the radiology patient archiving and communication system, and the electronic medical record. All data abstraction was performed by the same two radiologists who performed the CT reviews (K.W.K. and M.R.).
The collected data include subject age at diagnosis, sex, history of cancer diagnosis and treatment, location of the primary colorectal tumor, clinical and pathological cancer staging, tumor histologic grade, the presence or absence of mucinous histologic features, and the results of tumor KRAS genotype tests. In addition, the primary tumor was categorized as proximal or distal (divided at the middle of the transverse colon). The tumor differentiation was categorized as poorly differentiated versus not poorly differentiated based on the clinical pathology report. Survival data was provided by the oncology database, which references the Social Security Death Master File for up-to-date mortality data. Overall survival was calculated from diagnosis until death, or until data were censored, as of October 15, 2012.
KRAS testing was performed in a CLIA approved laboratory, including 52 subjects at our institution and 13 at outside institutions with formal reports reviewed by our oncologists. Testing at our laboratory included analysis of codons 12 and 13 in KRAS exon 2 and was performed using PCR on paraffin-embedded tumor tissue.
Based on our empirical clinical impression that patients with peritoneal disease have worse outcomes than those with liver-only disease, prior findings of peritoneal involvement as a risk factor by Shepherd et al., the recent assignment of peritoneal disease as M1b in the AJCC 7th edition TNM staging, and the recent support of this change by Kennecke et al., we also coded subjects as having isolated liver disease, isolated peritoneal disease, liver or peritoneal disease plus at least one other site of disease, or exclusively disease outside of the liver and peritoneum [2,8,9]. Only measurable lesions, as defined above, were considered in this categorization.
Statistical Analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC). All tests of significance were performed using an alpha of 0.05. Continuous variables were assessed for normality using the Shapiro-Wilk test.
Survival curves were drawn according to the Kaplan-Meier method, and univariate comparisons were made using the log-rank test. Cox proportional hazards models were constructed using stepwise selection among the factors that showed potential significance in univariate analysis. KRAS mutation status was included in the final model due to its relationship cetuximab treatment, which became commonplace during the follow-up period of our study and was a potential confounder. The proportionality of hazards assumptions and functional forms of the continuous variables were validated for the final models. For the analyses of stratified site(s) of metastasis, the group with isolated liver metastases was used as the reference.
RESULTS
Baseline characteristics of the cohort
The characteristics of the cohort are summarized in Table 1. Among 65 subjects with an average age of 56 years (range 8 – 115 months), there was a slight male predominance. Poorly differentiated histology and mucinous features were less common (29% and 26%, respectively). Subjects were similarly divided between KRAS wild type and mutant tumors (46 vs. 54%), and codon 12 mutations were more common among the KRAS mutant tumors (71%). Distal cancers were slightly more common than proximal tumors (61%).
Table 1. Baseline Characteristics of the Cohort.
| Characteristic | Cohort (n=65) |
|---|---|
| Age at diagnosis (years) a | 55.7 ± 11.7 |
| Sex | |
| Male | 37 (57) |
| Female | 28 (43) |
| Differentiation | |
| Well or moderate | 46 (71) |
| Poor | 19 (29) |
| Presence of mucin | |
| Non-mucinous | 48 (74) |
| Mucinous | 17 (26) |
| KRAS mutation status | |
| Wild type | 30 (46) |
| Mutant | 35 (54) |
| Codon 12 | 25 (71) |
| Codon 13 | 8 (23) |
| Unspecified | 2 (6) |
| Primary tumor location | |
| Proximal | 25 (39) |
| Distal | 40 (61) |
Unless otherwise specified, data are reported as count (percentage).
Age data are reported as means ± standard deviations.
Initial Distribution of Disease
Table 2 shows the baseline distribution of disease. The liver was involved in 77% of subjects and was the sole site of disease in 34%. The peritoneum was involved in 39% of subjects and was the sole site of disease in 8%. Lung metastases were present in 31%, and nodal metastases were present in 42%. Less common sites of metastasis included the ovaries, brain, and adrenal glands, but the counts were insufficient for inclusion in the analysis. No bone metastasis was observed in this cohort.
Table 2. Distribution of Disease.
| Disease Assessment | Cohort (n=65) |
|---|---|
| Sites of metastasis a | |
| Distant nodes | 27 (42) |
| Liver | 50 (77) |
| Lung | 20 (31) |
| Peritoneum | 25 (39) |
| Stratified site(s) of metastasis a | |
| Liver only | 22 (34) |
| Peritoneum only | 5 (8) |
| Liver or peritoneum plus another site | 27 (42) |
| Liver and peritoneum not involved | 11 (16) |
| Count of metastatic lesions >= 1cm b | |
| Retroperitoneal lymph nodes | 0 (1, 0-25) |
| Thoracic lymph nodes | 0 (0, 0-15) |
| Liver | 3 (7, 0-213) |
| Lung | 0 (0, 0-43) |
| Peritoneum | 0 (1, 0-13) |
Data are reported as count (percentage)
Data are reported as median (interquartile range, full range).
Our stratified location analysis showed that 34% of subjects had measurable metastases only in the liver, 8% had metastases only in the peritoneum, 42% had metastases in the liver or peritoneum and at least one additional site, and 17% had no measurable metastases in the liver and peritoneum.
Predictors of Overall Survival
Table 3 shows the results of the univariate and multivariate survival models. Among the 65 subjects, there were 52 deaths during a median follow-up and median overall survival of 39 months. Univariate analysis of survival demonstrated that the stratified site(s) of metastases and the counts of lesions in the liver, peritoneum, and retroperitoneal lymph nodes were statistically significant risk factors for mortality (p < 0.01). Age and sex were marginally significant (p=0.053 and 0.049 respectively). Thoracic metastases, including pulmonary metastases and thoracic adenopathy, tumor differentiation, mucin, and tumor location were not statistically significant predictors of survival (p > 0.05).
Table 3. Multivariate Model of Overall Survival for All Subjects.
| Variable | Univariate HR |
95% CI | Pe | Multivariate HR |
95% CI | Pf |
|---|---|---|---|---|---|---|
| Age (per 5 years) a | 0.88 | 0.77 to 1.00 | 0.053 | 0.88 | 0.77 to 1.01 | 0.063 |
|
| ||||||
| Sex | ||||||
| Female | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| Male | 1.77 | 1.00 to 1.77 | 0.049 | --b | -- | 0.63 |
|
| ||||||
| KRAS status | ||||||
| Wild type | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| Mutant | 0.89 | 0.52 to 1.54 | 0.683 | 0.88 | 0.46 to 1.68 | 0.69 |
|
| ||||||
|
Stratified site(s) of
metastasis |
0.0002c | |||||
| Liver only | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| Peritoneum only | 8.24 | 2.67 to 25.39 | 0.0002 d | 18.33 | 5.36 to 62.77 | < 0.0001 |
| Liver or peritoneum plus another site |
3.49 | 1.69 to 7.22 | 0.0008 d | 3.45 | 1.58 to 7.56 | 0.002 |
| Liver and peritoneum not involved |
1.70 | 0.67 to 4.32 | 0.261 d | 1.93 | 0.69 to 5.38 | 0.21 |
|
| ||||||
|
Count of liver lesions
(per 5 lesions) a |
1.11 | 1.05 to 1.17 | 0.0003 | 1.16 | 1.09 to 1.23 | < 0.0001 |
|
| ||||||
|
Count of retroperitoneal
adenopathy (per node) a |
1.11 | 1.03 to 1.19 | 0.008 | 1.09 | 0.99 to 1.10 | 0.069 |
|
| ||||||
|
Count of peritoneal lesions(per lesion) a |
1.15 | 1.05 to 1.26 | 0.0027 | --b | -- | 0.71 |
Hazard ratios for continuous variables are per unit change with unit listed in parentheses. The unit of change in the liver was chosen as five lesions to better reflect the clinical scale of the effect in this organ, where larger counts were more common. The per-lesion multivariate HR for the liver was 1.02.
Sex and count of peritoneal lesions were eliminated in stepwise selection for the multivariate model.
Test for equality over all strata.
Pairwise comparisons were made with respect to the reference stratum.
Log-rank test.
Wald chi-square statistic from Cox proportional hazards model.
After stepwise selection from among the potential covariates, the combined multivariate Cox proportional hazards model showed that the count of liver lesions and the stratified site(s) of metastasis remained as statistically significant independent risk factors for mortality (HR 1.11 per five lesions >= 1cm in the liver, 95% CI 1.05 to 1.17, p < 0.001; HR 18.3 for peritoneal metastasis only, 95% CI 5.4 to 62.8, p < 0.0001; and HR 3.45 for liver or peritoneum plus any additional site, 95% CI 1.6 to 7.6, p = 0.002). KRAS mutation status, age, sex, and counts of peritoneal and retroperitoneal disease did not persist as independent risk factors for mortality.
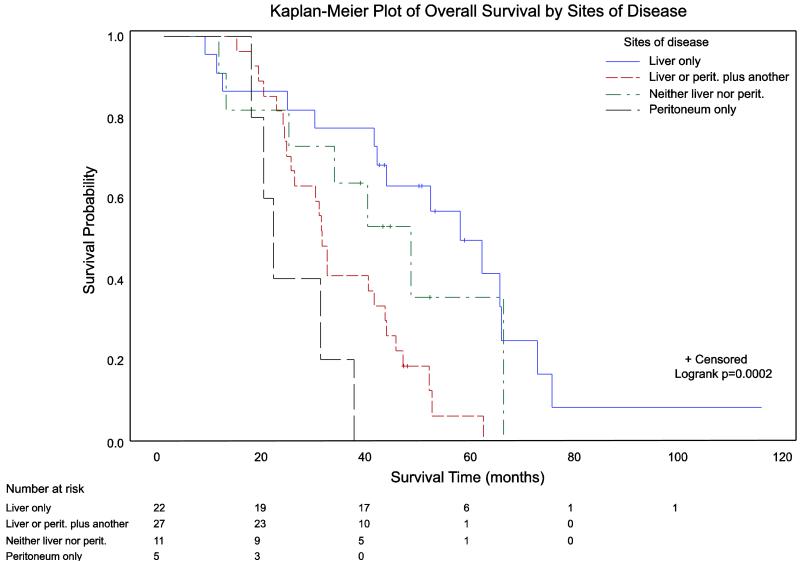
Figure 1 demonstrates the stratification of overall survival by site(s) of metastasis. Survival varied significantly across sites of metastatic involvement (p=0.0002 for equality across strata using log-rank test). Pairwise analyses showed that isolated peritoneal metastases and involvement of the liver or peritoneum plus at least one other site of disease were both associated with significantly worse overall survival than subjects who presented with liver metastases alone (p=0.0002 and 0.0008, respectively). Subjects without involvement of the liver or peritoneum had equivalent outcomes to those with isolated liver metastases (p=0.261).
Figure 1.
Kaplan-Meier plot of overall survival stratified by site(s) of metastasis. Survival varied significantly across sites of metastatic involvement using the log-rank test (p=0.0002 for equality across strata). Pairwise analyses showed that isolated peritoneal metastases and involvement of the liver or peritoneum plus at least one other site of disease were both associated with significantly worse overall survival than subjects who presented with liver metastases alone (p=0.0002 and 0.0008, respectively). Subjects without involvement of the liver or peritoneum had equivalent outcomes to those with isolated liver metastases (p=0.261). The number of at-risk subjects is listed at the bottom of the figure
Univariate analysis of the overall survival of subjects with KRAS mutations showed that subjects with KRAS mutations in codon 13 (n=8) had a trend toward better survival compared to those with KRAS mutations in codon 12 (n=25; median survival 61.4 months for codon 13 versus 30.3 months for codon 12, p=0.09 by log-rank test). After stepwise selection, the multivariate Cox proportional hazards model for this subgroup included KRAS codon mutation, stratified site(s) of metastasis, counts of liver lesions and retroperitoneal lymph nodes, and sex. KRAS codon mutation was a statistically significant factor in the multivariate model, with codon 12 mutations conferring a mortality hazard ratio of 4.55 (p=0.0064, CI 1.53-13.53). Counts of liver lesions, stratified site(s) of metastasis, and sex also remained as statistically significant risk factors (HRs 1.19 per 5 liver lesions [CI 1.09 – 1.31, p=0.0002], 11.10 for peritoneal disease only [CI 1.60 – 76.83, p=0.015], 6.42 for liver or peritoneum plus other sites of disease [CI 1.78 – 23.14, p=0.0045], 13.96 for sites other than liver or peritoneum [CI 1.20 – 162.35, p=0.035], and 2.80 for male sex [CI 1.04 – 7.57, p=0.043]).
DISCUSSION
Imaging, primarily with CT and PET-CT where appropriate, is the mainstay of systemic cancer staging. The National Comprehensive Cancer Network guidelines recommend staging CT of the chest, abdomen, and pelvis for all patients who present with colorectal cancer, and the American College of Radiology Appropriateness Criteria list CT as the best-supported diagnostic modality for pretreatment staging of colorectal cancer [10,11]. The main objective of our study was to assess whether the initial volume and distribution of metastatic disease, as detected by baseline staging CT, predicts the subsequent survival of subjects with stage IV colorectal cancer. Our analysis found two distinct independent predictors of overall survival: counts of liver lesions measuring at least 1 cm in size, and the measurable site(s) of disease involvement. In particular, isolated involvement of the peritoneum or involvement of the liver or peritoneum and at least one other site of disease were associated with significantly poorer outcomes than involvement of the liver alone. These factors may have utility in improving the existing risk stratification methods for stage IV colorectal cancers [12,2], but further research is needed to validate these factors in a larger, independent, and potentially prospective cohort.
In 2010, the seventh edition of the American Joint Committee on Cancer staging criteria newly divided patients with metastatic disease into two risk groups: M1a for a single involved organ or M1b if more than one organ is involved or if the peritoneum is involved [2]. The data supporting this change were not specifically cited in the published criteria, and the primary Surveillance, Epidemiology, and End Results (SEER) validation of the T and N stage changes for this edition did not address the changes in the M stage [5]. Our findings, which identify isolated peritoneal metastases as having the highest hazard ratio of any of the tested distributions of metastases, would support the classification of peritoneal disease as a high risk feature.
Other independent efforts to validate this division have produced conflicting results. An analysis of 2,049 patients from British Columbia by Kennecke et al. found a significant risk increase for the newly defined M1b versus M1a disease (HR 1.38, CI 1.22 – 1.55, p<0.0001), which appears compatible with our findings [9]. In contrast, Lan et al., in a single institution retrospective study from Taiwan that included 421 subjects with M1 disease, found that the new M1a and M1b distinctions in the AJCC 7th edition criteria did not provide statistically significant stratification of outcomes when isolated peritoneal disease was defined as M1b (p=0.16), but the stratification became significant when isolated peritoneal disease was redefined as M1a (p<0.001) [13]. This apparent disparity with our findings could be due to differences in the underlying patient populations, treatment strategies, or burden of disease at diagnosis.
Other authors have sought to identify factors that would predict outcomes in stage IV colorectal cancer. Zacharakis et al. found no multivariate association between overall survival and the binary presence or absence of hepatic and peritoneal disease, but did identify a number of clinical and laboratory prognostic factors [14]. The disparity been our findings and those of Zacharakis et al. may be due in part to the marked disparities in survival between the cohorts - 12.8 months for Zacharakis versus 39 months for our cohort – which suggests a qualitative difference in the underlying cohorts or subsequent interventions.
In 1997, Shepherd et al. found that local peritoneal invasion by the primary tumor was a negative prognostic factor. The authors stated that further work was necessary to confirm that broader peritoneal dissemination carries independent risk beyond the local tumor stage designation [8]. Our findings support that assertion, but specific analysis of isolated peritoneal metastases versus peritoneal invasion (T4a disease) would be necessary to exclude confounding by locally aggressive tumor growth.
With regard to peritoneal metastases, our univariate analyses showed that the risk factors for mortality included both the quantitative counts of peritoneal lesions at least one centimeter in size and the qualitative presence of measurable peritoneal carcinomatosis as the only site of metastasis (as part of the stratified site analysis). The quantitative counts of peritoneal lesions were no longer statistically significant in the multivariate model, which suggests that the qualitative presence of measurable peritoneal metastases, as captured by the stratified site analysis, was more important than the specific amount of tumor present in the peritoneum in this cohort. A larger cohort will be necessary to determine if the quantitative amount of peritoneal disease can offer any independent prognostic value beyond that of the qualitative presence of such disease.
Recently, Imamura et al. studied the prognostic effect of KRAS mutation in 1,261 subjects and found that KRAS codon 13 mutations are associated with better survival than KRAS codon 12 mutations or wild type status [15]. The results from our cohort support this finding and suggest that the factors that were significant in the overall analysis remained significant in this subgroup. A larger cohort will be needed to determine if the hazard ratios associated with these factors in the KRAS mutant group are significantly different than those in the KRAS wild type group, which could impact the prognostic use of these measures.
Our study has several limitations. First, the sample size of 65 patients resulted in relatively wide confidence intervals around some of our factors, including age, KRAS mutation status, and some of the lesion counts. While many of these factors were not statistically significant in the multivariate model, we have not excluded effects that may be clinically significant but too small to confirm in this cohort. Analysis of a larger cohort will be necessary for confirmation of our findings.
Second, we approximated the burden of disease using counts of lesions measuring at least 1 cm in size. We believe this to be a plausible compromise between RECIST’s limited lesion count and the time-intensive work of volumetric assessments, but further research is ongoing to understand the reproducibility and interreader variability of this approach. The ongoing work of the Quantitative Imaging Biomarkers Alliance of the Radiological Society of North America may also establish rigorous volumetric approaches to which this method should be compared. Our use of the 1 cm threshold, while practical and consistent with RECIST and clinical practice, also means that we have not assessed any potential impact that subcentimeter lesions would have on patient outcomes.
Third, we did not specifically analyze for oligometastatic disease and the potentially curative treatments thereof. There were only three subjects in our cohort who had oligometastatic disease at diagnosis, so this is not likely to affect the findings of our study, but this should be addressed in future work. Finally, this cohort was derived from therapeutic clinical trials that demanded good performance status for entry into the trials. This results in a selection bias against subjects with poor performance status, and the magnitude of that effect is not known in our cohort.
In conclusion, our study has shown that the site(s) of disease involvement, most notably isolated peritoneal disease, and the counts of liver lesions at least one centimeter in size, are independent risk factors for mortality in this cohort and could have utility in risk stratification of stage IV colorectal cancer. Further research is needed to confirm these findings in a larger, independent, and potentially prospective cohort.
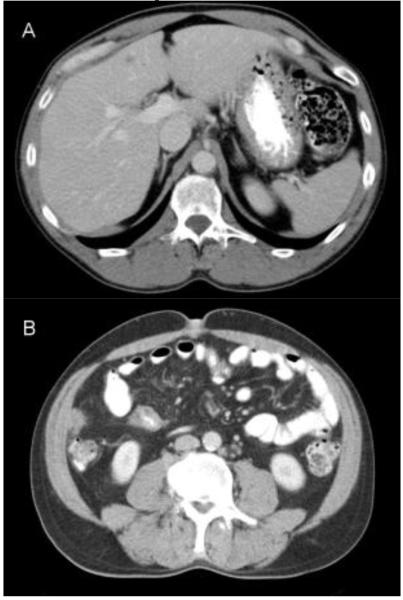
Figure 2.
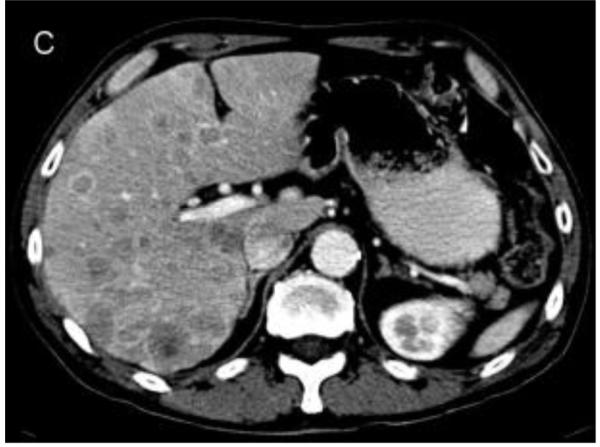
Representative images from cases in this cohort. a) 50 year old male with isolated liver metastases. The subject survived for 40.9 months. b) 49 year old male with liver and peritoneal metastases. The subject survived for 25.2 months. c) 63 year old male with extensive hepatic metastasis. The subject survived for 8.0 months
Acknowledgments
Funding: This work was supported in part by National Institutes of Health grants 5P50CA127003, R01CA149222, R01CA118553, and National Cancer Institute Cancer Center Support Grant 5P30CA06516.
Footnotes
Disclosures: The authors report no conflicts of interest.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AI, for the American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7th edn. Springer; New York: 2010. [Google Scholar]
- 3.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review, 1975-2011. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 5.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28(2):264–271. doi: 10.1200/JCO.2009.24.0952. doi:10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, Nordlinger B, Adam R, Gruenberger T, Choti MA, Bilchik AJ, Van Cutsem EJ, Chiang JM, D’Angelica MI. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26(29):4828–4833. doi: 10.1200/JCO.2008.17.6453. doi:10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd NA, Baxter KJ, Love SB. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112(4):1096–1102. doi: 10.1016/s0016-5085(97)70119-7. [DOI] [PubMed] [Google Scholar]
- 9.Kennecke HF, Yu J, Gill S, Cheung WY, Blanke CD, Cheifetz R, Woods R. Impact of M1a and M1b staging in patients (pts) with metastatic colorectal cancer. J Clin Oncol. 2013;31(15 (Supplement)) ASCO Annual Meeting, Abstract 3590. [Google Scholar]
- 10.Benson AB, III, for NCCN Guidelines Panel for Colon Cancer . NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. 2014. [Google Scholar]
- 11.Dewhurst C, Rosen M, Blake M, Baker M, Cash B, Fidler J, Greene FL, Hindman N, Jones B, Katz D, Lalani T, Miller F, Small W, Sudakoff G, Tulchinsky M, Yaghmai V, Yee J. ACR Appropriateness Criteria for pretreatment staging of colorectal cancer. American College of Radiology; [Accessed June 3, 2014]. http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/PretreatmentStagingColorectalCancer.pdf. [DOI] [PubMed] [Google Scholar]
- 12.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond MEH, Henson DE, Hutter RVP, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C. Prognostic Factors in Colorectal Cancer. Archives of Pathology & Laboratory Medicine. 2000;124(7):979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 13.Lan YT, Yang SH, Chang SC, Liang WY, Li AF, Wang HS, Jiang JK, Chen WS, Lin TC, Lin JK. Analysis of the seventh edition of American Joint Committee on colon cancer staging. Int J Colorectal Dis. 2012;27(5):657–663. doi: 10.1007/s00384-011-1366-6. doi:10.1007/s00384-011-1366-6. [DOI] [PubMed] [Google Scholar]
- 14.Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, Felekouras E, Antoniou E, Polyzos A, Sarantonis J, Syrios J, Zografos G, Papalambros A, Tsavaris N. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30(2):653–660. [PubMed] [Google Scholar]
- 15.Imamura Y, Morikawa T, Liao X, Lochhead P, Kuchiba A, Yamauchi M, Qian ZR, Nishihara R, Meyerhardt JA, Haigis KM, Fuchs CS, Ogino S. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res. 2012;18(17):4753–4763. doi: 10.1158/1078-0432.CCR-11-3210. doi:10.1158/1078-0432.CCR-11-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]