Abstract
Background
Stroke is a significant complication in patients supported with continuous-flow left ventricular assist devices (CF-LVAD) and hypertension (HTN) is a significant risk factor for stroke, but the association between blood pressure and stroke in LVAD patients is not well characterized.
Methods
We identified 275 consecutive patients who survived implant hospitalization between 1/2005 and 4/2013. Patients were divided into above and below median SBP (100 mmHg) groups based on their averaged systolic blood pressure (SBP) during the last 48 hours prior to discharge from implantation hospitalization. The groups were compared for the primary outcome of time-to-stroke.
Results
The above-median SBP (AM-SBP) group had mean SBP=110 mmHg and the below-median SBP (BM-SBP) group had mean SBP=95 mmHg. There were no significant between-group differences in BMI, smoking, vascular disease, HTN, atrial fibrillation, or prior stroke. During a mean follow-up of 16 months, stroke occurred in 16% of the AM-SBP group vs. 7% of the BM-SBP group, HR 2.38 (95% CI 1.11 - 5.11), with a similar proportion of hemorrhagic and ischemic strokes in each group. In Cox proportional hazard models adjusting for age, diabetes, or prior stroke, the HR remained statistically significant. SBP as a continuous variable predictor of stroke had an AUC of 0.64 in an ROC analysis.
Conclusion
In this large, CF-LVAD cohort, elevated SBP was independently associated with a greater risk of subsequent stroke. These results identify management of HTN as a potential modifiable risk factor for reducing the incidence of stroke in patients supported by CF-LVAD.
Background
Since their FDA approval continuous-flow left ventricular assist devices (CF-LVAD) have become the standard of care for end stage heart failure patients as bridges to transplant or destination therapy.1,2 In spite of improved technology and varying anticoagulation strategies, stroke remains a significant complication. In the original randomized controlled trial of CF-LVADs, the incidence of stroke (hemorrhagic and ischemic) was 18% at 2 years of followup.3 The Fifth INTERMACs annual report had a nearly identical 17% incidence of stroke at 2 years.4
Amongst the general population, hypertension is the most significant risk factor for stroke.5,6 Patients with hypertension have one and a half times the risk of having a stroke compared to those with normal blood pressure. Likewise, those with severe hypertension (systolic blood pressure >160) have a relative risk of stroke of nearly 4 when compared to normotensive patients.6
Earlier work suggests that cerebral blood flow autoregulation (CBFA) plays an important role in the pathology of neurological dysfunction in the short term after VAD implantation. Lietz et.al demonstrated a link between increased cardiac index after pulsatile flow VAD implantation and neurological dysfunction, manifest by encephalopathy, coma, seizure, and hemiplegia. 7 This was suspected to be due to hyperemia after acute restoration of normal blood flow overwhelming CBFA. Investigations have shown possible impairment in CBFA at 7 days with pulsatile flow VADs using transcranial Doppler. 8 However, more recently, combining transcranial Doppler and near-infrared spectcroscopy has suggested that CBFA was preserved in the immediate period postoperatively and on the day of endotrachial extubation after CF-LVAD implantation.9 The long term effects of continuous flow on CBFA after 7 days is less well studied. Further, although endothelial function appears to neither improve nor degrade for up to 4 months after CV-LVAD implantation long term function is poorly understood.10 Endothelial dysfunction and altered cerebral autoregulation in the setting of therapeutic anticoagulation could greatly influence the relation between systemic blood pressure and stroke risk.
The effects of elevated systemic blood pressure on CBFA and incidence of stroke in CF-LVAD patients have not been well characterized. There is an ongoing prospective trial among HeartWare HVAD® implants to compare neurological events in a cohort receiving optimal blood pressure control to those in previous trials who did not have specified blood pressure management, but no results are expected until late 2016. The mechanical support guidelines suggest a MAP of 80 mmHg for CF-LVADs, however, this is only based on expert opinion.11 In this retrospective analysis, we evaluate association between elevated discharge blood pressure and future stroke.
Methods
We retrospectively identified patients at least 18 years old who underwent implantation of a Heart Mate II ® (Thoratec Corp., Pleasanton, CA) or HVAD® (HeartWare Corp., Framingham, MA) between 1/2005 and 8/2013 at our center. Exclusion criteria included death or stroke during index hospitalization, repeat LVAD surgery (i.e. LVAD exchange), or incomplete data.
Of the 358 patients who were implanted with CF-LVAD during this time period, 308 were discharged alive and subsequently followed up at our institution. Twenty two patients were excluded as this was not their first LVAD, 16 patients were excluded for CVA during their index hospitalization, and 9 were excluded for incomplete records. The final cohort included 275 patients divided into two groups (above-median and below-median) based on their average systolic blood pressures taken during the last 48 hours prior to discharge. All blood pressures were obtained from nursing reports on the Electronic Medical Record at Barnes Jewish Hospital. All values were obtained non-invasively, predominantly by an automated blood pressure machine (Dinamap®). Patient characteristics and clinical outcomes were obtained through review of the medical record. Study data were collected and managed using REDCap electronic data capture tools hosted at Washington University.12 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies. The study was approved by our Institutional Review Board.
Follow-up and Clinical Outcomes
Follow-up was assessed for every patient by review of the medical record. The primary outcome of the study was hemorrhagic or ischemic stroke. Strokes were recorded by reviewing clinical notes, discharge summaries, and radiology records. To further review those without a listed stroke event a neurologist and a neuroradiologist queried a database of neurology consults for any patient with an LVAD, searched all ICD. 9 codes for stroke, seizure, TIA, and looked through all head CTs performed on a patient supported with an LVAD. Neurological deficits had to be present on physical exam for greater than 24 hours to be classified as a stroke. For subgroup analyses a stroke was defined as hemorrhagic or ischemic based on neurology assessment and head CT. Secondary analyses included time until death based on above or below median SBP.
Statistical Analysis
Categorical variables, including the number of stroke events, were compared using Fisher's exact tests. Continuous variables were compared with Student's two sample t-tests. For non-normal and ordinal variables, the median (min, max) were reported and a Kruskal-Wallis test was conducted to compare cohorts.
Time until stroke was evaluated through Kaplan Meier (KM) analysis and curves were compared with log rank tests. The start time was the device implant date and event-free patients were censored at time of death, transplant, pump replacement, or last follow-up, whichever occurred earliest. Univariable hazard ratios were created from Cox models for select variables. Multivariable Cox models were also developed to adjust a priori for age, history of Diabetes, Hypertension, and previous stroke.
SBP as a continuous variable predictor of the endpoint of stroke at any time post-discharge was analyzed by receiver operator curve (ROC). The optimal cut-point of SBP to discriminate stroke from non-stroke outcomes was determined using Youden's index, which equally weights sensitivity and specificity and maximizes the number of correctly classified patients.
A two-sided P-value <0.05 was considered significant for all analyses. All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Between January 1, 2005 and August 31, 2013, 358 patients underwent implantation of a LVAD at our center and 275 patients met inclusion criteria. Patients were divided into two cohorts based on the median discharge systolic BP of 100 mmHg (above-median and below-median) (Figure 1). The above-median cohort had a mean SBP of 110 mmHg prior to discharge, and the below-median cohort had a mean SBP of 95 mmHg. Patients in both groups were predominantly males (82%) with similar INTERMACS profile (≤ 2 in > 80%) at the time of LVAD implant (Table 1). The AM-SBP group was younger (mean age 53 vs. 57, p=0.008), but there were no significant between-group differences in BMI or prevalence of diabetes, smoking, ischemic etiology of heart failure, hypertension, atrial fibrillation, intra-cardiac thrombus, or stroke.
Figure 1. Histogram of Systolic Blood Pressures for below and above median blood pressure groups.
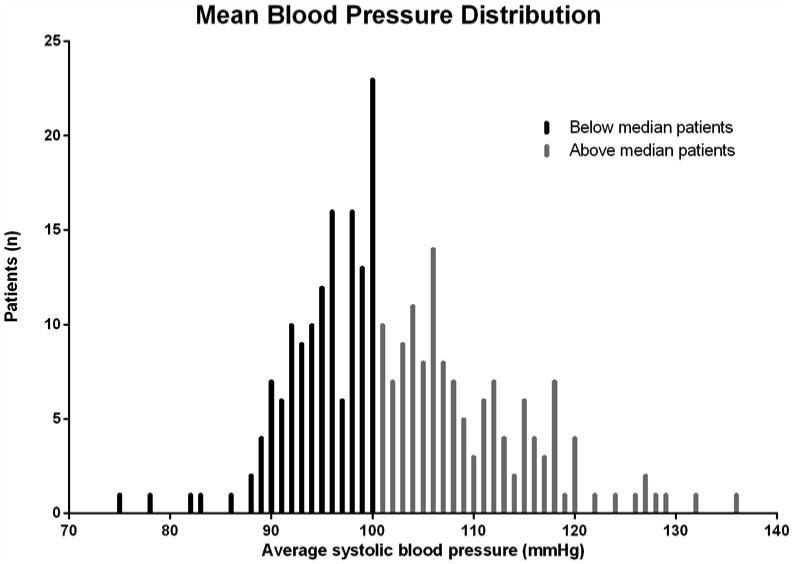
Table 1. Baseline Clinical Characteristics for the Study Population.
| Below median SBP (N = 139 patients) | Above median SBP (N = 136 patients) | p-value | |
|---|---|---|---|
|
| |||
| Age (years) | 57.07 ± 11.38 | 53.10 ± 13.23 | 0.008 |
| Male | 115 (83%) | 104 (76%) | 0.23 |
| Caucasian | 107 (77%) | 100 (74%) | 0.58 |
| BMI (kg/m2) | 28.48 ± 5.74 | 29.05 ± 6.56 | 0.44 |
| Medical history | |||
| Atrial fibrillation | 83 (40%) | 75 (55%) | 0.47 |
| Current smoker | 12 (9%) | 16 (12%) | 0.55 |
| CAD | 67 (48%) | 64 (47%) | 0.90 |
| DM | 59 (42%) | 49 (36%) | 0.32 |
| PAD | 19 (14%) | 28 (21%) | 0.15 |
| Intracardiac thrombus | 18 (15%) | 11 (11%) | 0.43 |
| Stroke | 11 (8%) | 18 (13%) | 0.39 |
| Hypertension | 66 (47%) | 64 (47%) | 1.00 |
| Bridge to transplant | 93 (68%) | 94 (69%) | 0.84 |
| Length of stay (days) | 34.9 ± 41.7 | 36.6 ± 29.1 | 0.72 |
| INTERMACS profile | 0.73 | ||
| 1 | 40 (29%) | 39 (29%) | |
| 2 | 80 (58%) | 73 (54%) | |
| 3 | 12 (9%) | 13 (10%) | |
| 4 | 6 (4%) | 10 (7%) | |
| Type of LVAD | 1.00 | ||
| Heartmate II | 123 (88%) | 121 (89%) | |
| Heartware HVAD | 16 (12%) | 15 (11%) | |
| RPM of Heartmate II | 9364 ± 321 | 9430 ± 301 | 0.13 |
| RPM of HVAD | 2631 ± 208 | 2810 ± 193 | 0.03 |
| Labs on discharge | |||
| Creatinine Clearance | 109.0 ± 59.6 | 103.5 ± 51.9 | 0.89 |
| Hgb (g/dL) | 9.64 ± 0.95 | 9.65 ± 1.02 | 0.97 |
| INR | 2.26 ± 0.55 | 2.25 ± 0.59 | 0.89 |
| Medications discharge | |||
| Statin | 65 (50%) | 53 (40%) | 0.17 |
| ACE-Inhibitor | 24 (17%) | 13 (17%) | 1.00 |
| Beta blocker | 31 (43%) | 54 (40%) | 0.13 |
| Warfarin | 133 (96%) | 129 (96%) | 0.77 |
| Clopidogrel | 7 (5%) | 3 (2%) | 0.33 |
| Aspirin | 133 (96%) | 134 (99%) | 0.21 |
| Aspirin dose | 0.22 | ||
| 325 mg daily | 63 (47%) | 53 (40%) | |
| 81 mg daily | 70 (53%) | 81 (60%) | |
Values are shown as absolute numbers (percentages), mean ± SD. BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LVAD, left ventricular assist device CrCl, creatinine clearance; Hgb, hemoglobin; INR, international normalized ratio
During a mean follow up of 16 months, stroke occurred in 16% of the above-median SBP cohort vs. 7% of the below-median-SBP cohort, HR 2.38 (95% CI 1.11 - 5.11, p=0.03) (Figure 2). In both groups 54% of the strokes were hemorrhagic and 46% were ischemic. The National Institute of Health Stroke Scale (NIHSS) was available in the record for 13 of the 32 patients. The mean NIHSS was 9.2 ± 6.3, with 4 of the 13 having a score >16 indicating a moderate to severe or severe stroke. In univariate analysis, stroke was not statistically associated with smoking, diabetes, atrial fibrillation, history of stroke, peripheral arterial disease, age, LVAD pump speed or LVAD device type (table 2). In a Cox proportional hazard model with adjustments for age, diabetes, and history of stroke the HR for SBP ≥ 100 mmHg and stroke was 2.51 (95% CI: 1.15 – 5.50, p = 0.022) (table 3).
Figure 2. Kaplan-Meier analysis of freedom from stroke between below and above median systolic blood pressure groups.
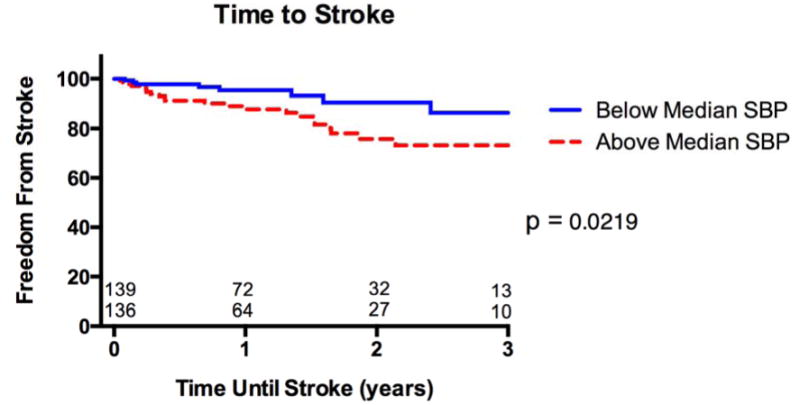
Table 2. Hazard ratio for stroke based on univariable Cox models.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
|
| |||
| SBP (per 5 units) | 1.189 | (1.03, 1.37) | 0.019 |
| SBP_above median vs. below median | 2.40 | (1.11, 5.20) | 0.026 |
| RPM (per 100 unit) HM II only | 1.00 | (0.88, 1.12) | 0.96 |
| Hx_PAD (vs. no) | 0.31 | (0.07, 1.29) | 0.11 |
| LVAD type HMII (vs. HW) | 0.88 | (0.27, 2.92) | 0.84 |
| Smoking | 1.34 | (0.65, 2.75) | 0.43 |
| Current_smoking (vs. no) | 1.44 | (0.54, 3.84) | 0.47 |
| Age (per 1 year) | 1.00 | (0.98,1.03) | 0.75 |
| Diabetes | 1.15 | (0.57, 2.34) | 0.69 |
| Hypertension | 0.95 | (0.47, 1.91) | 0.88 |
| Previous stroke | 1.39 | (0.48, 3.98) | 0.54 |
| Atrial Fibrillation | 1.05 | (0.52, 2.12) | 0.89 |
| Creatinine on discharge (per 1 unit) | 0.86 | (0.43, 1.74) | 0.68 |
SBP, systolic blood pressure; PAD, peripheral artery disease; LVAD, left ventricular assist device; HM II, Heartmate II; HW, HeartWare HVAD; CrCl, creatinine clearance;
Table 3. Hazard ratio for stroke based on multivariable Cox model.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
|
| |||
| SBP_above median vs. below median | 2.51 | (1.15, 5.45) | 0.022 |
| Age (per 1 year) | 1.01 | (0.98,1.04) | 0.54 |
| Diabetes | 1.10 | (0.54 2.26) | 0.80 |
| Previous stroke | 1.28 | (0.44, 3.70) | 0.65 |
SBP, systolic blood pressure
There was no difference in driveline infection rates between cohorts (23% of the below-median cohort vs. 29% of the above-median-SBP, p = 0.34). Additionally there was no difference in driveline infection rates amongst those with a stroke vs. those without a stroke (34% vs. 24%, p = 0.27). There was no difference in time until death between cohorts, log rank = 0.35 (data not shown). However, amongst those who had strokes mortality was high with 14 of the 32 patients not surviving one year after their event, and 10 of the 18 that survived having spent time in SNF or rehab facilities.
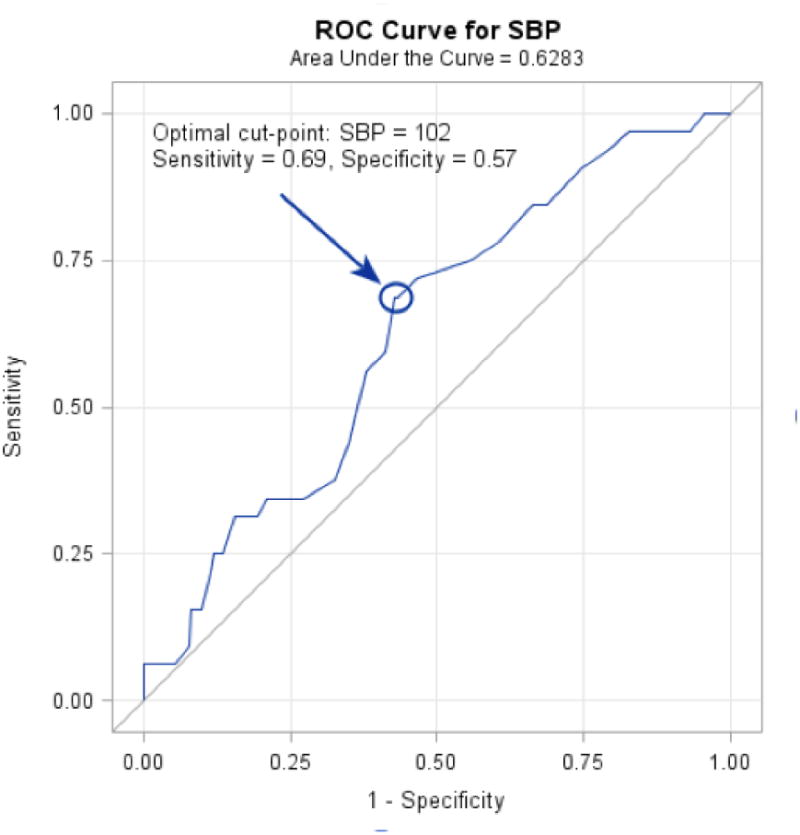
When systolic blood pressure was analyzed as a continuous variable, there was a 19% increase in the risk of stroke during follow up for every 5 mmHg increase in SBP (p = 0.02). Incorporating systolic blood pressure into a receiver operation curve as a predictor of stroke had an AUC of 0.64 (Figure 3). The ideal cut off value based on Youden's index was 102 mmHg, which gave a sensitivity of 69 percent and specificity of 58 percent. When dividing patients into cohorts based on the ROC derived cut-point of 102 mmHg the hazard ratio for stroke was 2.50 (95% CI 1.181 – 5.290, p = 0.017).
Figure 3. Receiver Operating Characteristic (ROC) analysis of SBP as a predictor of stroke.
To further elucidate the association between SBP and stroke, the competing risk of death and stroke was evaluated using Fine and Gray's extension of the Cox proportional hazards model. None of the findings were substantially different using this approach with the hazard of stroke remaining significantly greater for higher vs. lower SBP in both univariate and multivariable settings. (data not shown)
Discussion
Stroke is likely the most severe adverse event related to long term CF-LVAD support. In spite of advances in pump design and more aggressive antiplatelet and anticoagulation regimens, nearly 1 in 5 LVAD patients will have a clinical stroke during two years of support. Elevated blood pressure is known to increase stroke risk amongst the general population; the purpose of our study was to investigate associations with elevated SBP and future stroke after LVAD implantation.
In this cohort of patients with a CF-LVAD, elevated systolic blood pressure at hospital discharge was associated with a 2.5 times increase in subsequent stroke. Likewise for every 1 mmHg increase in average systolic blood pressure, risk of subsequent stroke increased by 3.5 percent. These findings are consistent with literature on stroke risk factors amongst the general population; however, the threshold at which blood pressure influenced outcomes was much lower. In those studies, increased risk of stroke was found to occur at a SBP of 140 mmHg.
These findings suggest that the noninvasive blood pressure (NIBP) cuff measure of systolic blood pressure in LVAD supported patients may underestimate true systolic pressure and more closely represent a mean arterial pressure. Consistent with this, Myers and colleagues found systolic blood pressure measured on automated cuff in 35 patients supported with CF-LVAD to be 89.7 mmHg and this was in the middle of the MAP (81.4 mmHg) and systolic pressure (96.9 mm Hg) of simultaneous arterial line measurements.13 In published literature comparing automated blood pressure measurements to arterial line measurements in patients with CF-LVADs the automated machine was only able to detect blood pressure 50-60% of the time. However, the automated systolic pressure values are very strongly correlated with arterial pressure, r = 0.87, (p < 0.0001). 14 While our noninvasive systolic blood pressure measurements are likely falsely low, the strong correlation with arterial pressure suggests the association with higher blood pressures and stroke is almost certainly accurate, though further investigation would be needed to find more accurate therapeutic targets. One major hurdle to defining blood pressure in CF-LVAD patients is a lack of standardized techniques to measure pressure. The current gold standard is considered using Doppler, however, even this is controversial.13
Given its retrospective nature our analysis is not able to decipher whether elevated blood pressure is an actual cause of neurological events in our cohort or is simply a marker of a phenotype at higher risk of stroke. A recently published blood pressure management protocol in CF-LVAD patients from the University of Pittsburgh targeted a MAP of 80 mmHG.15 In the analysis it was shown that the majority of patients 74% required at least 1 agent to achieve this goal. While there was no difference in hemorrhagic stroke between those patients requiring antihypertensive medications and those who did not, there was a lower rate of cumulative neurological events (TIAs, ischemic stroke or hemorrhagic stroke) in the group on antihypertensives.
Our results demonstrate a significant increase in subsequent stroke among those with higher systolic blood pressures after LVAD insertion. Further study is needed to determine whether this is a therapeutic target and to define an optimal blood pressure range. Nevertheless, these findings suggest that post-operative management of hypertension may have a favorable impact in reduction of stroke risk in patients supported by CF-LVAD.
Acknowledgments
Funding Sources: This study was supported in part by research funds from the National Institutes of Health (NIH grant U10 HL110309, Heart Failure Network), and Grant # UL1 TR000448
No relationships with industry.
Footnotes
Disclosures: Michael E. Nassif - None
Gregory A. Ewald - Thoratec
Akinobu Itoh – None
Shane J. LaRue – None
Eric Novak - None
Justin M Vader – None
Anjan Tibrewala - None
David S. Raymer – None
Adam Andruska - None
Scott C. Silvestry - Thoratec, HeartWare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001 Mar;71(3 Suppl):S116–120. doi: 10.1016/s0003-4975(00)02639-4. discussion S114-116. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013 Feb;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Sacco RL, Smith DB, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA. 1999 Mar 24-31;281(12):1112–1120. doi: 10.1001/jama.281.12.1112. [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Benjamin EJ, Broderick JP, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997 Jul;28(7):1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 7.Lietz K, Brown K, Ali SS, et al. The role of cerebral hyperperfusion in postoperative neurologic dysfunction after left ventricular assist device implantation for end-stage heart failure. The Journal of thoracic and cardiovascular surgery. 2009 Apr;137(4):1012–1019. doi: 10.1016/j.jtcvs.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Bellapart J, Chan GS, Tzeng YC, et al. The effect of Ventricular Assist Devices on cerebral autoregulation: A preliminary study. BMC anesthesiology. 2011;11:4. doi: 10.1186/1471-2253-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono M, Joshi B, Brady K, et al. Cerebral blood flow autoregulation is preserved after continuous-flow left ventricular assist device implantation. Journal of cardiothoracic and vascular anesthesia. 2012 Dec;26(6):1022–1028. doi: 10.1053/j.jvca.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lou X, Templeton DL, John R, Dengel DR. Effects of continuous flow left ventricular assist device support on microvascular endothelial function. Journal of cardiovascular translational research. 2012 Jun;5(3):345–350. doi: 10.1007/s12265-011-9321-z. [DOI] [PubMed] [Google Scholar]
- 11.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013 Feb;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers TJ, Bolmers M, Gregoric ID, Kar B, Frazier OH. Assessment of arterial blood pressure during support with an axial flow left ventricular assist device. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009 May;28(5):423–427. doi: 10.1016/j.healun.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MK, Roberts CA, Dordunoo D, Shah A, Russell SD. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010 May;29(5):593–594. doi: 10.1016/j.healun.2009.11.604. [DOI] [PubMed] [Google Scholar]
- 15.Lampert BC, Eckert C, Weaver S, et al. Blood pressure control in continuous flow left ventricular assist devices: efficacy and impact on adverse events. Ann Thorac Surg. 2014 Jan;97(1):139–146. doi: 10.1016/j.athoracsur.2013.07.069. [DOI] [PubMed] [Google Scholar]


