Abstract
Objective
To evaluate possible mechanisms for functional improvement and compare ambulation training with surface peroneal nerve stimulation (PNS) versus usual care (UC) via quantitative gait analysis.
Design
Randomized controlled clinical trial.
Setting
Teaching hospital of academic medical center.
Participants
110 chronic stroke survivors (> 12-wks post-stroke) with unilateral hemiparesis.
Interventions
Subjects were randomized to a surface PNS device or UC intervention. Subjects were treated for 12-wks and followed for 6-months post-treatment.
Main Outcome Measures
Spatiotemporal, kinematic, and kinetic parameters of gait.
Results
Cadence (F3,153=5.81, p=.012), stride length (F3,179=20.01, p<.001), walking speed (F3,167=18.2, p<.001), anterior posterior ground reaction force (F3,164=6.61, p=.004), peak hip power in pre-swing (F3,156=8.76, p<.001), and peak ankle power at push-off (F3,149=6.38, p=.005) all improved with respect to time. However, peak ankle DF in swing (F3,184=4.99, p=.031) worsened. In general, the greatest change for all parameters occurred during the treatment period. There was no significant treatment group by time interaction effects for any of the spatiotemporal, kinematic, or kinetic parameters.
Conclusions
Gait training with PNS and usual care was associated with improvements in peak hip power in pre-swing and peak ankle power at push-off, which may have resulted in improved cadence, stride length, and walking speed; however, there were no differences between treatment groups. Both treatment groups also experienced a decrease in peak ankle DF in swing, though the clinical implications of this finding are unclear.
Keywords: Hemiparesis, peroneal nerve, gait
INTRODUCTION
Several recent studies have suggested that the daily use of a peroneal nerve stimulator (PNS) may facilitate motor recovery of the lower limb in patients with post-stroke hemiparesis1–3. However, in our recently published randomized controlled trial of 12-weeks of ambulation training with a surface PNS versus usual care (UC) in chronic hemiparesis, there was no evidence of a therapeutic effect as measured by the Fugl-Meyer (FMA) score, the primary measure of lower limb motor impairment4. However, both PNS and UC treatment groups demonstrated significant improvement in functional mobility at the end of treatment, as measured by the tasks of the modified Emory Functional Ambulation Profile (mEFAP), which was maintained at 6-months follow-up. The explanation for improvement on a measure of activity limitation in response to a treatment intervention, in the absence of improvement on the primary motor impairment measure, is unclear. It is possible that both the PNS and UC treatments conveyed focal therapeutic effects not detectable by the FMA score. Alternatively, compensatory strategies, not specific to the treatment intervention, may have been acquired during the treatment period resulting in sustained improvement in functional mobility. Examples of compensatory strategies include a change in proximal kinematics or kinetics of the paretic lower limb. Further, the improvement in functional mobility, in both PNS and UC groups, may have been induced by triggering either the same or different strategies for motor recovery and/or compensatory behaviors. If different strategies were employed, then one of the two treatments may produce a more effective response to facilitate long-term motor recovery and may thus be preferable. The primary objective of this analysis was to evaluate possible mechanisms responsible for the improvement in functional mobility, which was evident in both the PNS and UC groups following 12-wks of ambulation training, by comparing the effect of treatment on spatiotemporal, kinematic, and kinetic parameters of gait.
METHODS
Study Design
A randomized controlled trial was performed comparing ambulation training with a surface PNS to UC. Chronic hemiparetic stroke subjects were treated for 12-wks (Device Usage period) and followed for a total of 6-months post-treatment. Outcome assessments were performed at baseline (t1), end of the Device Usage period (t2), and at 12-wks (t3) and 24-wks (t4) post-treatment. All outcome assessments, including quantitative gait analysis (QGA), were performed while the subject was not wearing the treatment device.
Subjects
Subjects were recruited from and treated at an academic medical center. Quantitative gait analyses were conducted in the Motion Studies Laboratory at a Veterans Affairs Medical Center. The institutional review boards of both institutions approved the study protocol, and all participants signed informed consent. Inclusion criteria were age ≥ 18 years, ≥ 12-wks post-stroke with unilateral hemiparesis, and ankle dorsiflexion (DF) strength of ≤ 4/5 on the Medical Research Council (MRC) scale. Subjects were required to ambulate ≥ 30-ft without an ankle foot orthosis (AF0) and score ≥ 24 on the Berg Balance Scale. Subjects were excluded for lower extremity edema, knee hyperextension during stance phase of gait, skin breakdown, or absent sensation; serious cardiac arrhythmias, pacemakers or other implanted electronic systems; pregnancy; uncontrolled seizure disorder; concomitant lower motor neuron dysfunction and non-stroke upper motor neuron dysfunction; uncompensated hemineglect; sensory or motor peripheral neuropathy; fixed ankle plantarflexor contracture; or lower extremity (LE) botulinum toxin injection within the 3-months prior to enrollment. All of the subjects were trialed with the PNS device, prior to enrollment, to ensure that the subject could safely and effectively use the device if randomized to the PNS treatment group.
Randomization Procedure
As post-stroke motor outcomes may be affected by baseline motor function5–6, eligible subjects were first stratified on the basis of presence (≥ 1/5) or absence (0/5) of volitional ankle DF prior to being randomized to the PNS or UC group. The randomization sequence was concealed in consecutively numbered envelopes, which were allocated once eligibility was determined. Within the UC treatment group, the treatment device (AFO or no device) assigned to each subject was determined using standard of care clinical decision-making based on observational gait pattern with subjects with milder DF weakness generally assigned to no device and subjects with more significant DF weakness being assigned an AFO.
Devices
The PNS device was the Odstock Dropped-Foot Stimulator (ODFS)7–9 (Odstock Medical Limiteda, United Kingdom), a single-channel surface stimulator which detects heel rise at pre-swing via a 3-mm insole pressure sensing footswitch. The custom molded hinged AFO with plantarflexion block was fabricated using conventional techniques (G.A. Guilford & Son Ltdb, Brookpark, OH).
Intervention
The 12-wk Device Usage (treatment) Period consisted of a Functional Training phase (two 1-hr therapy sessions per wk × 5-wks) and a Post-Functional Training phase (three additional 1-hr therapy sessions over the remaining 7 wks). During the Functional Training phase, subjects were trained to use their treatment devices for home and community mobility with an assistive device (AD), such as a straight cane, quad cane, or walker, if needed. The content of the Device Usage therapy sessions was standardized across treatment groups with the only between-group difference being the treatment device used in the therapy session. Therapy content included passive and active range of motion exercises, lower extremity strengthening (supine and standing), standing balance activities, weight-shifting activities to the affected limb using parallel bars with transition to least restrictive gait assistive device, and refinement of a reciprocal gait pattern (visual and manual cues were given). Exercises were done with multiple repetitions with increase in difficulty and decrease in cues, with and without the assigned treatment device, as appropriate. A focus of the therapy sessions was on higher level gait activities including functionally relevant movement tasks such as stair climbing, walking on various surfaces (tile, carpet, ramps), negotiation of obstacles, community stepping (curbs), and treadmill training, as appropriate Both treatment groups received the same total amount of therapy hours during the 12-week Device Usage period.
Subjects independently used their treatment devices up to 8 hours per day during the Device Usage Period once device safety was demonstrated. At each therapy session, treatment device function, application, and usage guidelines were reviewed with the subject to maximize device compliance. At completion of the Device Usage Period, all subjects discontinued use of the assigned treatment device.
Outcome Assessments
Quantitative Gait Analysis: QGA was performed using a Vicon system (Vicon Motion Systems Limited, Oxford, United Kingdom), a motion measurement and analysis system that tracked the trajectories of reflective markers in the field of view of multiple cameras mounted around the periphery of the lab. Retro-reflective markers were adhered to the skin at anatomical locations following a modified Helen Hayes marker set7–8. Subjects were asked to ambulate 10 meters at a self-selected comfortable rate while not wearing the treatment device. A minimum of 20 strides (approximately 10 trials) wascollected at each outcome assessment. Infra-red strobe lights mounted on each camera illuminated the measurement space. AMTI Biomechanics Platforms (Advanced Mechanical Technology, Inc., Watertown, MA) were embedded in the walkway of the laboratory. Joint motion was measured about 3 axes using standard Vicon plug-in-gait nomenclature. Illumination, motion capture data, and analog to digital conversion of transducer input were synchronized and controlled by the Vicon system, which was in turn controlled by a Pentium based PC. Data were processed using the Vicon Plug-In-Gait biomechanical model in Vicon supplied to generate joint angles, moments, powers, and spatiotemporal parameters of gait. Midway through the study (after enrollment of 48 subjects), the gait laboratory Vicon system underwent a hardware update from Vicon 370 to Vicon MX, which resulted in the camera sampling frequency increasing from 60 to 100 Hz, and a software change from Workstation to Nexus. Following the Vicon system upgrade, all methods of data collection and analysis were kept consistent by following the standard Vicon Plug-In Gait model methods. At each QGA assessment, subjects used the AD (no device, straight cane, quad cane, or walker) determined to be most clinically appropriate for gait safety and stability. However, all QGAs were preferentially performed without an AD and within-subject AD consistency was maintained across testing sessions if appropriate. All QGA sessions were performed under the direction of the same gait laboratory engineer.
For purposes of the primary analyses, specific spatiotemporal, kinematic, and kinetic parameters of gait were identified a priori based on clinical relevance to treatment intervention. We report the following spatiotemporal parameters: cadence (steps/min), double support time (s), stride length (m), and walking speed (m/s). We report the following proximal kinematic parameters: peak hip flexion angle in swing (deg) and peak knee flexion angle in swing (deg) of the paretic lower limb. For purposes of a secondary analysis (see below), peak hip extension of the paretic limb during stance was additionally recorded. The 3-dimensional movement of the ankle joint, which characterized foot position, was recorded as the degree of movement away from the neutral position in the sagittal (ankle dorsiflexion/plantarflexion), coronal (ankle abduction/adduction), and transverse (ankle external/internal rotation) planes. We report the following distal kinematic parameters of the paretic ankle: DF angle at initial contact (deg), peak ankle DF angle in swing (deg), peak ankle abduction angle in swing (deg), and peak ankle external rotation angle in swing (deg). Lastly, we report the following kinetic parameters of gait: peak anterior-posterior (AP) ground reaction force (GRF) (N/kg), peak hip power in pre-swing (W/kg), and peak ankle power at push-off (W/kg) of the paretic lower limb.
Activity Monitor: The ActivPAL™ monitor (PAL Technologies Ltd, Glasgow, UK) was used to record subject activity over a 3-day consecutive period at each Outcome Assessment. The ActivPAL™ is an accelerometer-based activity logger which records the time spent standing, time spent stepping, and total number of steps taken over a given time period. Each subject was issued an ActivPAL™ device at the time of each outcome assessment and given standardized instruction on application and 3-day usage of the monitoring device. In this study, average daily time spent standing (mins), average daily time spent stepping (mins), and average total steps per day were evaluated and used as a proxy for overall activity level at each time point.
Statistical Analysis
The original RCT was designed as a 2 × 2 factorial design with treatment group (PNS vs. UC) and DF status (present vs. absent) as between subject factors. The study was powered using the Fugl-Meyer score, which was the primary motor impairment outcome measure. However, during subject accrual, we experienced uneven recruitment with only 26% of subjects assigned to the DF absent group. Thus, the study was converted to a single factor design (PNS versus UC) with anticipated difference in Fugl-Meyer score (primary motor impairment outcome measure) between groups of 5 points (0.83 S.D), which increased the power of the study to 99%. The stratification on DF status was maintained during randomization to ensure even distribution of baseline motor function. All analyses were performed as intent-to-treat. Pretreatment gait parameters were evaluated for mean and standard deviation. Each outcome was modeled using a linear mixed effects approach to evaluate the mean change in the outcome measures with treatment group. Time was considered discrete, since measurements were made at the four time periods (0, 12, 24, and 36-wks). However, since there was some variation in the exact date that individual measurements took place, we allowed for different growth rates for individuals by including a random intercept and slope in the models. Mixed effects models are well-suited for handling correlated repeated measurements, missing data and dropouts in longitudinal studies.12
In this study, the models yielded estimates of the treatment group main effect, treatment group by time effect, and time effects while permitting us to control for potential confounders. We adjusted for potential confounders including age, gender, interval post-stroke, involved hemisphere, and stroke type (ischemic versus hemorrhagic). Plots of least-square (LS) means over time were generated for each of the gait variables providing correction for missing data, estimating the marginal means for a balanced population, while adjusting for the confounders in the model.
In order to assess the effect of the treatment intervention on spatiotemporal, kinematic, and kinetic parameters over time, of primary interest was the two-way interaction between treatment group and time. We used an unstructured covariance structure, which made no assumptions about the variances and covariances, and allowed for differences in variability of the measurements at each time point. Model estimation was performed via Restricted Maximum Likelihood (REML) using PROC MIXED in SAS Version 9.2.13 We used a Bonferroni correction to control the family-wise error rate and report our adjusted p-values. We also modeled all outcomes using a more robust estimator for the standard error (EMPIRICAL option in PROC MIXED), which would confirm that parametric assumptions of the model were not violated.14 Three secondary analyses were performed using the same statistical methods as reported above, after review of the primary analyses suggested additional research hypotheses (see Discussion). These analyses included 1) the effect of treatment device on hip range of motion (approximated by the difference between peak hip flexion during swing and peak hip extension in stance measured via QGA); 2) an exploratory analysis of the differential effect of treatment device on peak DF angle in swing within the UC group (AFO=48 subjects, ND = 8 subjects); and 3) an analysis of spatiotemporal, kinematic, and kinetic data, limited to the subset of subjects who used no AD (straight cane, quad canes, or walker) at any of their OAs, to eliminate the potential confound of an AD, particularly on the kinetic data.
RESULTS
Participants and Baseline Characteristics
A total of 110 stroke survivors were enrolled in the study. Fifty-four were randomized to the PNS group and 56 to the UC group. Forty-eight subjects (86%) randomized to UC were treated with an AFO; eight subjects (14%) were treated with no device. Subject drop-out rates at t1, t2, t3, and t4 were 2%, 13%, 15% and 24%, respectively4. As previously reported, there were no significant differences in baseline characteristics (age, gender, interval post-stroke, stroke type, hemisphere, lower extremity FM score, or performance on the mEFAP) between the treatment groups.4 Table 1 presents the mean ± standard deviation of all quantitative gait analysis parameters by treatment group at baseline (t1), end of the Device Usage period (t2), and at 12-wks (t3) and 24-wks (t4) post-treatment. Treatment group main effect, treatment group × time effect, and time effect by gait parameter, as determined by the REML mixed linear effects model previously described, are shown in Table 2. Model estimation using the more robust estimator for the standard error yielded the same results as the REML model, confirming that parametric assumptions were most likely not violated. Lastly, we used LS means in all figures to provide a visual representation of the data for better reader interpretation of the data, with the caveat that LS means provides a correction of the mean for missing data, estimating the marginal means for a balanced population, while adjusting for the confounders in the model.13
Table 1.
Mean ± standard deviation of quantitative gait analysis parameters by treatment group at baseline (t1), end of the Device Usage period (t2), and at 12-wks (t3) and 24-wks (t4) post-treatment. Subject drop-out rates at t1, t2, t3, and t4 were 2%, 13%, 15% and 24%, respectively.
| PNS Treatment | Usual Care Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| QGA Parameter | t1 | t2 | t3 | t4 | t1 | t2 | t3 | t4 |
| Cadence (steps/min) |
65.0± 22.0 | 67.4± 21.5 | 69.3± 26.4 | 70.8± 26.8 | 66.7± 22.7 | 72.6± 22.6 | 72.0± 23.2 | 73.7± 22.7 |
| Double support (seconds) |
1.14± 0.76 | 1.05± 0.75 | 1.08± 0.87 | 1.02± 0.77 | 1.15± 0.87 | 0.92± 0.72 | 0.97± 0.74 | 0.91± 0.68 |
| Stride length (m) |
0.62± 0.24 | 0.68± 0.27 | 0.71± 0.28 | 0.71± 0.28 | 0.67± 0.24 | 0.74± 0.22 | 0.72± 0.24 | 0.73± 0.23 |
| Walking speed (m/sec) |
0.35± 0.20 | 0.40± 0.25 | 0.44± 0.28 | 0.44± 0.28 | 0.40± 0.24 | 0.47± 0.24 | 0.46± 0.25 | 0.47± 0.24 |
| Peak hip flexion in swing (deg) |
32.5± 8.0 | 33.2± 11.3 | 32.8± 9.6 | 34.5± 10.1 | 35.3± 9.3 | 35.1± 9.4 | 36.2± 9.2 | 35.4± 8.6 |
| Peak knee flexion in swing (deg) |
25.1± 13.1 | 27.6± 14.1 | 27.8± 15.9 | 29.6± 16.4 | 30.5± 15.1 | 31.3± 16.6 | 31.6± 17.0 | 31.3± 15.5 |
| Ankle DF at initial contact (deg) |
−7.3± 9.4 | −6.9± 9.3 | −5.9± 8.0 | −6.8± 8.4 | −5.9± 8.1 | −8.2± 10.2 | −9.3± 11.4 | −6.7± 9.4 |
| Peak ankle DF in swing (deg) |
2.1± 7.7 | 1.0± 8.5 | 1.8± 8.5 | 1.1± 8.5 | 2.6± 6.7 | −0.3± 7.9 | −1.2± 10.2 | 0.1± 8.8 |
| Peak ankle abduction in swing (deg) |
−4.1± 6.2 | −3.8± 4.5 | −3.8± 4.5 | −4.2± 5.3 | −7.5± 17.6 | −6.1± 7.7 | −6.0± 6.0 | −4.7± 4.9 |
| Peak ankle ext rot in swing (deg) |
0.1± 18.0 | 1.6± 19.3 | −0.8± 16.5 | 1.2± 20.3 | 4.4± 20.1 | 3.4± 17.8 | 4.7± 19.9 | 0.8± 17.9 |
| Peak AP GRF (N/kg) |
0.51± 0.28 | 0.60± 0.29 | 0.64± 0.38 | 0.74± 0.56 | 0.55± 0.30 | 0.69± 0.33 | 0.64± 0.27 | 0.67± 0.31 |
| Peak hip power in pre-swing (W/kg) |
0.30± 0.21 | 0.45± 0.43 | 0.48± 0.41 | 0.53± 0.52 | 0.38± 0.31 | 0.50± 0.44 | 0.53± 0.48 | 0.59± 0.61 |
| Peak ankle power at push-off (W/kg) |
0.42± 0.41 | 0.54± 0.49 | 0.56± 0.54 | 0.62± 0.63 | 0.51± 0.58 | 0.66± 0.65 | 0.64± 0.64 | 0.64± 0.64 |
PNS: Peroneal nerve stimulation; DF: dorsiflexion; ext rot: external rotation; AP GRF: anterior-posterior ground reaction force; N: Newton; W: Watt; kg: kilogram.
Table 2.
Primary analysis of spatiotemporal, kinematic, and kinetic data of all subjects (n=110). P-values for treatment group main effect, treatment group × time effect, and time effect by gait parameter are presented. The time effect at t2, t3, and t4 is relative to t1.
| Gait Parameter | Treatment Group main effect | Treatment Group × Time Effect | Time effect | Time effect at t2 | Time effect at t3 | Time effect at t4 |
|---|---|---|---|---|---|---|
| Spatiotemporal | ||||||
| Cadence (steps/min) | <0.001* | >0.999 | 0.012* | 0.002* | 0.003* | <0.0001* |
| Double support (sec) | >0.999 | >0.999 | 0.159 | |||
| Stride length (m) | 0.998 | >0.999 | <0.001* | <0.0001* | 0.003* | 0.0003* |
| Walking speed (m/sec) | >0.999 | >0.999 | <0.001* | <0.0001* | 0.001* | <0.0001* |
| Kinematic | ||||||
| Peak hip flex swing (deg) | 0.350 | >0.999 | >0.999 | |||
| Peak knee flex swing (deg) | >0.999 | >0.999 | >0.999 | |||
| Peak ankle DF swing (deg) | 0.293 | >0.999 | 0.031* | 0.002* | 0.0001* | 0.058 |
| Ankle DF at IC (deg) | >0.999 | 0.181 | >0.999 | |||
| Peak ankle abd swing (deg) | 0.464 | >0.999 | >0.999 | |||
| Peak ankle ext rot swing (deg) | >0.999 | >0.999 | >0.999 | |||
| Kinetic | ||||||
| AP GRF (Nm) | >0.999 | >0.999 | 0.004* | 0.005* | 0.097 | 0.032* |
| Peak hip power pre-swing (W/kg) | 0.003* | >0.999 | <0.001* | 0.004* | <0.0001* | <0.0001* |
| Peak ankle power at push-off (W/kg) | >0.999 | >0.999 | 0.005* | 0.002* | 0.004* | 0.003* |
DF: dorsiflexion; IC: initial contact; abd: abduction; ext: external; AP GRF: anterior-posterior ground reaction force
Statistically significant value, p<0.05.
Primary Analyses
Spatiotemporal parameters
The treatment group main effect, which reflects pretreatment between-group differences, was significant for cadence (F1,187=20.06, p<0.001) (cadence being greater in the UC group) but not double support time, stride length, or walking speed. The treatment group by time interaction effect was not significant for any of the four spatiotemporal parameters. The time effect for double support time was also not significant. However, the time effect was significant for cadence (F3,153=5.81, p=0.012), stride length (F3,179=20.01, p<.001), and walking speed (F3,167=18.2, p<.001). Post-hoc analysis showed significant improvements at t2, t3, and t4 relative to t1 for all three parameters. Plots of LS means over time for cadence (Figure 1), stride length (Figure 2), and walking speed (Figure 3) are presented.
Figure 1.
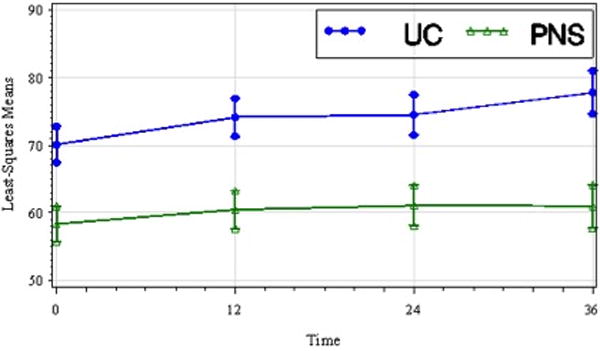
Least-squares means of cadence (steps/min) by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Cadence improved similarly over time in both treatment groups despite a baseline difference that favored the UC treatment group. The between group difference was not significant.
Figure 2.
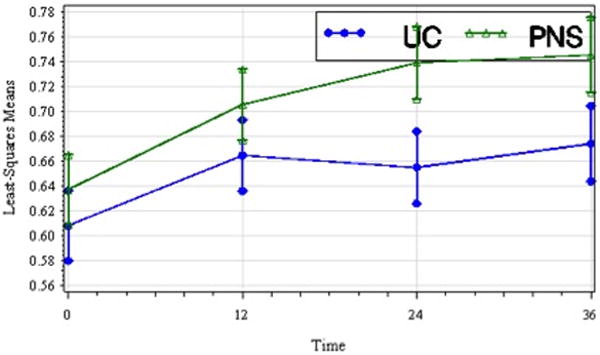
Least-squares means of stride length (m) by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Stride length improved over time in both treatment groups though the between group difference was not significant.
Figure 3.
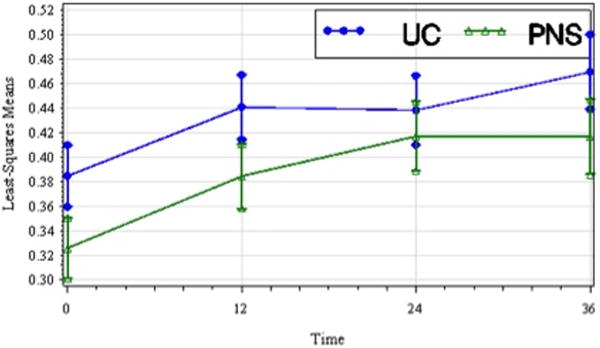
Least-squares means of walking speed (m/sec) by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Walking speed improved over time in both treatment groups though the between groups difference was not significant.
Kinematic parameters
The treatment group main effect was not significant for any of the kinematic parameters. Treatment group by time interaction effect and time effect were not significant for peak hip flexion angle during swing, peak knee flexion angle during swing, ankle DF angle at initial contact, peak ankle abduction angle during swing, or peak ankle external rotation during swing. Treatment group by time interaction effect for peak ankle DF angle during swing was also not significant; however, there was a significant time effect (F3,184=4.99, p=.031) with subjects in both groups experiencing a reduction in peak ankle DF angle during swing. Post-hoc analysis showed significant decreases in peak DF angle during swing at t2 and t3, both relative to t1. Figure 4 shows the plot of LS means over time for peak ankle DF angle in swing.
Figure 4.
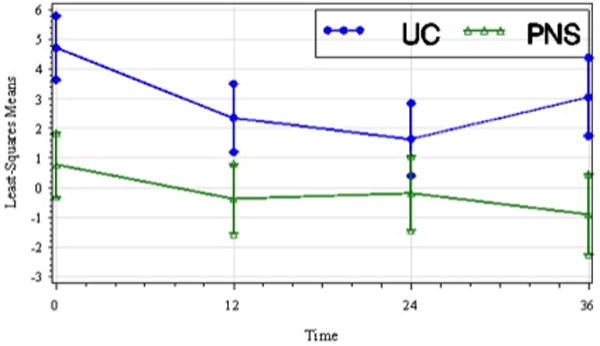
Least-squares means of peak ankle dorsiflexion in swing (deg) of the paretic lower limb by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Peak ankle dorsiflexion in swing decreased in both groups over time though the between group difference was not significant.
Kinetic parameters
The treatment group main effect was significant for peak hip power in pre-swing (p=0.003) (pretreatment peak hip power was greater in the UC group) but not AP GRF or peak ankle power at push-off. There was no significant treatment group by time interaction effect for peak AP GRF, peak hip power in pre-swing, or peak ankle power at push-off. The time effect was significant for peak AP GRF(F3,164=6.61, p=0.004) with post-hoc analysis showing significant improvement at both t2 and t4 relative to t1. The time effect was significant for peak hip power in pre-swing (F3,156=8.76, p<.001) and post-hoc analysis showed significant improvement at t2, t3, and t4relative to t1. Lastly, the time effect was significant for peak ankle power at push-off (F3,149=6.38, p=0.005), and post-hoc analysis showed significant improvement at t2, t3, and t4 relative to t1. Figures 5 and 6 are plots of LS means over time for peak hip power in pre-swing, and peak ankle power at push-off, respectively.
Figure 5.
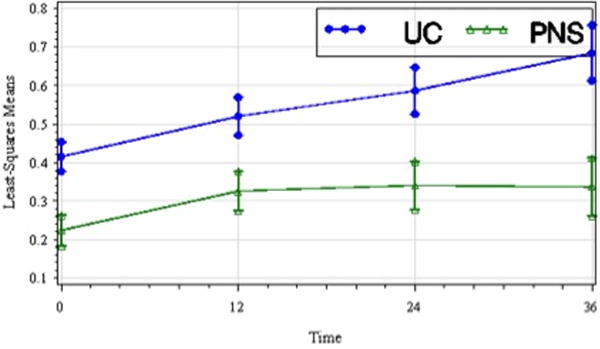
Least-squares means of peak pre-swing hip power (W/kg) of the paretic lower limb by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Peak hip power increased in both groups over time though the between group difference was not significant.
Figure 6.
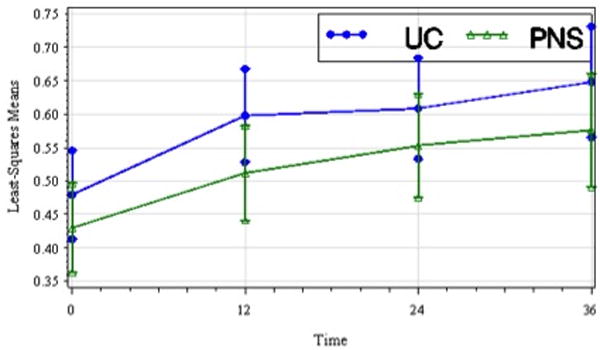
Least-squares means of peak ankle power (W/kg) of the paretic lower limb by treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). Peak ankle power improved in both groups over time though the difference between groups was not significant.
Activity Level
Table 3 presents the mean ± standard deviation of the three activity parameters measured by the ActivPAL™ monitor by treatment group at baseline (t1), end of the Device Usage period (t2), and at 12-wks (t3) and 24-wks (t4) post-treatment. For all parameters, the treatment group main effect was not significant. The time effects for average time standing (F3,151= 1.05), average time walking (F3,148= 0.54), and average number of steps per day (F3,153= 0.78) were not significant (p>.999). Similarly, the treatment group by time effects for average time standing(F3,151= 0.57), average time walking (F3,148= 1.13), and average number of steps (F3,153= 0.78) were not significant (p>.999).
Table 3.
Assistive device usage at each QGA assessment by treatment group. Within-subject assistive device usage was consistent for 41 subjects in the PNS group (77%) and 44 subjects in the UC group (80%).
| PNS Treatment | Usual Care Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Activity Parameter | t1 | t2 | t3 | t4 | t1 | t2 | t3 | t4 |
| Average time standing/day (mins) | 153.5 ± 87.2 | 170.6 ± 78.6 | 156.4 ± 79.2 | 173.2 ± 115.2 | 127.3 ± 75.5 | 127.1 ± 71.7 | 138.1 ± 91.5 | 157.5 ± 115.5 |
| Average time walking/day (mins) | 67.8 ± 58.6 | 67.3 ± 58.5 | 77.5 ± 56.1 | 71.8 ± 54.1 | 65.9 ± 53.6 | 67.5 ± 48.2 | 71.1 ± 46.8 | 78.8 ± 50.7 |
| Average number steps/day | 3223 ± 3134 | 3383 ± 3470 | 3991 ± 3397 | 3738 ± 3211 | 3270 ± 2947 | 3555 ± 2951 | 3734 ± 2820 | 4038 ± 2848 |
PNS: Peroneal nerve stimulation.
Assistive Devices
AD designations were the following: no device, straight cane, quad cane, or walker. Table 4 details AD usage across QGA assessments. Of the 54 subjects in the PNS treatment group, 41 subjects used the same AD for all of the QGA assessments. Within this group of 41 subjects, 27 used no device for all QGA assessments. Of the 56 subjects in the UC treatment group, 44 subjects used the same AD for all of the QGA assessments. Within this group of 44 subjects, 25 used no device for all QGA assessments. Table 5 presents interval change in AD usage for subjects who used a different device at any of their QGA assessments.
Table 4.
Mean ± standard deviation of each activity parameter, measured using the ActivPAL™ monitor, by treatment group at baseline (t1), end of the Device Usage period (t2), and at 12-wks (t3) and 24-wks (t4) post-treatment.
| PNS | UC | |
|---|---|---|
| Subjects using the same AD at each QGA assessment No Device Straight cane Quad cane Walker |
27 6 6 2 |
25 11 8 0 |
| Subjects using a different AD at any QGA assessment | 12 | 11 |
AD: assistive device; QGA: quantitative gait analysis.
Table 5.
Change in the assistive device by treatment interval for those subjects who used a different device at any of their QGA assessments (PNS=12, UC=11).
| Interval t0 to t1 PNS: US |
Interval t1 to t2 PNS: UC |
Interval t2 to t3 PNS: UC |
|
|---|---|---|---|
| Change: QC to cane | 7:3 | 1:1 | 0:1 |
| Change: cane to ND | 0:1 | 0:1 | 0:2 |
| No change in AD | 3:6 | 9:8 | 8:5 |
| Change: ND to cane | 1:1 | 0:1 | 1:3 |
| Change: cane to QC | 1:0 | 0:0 | 1:0 |
| Absent data | 0:0 | 2:0 | 2:0 |
QD: quad cane; ND: no device; AD: assistive device.
Secondary analyses
An analysis of hip range of motion (approximated by the difference between peak hip flexion during swing and peak hip extension measured via QGA) demonstrated a significant time effect (F3,198=9.4, p<0.0001) with both treatment groups gaining approximately 3 degrees of active range of motion by t4, though a treatment group by time interaction effect was not found (F3,205=2.12, p=0.099).
An exploratory analysis limited to the UC group (AFO=48 subjects, ND = 8 subjects) showed a significant treatment group (AFO versus no device) by time interaction effect (F3,87=3.32, p=.023) favoring the no device group; post-hoc analysis found the difference to be significant at t2 and t3 relative to t1. Figure 7 shows the plot of LS means over time of peak DF angle in swing within the UC treatment group.
Figure 7.
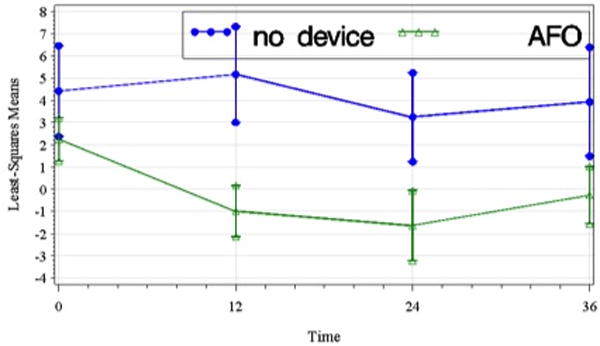
Least-squares means of peak ankle dorsiflexion during swing (deg) of the paretic lower limb within the UC treatment group over time including standard error bars (t1, pretreatment; t2, 12 wks; t3, 24 wks; t4, 36 wks). The difference between groups was significant though the small and uneven sample size (no device = 8, AFO = 480) precludes definitive statistical conclusions.
An analysis of the subset of subjects who used no assistive device for all of their QGA assessments (PNS n=27, UC n=25) (Table 6) showed no significant group effect or treatment group by time interaction for any of the spatiotemporal, kinematic, and kinetic parameters. Time effect was significant for cadence (F3,73=5.34, p=0.029), stride length (F3,78=12.72, p<.001), and walking speed (F3,74=12.01, p<.001). Post-hoc analysis showed significant differences at t2, t3, and t4 relative to t1 for both cadence and walking speed and a significant difference at t4 relative to t1 for stride length. Time effect was not significant for the kinematic parameters. Time effect was significant for AP GRF (F3,101=7.05, p=0.003) and peak hip power in pre-swing (F3,81=13.29, p<.001). Post-hoc analysis showed significant improvements at t2, t3, and t4 relative to t1 for peak hip power in pre-swing and at t4 relative to t1 for AP ground reaction force.
Table 6.
Secondary analysis of subgroup of subjects (n=52) who did not use an assistive device during any of the QGAs. P-values for treatment group main effect, treatment group × time effect, and time effect by gait parameter are presented. Time effect at t2, t3, and t4 is relative to t1.
| Gait Parameter | Treatment Group main effect | Treatment Group × Time Effect | Time Effect | Time effect at t2 | Time effect at t3 | Time effect at t4 |
|---|---|---|---|---|---|---|
| Spatiotemporal | ||||||
| Cadence (steps/min) | >0.999 | >0.999 | 0.029* | 0.015* | 0.033* | 0.015* |
| Double support (sec) | >0.999 | >0.999 | >0.999 | |||
| Stride length (m) | >0.999 | 0.073 | <0.001* | 0.107 | 0.104 | 0.026* |
| Walking speed (m/sec) | >0.999 | 0.601 | <0.001* | 0.030* | 0.039* | 0.006* |
| Kinematic | ||||||
| Peak hip flex swing (deg) | >0.999 | >0.999 | >0.999 | |||
| Peak knee flex swing (deg) | >0.999 | >0.999 | 0.244 | |||
| Peak ankle DF swing (deg)‡ | 0.823 | >0.999 | 0.256 | |||
| Peak ankle DF at initial contact (deg) | >0.999 | 0.984 | >0.999 | |||
| Peak ankle abd swing (deg) | >0.999 | >0.999 | >0.999 | |||
| Peak ankle ext rot swing (deg) | >0.999 | >0.999 | >0.999 | |||
| Kinetic | ||||||
| AP Ground Reaction Force (Nm) | >0.999 | >0.999 | 0.003* | 0.246 | 0.231 | 0.032* |
| Peak hip power pre-swing (W/kg) | >0.999 | >0.999 | <0.001* | 0.031* | 0.007* | <0.0001* |
| Peak ankle power at push-off (W/kg) | >0.999 | >0.999 | 0.075 | 0.121 | 0.188 | 0.098 |
Statistically significant value, p<0.05
DISCUSSION
A primary finding of this study was that both PNS and UC groups experienced significant improvements in peak hip power in pre-swing and peak ankle power at push-off of the paretic lower extremity during the treatment phase, which was maintained at 6-months post-treatment. Given that the change was most apparent at the end of the Device Usage period (t2), a reasonable explanation is that the therapy session activities, which were common to both groups and performed during the Device Usage period, increased hip and ankle muscle strength, muscle mass, and/or motor control, resulting in improved hip and ankle power. Alternatively, study participation and/or prescription of either DF-assist device may have inspired a general increase in walking, exercise, or overall activity level during the Device Usage period and beyond, which resulted in improved hip and ankle power in both groups. This second explanation is less likely because the ActivPAL™ data, which was recorded at each outcome assessment and used as a proxy for overall level of activity, show no change in activity level over the course of the study. Of note, improvements were noted in both treatment groups despite a treatment group main effect that favored the UC group for peak pre-swing hip power at baseline. Though a significant treatment group main effect was unanticipated given the subject stratification, this finding suggests that a higher level of motor impairment may not preclude a positive response to an ambulation training intervention in chronic stroke.
An interesting observation is that paretic ankle power at push-off improved at end of the Device Usage period in the UC group, despite relative ankle movement restriction imposed by an AFO in most of the UC subjects. While the small subject numbers preclude definitive statistical conclusions, this observation poses additional research hypotheses about a theoretical clinical concern that restriction of ankle movement within an AFO may be detrimental to residual paretic ankle strength and/or increase the metabolic cost of walking15. In this study, each AFO had a hinge that allowed limited ankle dorsiflexion range for those subjects in whom it was clinically appropriate. If, in fact, ankle strength improved in the subset of UC subjects who used an AFO, it is possible that eccentric contraction during mid-stance (prior to heel-off) and concentric contraction at beginning push-off of the gastrocsoleus muscles, within the allowable AFO ankle range and in the setting of a 12-wk period of gait training, was sufficient to mitigate deleterious effects on ankle strength or power that might have been observed with a solid ankle AFO. Alternatively, ankle power during the Device Usage period may have improved in the UC group if AFO compliance was poor, which in the absence of reliable usage data, cannot be known.
Subjects in both treatment groups experienced significant improvements in cadence, stride length, and walking speed during the Device Usage period, which was maintained at 6-months post-treatment. However, despite subject stratification based on motor impairment, a treatment group main effect was noted for cadence favoring the UC group at baseline. In order to test the hypothesis that an improvement in hip range of motion may have contributed to increased cadence and stride length, a secondary analysis of hip range of motion (approximated by the difference between peak hip flexion during swing and peak hip extension measured via QGA) was performed which demonstrated a significant time effect with both treatment groups gaining approximately 3 degrees by t4, though a treatment group by time interaction effect was not found. Lastly, improvements in walking speed may be primarily related to the improvement noted in pre-swing hip and ankle push-off power. Jonkers et al16 found that, similar to able-bodied controls, higher functioning stroke survivors increased their walking speeds by increasing both paretic hip and ankle power generation. Similarly, Mulroy et al17 found that improved walking speeds during post-stroke treadmill training with body-weight support were associated with increased paretic hip flexion power. Multiple studies, which have evaluated a variety of post-stroke gait interventions, including task-oriented biofeedback18, virtual reality training19, task-oriented physical therapy with and without rehabilitation technology20, robotic gait training21, and treadmill gait retraining augmented via visual EMG-biofeedback22 have also concluded that intervention-associated functional gains were largely attributable to an increase in paretic ankle power. Based on these data, the best explanations for the improvement in functional mobility reported in our earlier study4 are improvements in peak hip power in pre-swing and peak ankle power at push-off.
An unanticipated finding, that merits further investigation in a future, appropriately powered study, was that both PNS and UC groups experienced worsening of peak DF angle during swing during the treatment period, which persisted at 3-mos post-treatment but not at 6-mos post treatment. This finding suggests that wearing either device may have a detrimental effect on peak DF in swing. If this hypothesis were true, one possible explanation might be that the daily use of either device reduces the need for volitional drive to dorsiflex the ankle during swing, albeit through different mechanisms. The effect of functional electrical stimulation on motor cortical excitability depends on the concurrent motor cortical drive present at the time of stimulation, and the combination of these factors is proposed to modulate neural excitability.23 It can be hypothesized that repetitive surface peroneal nerve stimulation used during walking decreases the concurrent motor cortical drive which might otherwise be present and necessary to achieve maximal DF during the swing phase of gait.
Speculating further, an AFO may reduce peak DF via several mechanisms. Similar to PNS, the magnitude of concurrent motor cortical drive may be less when wearing an AFO due to the AFO functioning to keep the ankle at a neutral angle throughout the gait cycle. It is also possible that inhibition of active contraction of the anterior tibialis by the rigidity of an AFO results in loss of DF muscle mass and/or strength. In order to further explore the hypothesis that the continual use of an AFO reduces peak DF angle, an additional exploratory analysis of the UC group (AFO=48 subjects, ND = 8 subjects) was performed using the previously noted robust statistical methods. The analysis showed a significant treatment group (AFO versus no device) by time interaction effect that was significant at both t2 and t3, and approached significance at t4. Consistent with our hypothesis, Figure 5 shows that peak DF angle in swing decreased for the AFO subset during the Device Usage period, but modestly increased for the no device subset. This additional analysis should be interpreted as exploratory and hypothesis-generating only due to statistical limitations associated with the small and unequal sample sizes within the UC treatment group.
Regardless of mechanism, these results suggest that extended DF-assist device usage (PNS or AFO) may be detrimental, though the clinical significance of an approximate 3 degree loss of peak ankle DF during swing is unclear as other clinically relevant gait parameters improved in both groups (stride length, cadence, walking velocity). The absence of treatment by time effect on DF angle at initial contact, ankle external rotation, or ankle abduction during swing additionally suggests that neither treatment group benefitted from a therapeutic effect or compensatory strategy which specifically improved ankle positioning either at heelstrike or throughout the gait cycle.
An important potential confound in this trial was the unknown effect of any assistive device (straight cane, quad cane, or walker) used during the QGA session, particularly on the kinetic data recorded. While all QGAs were preferentially performed without an assistive device and within-subject assistive device consistency maintained across testing sessions as possible, the use of an assistive device to provide gait stability and safety during the QGA session was ultimately a clinical decision, made by the research staff present at each outcome assessment. Within-subject assistive device consistency was 77% and 80% in the PNS and UC treatment groups, respectively (Table 3). Of those subjects who used a different assistive device at any given QGA session (PNS n=12, UC n=11), most subjects in both treatment groups showed improvement with subsequent sessions, as demonstrated by transitioning from a greater to lesser device (i.e. quad cane to straight cane or straight cane to no device)(Table 4). A secondary analysis, which was limited to subjects who did not use an assistive device for any of their QGA assessments (n=52), showed similar results to the primary analysis (n=110)(Table 5). There was no evidence of a treatment effect favoring one DF-assist device over the other, though improvements were noted in cadence, stride length, and walking speed in both treatment groups, most pronounced at end of the Device Usage period (t2), also associated with an increase in peak hip power in pre-swing. In contrast to the larger analysis, the time effect was not significant for peak ankle power at push-off in this subset of subjects. This finding is consistent with a recent study by Polese et al24 which demonstrated an increase in ankle power generation with the use of a cane (“walking stick”) in post-stroke survivors. The lack of a time effect suggests that the increase in peak ankle power at push-off in both treatment groups in the larger analysis may be in part related to use of an assistive device. Definitive analysis of the contribution of an AD on the kinetics of gait in this study would have required that QGA be performed under multiple AD conditions at each outcome assessment.
In summary, this study failed to demonstrate the superiority of PNS over UC in reducing LE motor impairment and activity limitation in chronic stroke survivors. However, subjects in both treatment groups exhibited improvements in paretic ankle push-off power and specifically, pre-swing hip power which likely contributed to the improvements in the spatiotemporal parameters in the present report and improvement in functional mobility (mEFAP scores) described in our earlier publication4. It appears that gait training with either PNS or UC conveyed focal therapeutic effects, detectable by quantitative gait analysis, which were not detectable by the FMA, a global measure of motor impairment. This study also adds to the growing body of evidence25–26 that rehabilitation interventions in the chronic phase of stroke can be clinically relevant and further contributes to the debate27 regarding specificity of post-stroke gait treatment. Study limitations are primarily related to study design and include 1) the inability to determine role of baseline DF function due to a single factor design, 2) the lack of clear knowledge of the minimal clinically significant difference for each of the gait parameters, 3) lack of accurate treatment device usage data, 4) unknown optimal treatment device duration, 5) potential confound of DF-assist device used, if any, between the end of the Device Usage period (t2) and the 6-mos post-treatment (t4) assessments, 6) potential confound of offset between different assessments (marker placement) which may influence kinematic data, and 7) relatively large drop-out rate of 24% by 6-mos, which may have compromised internal validity. Lastly, a final important consideration is that compensatory strategies may have been acquired during the treatment period related to biomechanics of the nonparetic limb. An analysis of the kinematic and kinetic data from the nonparetic limb, while outside the scope of this present analysis, may provide further understanding of compensatory mechanisms in post-stroke gait recovery.
CONCLUSIONS
Both PNS and UC treatment groups demonstrated increased paretic pre-swing hip power and ankle power at push-off (measures of motor impairment) which translated into significant improvement in stride length, cadence, and walking velocity (measures of functional ambulation) which were sustained at 6-mos post-treatment. Thus, quantitative gait analysis was able to demonstrate a therapeutic effect in both groups that was not previously evident using only the FMA. These results suggest that the same mechanism for motor recovery is responsible for the improvement in functional ambulation that was evident in both groups of chronic hemiparetic stroke survivors at end of the Device Usage period. However, there was no evidence of a treatment effect on any of the spatiotemporal, kinematic, or kinetic gait parameters that would make either the PNS or UC treatments preferable to the other. The decrease in peak ankle DF during swing in both the PNS group and the AFO subjects within the UC group merits further investigation.
Acknowledgments
Funded by the National Institute for Child Health and Human Development grants R01HD44816 (PI: Chae), K23HD060689 (PI:Sheffler), and K24HD054600 (PI:Chae); and the National Center for Research Resources Clinical and Translational Science Collaborative of Cleveland (grant UL1RR024989).
The authors certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified above on the title page of the manuscript.
PAL Technologies Ltd via Endolite North America Ltd (activePAL), Douglas Maxwell (PAL Tech), 141 St James Road, Glasgow G4 0LT, United Kingdom, + 44(0)141 303 8380; Jeff Livingston (Endolite North America), 105 Westpark Road, Centerville, OH 45459
Footnotes
Clinical Trial Registration Information: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00148343.
The authors certify that one author has an affiliation with or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants and patents received or pending, royalties) with an organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript AND all such affiliations and involvements are disclosed on this title page of the manuscript.
Disclosures
Author PT is the co-inventor of the PNS device evaluated in this study and holds the patent for the device.
Authors LS, SNB, DG, JB, MIJ, JC – none
Salisbury District Hospital and/or Odstock Medical Limited (NDI was US distributor early on) Paul Taylor, Salisbury, Wiltshire, UK, SP2 8BJ, +44(0)1722 439540
G A Guilford & Son Ltd, Skip Guilford, 13515 Brookpark Rd, Cleveland, OH 44142, 216-362-1350
References
- 1.Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–67. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 2.Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32:1594–603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 3.Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, Groothuis-Oudshoorn CG, Ijzerman MJ. Therapeutic effect of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized controlled trial. Phys Ther. 2008;88:437–48. doi: 10.2522/ptj.20070035. [DOI] [PubMed] [Google Scholar]
- 4.Sheffler LR, Taylor PN, Gunzler DD, Buurke JH, Ijzerman MJ, Chae J. Randomized controlled trial of surface peroneal nerve stimulation for motor relearning in lower limb hemiparesis. Arch Phys Med Rehabil. 2013 Jun;94(6):1007–14. doi: 10.1016/j.apmr.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin KC, Huang YH, Hsieh YW, Wu CY. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23:336–42. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- 6.Park SW, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE Trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22:486–93. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–10. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–83. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- 9.Wright PA, Mann GE, Swain ID. Training effects of electrical stimulation and the conventional ankle foot orthosis in the correction of drop foot following stroke; Paper presented at: The First Annual Conference of FESnet; Sept 2–3, 2002; Glasgow, Scotland. [Google Scholar]
- 10.Davis RB, O S, Tyburski D, Gage JR. A Gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–87. [Google Scholar]
- 11.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–92. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 12.Fitzmaurice GM, L N, Ware JH. Applied Longitudinal Analysis. New York: John Wiley and Sons; 2004. [Google Scholar]
- 13.Enhancements in SAS/STAT 9.2 Software. Cary, North Carolina: SAS Institute Inc; 2008. [Google Scholar]
- 14.Diggle P, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford Science; 1994. [Google Scholar]
- 15.Wutzke CJ, Sawicki GS, Lewek MD. The influence of a unilateral fixed ankle on metabolic and mechanical demands during walking in unimpaired young adults. J Biomech. 2012;45:2405–10. doi: 10.1016/j.jbiomech.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2009;29:129–37. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ. Gait parameters associated with responsiveness to treadmill training with body-weight support after stroke: an exploratory study. Phys Ther. 2010;90:209–23. doi: 10.2522/ptj.20090141. [DOI] [PubMed] [Google Scholar]
- 18.Jonsdottir J, Cattaneo D, Recalcati M, Regalo A, Rabuffetti M, Ferrarin M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil Neural Repair. 2010;24:478–85. doi: 10.1177/1545968309355986. [DOI] [PubMed] [Google Scholar]
- 19.Mirelman A, Patritti BL, Bonato P, Deutsch JE. Effects of virtual reality training on gait biomechanics of individuals post-stroke. Gait Posture. 2010;31:433–7. doi: 10.1016/j.gaitpost.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Richards CL, Malouin F, Bravo G, Dumas F, Wood-Dauphinee S. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2004;18:199–211. doi: 10.1177/1545968304269397. [DOI] [PubMed] [Google Scholar]
- 21.Brincks J, Nielsen JF. Increased power generation in impaired lower extremities correlated with changes in walking speeds in sub-acute stroke patients. Clin Biomech (Bristol, Avon) 2012;27:138–44. doi: 10.1016/j.clinbiomech.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Aiello E, Gates DH, Patritti BL, Cairns KD, Meister M, Clancy EA, et al. Visual EMG Biofeedback to Improve Ankle Function in Hemiparetic Gait. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7703–6. doi: 10.1109/IEMBS.2005.1616297. [DOI] [PubMed] [Google Scholar]
- 23.Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res. 2005;162:497–502. doi: 10.1007/s00221-004-2153-1. [DOI] [PubMed] [Google Scholar]
- 24.Polese JC1, Teixeira-Salmela LF, Nascimento LR, Faria CD, Kirkwood RN, Laurentino GC, et al. The effects of walking sticks on gait kinematics and kinetics with chronic stroke survivors. Clin Biomech. 2012 Feb;27(2):131–7. doi: 10.1016/j.clinbiomech.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–15. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornby TG, Straube DS, Kinnaird CR, Holleran CL, Echauz AJ, Rodriguez KS, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil. 2011;18:293–307. doi: 10.1310/tsr1804-293. [DOI] [PubMed] [Google Scholar]


