TO THE EDITOR
Accurate diagnosis of melanoma is valuable in reducing melanoma mortality and morbidity. A number of candidate biomarkers have been developed for melanoma (Mandala and Massi, 2014); however, none has been accepted as being accurate, easily interpretable, and practical in diagnosing histologically challenging melanocytic lesions. Recently, it was found that primary cilia exist in melanocytes and melanoma cells and that primary cilia were frequently lost in melanomas (Kim et al., 2011; Le Coz et al., 2014), suggesting that the primary cilium may be used as a biomarker for melanoma. However, it remained unclear whether the loss of cilia in melanoma was due to increased proliferation or cell cycle progression. The statistical power of using primary cilium as a biomarker also remained to be established.
Here, we independently evaluated primary cilia by immunofluorescence microscopy on 87 cases of melanocytic nevi and melanomas obtained from the archival collections of the Department of Pathology of Stony Brook Medicine between 2003 and 2011. This study was approved by the IRB of Stony Brook University. ARL13B (Proteintech), acetylated α-tubulin (Sigma), and ACIII (Santa Cruz) were used to detect primary cilia; gamma-tubulin and chibby (CBY1, (Takemaru et al., 2003)) were used to detect basal body; SOX10 (Santa Cruz) and S100 (BioCare Medical) were used to detect melanocytes and melanoma cells, as described previously (Dai et al., 2011). Comparable results were obtained from all antibodies of each category (Supplemental Figure 1 and 2). An average of 366 (97 to 959) cells were evaluated for each specimen.
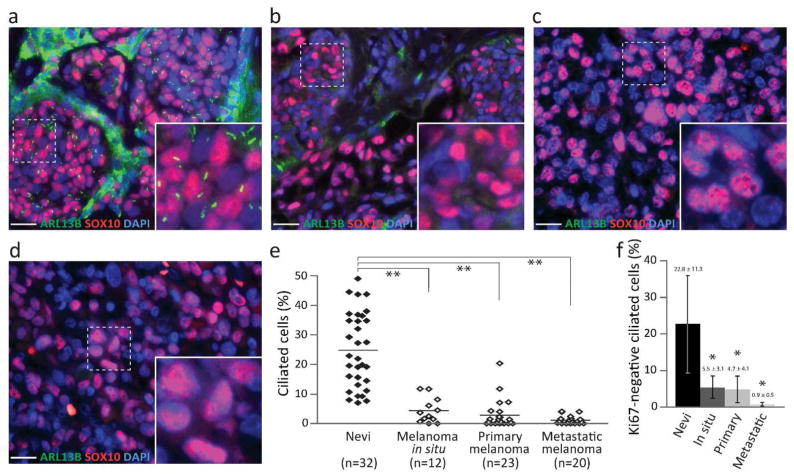
Remarkable decrease in ciliated cells was found in all types of melanoma when compared with nevi. Specifically, 24.85 ± 6.33% of melanocytes in nevi were ciliated (Figure 1a). In contrast, percentages of ciliated cells were reduced to 4.36 ± 2.13, 2.85 ± 2.43, and 1.16 ± 0.70% in melanoma in situ, primary melanoma, and metastatic melanoma, respectively (Figure 1b–d). The decrease was statistically significant (P = 2.50 ×10−17 by two-way ANOVA) (Figure 1e). In comparison to nevi, all types of melanoma demonstrated highly significant reduction in ciliated cells (P < 1 × 10−9 by Tukey test).
Figure 1. Primary cilia are frequently lost in melanoma.
(a–d) Primary cilia and melanocytes/melanoma cells were labeled with ARL13B (green) and SOX10 (red), respectively, in compound dysplastic nevus (a), melanoma in situ (b), primary melanoma (c), and metastatic melanoma (d). Scale bar: 20 μm. (e) Ciliated SOX10-positive cells expressed as a mean ± SD. Two-way ANOVA, P = 2.50 × 10−17. Asterisks (**) represent statistical significance when compared with nevi (Tukey test, P = 1.01 × 10−9, 5.34 × 10−13, 5.21 × 10−13, respectively). Samples were analyzed independently by two investigators with similar results. Results obtained by one investigator are presented. (f) Ciliated Ki67-negative melanocytes/melanoma cells. Asterisk (*) represents statistical significance when compared with nevi (Tukey test, P < 0.002).
Proliferating cells typically show higher rates of cilia formation than non-proliferating cells (Ishikawa and Marshall, 2011). To determine whether the loss of cilia was a result of increased proliferation, we examined cilia in non-proliferating (Ki67-negative) melanoma cells. A statistically significant loss of primary cilia was observed in non-proliferating melanoma cells (Figure 1f and Supplemental Figure 3), suggesting that the loss of primary cilia in tumor cells was not a direct result of proliferation.
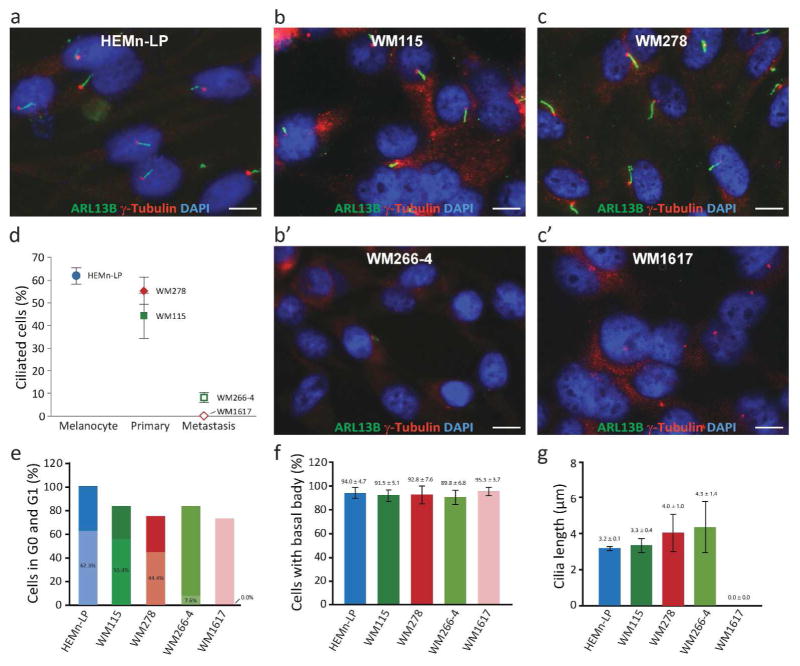
To determine whether the loss of cilia in tumor cells was caused by cell cycle progression, we examined ciliogenesis in two paired melanoma cell lines, i.e. cell lines derived from primary tumors (WM115 and WM278) and metastatic tumors (WM266-4 and WM1617, respectively) of the same patients. Primary melanocytes (HEMn-LP) were used as controls. Primary cilia and basal bodies were immunolabeled with ARL13B (NeuroMab) and γ-tubulin (Abcam), respectively. An average of 1,829 (753 to 3,000) cells were evaluated for each cell line. Two-days after serum starvation, HEMn-LP contained 62.00 ± 3.51% ciliated cells; WM115 and WM278 contained 44.44 ± 5.91 and 55.44 ± 10.08% ciliated cells; whereas, WM266-4 and WM1617 contained significantly fewer ciliated cells (7.56 ± 2.04 and 0.00 ± 0.00%, respectively, P < 6.9 × 10−5) (Figure 2a–d). Interestingly, after 2-day serum starvation, the proportion of cells in G0/G1 were comparable among tumor cells (Figure 2e), suggesting that the loss of primary cilia in melanoma cells was unlikely a result of cell cycle progression.
Figure 2. Loss of primary cilia in melanoma cell lines.
(a–c) Immunofluorescence labeling of primary cilia and basal bodies with ARL13B (NeuroMab) and γ-tubulin (Abcam), respectively, after 2-day serum-starvation. Scale bar: 10 μm. (d) Percentage of ciliated melanocytes, primary melanoma cells (WM115 and WM278), and metastatic melanoma cells (WM266-4 and WM1617). Metastatic melanoma cells contained significantly reduced percentages of ciliated cells when compared with paired primary melanoma cells (WM115 and WM278) (P = 0.0009 and 0.0025, respectively). (e) Percentage of G0/G1 cells by PI staining after 2-day serum-starvation. Shaded areas represent proportions of ciliated cells in percentage. (f) Percentage of cells with basal body as determined by γ-tubulin and chibby. (g) Length of ciliary axoneme determined after 2-day serum starvation.
To gain insight into the mechanism underlying the loss of cilia in melanoma cells, we examined basal body formation and found that the presence of basal body in serum-starved cells was indistinguishable across all cell lines (Figure 2f and supplemental Figure 4). The length of the ciliary axoneme was also comparable among all cell lines (Figure 2g). These observations suggest that the loss of cilia is likely intrinsic to melanoma cells. However, the precise molecular mechanism underlying reduced ciliogenesis or increased ciliary retraction remains to be determined.
Primary cilia perform diverse biological functions (Oh and Katsanis, 2012; Satir et al., 2010). The functions of primary cilia in melanocytes and melanoma cells are unknown. Defining the functions of primary cilia in melanocytic cells will undoubtedly contribute to our understanding of whether loss of primary cilia in melanoma is causative, concomitant, or consequential to malignant transformation or invasion. Because of the pivotal role of primary cilia in hedgehog signaling (Goetz and Anderson, 2010; Singla and Reiter, 2006), it will be interesting to determine whether therapeutic potential of inhibiting the hedgehog signaling pathway, such as previously reported in (Jalili et al., 2013; Stecca et al., 2007), is dependent on the presence of primary cilia in melanoma cells. Correlating primary cilia formation with mutations in the BRAF, PTEN, and NRAS genes may also shed light on the value of using primary cilia to inform melanoma risk or survival.
In summary, this study demonstrates that the loss of primary cilia is strongly correlated with melanoma. Statistical significance demonstrated the potential of using primary cilium as a biomarker for melanoma. Data obtained herein also suggested that the loss of cilia in melanoma is unlikely caused by increased proliferation, cell cycle progression, or absence of basal body. Further investigation on the molecular mechanism underlying loss of primary cilia in melanoma may help to understand malignant transformation and invasion, and identify novel therapeutic avenues to reduce melanoma-related morbidity and mortality.
Supplementary Material
Acknowledgments
We thank Mallory Korman and Stephanie Burke for histology assistance; Ken-Ichi Takemaru for CBY1 antibody. This study is supported by the Research Histology Core Laboratory of the Department of Pathology, the Cancer Center of Stony Brook University, and a research grant from NIH/NIAMS (AR061485) to JC.
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Dai D, Zhu H, Wlodarczyk B, et al. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–10. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–34. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jalili A, Mertz KD, Romanov J, et al. NVP-LDE225, a potent and selective SMOOTHENED antagonist reduces melanoma growth in vitro and in vivo. PLoS One. 2013;8:e69064. doi: 10.1371/journal.pone.0069064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dabiri S, Seeley ES. Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One. 2011;6:e27410. doi: 10.1371/journal.pone.0027410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coz M, Benmerah A, Larue L. Quiescent melanocytes form primary cilia. Exp Dermatol. 2014;23:426–7. doi: 10.1111/exd.12426. [DOI] [PubMed] [Google Scholar]
- Mandala M, Massi D. Tissue prognostic biomarkers in primary cutaneous melanoma. Virchows Arch. 2014;464:265–81. doi: 10.1007/s00428-013-1526-x. [DOI] [PubMed] [Google Scholar]
- Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–8. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Stecca B, Mas C, Clement V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K, Yamaguchi S, Lee YS, et al. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–9. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.