Abstract
Pressure ulcers (PUs) are serious skin injuries whereby the wound healing process is frequently stalled in the inflammatory phase. Myeloid-derived suppressor cells (MDSCs) accumulate as a result of inflammation and promote cutaneous wound healing by mechanisms not fully understood. Recently, MDSCs have been shown to differentiate into fibrocytes which serve as emerging effector cells that enhance cell proliferation in wound healing. We postulate that in wound healing, MDSCs not only execute their immunosuppressive function to regulate inflammation, but also stimulate cell proliferation once they differentiate into fibrocytes. In the current study, using full thickness and pressure ulcer mouse models, we found that KLF4 deficiency resulted in decreased accumulation of MDSCs and fibrocytes and wound healing was significantly delayed. Conversely, KLF4 activation by the plant-derived product, Mexicanin I, increased the numbers of MDSCs and fibrocytes and accelerated wound healing. Collectively, our study revealed a previously unreported function of MDSCs in cutaneous wound healing and identified Mexicanin I as a potential agent to accelerate PU wound healing.
INTRODUCTION
A pressure ulcer (PU) is defined as an injury caused by unrelieved pressure resulting in damage to the skin and underlying tissue (Black et al., 2011). Wound healing is a complex process that includes an early inflammatory phase and subsequent proliferative and maturative phases (Kondo and Ishida, 2010; Li et al., 2005; Shih et al., 2010). However, this process is frequently stalled in the inflammatory phase in PU (Gist et al., 2009) resulting in chronic wounds. Therefore, a better understanding of how to control inflammation in wound healing will lead to therapeutic strategies that will potentially improve the quality of life of PU patients.
Myeloid derived suppressor cells (MDSCs) are a heterogeneous population of bone marrow derived cells that bear monocyte markers and possess phenotypic plasticity (Manjili, 2012). They are broadly defined as Ly6G+CD11b+ cells in mice, with a wider range of markers in humans. MDSCs are characterized by their potent ability to suppress immune responses, especially T-cell proliferation and cytokine production (Gabrilovich and Nagaraj, 2009). While MDSCs have been shown to be beneficial to the wound healing process (Cuenca et al., 2011; Mahdipour et al., 2011; Zhang et al., 2011), the mechanisms remain unknown. Recent reports indicated that MDSCs have a high potential of differentiating into fibrocytes (Niedermeier et al., 2009). Fibrocytes are emerging effector cells in chronic inflammation (Reilkoff et al., 2011) which enhance keratinocyte proliferation in wound healing (Kao et al., 2011). Therefore, we postulate that in wound healing, the recruited MDSCs not only execute their immunosuppressive function to keep inflammation in check, but also stimulate cell proliferation once they differentiate into fibrocytes as suggested by the presence of fibrocytes during the latter phase of the inflammatory process (Fan et al., 2011; Mori et al., 2005).
Kruppel like factor 4 (KLF4) is a transcription factor (Garrett-Sinha et al., 1996; Shields et al., 1996; Yet et al., 1998) critical to monocyte differentiation (Alder et al., 2008; Feinberg et al., 2007). It promotes cutaneous wound healing by mechanisms not clearly delineated (Li et al., 2012; Liao et al., 2011). We recently reported that KLF4 regulates the recruitment of MDSCs and the generation of fibrocytes in cancer development (Shi et al., 2014; Yu et al., 2013). However, whether KLF4 regulates cutaneous wound healing in a MDSC- and fibrocyte-dependent manner is not clear. In this study, using full thickness and pressure ulcer mouse models, we show that KLF4 promotes wound healing by regulating the recruitment of MDSCs and their subsequent differentiation into fibrocytes.
RESULTS
KLF4 ablation resulted in delayed wound healing in a full thickness mouse wound healing model
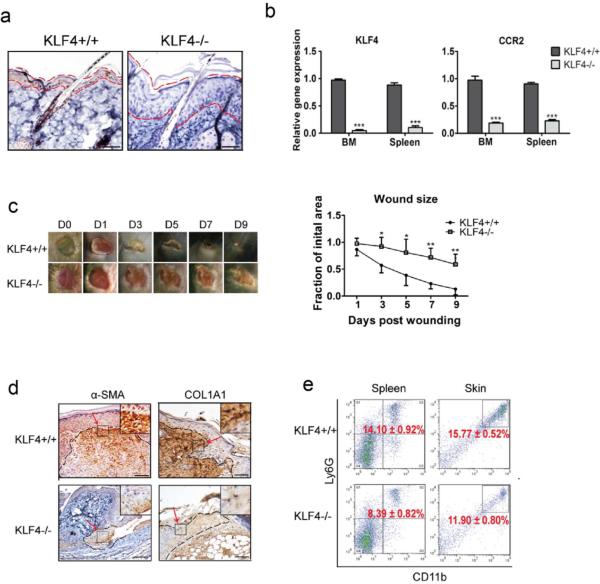
To confirm our observation that KLF4 ablation delayed cutaneous wound healing in tamoxifen-inducible KLF4-CreER/KLF4(flox) mice (Li et al., 2012), we utilized RosaCre26ER/KLF4(flox) double transgenic mice, in which KLF4 was knocked out following tamoxifen induction in all cells. As shown in Figure 1a and 1b, successful KLF4 knockout after tamoxifen induction was confirmed by immunohistochemical staining of KLF4 in the skin and by qRT-PCR analysis of the expression of KLF4 and its downstream target CCR2 (Alder et al., 2008) in bone marrow (BM) and spleen. Consistent with a growth inhibitory role of KLF4, the superbasal layer of the skin in KLF4 knockout mice (KLF4−/− mice) was significantly thicker than that in the un-induced mice (KLF4+/+ mice). We then performed the wound healing experiment in RosaCre26ER/KLF4 (flox) mice by placing full-thickness wounds (8mm in diameter) with a puncher as previously described (Li et al., 2012). Wound closure in the KLF4−/− group was significantly delayed from day 3 to day 9 when compared to that in the KLF4+/+ group (Figure 1c). We also used immunohistochemistry (IHC) to examine the expression of α-SMA (a myofibroblast marker) and COL1A1 (a fibroblast marker) in granulation tissues on day 3. As expected, their immunoreactivities were both significantly reduced in the KLF4−/− group compared to that in the KLF4+/+ group (Figure 1d).
Figure 1. KLF4 ablation delayed cutaneous wound healing accompanied by decreased accumulation of MDSCs.
(a). KLF4 IHC staining in skin of RosaCreER/KLF4(flox) mice without (KLF4+/+) and with tamoxifen induction (KLF4−/−). The areas between two dotted red lines delineate skin suprabasal layers. (b). qRT-PCR to analyze expression of KLF4 and CCR2. (c). Wound healing phenotypes (left, n=10) and the wound size quantification (right). (d). IHC staining of α–SMA and COL1A1 in KLF4+/+ and KLF4−/− mice at Day 3 after wound induction. (e). Flow cytometry using anti-Ly6G and anti-CD11b antibodies with spleen cells and single skin cells. Representative images from one of five mice in each group are shown (a, c, and d). Scale bars: 50 μm. Mean ± SEM. *p< 0.05, **p< 0.01, ***p< 0.001.
To examine whether KLF4 deficiency reduces the recruitment of MDSCs in wound healing at Day 3, we performed flow cytometric analysis using cells purified from BM, spleen and the wound site (specific tissue from its edge and subsurface granulation tissue). While the percentage of MDSCs in BM showed no significant difference between the KLF4+/+ and KLF4−/−group (data not shown), it was dramatically less in the spleen and the skin wound site in the KLF4 knockout (KLF4−/− 8.39±0.82% vs. KLF4+/+ 14.10±0.92% in spleen, p<0.01; KLF4−/− 11.90±0.80% vs. KLF4+/+ 15.77±0.52% in skin, p<0.05) (Figure 1e).
KLF4 ablation in bone marrow delayed cutaneous wound healing accompanied by decreased accumulation of CCR2+ MDSCs and fibrocytes in the wound site
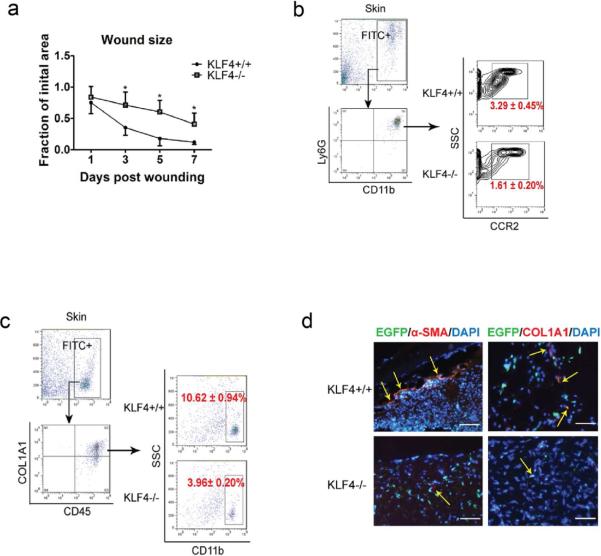
To test the possibility that the KLF4 deficiency-induced delay of cutaneous wound healing is attributable to bone marrow cells, we performed full-thickness wound healing experiments using chimeric mice. These mice were generated by transplanting bone marrow cells from RosaCre26ER/KLF4(flox)/β-actin-EGFP triple transgenic mice into wild type C57BL/6 mice. The percentage of EGFP positivity in circulating leukocytes of the chimeric mice was more than 90% as assessed by flow cytometry in tamoxifen-induced mice (data not shown), suggesting a successful bone marrow reconstitution. The wound-closure kinetics showed that wound healing was significantly delayed in the bone marrow KLF4−/− group (Figure 2a). In addition, while the total population of MDSCs in BM, spleen and the skin wound site was not significantly different before and after KLF4 bone marrow knockout by tamoxifen induction (data not shown), CCR2+ MDSCs in the skin wound site decreased from 3.29±0.45% in the BM KLF4+/+ group to 1.61±0.20% in the BM KLF4−/− group (p<0.05, Figure 2b).
Figure 2. Delayed wound healing in bone marrow KLF4 knockout mice and compromised accumulation of CCR2+MDSCs and fibrocytes.
(a). Chimeric mice receiving bone marrow cells from Rosa26CreER/KLF4(flox)/β-actin-EGFP donor mice were used. Quantification of the wound size in each group of mice is shown (n=10). (b). Single cells from the skin wound were gated by EGFP. They were examined by CD11b and Ly6G antibodies, followed by further analysis using a CCR2 antibody. Representative contour plots in each group are shown. (c). Similar to (b) except COL1A1, CD45, and CD11b antibodies were used to analyze the fibrocytes. (d). Representative immunofluorescent staining of the wounds with α-SMA and COL1A1 antibodies. Yellow arrows indicate EGFP/α-SMA or EGFP/COL1A1 co-expressing cells (Scale bars: 50 μm).
The generation of fibrocytes from MDSCs shown in recent studies prompted us to examine the fibrocyte population in our wound healing model. The results from flow cytometry revealed that the percentage of bone marrow-derived fibrocytes, characterized as EGFP+COL1A1+CD45+CD11b+, decreased from 10.62±0.94% in the KLF4+/+ group to 3.96±0.20% in the KLF4−/− group (p<0.01, Figure 2c). This finding was further confirmed by immunofluorescent staining of skin cryosections from the wound site. That is, the numbers of COL1A1/EGFP and α-SMA/EGFP co-expressing cells in the KLF4−/− group were significantly reduced compared to those in the control group (Figure 2d).
KLF4-expressing bone marrow cells integrated into the wound healing tissue and adapted fibroblast morphology in a mouse wound healing model
To further examine whether KLF4-expressing bone marrow cells are involved in cutaneous wound healing, we transplanted bone marrow cells from our KLF4/EGFP transgenic mice, in which KLF4-expressing cells are labeled with EGFP (Li et al., 2012), to wild type mice and performed full thickness wound healing experiments. In control mice, bone marrow cells from the wild type C57BL/6 mice were transplanted. As shown in Figure 3, EGFP+ cells were observed in the healing tissue of chimeric mice receiving KLF4/EGFP bone marrow cells, but not in the control mice. Interestingly, KLF4/EGFP cells adapted an elongated morphology and expressed α-SMA, a marker of myofibroblasts that play a critical role in wound healing (Desmouliere et al., 2005; Hinz et al., 2012).
Figure 3. KLF4-expressing bone marrow cells are integrated into the healing tissue and co-localized with α-SMA-expressing cells.
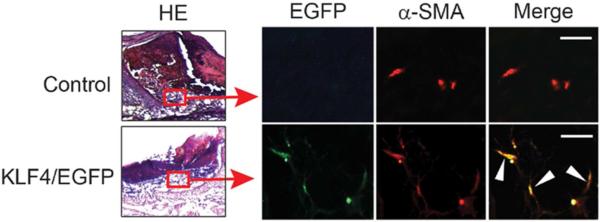
Chimeric mice were generated by bone marrow transplantation using bone marrow cells from C57BL/6 mice (Control) or KLF4/EGFP mice into C57BL/6 recipient mice. 8mm diameter full thickness wound was placed and the wound beds were collected 4 days later followed by immunofluorescence staining with an anti-α-SMA antibody. Representative co-localization of EGFP cells with red α-SMA-expressing cells in the healing tissue (left) are indicated by white arrowheads (n=5). Scale bars: 25 μm.
KLF4 deficiency in CCR2+ MDSCs decreased fibrocyte generation in FSP-1-Cre/KLF4(flox) mice
Since KLF4 deficiency in bone marrow resulted in delayed wound healing accompanied by decreased CCR2+MDSCs (Figure 2b), we postulated that KLF4 in CCR2+ MDSCs directly regulates cutaneous wound healing. To test this possibility, we generated a mouse model in which KLF4 was specifically ablated in CCR2+ MDSCs by crossing KLF4(flox) mice with FSP-1-Cre mice, because FSP-1 is highly expressed in bone marrow CCR2+ MDSCs as shown in our recent studies (Shi et al., 2014).
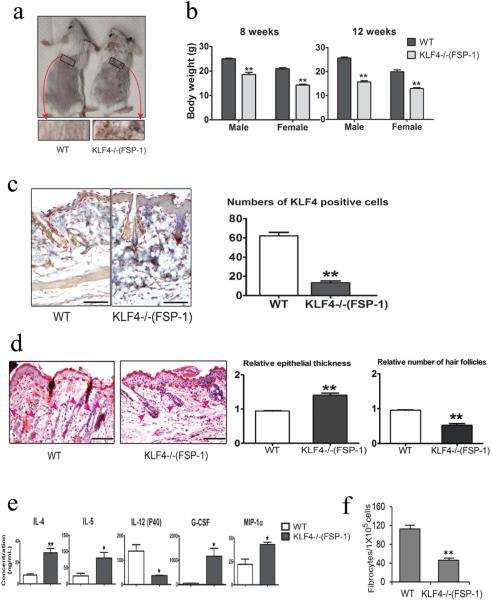
FSP-1-Cre/KLF4(flox) mice showed no obvious abnormalities from birth to 8 weeks of age. After 8 weeks of age, significant hair and weight loss was observed in these KLF4 deficient mice when compared to WT mice (Figure 4a and 4b). Successful KLF4 knockout in monocytes in KLF4 deficient mice (designated as KLF4−/−(FSP-1)) was confirmed by qRT-PCR (Supplementary Figure 1). IHC staining showed that the number of KLF4 positive cells was decreased by 84% in KLF4−/−(FSP-1) mice (Figure 4c). H&E staining showed that the hair follicle density decreased by about 50%, and the suprabasal layer was thicker in KLF4−/−(FSP-1) mice (Figure 4d). To examine the potential immunological defects in FSP-1-Cre/KLF4 (flox) mice, we measured the cytokine/chemokine levels in mouse sera. Expression levels of IL4 and IL5 were significantly increased, but the level of IL-12 (P40), a shared subunit of IL-12 and IL-23 (Quatresooz et al., 2012), was decreased in KLF4−/−(FSP-1) mice (Figure 4e). Interestingly, expression levels of granulocyte colony-stimulating factor (G-CSF) and macrophage inflammatory protein 1 alpha (MIP-1α) were also significantly increased in KLF4−/−(FSP-1) mice (Figure 4e). Consistent with the importance of CCR2+ MDSCs in fibrocyte generation (Shi et al., 2014), the number of fibrocytes generated from spleen cells decreased from 112±8 per 105 cells in the WT mice to 46±4 per 105 cells in FSP-1-Cre/KLF4 (flox) mice (Figure 4f).
Figure 4. Hair loss and decreased fibrocyte generation in FSP-1-Cre/KLF4(flox) mice.
(a). Representatives of the wild type (WT) and FSP=1-Cre/KLF4(flox) (KLF4−/−(FSP-1)) mice. Black squares indicate an area in which a severe hair loss was seen in KLF4−/−(FSP-1) mice. (b). Body weights of male and female WT and KLF4−/−(FSP-1) mice. (c). Representative images of KLF4 staining of skin (left) and measurement of KLF4 positive cells (right). (d). Left, representative images of HE staining of skin. The areas between two dotted red lines represent skin suprabasal layers and the red arrow heads are pointing to hair follicles. Right, measurement of epithelial thickness and hair follicles. (e). Measurement of serum cytokines by ELISA (n=3). (f). Quantification of fibrocyte generation (n=5) Scale bars: 100 μm. *p<0.05, **p<0.01.
Compromised wound healing in FSP-1-Cre/KLF4(flox) mice was associated with decreased accumulation of CCR2+ MDSCs and fibrocytes in a mouse pressure ulcer model
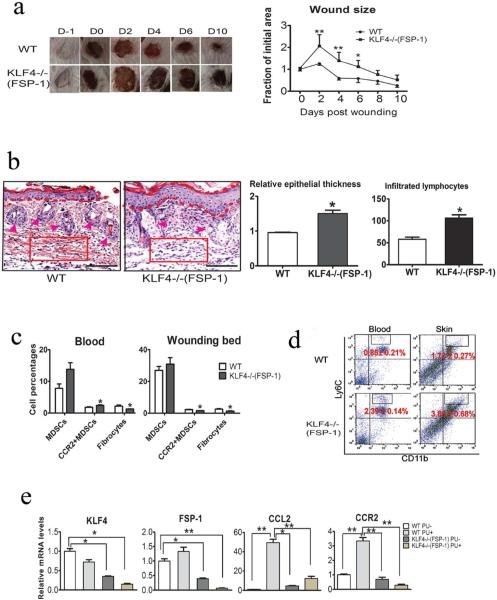
We used a recently established mouse PU model (Stadler et al., 2004) to further examine the role of KLF4 in wound healing with our FSP-1-Cre/KLF4(flox) mice. In this model, ulcers were typically formed at the end of the third ischemia/reperfusion (I/R) cycle and were accompanied by full-thickness loss of skin. The detached ulcerated skin was removed right after the third I/R circle. As shown in Figure 5a, one day after the ulcerated skin was removed, the open areas were increased in both WT and KLF4−/−(FSP-1) mice; in all likelihood this was related to an acute response. From Day 2 to Day 10, the wounds were gradually healed in WT mice, but the healing was significantly delayed in KLF4−/−(FSP-1) mice. HE staining showed increases in suprabasal layer of the skin and decreases in hair follicle density. In addition, the infiltrated lymphocytes in the granule tissue of the skin in KLF4−/−(FSP-1) mice were almost doubled that in WT mice, suggesting an increased inflammatory status in KLF4−/−(FSP-1) mice (Figure 5b). Consistent with decreased populations of CCR2+ MDSCs and COL1A1+CD45+CD11b+ fibrocytes in the bone marrow KLF4 knockout mice with a full-thickness wound, the numbers of these cells were also reduced in FSP-1-Cre/KLF4(flox) mice in the PU model (Figure 5c). In parallel with the increased inflammation in KLF4−/−(FSP-1) mice, the populations of CD11b+Ly6C++ cells, which may represent inflammatory monocytes (Robbins and Swirski, 2010), in blood and the skin wound were increased compared to those in WT mice (Figure 5d). In addition, the fact that FSP-1 expression was significantly reduced in the wound in KLF4−/−(FSP-1) mice (Figure 5e) is consistent with KLF4 role in regulating FSP-1 (Shi et al., 2014).
Figure 5. Compromised wound healing of PU in FSP-1-Cre/KLF4(flox) mice associated with decreased CCR2+MDSCs and fibrocytes.
(a). Similar to Figure 1c, except the WT and KLF4−/−(FSP-1) mice were used in the PU model (n=10). (b). Left: Representative images of HE staining of skin wounds in PU model. Right: Quantification of the epithelial thickness and the numbers of infiltrated lymphocytes in arbitrary red squares with the same sizes as the left (n=5). (c). Flow cytometry analysis to examine MDSCs and fibrocytes in mouse blood and skin wounds. (d). Similar to c, CD11b and Ly6C antibodies were used to examine inflammatory monocytes. (e). qRT=PCR to analyze expression of KLF4, FSP-1, CCL2, and CCR2 in the skin wounds. Scale bars: 100 μm, *p<0.05, **p<0.01.
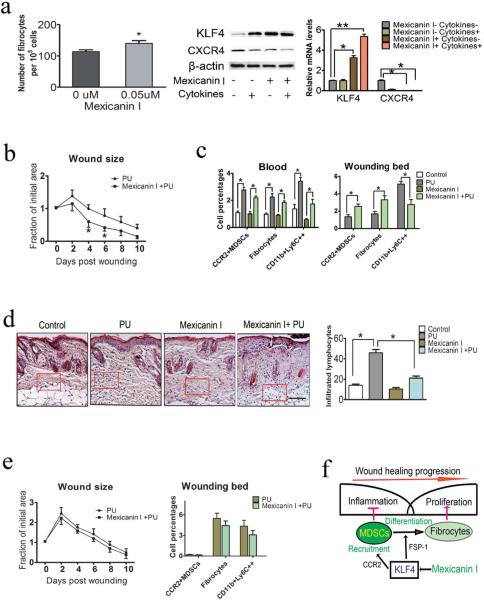
Activation of KLF4 by Mexicanin I increased CCR2+ MDSCs and fibrocytes in the mouse PU model accompanied by elevated gene expression of CCR2 and FSP-1
To further confirm the role of KLF4 in the PU healing process, we tested the plant-derived sesquiterpene lactone, Mexicanin I, which is known to be a c-Myb inhibitor (Bujnicki et al., 2012). c-Myb activates the proto-oncogene c-Myc (Neiman et al., 2003) that may inhibit the tumor suppressor KLF4. As shown in Figure 6a, Mexicanin I, at a 50 nM concentration, promoted fibrocyte generation from mouse spleen cells, which was accompanied by up-regulation of KLF4. In the mouse PU model, we found that Mexicanin I significantly accelerated wound healing (Figure 6b). In addition, while the total populations of MDSCs or CCR2+ MDSCs in blood with and without Mexicanin I treatment in control mice did not show any significant differences (Figure 6c and data not shown), COL1A1+CD45+CD11b+ fibrocytes and CD11b+Ly6C++ inflammatory monocytes were increased following PU induction in naïve and Mexicanin I treated mice. Importantly, in the skin wounds, CCR2+ MDSCs and fibrocytes were increased in the Mexicanin I treated mice from 1.36% to 2.55% for CCR2+ MDSCs and from 1.70% to 3.31% for fibrocytes. In line with the accelerated wound healing, the CD11b+Ly6C++ monocytes in the skin were decreased from 5.11% in the control mice to 2.75% in the Mexicanin I treated mice (Figure 6c). Consistently, there was minimal change of infiltrated lymphocytes in Mexicanin I-treated mice following PU induction but a significant increase in untreated control mice (Figure 6d). In parallel with the changes of CCR2+ MDSCs and fibrocytes, expression levels of KLF4, FSP-1, CCL2 and CCR2 were all significantly increased in the wounds after Mexicanin I treatment (Supplementary Figure 2). Most importantly, there were no significant differences in the wound healing kinetics, numbers of CCR2+ MDSCs, fibrocytes and CD11b+Ly6C++ monocytes between Mexicanin I-treated mice and untreated mice in KLF4−/−(FSP-1) mice following PU induction (Figure 6e).
Figure 6. KLF4 activation by Mexicanin I accelerated PU wound healing accompanied by increased CCR2+MDSCs and fibrocytes.
(a). Fibrocytes generation in the presence of 50 nM Mexicanin I (left), KLF4 expression detected by Western blotting (middle) and qRT=PCR (right). CXCR4 and β-actin were used as controls. (b). Measurement of wound healing kinetics after Mexicanin I treatment (n=10). (c): Flow cytometry analysis to examine different cell types. (d). Representatives of HE staining of the skin wounds (left) and numbers of infiltrated lymphocytes in the arbitrary squares with the same sizes from the left (right). (e). Left: measurement of wound healing kinetics in KLF4−/−(FSP-1) mice (n=10) and Right: flow cytometry analysis. (f). Proposed function of KLF4-mediated fibrocyte generation in wound healing. Scale bar: 100 μm, *p<0.05.
DISCUSSION
In this study, using two different mouse models of wound healing, we demonstrated that KLF4 promotes healing by regulating the recruitment of monocytic MDSCs and their subsequent differentiation into fibrocytes. This concept is consistent with the current wound healing model in which the inflammatory response wanes before the proliferation of fibroblasts and keratinocytes. Thus we have shown that MDSCs have a dual role by regulating the inflammation as an immunosuppressive and by promoting cell proliferation after they differentiate into fibrocytes (Figure 6f).
Initially, the decreased recruitment of CCR2+ MDSCs into the wound of KLF4 knockout mice (Figure 2b) raised a concern that the decreased number of fibrocytes may not be due to the subsequent transdifferentiation of these MDSCs. That is, the relative importance of transdifferentiation was questionable. However, our data suggest that KLF4 controls both the recruitment of MDSCs and their subsequent transdifferentiation. Firstly, KLF4 deficiency in bone marrow decreased expression of CCR2 ((Alder et al., 2008) and Figure 1b). It is known that CCL2/CCR2 signaling plays a critical role in cutaneous wound healing (Gillitzer and Goebeler, 2001) and confirmed by elevated CCL2 expression following PU induction in our study (Figure 5e). Secondly, we recently reported that KLF4 deficiency in CCR2+ MDSC attenuated fibrocyte generation and resulted in compromised tumor metastasis (Shi et al., 2014). The role of KLF4 in fibrocyte generation was also supported by Mexicainin I-mediated KLF4 activation and fibrocyte generation (Figure 6a). Thirdly and most importantly, in FSP-1-Cre/KLF4(flox) mice, the basal level of CCR2+ MDSCs in the blood was increased (Figure 5c). However, this population was significantly decreased in the wound. The increased basal level of CCR2+ MDSCs most likely reflected the developmental regulation of these cells by KLF4. It is also supported by our ELISA data (Figure 4e). Levels of G-CSF, MIP-1α, IL4, and IL5 were increased, but IP-12(P40) level was decreased in KLF4 knockout mice. High levels of G-CSF are correlated with the conversion of bone marrow derived MDSCs from CCR2+ MDSCs to neutrophils (Sawanobori et al., 2008). High MIP-1α expression was associated with CD49d (a marker of CCR2+ MDSCs (Haile et al., 2010)) mediated monocyte migration (Chuluyan et al., 1995). Increased G-CSF and MIP-1α suggests that KLF4 regulates the development of CCR2+ MDSCs. In addition, given the role of IL4 and IL5 in the polarization of Th2 cells (Ansel et al., 2006) and the role of IL-12(P40) in the polarization of Th1 cells (Torti and Feldman, 2007), alterations in the levels of these cytokines suggest that KLF4 ablation promotes Th2 cell polarization.
The critical role of KLF4 in monocytic MDSCs in cutaneous wound healing is not only supported by using the mouse model with KLF4 deficiency in the bone marrow (Figure 2), but also by using the FSP-1-Cre/KLF4(flox) mice with KLF4 deficiency in CCR2+ MDSCs (Figure 5). However, there is one limitation in our FSP-1-Cre/KLF4(flox) mouse model. FSP-1 is expressed in multiple cell types including monocytes (Cabezon et al., 2007) and fibroblasts (Strutz et al., 1995) and, therefore, we cannot exclude the contribution of KLF4-regulated fibroblasts in wound healing. Future experiments will be performed to specifically test the effect of bone marrow CCR2+ MDSCs on wound healing. Similarly, we plan to test the fibroblast-specific effect of KLF4 on wound healing. In addition, the molecular mechanisms by which KLF4 regulates FSP-1 expression will be further determined.
Our data showed that Mexicanin I activated KLF4 and accelerated PU wound healing (Figure 6). Although we favored a KLF4 and fibrocyte-mediated mechanism, KLF4-independent effects of Mexicanin I should also be considered. Furthermore, pharmacological kinetics of Mexicanin I will be determined in wound healing.
Collectively, this study not only confirms a critical role of KLF4 in wound healing, but also warrants the future development of KLF4-based therapeutic strategies such as activation of KLF4 by Mexicanin I in the treatment of patients with PUs.
MATERIALS AND METHODS
Generation of KLF4 deficient Mice
Rosa26CreER/KLF4(flox) mice and Fsp-1-Cre/KLF4 (flox) mice were generated by crossing Rosa26CreER (NCI, Stock#: 01XAB) and Fsp-1-Cre (Jackson, Stock#: 012641) with KLF4(flox) mice respectively. Rosa26CreER/KLF4(flox)/β-actin-EGFP mice were generated by crossing Rosa26CreER/KLF4(flox) mice with β-actin-EGFP mice (Jackson, Stock#: 006567). KLF4 knockout in RosaCre26ER/KLF4(flox) mice was induced by daily intraperitoneal injection of tamoxifen (TAM, Sigma, 50 mg/kg) for 5 consecutive days. Sunflower seed oil was used as a control. Mice were bred and used in specific pathogen-free facilities according to the Animal Care and Use Committee Guidelines of the University of South Carolina. In the wound healing, the control and mutant mice were littermates with mixed sex at a similar six to eight week age range.
Bone marrow transplantation
Six-week old C57BL/6 recipient mice were lethally irradiated (900 rad). The donor cells were harvested from the long bones of RosaCre26ER/KLF4 (flox)/β-actin-EGFP or KLF4/EGFP mice, and 3×106 nucleated cells were injected retro-orbitally into each recipient mouse. After repopulation success was established, KLF4 knockout was induced as described above.
Wound healing mouse models and Mexicanin I treatment
The full-thickness wound healing model was created as previously described (Li et al., 2012). Before the placement of the wound, the mice were anesthesia by IP injection of ketamine (100mg/kg) and xylazine (12.5mg/kg). The time when tissue was removed was considered day 0 and the healing wounds were photographed on days 1, 3, 5, 7 and 9. The edges of the wound were circled and quantified by Image J and expressed as a fraction of initial area. 10 mice each in KLF4+/+ and KLF4−/− groups were used to measure the relative wound areas at different time points.
The mouse PU model was created as reported (Stadler et al., 2004). Mexicanin I treatment consisted of an intra-peritoneal injection every other day at a concentration of 0.38mg/kg mice (Jodynis-Liebert et al., 2000). The injection was started one day before the first I/R cycle until the end of third I/R when the mice were sacrificed.
Fibrocytes generation
The splenocytes of Babl/c WT mice were purified and subjected to fibrocyte generation using a developed approach with the application of IL-13 (50 ng/ml) and M-CSF (25 ng/ml) (Crawford et al., 2010). Three days later, the cells were stained with a Hema 3 staining kit (Fisher) and the numbers of fibrocytes were counted. 50nM Mexicanin I was used in the experimental group and DMSO (Sigma-Aldrich) used in the control group.
Immunohistochemistry (IHC) and immunofluorescence (IF) staining
IHC and IF were performed using standard protocols with sections from the skin wounds. The following antibodies were used: rabbit anti-mouse KLF4 antibody (1:100), rabbit anti-mouse FSP-1 antibody (1:100) and rabbit anti-mouse α-SMA antibody (1:100, both from Abcam, ) and rabbit anti-mouse Collagen Type I alpha 1 antibody (COL1A1, 1:200, Rockland).
Western blotting
Protein from skin tissues and cultured cells were extracted for Western blotting as described (Ai et al., 2007). Primary antibodies were rabbit anti-mouse KLF4 (1:1000, GenSpin,), rabbit anti-mouse CXCR4 (1:1000, eBioscience) and rat anti-mouse β-actin (1:1000, Sigma-Aldrich).
RNA extraction and real-time PCR analysis
Total RNA was prepared using Trizol Reagent (Invitrogen,) according to the manufacturer's instructions. First-strand cDNA synthesis and real-time PCR were carried out as described (Li et al., 2012). Primer sequences for real-time PCR are listed in Supplementary Table S1.
Flow cytometry
Spleen and bone marrow were prepared as described previously (Yu et al., 2013). The peripheral blood monocytes (PBMCs) were isolated using Histoplaque (Sigma). Wound sites were minced and digested with 1.0 mg/ml collagenase (Sigma) to generate a single cell suspension and purified using a Percoll gradient (Sigma). These dissociated single cells were stained with fluorochrome-conjugated antibodies specific for mouse CD11b, Ly6G, CD45 (eBioscience) and CCR2 (R&D). For COL1A1 intracellular staining, cells were harvested, stained with surface markers, fixed with a fixation solution (eBioscience) for 15 min, permeabilized with ice-cold pure methanol for 30 min and incubated with biotin-conjugated mouse COL1A1 antibody (Rockland) and fluorochrome-conjugated streptavidin (eBioscience). Flow cytometry was performed using FACS Aria II (BD) and data analyzed with Flowjo.
Quantification and statistical analysis
KLF4 positive brown cells in suprabasal layers of each arbitrarily defined high magnified field (HMF) were counted by Image J. Three different HMFs were counted for each animal. Each group consisted of five mice. Epidermal thickness, numbers of hair follicles, and infiltrated lymphocytes were similarly quantified. Data was represented as mean ± SEM. Data were analyzed using the following tests, t test (two-group comparison), one-way ANOVA (multi-group comparison). A p-value < 0.05 was considered to indicate statistical significance.
ACKNOWLEDGMENTS
This work was supported by NIH grant (R03AR060987) and ASPIRE-I from University of South Carolina to WA. We thank Dr. Udai P. Singh and Mr. Kyle McCloskey for technical assistance and Dr. Joseph S. Janicki for his editing of the manuscript.
Abbreviations
- MDSC
myeloid-derived suppressor cell
- PU
pressure ulcer
- BM
bone marrow
- WT
wild type
- KLF4
Kruppel like factor 4
Footnotes
CONFLICT OF INTEREST
The authors state no potential conflicts of interests.
REFERENCES
- Ai W, Zheng H, Yang X, et al. Tip60 functions as a potential corepressor of KLF4 in regulation of HDC promoter activity. Nucleic Acids Res. 2007;35:6137–49. doi: 10.1093/nar/gkm656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Georgantas RW, 3rd, Hildreth RL, et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–52. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Djuretic I, Tanasa B, et al. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Black JM, Edsberg LE, Baharestani MM, et al. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manage. 2011;57:24–37. [PubMed] [Google Scholar]
- Bujnicki T, Wilczek C, Schomburg C, et al. Inhibition of Myb-dependent gene expression by the sesquiterpene lactone mexicanin-I. Leukemia. 2012;26:615–22. doi: 10.1038/leu.2011.275. [DOI] [PubMed] [Google Scholar]
- Cabezon T, Celis JE, Skibshoj I, et al. Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int J Cancer. 2007;121:1433–44. doi: 10.1002/ijc.22850. [DOI] [PubMed] [Google Scholar]
- Chuluyan HE, Schall TJ, Yoshimura T, et al. IL-1 activation of endothelium supports VLA-4 (CD49d/CD29)-mediated monocyte transendothelial migration to C5a, MIP-1 alpha, RANTES, and PAF but inhibits migration to MCP-1: a regulatory role for endothelium-derived MCP-1. J Leukoc Biol. 1995;58:71–9. doi: 10.1002/jlb.58.1.71. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Pilling D, Gomer RH. Improved serum-free culture conditions for spleen-derived murine fibrocytes. J Immunol Methods. 2010;363:9–20. doi: 10.1016/j.jim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Fan YY, Guan DW, Wang T, et al. [Time-dependent recruitment and differentiation of fibrocytes in mouse skin wound healing]. Fa Yi Xue Za Zhi. 2011;27:246–9. [PubMed] [Google Scholar]
- Feinberg MW, Wara AK, Cao Z, et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. Embo J. 2007;26:4138–48. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, et al. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–90. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- Gist S, Tio-Matos I, Falzgraf S, et al. Wound care in the geriatric client. Clin Interv Aging. 2009;4:269–87. doi: 10.2147/cia.s4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile LA, Gamrekelashvili J, Manns MP, et al. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodynis-Liebert J, Murias M, Bloszyk E. Effect of sesquiterpene lactones on antioxidant enzymes and some drug-metabolizing enzymes in rat liver and kidney. Planta Med. 2000;66:199–205. doi: 10.1055/s-2000-8566. [DOI] [PubMed] [Google Scholar]
- Kao HK, Chen B, Murphy GF, et al. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg. 2011;254:1066–74. doi: 10.1097/SLA.0b013e3182251559. [DOI] [PubMed] [Google Scholar]
- Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203:93–8. doi: 10.1016/j.forsciint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng H, Wang J, et al. Expression of Kruppel-Like Factor KLF4 in Mouse Hair Follicle Stem Cells Contributes to Cutaneous Wound Healing. PLoS One. 2012;7:e39663. doi: 10.1371/journal.pone.0039663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dasgeb B, Phillips T, et al. Wound-healing perspectives. Dermatol Clin. 2005;23:181–92. doi: 10.1016/j.det.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Liao X, Sharma N, Kapadia F, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdipour E, Charnock JC, Mace KA. Hoxa3 promotes the differentiation of hematopoietic progenitor cells into proangiogenic Gr-1+CD11b+ myeloid cells. Blood. 2011;117:815–26. doi: 10.1182/blood-2009-12-259549. [DOI] [PubMed] [Google Scholar]
- Manjili MH. Phenotypic plasticity of MDSC in cancers. Immunol Invest. 2012;41:711–21. doi: 10.3109/08820139.2012.673670. [DOI] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey MA, et al. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Neiman PE, Grbic JJ, Polony TS, et al. Functional genomic analysis reveals distinct neoplastic phenotypes associated with c-myb mutation in the bursa of Fabricius. Oncogene. 2003;22:1073–86. doi: 10.1038/sj.onc.1206070. [DOI] [PubMed] [Google Scholar]
- Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106:17892–7. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatresooz P, Hermanns-Le T, Pierard GE, et al. Ustekinumab in psoriasis immunopathology with emphasis on the Th17-IL23 axis: a primer. J Biomed Biotechnol. 2012;2012:147413. doi: 10.1155/2012/147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–35. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 2010;67:2685–93. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–66. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ou L, Han S, et al. Deficiency of Kruppel-like factor KLF4 in myeloid-derived suppressor cells inhibits tumor pulmonary metastasis in mice accompanied by decreased fibrocytes. Oncogenesis. 2014;3:e129. doi: 10.1038/oncsis.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–17. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih B, Garside E, McGrouther DA, et al. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139–53. doi: 10.1111/j.1524-475X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- Stadler I, Zhang RY, Oskoui P, et al. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J Invest Surg. 2004;17:221–7. doi: 10.1080/08941930490472046. [DOI] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti DC, Feldman SR. Interleukin-12, interleukin-23, and psoriasis: current prospects. J Am Acad Dermatol. 2007;57:1059–68. doi: 10.1016/j.jaad.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Yet SF, McA'Nulty MM, Folta SC, et al. Human EZF, a Kruppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J Biol Chem. 1998;273:1026–31. doi: 10.1074/jbc.273.2.1026. [DOI] [PubMed] [Google Scholar]
- Yu F, Shi Y, Wang J, et al. Deficiency of Kruppel-like factor KLF4 in mammary tumor cells inhibits tumor growth and pulmonary metastasis and is accompanied by compromised recruitment of myeloid-derived suppressor cells. Int J Cancer. 2013;133:2872–83. doi: 10.1002/ijc.28302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sarkar K, Rey S, et al. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med (Berl) 2011;89:985–95. doi: 10.1007/s00109-011-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]