Abstract
Chronic hyperglycemia is an important risk factor involved in the onset and progression of diabetic retinopathy (DR). Among other effectors, aldose reductase (AR) has been linked to the pathogenesis of this degenerative disease. The purpose of this study was to investigate whether the novel AR inhibitor, beta-glucogallin (BGG), can offer protection against various hyperglycemia-induced abnormalities in human adult retinal pigment epithelia (ARPE-19) cells. AR is an enzyme that contributes to cellular stress by production of reactive oxygen species (ROS) under high glucose conditions. A marked decrease in cell viability (from 100% to 78%) following long-term exposure (4 days) of RPE cells to high glucose (HG) was largely prevented by siRNA-mediated knockdown of AR gene expression (from 79% to 97%) or inhibition using sorbinil (from 66% to 86%). In HG, BGG decreased sorbitol accumulation (44%), ROS production (27%) as well as ER stress (22%). Additionally, we demonstrated that BGG prevented loss of mitochondrial membrane potential (MMP) under HG exposure. We also showed that AR inhibitor pretreatment reduced retinal microglia-induced apoptosis in APRE-19 cells. These results suggest that BGG may be useful as a therapeutic agent against retinal degeneration in the diabetic eye by preventing RPE cell death.
Keywords: Aldose reductase, High glucose, ER stress, Retinal pigment epithelium, Mitochondria, Retinal microglia
1. Introduction
In 2010, an estimated 227 to 285 million people in the world had diabetes mellitus and its associated chronic hyperglycemia and secondary complications [1]. Hyperglycemia or high blood glucose (126 mg/dl when fasting or 200 mg/dl 2 hours after meals) may cause damage to peripheral nerves as well as to cells of the renal and cardiovascular systems [2–4]. Diabetes is associated with increased risk for glaucoma, cataract, and retinopathy and is considered the leading cause of blindness among adults [5]. Long-term diabetes leads to retinal edema [6–8]. and increased apoptosis in a range of retinal cells including pigment epithelial cells, endothelial cells and pericytes [9–11]. Among the diabetic stressors, reactive oxygen species (ROS), endoplasmic reticulum (ER) stress as well as mitochondrial fragmentation are thought as the important causes of apoptosis [12–16]. Trudeau and colleagues have hypothesized that high glucose (HG) triggers mitochondrial morphological change and cytochrome c release in retinal endothelial cells (RECs) as well as retinal pericytes, further potentiating cell apoptosis [10, 11, 17], even leading to DR [18]. Extensive investigation on the role of ER stress in hyperglycemia has been shown that high glucose is linked to the unfolded protein response (UPR), production of ROS, and increased apoptosis [14].
Aldose reductase (AR), a member of aldo-keto reductase super family, catalyzes the conversion of glucose to sorbitol as the first step in the polyol pathway [19]. In a variety of diabetic target tissues, AR is linked to pro-inflammatory responses [20, 21]. In the eye, AR is linked to diabetic complication such as cataract and posterior capsule opacification (PCO) [22, 23]. Additionally, experimental studies demonstrated that genetic or pharmacological blockade of AR prevents the onset and progression of many of the retinal sequellae of diabetes including pericyte loss [24], capillary degeneration [21] and increased markers of oxidative stress [25]. As byproducts of long-term hyperglycemia, advanced glycation endproducts (AGEs) accumulate in the diabetic retina and induce expression of inflammatory molecules in a variety of cell populations including retinal microglia (RMG) [26]. Our previous studies showed that blockade of AR by AR inhibitors (ARIs) or genetic ablation alleviated ocular inflammatory responses such as cytokines secretion and ROS production as well as cell migration [27, 28]. Therefore, AR may be involved in AGE-induced ocular inflammation in diabetic patients. Additionally, HG triggers up-regulation of AR in human retinal pigment epithelial (hRPE) cells and peripheral blood mononuclear cells (PBMCs) [29, 30], which may enhance glucose metabolism through the polyol pathway and increased production of ROS.
β-glucogallin (BGG), a novel ARI isolated from Indian gooseberry (Emblica officinalis) [31], inhibits AR activity in cells and ocular tissues [27, 32]. Lens culture in HG condition demonstrated the inhibitory activity of BGG as evidenced by decreased accumulation of sorbitol [31], an inducer of osmotic stress associated with diabetic cataract development [33]. BGG has low cytotoxicity and is capable of reducing ROS production and mitogen-activated protein kinase (MAPK) activation triggered by endotoxin [27]. However, the effect of BGG on preventing of hyperglycemia-induced stresses in ocular cells is still unknown. To determine whether BGG holds promise as an effective agent in prevention of hyperglycemic defects, we conducted studies using BGG in RPE with HG conditions. We explored the efficacy of BGG in alleviation of HG-induced cell growth inhibition, ROS production and ER responses as well as mitochondrial damage in adult retinal pigment epithelial (ARPE-19) cells. We further probed the ability of ARI treatment in activated RMG to prevent apoptosis of ARPE-19 in a co-culture model. Our results demonstrate the role of AR in hyperglycemia and suggest that BGG is a potent natural agent to prevent some of the ocular complications of diabetes.
2. Materials and Methods
2.1. Materials and cell culture
BGG was obtained as previously reported [32]. Sorbinil was generously provided by Pfizer Central Research (Groton, CT, USA). Adult retinal pigment epithelium (ARPE-19) cells were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Corning Cellgro, Manassas, VA, USA) containing low glucose (1g/L) and supplemented with 4 mM L-glutamine, 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified incubator containing 5% carbon dioxide at 37 °C.
2.2. Cell viability assay
The effect of high glucose (30 mM) on cell viability was determined by a colorimetric assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) as described [34]. Briefly, ARPE-19 cells (105) were seeded into individual wells of a 24-well tissue culture plate and were incubated overnight at 37°C, 5% CO2. Cells were then treated with BGG under low or high glucose for either 2 or 4 days. After treatment with indicated time, 50 μl MTT (5 mg/ml in PBS) was combined with 450 μl of culture medium from each well and incubated for 4 h. Levels of MTT formazan reaction product were determined by measuring the absorbance at 570 nm by using BioTek Synergy™ 4 Hybrid Microplate Reader.
2.3. Small interfering RNA (siRNA) transfection
Control siRNA and AKR1B1 (AR) siRNA were purchased from Qiagen (Valencia, CA, USA). Transient transfection of siRNA was performed using HiPerFect transfection reagent (Qiagen) according to the manufacturer’s protocol. HLE B3 cells (106 cells) were seeded in a 100 mm culture dish. After 16 h cells were ~ 70% confluent and cells were transfected with control or AR siRNA (10 nM) and cultured for an additional 72 h. Efficiency of AR knockdown was confirmed by Western blot.
2.4. Western blotting
Lysates were prepared by suspending cells in Laemmli sample buffer (Sigma-Aldrich) and heated to 100 °C for 10 min, and resolved by SDS-PAGE (Bio-Rad, Hercules, CA, USA). Proteins were transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and primary antibodies were used for immunodetection: rabbit anti-AR (1:1000) [28] or mouse anti-actin (1:4000, Sigma-Aldrich) or rabbit anti-p-JNK, JNK, p-ERK, ERK, p-p38, p38 and Bip/GRP78 (1:1000, Cell signaling Technology, Inc., Danvers, MA, USA). Secondary anti-mouse and anti-rabbit antibodies conjugated to horseradish peroxidase (1:5000, Millipore, Bedford, MA, USA), as well as the Western Blot Substrate kit (Bio-Rad) were used to detect chemiluminescence using a BioRad ChemiDoc™ XRS+ imaging system.
2.5. Sorbitol colorimetric assay
ARPE-19 Cells (106 cells) were incubated in 100 mm dish. After treatment with BGG and low or high glucose, cells were collected and washed with cold PBS twice. The cell lysates were followed by deproteinization with Deproteinizing Sample Preparation Kit (BioVision, Milpitas, CA, USA). Sorbitol detection with neutralized samples was performing using a D-Sorbitol Colorimetric Assay Kit and protocol (BioVision).
2.6. Detection of ROS levels
ARPE-19 cells (5 × 103 cells) were incubated in a 96-well plate and treated with BGG and low or high glucose followed by incubation with ROS-sensitive dye fluorophore 2′, 7′-dichlorofluorescencein diacetate (Sigma-Aldrich) for 30 min. The fluorescence was measured with a BioTek Synergy™ 4 Hybrid Microplate Reader at excitation of 485 nm and emission of 528 nm.
2.7. Detection of mitochondrial membrane potential
This method was followed as previous described [34]. To determine MMP, the detached ARPE-19 cells were stained with 100 nM MitoTracker Red CMXRos (Invitrogen, Carlsbad, CA, USA) in DMEM medium for 20 min at 37°C in the dark. After typsinization, the stained cells were analyzed by fluorescence-activated cell sorting (FACS). All data was evaluated using Cell Quest software (BD Biosciences, San Jose, CA, USA).
2.8. Apoptosis of ARPE-19 cells
Primary mouse RMG were obtained as previous described [28]. RMG were first activated by LPS (Sigma-Aldrich) exposure for 6 h. Activated RMG were seeded in the upper chamber of a transwell device and ARPE-19 cells were seeded in the bottom chamber. After co-culture for 2 days, apoptosis of RPE cells was measured using an Annexin-V-FITC Apoptosis Detection kit (BD Biosciences) as described [34]. Apoptotic cells were analyzed by FACS. All data was evaluated using Cell Quest software (BD Biosciences).
2.9. Statistical analysis
Results are shown as the Means ± SEM of at least three experiments. Data were analyzed by Student’s t test with P value of <0.05 considered significant.
3. Results
3.1. Aldose reductase ablation protects the viability and decreases sorbitol accumulation in RPE cells under HG conditions
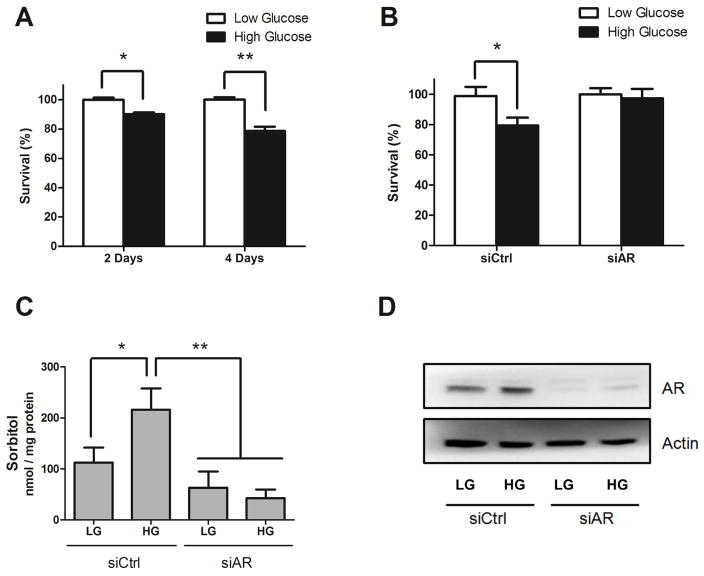
ROS, a by-product of the polyol pathway, are a robust inducer of apoptosis [35]. Previous studies showed that an increase of ROS production under HG condition caused apoptosis in retinal cells [10, 13]. Therefore, we first investigated a role for AR in HG-induced cell death by downregulating expression of the AR gene. Growth of ARPE-19 cells under HG conditions results in 10% and 22% fewer cells as compared with low glucose condition when measured at 2 and 4 days, respectively (Fig. 1A). Genetic ablation of AR using siRNA essentially prevented cell loss observed under HG growth conditions (Fig. 1B). Increased sorbitol accumulation, mediated in large part by AR in the polyol pathway, has been reported to correlate with increased levels of cell death [36]. HG led to a significant increase of sorbitol accumulation and AR ablation prevented it (Fig. 1C). We further confirmed downregulation of AR protein level in cells by using Western blot (Fig. 1D). We also found that AR was slightly induced by HG exposure which is consistent with previous study [29].
Fig. 1. AR knockdown prevents cell growth inhibition under HG exposure.
ARPE-19 cells were cultured under low glucose (LG, 5.5 mM) or high glucose (HG, 30 mM) conditions for 2 to 4 days (A). Cells were further transfected with control (siCtrl) or AKR1B1 siRNA (siAR) for 72 h and followed by low or high glucose exposure for 4 days for cell viability (B) or for 2 days for sorbitol measurements (C). Cell viability was determined by MTT assay, while sorbitol level in cell lysates was measured by using a sorbitol colorimetric assay. The efficiency of AR knockdown was probed confirmed by Western blot using an AR antibody (D). Data shown are means ± SEM (N = 3). *P < 0.05; **P < 0.01.
3.2. AR inhibition prevents HG-induced cell death, sorbitol accumulation, ROS production, and ER response
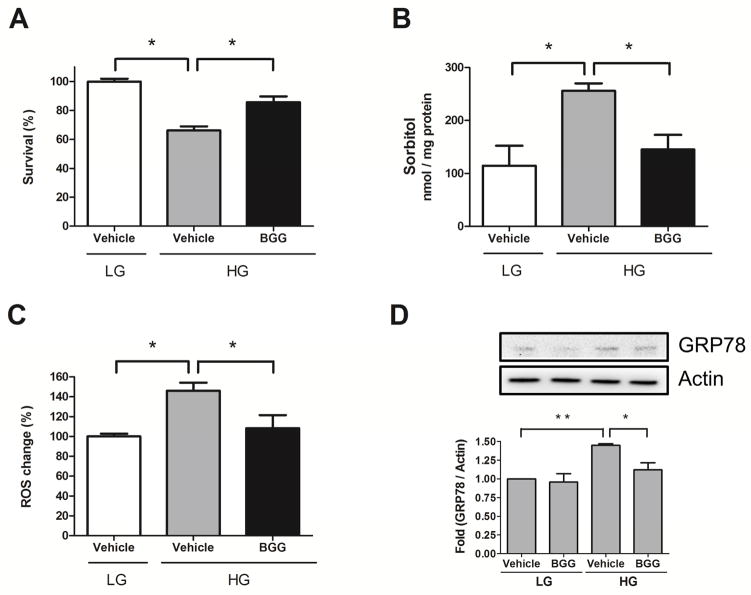
As a complement to our genetic studies, we used a novel AR inhibitor to probe the potential role for AR in HG-induced imbalances in RPE cells. BGG (Fig. 2) is a novel ARI isolated from Indian gooseberry fruits (Emblica officinalis), which we previously demonstrated to have ARI activity in vitro and in a lens culture system [27, 32]. BGG significantly prevented cell loss (Fig. 3A), reduced sorbitol accumulation (Fig. 3B), and attenuated ROS production (Fig. 3C) in ARPE-19 cells cultured under HG conditions. GRP78, an indicator for ER stress [37], increased 1.5-fold in ARPE-19 cultured in HG, whereas it increased only 1.1-fold when BGG was included (Fig. 3D). Taken together, these results demonstrate that BGG was effective in suppressing HG-induced stresses in ARPE-19 cells.
Fig. 2.

Schematic structure of β-glucogallin (BGG).
Fig. 3. BGG rescues HG-induced cell growth inhibition by attenuating sorbitol accumulation, ROS production and ER stress.
ARPE-19 cells were treated with vehicle or BGG (50 μM) followed by low glucose (LG, 5.5 mM) or high glucose (HG, 30 mM) exposure for 4 days for cell viability (A) or for 2 days for sorbitol measurements (B) and GRP78 detection (D) or for 1 day for ROS production (C). The fold activation of GRP78 was normalized to actin. Data shown are means ± SEM (N = 3). *P < 0.05; **P < 0.01.
3.3. AR inhibition prevents HG-induced loss of mitochondrial membrane potential
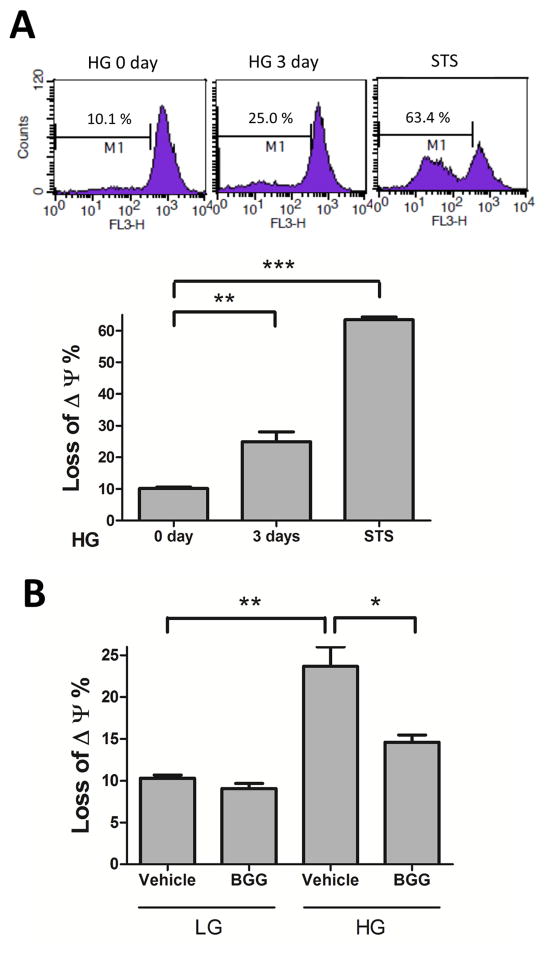
ROS production results in a loss of mitochondrial membrane potential (MMP) [38], which leads to cell death [39]. Incubation in HG for 3 days resulted in an increase in the loss of MMP (Fig. 4A). For comparison, incubation with staurosporine as a positive control resulted in 63% loss of MMP (Fig. 4A). We further investigated the effect of AR inhibition on loss of MMP under HG exposure for 3 days. In low glucose there was no appreciable difference in MMP between vehicle and BGG groups. However, HG caused a significant loss of MMP (24%) which was substantially prevented by BGG (Fig. 4B). Thus, BGG plays a protective role against HG-induced mitochondrial dysfunction.
Fig. 4. BGG prevents HG-induced loss of MMP in ARPE-19 cells.
The MMP of ARPE-19 cells, stained with Mito Tracker (Red CMXRos), represents the percentage of damage in mitochondria. ARPE-19 cells were treated with high glucose (HG, 30 mM) for 3 days or treated with staurosporine (STS, 1μM) for 1 day (A). ARPE-19 cells were treated with vehicle or BGG (50 μM) followed by low glucose (LG, 5.5 mM) or high glucose (HG, 30 mM) exposure for 3 days for MMP assay (B). After treatment, the MMP of cells was measured by FACS. We gated M1 for calculation of intensity shift. Δψ represents the membrane potential. Data shown are means ± SEM (N = 3). *P < 0.05; **P < 0.01; ***P < 0.005.
3.4. AR inhibition attenuates apoptosis of ARPE-19 caused by activated retinal microglia
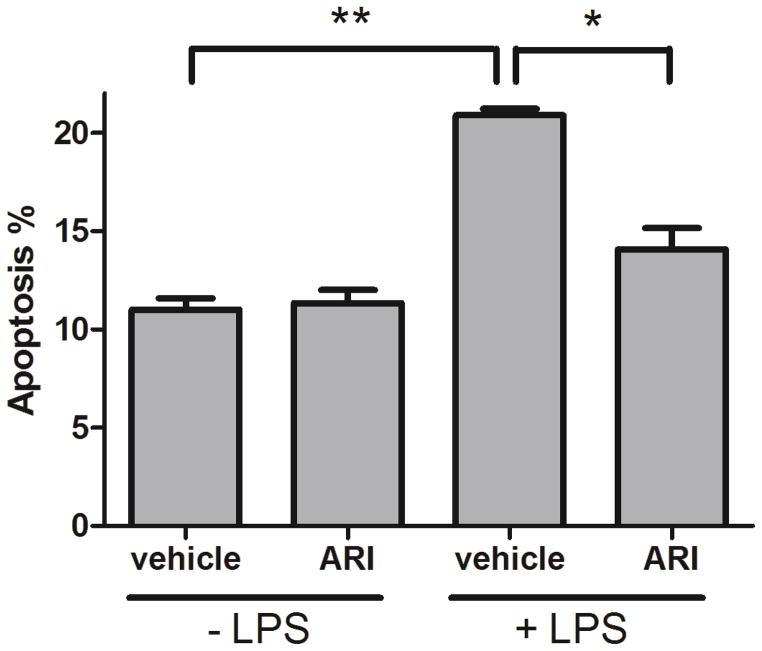
Cytokines released from activated RMG can induce photoreceptor degeneration [40]. Our previous study showed that AR inhibition reduces TNF-α secretion from RMG [28]. In the current study, we used a co-culture system to determine if inhibition of AR in activated RMG results in protection of RPE against apoptosis. In the absence of LPS-induced RMG activation, treatment of RMG with sorbinil as an ARI had virtually no effect on apoptosis of co-cultured RPE cells (Fig. 5). However, endotoxin-induced activation of RMG caused an approximate 100% increase in apoptosis of RPE in the co-culture system. In contrast, treatment of RMG with Sorbinil prior to exposure to endotoxin resulted in almost complete protection of RPE cells against apoptosis, indicating that AR plays a role in the secretion of paracrine factors from activated RMG (Fig. 5).
Fig. 5. Effect of ARI on activated RMG-induced apoptosis.
RMG were incubated with vehicle (DMSO, 0.1 % v/v) or ARI (Sorbinil, 10 μM) in the absence or presence of LPS (100 ng/ml) for 6 h and then transferred for co-culture with ARPE-19 cells for 2 days. Apoptosis was measured by FACS with Annexin-V-FITC kit. Data shown are means ± SEM (N = 3). *P < 0.05; **P < 0.01.
4. Discussion
RPE is an important densely pigmented layer in retina that fulfills many critical functions in the visual cycle, including isomerization of retinoids, phagocytosis of photoreceptor outer segments, and various metabolic and neurotrophic support functions [41]. Many studies have shown that RPE cells play a crucial role in the pathogenesis of DR [12, 42, 43]. Hyperglycemia is one of the primary factors in the development of DR causing retinal injury [44, 45]. HG induces ROS production as well as p38 and extracellular signal-regulated kinase (ERK) activation in RPE cells which cause apoptosis [12, 16]. In hyperglycemic conditions, mitochondrial damage and ER stress are major factors that trigger apoptosis leading to death of a several different cell types in the retina including pericytes, vascular endothelial cells, and cells of the pigmented epithelium [10, 11, 14, 15]. ROS is thought to be involved in mitochondrial damage and ER stress [15, 38, 46]. Therefore, prevention of ROS production might be a way to alleviate HG-induced effects.
AR inhibition was shown to reduce oxidative stress and cell death under HG conditions [47, 48]. Despite the failure of several different ARIs in clinical trials against diabetic complications, research on development of newer generations of inhibitors is still being pursued [49]. In the present study, we conducted experiments using a natural ARI, BGG, which was purified from Indian gooseberry [31]. We investigated the BGG activity for its role in protecting RPE cells from HG-induced damage. Our previous studies demonstrated that BGG, which has low cytotoxicity, is effective at preventing endotoxin-induced inflammatory responses including p38 and ERK activation, cytokines secretion, cell migration as well as ROS production [27, 28]. Here, we further demonstrated the protective roles of BGG in HG-induced stresses such as reductions of ROS production, ER stress and loss of MMP. In addition, sorbitol accumulation can lead to osmotic stress and cell death [36]. In our study, we observed that pharmacological inhibition (BGG) or genetic ablation (siRNA) of AR reduces sorbitol accumulation under HG conditions, consistent with a potential therapeutic role for BGG against HG-induced cell death.
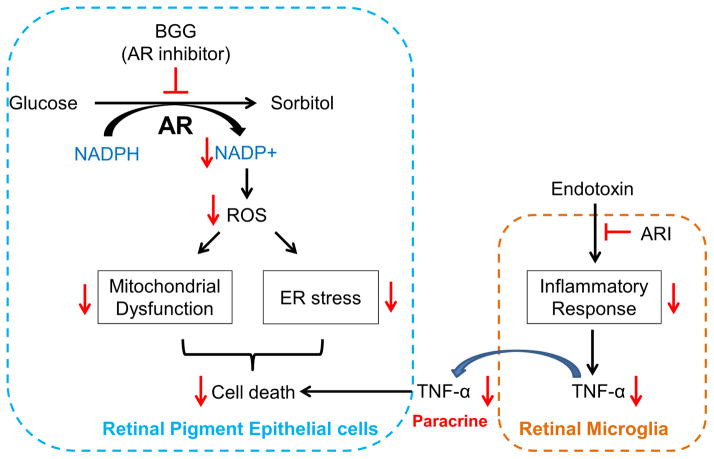
RMG have functional similarities to macrophages that are involved with tissue surveillance. Increased number of activated RMG have been observed in human eyes with DR [50] and in various experimental animal models of diabetic retinopathy [51, 52]. TNF-α is a cytokine that can cause apoptosis. Activated RMG contribute to increased levels of TNF-α in retinas of diabetic mice [53]. Our previous study showed that downregulation of AR decreases TNF-α secretion in RMG [28]. In different cell type, Ramana and colleagues have demonstrated that AR inhibition prevents HG-induced TNF-α secretion [54]. In the current study, we tested the hypothesis that AR inhibition can attenuate the apoptosis of ARPE-19 cells induced by activated RMG. Pretreatment of RMG with ARIs resulted in lower levels of apoptosis in ARPE-19 cells in our co-culture system. These results suggest that AR inhibition could play a protective role in the pathogenesis of DR though decreased release of TNF-α. A scheme illustrating the impact of AR inhibition in RPE and RMG under high glucose conditions is given in Figure 5. Metabolism of glucose by AR results in NADP+ production, which further induces ROS production, ER stress, mitochondrial damage, and ultimately cell death. AR inhibition rescues cell death by attenuating glucose metabolism through the AR polyol pathway and its downstream effects. We hypothesize that AR inhibition in RMG suppresses TNF-α production and thereby prevents apoptosis in RPE cells in the diabetic retina. Taken together, AR inhibition might be a potential therapeutic strategy against both hyperglycemia-induced cell death as well as inflammatory responses in the retina (Fig. 6).
Fig. 6. Flow chart of ARIs effects on AR polyol pathway and anti-apoptotic response.
Metabolism of excess glucose through the AR polyol pathway induces ROS production, leading to ER stress, mitochondria damage, and RPE cell death. Inhibition of AR by BGG protects against these downstream effects. Furthermore, blockade of AR in RMG reduces the production of TNF-α, which could otherwise induce apoptosis of RPE through a paracrine mechanism.
The Indian gooseberry (Emblica officinalis) is used in the practice of Indian traditional medicine to alleviate diabetic complications [55]. Our previous study elucidated BGG as an active component for AR inhibition in gooseberry [31]. Although BGG has been efficacious in cell model experiments, its potential role in animal model experiments is still unclear. In light of the low cytotoxicity and strong efficacy against experimental uveitis in mice [27], BGG may be an attractive therapeutic against the various stress associated pathways that are thought to play a role in the pathogenesis of diabetic eye disease [56]. Encouraging results from cell culture experiments reported in this paper provide a strong justification for moving forward with evaluation of BGG in a diabetic mouse model as the next step in the development of this naturally occurring compound for human therapy.
Supplementary Material
Acknowledgments
This study is supported by NIH grants EY005856 (JMP) and EY021498 (JMP, DVL).
Abbreviations
- AGEs
advanced glycation endproducts
- AR
aldose reductase
- ARI
aldose reductase inhibitor
- BGG
beta-glucogallin
- DR
diabetic retinopathy
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- HG
high glucose
- MAPK
mitogen-activated protein kinase
- MMP
mitochondrial membrane potential
- PCO
posterior capsule opacification
- PBMCs
peripheral blood mononuclear cells
- RECs
retinal endothelial cells
- RMG
retinal microglia
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- UPR
unfolded protein response
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Abdo S, Shi Y, Otoukesh A, Ghosh A, Lo CS, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase Overexpression Prevents Nuclear Factor Erythroid 2-Related Factor 2 Stimulation of Renal Angiotensinogen Gene Expression, Hypertension and Kidney Injury in Diabetic Mice. Diabetes. 2014 doi: 10.2337/db13-1830. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang JY, Li LT, Wang H, Liu SS, Lu YM, Liao MH, Tao RR, Hong LJ, Fukunaga K, Chen Z, Wilcox CS, Lai EY, Han F. In Vivo Two-photon Fluorescence Microscopy Reveals Disturbed Cerebral Capillary Blood Flow and Increased Susceptibility to Ischemic Insults in Diabetic Mice. CNS Neurosci Ther. 2014 doi: 10.1111/cns.12268. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59:484–489. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarr JM, Kaul K, Wolanska K, Kohner EM, Chibber R. Retinopathy in diabetes. Advances in experimental medicine and biology. 2012;771:88–106. doi: 10.1007/978-1-4614-5441-0_10. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Tocino H, Alvarez-Vidal A, Maldonado MJ, Moreno-Montanes J, Garcia-Layana A. Retinal thickness study with optical coherence tomography in patients with diabetes. Investigative ophthalmology & visual science. 2002;43:1588–1594. [PubMed] [Google Scholar]
- 7.Schaudig UH, Glaefke C, Scholz F, Richard G. Optical coherence tomography for retinal thickness measurement in diabetic patients without clinically significant macular edema. Ophthalmic Surg Lasers. 2000;31:182–186. [PubMed] [Google Scholar]
- 8.Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 9.Lim SK, Park MJ, Lim JC, Kim JC, Han HJ, Kim GY, Cravatt BF, Woo CH, Ma SJ, Yoon KC, Park SH. Hyperglycemia induces apoptosis via CB1 activation through the decrease of FAAH 1 in retinal pigment epithelial cells. Journal of cellular physiology. 2012;227:569–577. doi: 10.1002/jcp.22756. [DOI] [PubMed] [Google Scholar]
- 10.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. The American journal of pathology. 2010;177:447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trudeau K, Molina AJ, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Investigative ophthalmology & visual science. 2011;52:8657–8664. doi: 10.1167/iovs.11-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Z, Feng W, Hong J, Zheng Q, Shuai J, Ge Y. p38MAPK and ERK promote nitric oxide production in cultured human retinal pigmented epithelial cells induced by high concentration glucose. Nitric Oxide. 2009;20:9–15. doi: 10.1016/j.niox.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Castilho A, Aveleira CA, Leal EC, Simoes NF, Fernandes CR, Meirinhos RI, Baptista FI, Ambrosio AF. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PloS one. 2012;7:e42428. doi: 10.1371/journal.pone.0042428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Investigative ophthalmology & visual science. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 15.Yao J, Tao ZF, Li CP, Li XM, Cao GF, Jiang Q, Yan B. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem. 2014;33:107–116. doi: 10.1159/000356654. [DOI] [PubMed] [Google Scholar]
- 16.Xie P, Fujii I, Zhao J, Shinohara M, Matsukura M. A novel polysaccharide compound derived from algae extracts protects retinal pigment epithelial cells from high glucose-induced oxidative damage in vitro. Biol Pharm Bull. 2012;35:1447–1453. doi: 10.1248/bpb.b110706. [DOI] [PubMed] [Google Scholar]
- 17.Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Investigative ophthalmology & visual science. 2012;53:6675–6681. doi: 10.1167/iovs.12-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 19.Petrash JM. All in the family: aldose reductase and closely related aldo-keto reductases. Cellular and molecular life sciences: CMLS. 2004;61:737–749. doi: 10.1007/s00018-003-3402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicology and applied pharmacology. 2013;267:16–29. doi: 10.1016/j.taap.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Du Y, Petrash JM, Sheibani N, Kern TS. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PloS one. 2013;8:e62081. doi: 10.1371/journal.pone.0062081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivelli JF, Santander VS, Peretti SO, Monesterolo NE, Nigra AD, Previtali G, Amaiden MR, Arce CA, Primo E, Lisa AT, Pie J, Casale CH. Activation of aldose reductase by interaction with tubulin and involvement of this mechanism in diabetic cataract formation. Diabetes. 2014 Epub ahead of print. [Google Scholar]
- 23.Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Investigative ophthalmology & visual science. 2009;50:752–759. doi: 10.1167/iovs.08-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 25.Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang KC, Laffin B, Ponder J, Enzsoly A, Nemeth J, Labarbera DV, Petrash JM. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chemico-biological interactions. 2013;202:283–287. doi: 10.1016/j.cbi.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KC, Ponder J, Labarbera DV, Petrash JM. Aldose reductase inhibition prevents endotoxin-induced inflammatory responses in retinal microglia. Investigative ophthalmology & visual science. 2014;55:2853–2861. doi: 10.1167/iovs.13-13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry DN, Del Monte M, Greene DA, Killen PD. Altered aldose reductase gene regulation in cultured human retinal pigment epithelial cells. The Journal of clinical investigation. 1993;92:617–623. doi: 10.1172/JCI116629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B, Hodgkinson A, Oates PJ, Millward BA, Demaine AG. High glucose induction of DNA-binding activity of the transcription factor NFkappaB in patients with diabetic nephropathy. Biochimica et biophysica acta. 2008;1782:295–302. doi: 10.1016/j.bbadis.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Puppala M, Ponder J, Suryanarayana P, Reddy GB, Petrash JM, LaBarbera DV. The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PloS one. 2012;7:e31399. doi: 10.1371/journal.pone.0031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Chang KC, Zhou Y, Shieh B, Ponder J, Abraham AD, Ali H, Snow A, Petrash JM, LaBarbera DV. Design of an amide N-glycoside derivative of beta-glucogallin: a stable, potent, and specific inhibitor of aldose reductase. Journal of medicinal chemistry. 2014;57:71–77. doi: 10.1021/jm401311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang P, Jiang Z, Teng S, Wong YC, Frohman MA, Chung SK, Chung SS. Synergism between phospholipase D2 and sorbitol accumulation in diabetic cataract formation through modulation of Na,K-ATPase activity and osmotic stress. Experimental eye research. 2006;83:939–948. doi: 10.1016/j.exer.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Chang KC, Lo CW, Fan TC, Chang MD, Shu CW, Chang CH, Chung CT, Fang SL, Chao CC, Tsai JJ, Lai YK. TNF-alpha mediates eosinophil cationic protein-induced apoptosis in BEAS-2B cells. BMC cell biology. 2010;11:6. doi: 10.1186/1471-2121-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocrine reviews. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 36.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN ophthalmology. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. The Biochemical journal. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao VK, Carlson EA, Yan SS. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochimica et biophysica acta. 2014;1842:1267–1272. doi: 10.1016/j.bbadis.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SH, Shih YL, Lee CC, Chen WL, Lin CJ, Lin YS, Wu KH, Shih CM. The role of endoplasmic reticulum in cadmium-induced mesangial cell apoptosis. Chemico-biological interactions. 2009;181:45–51. doi: 10.1016/j.cbi.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Zeng HY, Zhu XA, Zhang C, Yang LP, Wu LM, Tso MO. Identification of sequential events and factors associated with microglial activation, migration and cytotoxicity in retinal degeneration in rd mice. Investigative ophthalmology & visual science. 2005;46:2992–2999. doi: 10.1167/iovs.05-0118. [DOI] [PubMed] [Google Scholar]
- 41.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Current molecular medicine. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Experimental eye research. 2009;89:913–920. doi: 10.1016/j.exer.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. High glucose concentration leads to differential expression of tight junction proteins in human retinal pigment epithelial cells. Endocrinologia y nutricion: organo de la Sociedad Espanola de Endocrinologia y Nutricion. 2009;56:53–58. doi: 10.1016/S1575-0922(09)70552-2. [DOI] [PubMed] [Google Scholar]
- 44.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free radical biology & medicine. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Casson RJ, Chidlow G, Wood JP, Osborne NN. The effect of hyperglycemia on experimental retinal ischemia. Archives of ophthalmology. 2004;122:361–366. doi: 10.1001/archopht.122.3.361. [DOI] [PubMed] [Google Scholar]
- 46.Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. The Journal of biological chemistry. 2008;283:4252–4260. doi: 10.1074/jbc.M705951200. [DOI] [PubMed] [Google Scholar]
- 47.Fu J, Tay SS, Ling EA, Dheen ST. Aldose reductase is implicated in high glucose-induced oxidative stress in mouse embryonic neural stem cells. Journal of neurochemistry. 2007;103:1654–1665. doi: 10.1111/j.1471-4159.2007.04880.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Liu P, Tao S, Deng Y, Li X, Lan T, Zhang X, Guo F, Huang W, Chen F, Huang H, Zhou SF. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Archives of biochemistry and biophysics. 2008;475:128–134. doi: 10.1016/j.abb.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Experimental diabetes research. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Archives of ophthalmology. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 51.Kezic JM, Chen X, Rakoczy EP, McMenamin PG. The effects of age and Cx3cr1 deficiency on retinal microglia in the Ins2(Akita) diabetic mouse. Investigative ophthalmology & visual science. 2013;54:854–863. doi: 10.1167/iovs.12-10876. [DOI] [PubMed] [Google Scholar]
- 52.Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Investigative ophthalmology & visual science. 2000;41:1971–1980. [PubMed] [Google Scholar]
- 53.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Survey of ophthalmology. 2002;47(Suppl 2):S253–262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 54.Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK. Aldose reductase-regulated tumor necrosis factor-alpha production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology. 2007;148:4371–4384. doi: 10.1210/en.2007-0512. [DOI] [PubMed] [Google Scholar]
- 55.Akhtar MS, Ramzan A, Ali A, Ahmad M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. International journal of food sciences and nutrition. 2011;62:609–616. doi: 10.3109/09637486.2011.560565. [DOI] [PubMed] [Google Scholar]
- 56.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.