Abstract
Seasonal variation in immune function putatively maximizes survival and reproductive success. Day length (photoperiod) is the most potent signal for time of year. Animals typically organize breeding, growth, and behavior to adapt to spatial and temporal niches. Outside the tropics individuals monitor photoperiod to support adaptations favoring survival and reproductive success. Changes in day length allow anticipation of seasonal changes in temperature and food availability that are critical for reproductive success. Immune function is typically bolstered during winter, whereas reproduction and growth are favored during summer. We provide an overview of how photoperiod influences neuronal function and melatonin secretion, how melatonin acts directly and indirectly to govern seasonal changes in immune function, and the manner by which other neuroendocrine effectors such as glucocorticoids, prolactin, thyroid, and sex steroid hormones modulate seasonal variations in immune function. Potential future research avenues include commensal gut microbiota and light pollution influences on photoperiodic responses.
1. Introduction
Although most neuroendocrinologists consider Darwinian fitness in terms of reproductive success, the concept of fitness comprises both survival and reproductive functions. Production of successful offspring (i.e., production of grand offspring) is certainly the primary measure of fitness; however, outliving competitors also increases fitness because, all things being equal, individuals that survive longer have more opportunities to produce additional offspring. However, with some notable exceptions such as salmon (Oncorhynchus nerka) or brown marsupial mice (Antechinus stuartii) and laboratory animals, most extant animals do not continuously reproduce from reproductive maturity until they die. Individuals grow and develop and generally limit reproduction to specific times of year. Importantly, producing offspring can use energy necessary for other physiological priorities. Thus, adaptations have evolved to allow individuals to maintain a careful balance among reproduction and other survival priorities. Adaptations are often considered in the context of spatial niches, however, animals have also adapted to temporal niches (Nelson, 1987). Environmental conditions can change substantially across the day and across the seasons, especially in temperate or boreal habitats. In response, individuals of many species display separate adaptations to cope with the fluctuating seasonal conditions that favor investing in reproduction at one time of year, and investing more heavily in survival mechanisms at other times of year. Seasonal changes in neuroendocrine function often serve as the physiological switch in mediating many of these seasonal adaptations.
One important proxy for survival is optimal immune function. Maintaining full energetic investment in both the immune and reproductive systems is energetically costly (Demas et al., 1997; Hanssen et al., 2005; Martin et al., 2003; Speakman, 2008); indeed, for many post-pubertal small mammals and birds maximal investment in immune and reproductive functions are energetically incompatible. Energetically demanding processes associated with successful reproduction including the defense of territory, competing for access to mates, pregnancy, and lactation may preclude investment in optimal immune defenses. However, when environmental conditions reduce the possibility of successful breeding, investment in survival (i.e., immune function) may be favored. Thus, natural selection has not favored simultaneous and maximal investment in both the reproductive and immune systems, but rather appears to favor investment in these systems during specific seasonal niches (Nelson & Demas, 1997). This phenotypic plasticity likely evolved to promote survival despite significant, yet predictable, seasonal variation in temperature, weather, predation pressure, and food availability. Importantly, to orchestrate seasonal investments in competing physiological priorities, the neuroendocrine system is required to both transduce seasonal cues and coordinate the activity of target tissues. In this review, we will describe how neuroendocrine function coordinates photoperiodic changes in immune defenses.
Photoperiodism is the biological ability to measure day length. The annual cycle of day lengths (photoperiod) provides one of the most reliable environmental cues for time of year. Animals can gauge time of year with just two pieces of information: (1) the photoperiod, and (2) whether day lengths are increasing or decreasing (Goldman, 2001). Changes in pelage, sleep, body mass, adiposity, reproduction, and immune function are gated by photoperiodic information ensuring their optimization within the relevant adaptive time frame (Prendergast et al., 2009). These physiological changes require substantial time to initiate and terminate; thus, anticipatory cues encoded by the annual cycle of changing photoperiods remain an important mechanism to ensure proper timing of development of seasonal adaptations.
Because many seasonal physiological adaptations require substantial time to develop, (e.g., changes in adiposity, reproductive regression, alterations in immune function) neuroendocrine adjustments often predate other physiological changes (e.g., reduced gonadotropin pulse frequency and amplitude lead to gonadal regression) (Wingfield, 2008). Seasonal variation in environmental conditions are generally predictable, but they are neither consistent in timing nor magnitude. Thus, the environmental conditions themselves (i.e., rainfall, temperature, barometric pressure, food availability) are of little predictive value. Natural selection has favored a strategy of monitoring a relatively neutral geophysical cue, day length, to predict changes in conditions that are more directly relevant to reproduction and survival.
To maintain a sustainable energetic budget, investments in competing physiological priorities are adjusted on a seasonal basis. For most small mammals that breed during long days investments in growth, reproductive physiology and behavior appear to be favored in the long days of spring and summer when resources are abundant and optimal for rearing offspring (Nelson & Demas, 1996). In contrast, during the short days of late autumn and winter when the odds of survival for new offspring are low many species invest more heavily in immunological defenses presumably to increase the probability of surviving to the next breeding season (Nelson et al., 2010). Laboratory – based studies have mostly reported that immune defenses are increased during short winter-like day lengths when all other conditions are held constant (Bilbo et al., 2002a; Navara et al., 2007). Increased immune investments may represent an attempt to buffer individuals from the immune suppression associated with the energetic bottlenecks of the winter when increased thermoregulatory requirements coincide with reduced nutrient availability (Martin et al., 2006; Martin et al., 2008).
1.1. Immune System
The vertebrate immune system broadly comprises two categories: innate and adaptive (Parham, 2009). The innate immune system is responsible for the immediate response to pathogen infiltration and will ultimately induce inflammation. Inflammatory processes are initiated by pathogen recognition, complement activation, release of antimicrobial peptides, and recruitment of various types of effector cells, including macrophages, granulocytes, and natural killer cells. Toll-like receptors (TLRs) expressed on various cell types throughout the body mediate signaling in response to microbial products (Janeway et al., 2001). Macrophage TLR4 activation induces gene expression and release of inflammatory cytokines. Innate immune system activation is often assessed by treatment with the endotoxin, lipopolysaccharide (LPS), a component of gram-negative bacteria cell walls which acts as a ligand of TLR4. Secretion of cytokines, from infection or LPS treatment, coincident with various physiological and behavioral responses, forms a coordinated adaptive recovery reaction (Ashley & Wingfield, 2012; Hart, 1988). Mounting an innate immune response is relatively nonspecific, energetically costly (Bonneaud et al., 2003), and generally compromised during the winter (Navara et al., 2007).
The adaptive immune system is a focused response to a specific pathogen only recruited if the innate immune response fails to eliminate pathogen infiltration (Parham, 2009). An adaptive immune response requires recruitment of lymphocytes. Pathogen specificity is encoded in the variety of antigen receptors expressed by lymphocytes. T lymphocytes are responsible for specific antigen recognition to defend against both intracellular (cytotoxic T cell) and extracellular (helper T cell) infection. B-lymphocytes mediate pathogen recognition and endocytosis, as well as release of appropriate antibodies. B and T cells create ‘memory’ cells that ensure future infections with the same pathogen will elicit a faster and more powerful response from the immune system. Although more specific, an initial adaptive immune response requires several days to generate sufficient antigen-specific B and T cells, and is also energetically costly to mount and develop although there is some debate about the relative costs of the different arms of the immune system (Bonneaud et al., 2003; Hanssen et al., 2004; Klasing, 2004).
The immune system does not exist in isolation; recent studies have outlined functional bidirectional interactions among the immune, endocrine and nervous systems (Haddad et al., 2002). Peripheral cytokine release, as a signal of infection, can be relayed to the brain directly by crossing the blood brain barrier at circumventricular organs or indirectly through vagal afferents (Haddad, 2008). Cytokine signaling to the CNS can affect neuronal excitability, pituitary development, and hormone release from the anterior pituitary (Bumiller et al., 1999; Haddad et al., 2002). Likewise, central cytokine release can affect peripheral immunity through the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis activation (Woiciechowsky et al., 1999). As one of the largest circumventricular organs and the primary site of synthesis and secretion of melatonin, the hormone largely responsible for transduction of day length, the pineal gland stands at the interface among the circadian, immune, and endocrine systems. In the next sections we outline the influence of photoperiod on seasonal changes of neuroendocrine systems and overall immune function.
2. Pineal Gland, Melatonin, and Other Hormones
2.1 Transduction of Day Length Information
The pineal gland acts as the key structure responsible for conveying photoperiodic information to peripheral tissues via nocturnal secretion of the indoleamine, melatonin (N-acetyl-5-methoxytryptamine), directly into blood and cerebrospinal fluid. An endogenous signal of darkness (Tan et al., 2010), melatonin is secreted from the pineal gland during the night in both diurnal and nocturnal vertebrates (Challet, 2007). Therefore, changes in day length throughout the year coincide with changes in the duration of nightly melatonin. It communicates both circadian and photoperiodic information to peripheral tissues to regulate responses at multiple temporal levels; melatonin rhythms can be considered to serve as both a ‘clock and a calendar’ to individuals (Hazlerigg, 2012; Reiter, 1993a; Zawilska et al., 2009).
Pinealectomy blocks all photoperiod-induced phenotypic changes in most mammalian species studied thus far (Hazlerigg & Wagner, 2006). Furthermore, pinealectomized animals treated with exogenous melatonin either tonically or via timed daily injections display phenotypes similar to pineal-intact animals maintained in short photoperiods (Bartness et al., 1993; Goldman, 2001; Hiebert et al., 2006; Walton et al., 2013), suggesting that melatonin alone is sufficient to elicit a photoperiodic response. However, melatonin may not be necessary for this response (Hiebert et al., 2000; Lincoln et al., 1989; Monecke et al., 2013), and a pineal independent photoperiodic pathway may exist. Importantly, avian reproductive photoperiodism is largely independent of melatonin signaling (Bentley, 2001).
In mammals, photic information reaches the pineal gland through a polysynaptic pathway beginning with a diverse population of melanopsin expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) located in the retina (Berson, 2003; Schmidt et al., 2011a). These cells play a primary role in circadian entrainment and only a marginal role in low-acuity vision (Schmidt et al., 2011b). Their axons travel along the non-visual retinohypothalamic tract and provide glutamatergic input to cells in the suprachiasmatic nuclei (SCN) of the hypothalamus (Baver et al., 2008). Collateral axons also innervate the intergeniculate leaflet (IGL) and olivary pretectal nucleus (OPN) to encode ambient light levels, and mediate the pupillary light reflex (Chen et al., 2011b). The SCN, composed of mainly GABAergic cells within the anterior hypothalamus, act as the primary circadian pacemakers for peripheral tissues in mammals (Bass & Takahashi, 2010; Moore, 1983). SCN cells display autonomous rhythmicity in vitro (Welsh et al., 2010) and lesions of the SCN abolish circadian rhythms in physiology and behavior (Stephan & Zucker, 1972). Transplantation of donor SCN tissue into the 3rd ventricle restores these rhythms (Ralph et al., 1990). The SCN modulate ipRGC input and project to the nearby paraventricular nucleus, to the interomediolateral cell column of the cervical spinal cord, and then onto the superior cervical ganglion. From there, sympathetic post-ganglionic noradrenergic projections innervate the pineal gland to modulate melatonin secretion (Teclemariam-Mesbah et al., 1999).
2.2 Melatonin
Melatonin is widely distributed in plants, unicellular organisms, algae, bacteria, invertebrates, and vertebrates (Hardeland & Poeggeler, 2003; Iriti et al., 2010; Stehle et al., 2011). This wide distribution reflects its pleiotropic functions, acting through several signaling pathways (Celinski et al., 2011; Gomez-Moreno et al., 2010; Jung-Hynes et al., 2010; Paradies et al., 2010; Reiter, 1991; Tan et al., 2010).
Photoperiod-responsive melatonin production is primarily reserved to the pineal gland in mammals, but extra-pineal melatonin is produced in the retina, skin, gut, and immune competent cells (Chen et al., 2011a; Huang et al., 2013; Kleszczynski & Fischer, 2012; Maldonado et al., 2010). Melatonin is synthesized in a two-step process from its precursor serotonin via N-acetyltransferase (AANAT) and then hydroxyindole-O-methyltransferase (HIOMT). Its production is driven by noradrenergic input from the superior cervical ganglion, with adrenergic receptor signaling-induced cAMP formation leading to downstream expression of AANAT (Carpentieri et al., 2012; Konturek et al., 2007). In many non-mammalian vertebrates, AANAT expression in the pineal is under the direct control of circadian clock genes (Hardeland & Poeggeler, 2003).
By using a radioiodinated ligand, 2-[125I]iodomelatonin, melatonin binding sites were discovered throughout the mammalian central nervous system (Dubocovich & Markowska, 2005) and periphery (Slominski et al., 2012). Melatonin signals via multiple membrane bound G-protein linked receptors (MT1 and MT2) and a receptor independent pathway via quinone reductase II enzyme binding (previously identified as MT3). MT1 and MT2 can homo-or heterodimerize, but the role these receptor dimer species play in melatonin signaling outside the retina are unclear (Ayoub et al., 2002; Ayoub et al., 2004; Baba et al., 2013). A feedback loop mediating the mid-night peak and pre-dawn decline in melatonin secretion is thought to be governed by MT1 but not MT2 (Bedrosian et al., 2013b). More controversially, melatonin has also been shown to be synthesized directly by T lymphocytes and act as a ligand of the retinoic acid-related orphan receptor alpha (ROR-α) and other nuclear receptors ((Carrillo-Vico et al., 2004; Lardone et al., 2011; Slominski et al., 2012). Intracellularly, the melatonin signal is largely transduced to influence downstream gene transcription via Gi and Gq activation, which leads to a reduction in cAMP via adenylyl cyclase inhibition (Gi) and in some cells increases Ca2+ via phospholipase-C activation (Gq) (Carrillo-Vico et al., 2004; Lardone et al., 2011; Slominski et al., 2012). MT1 is the receptor subtype primarily responsible for the transduction of photoperiodic information by melatonin (Pelletier et al., 2000; Prendergast, 2010; Walton et al., 2011; Weaver et al., 1996; Yasuo et al., 2009), and several photoperiodic species do not express MT2 (e.g., Siberian hamsters (“nature’s knockout”) and sheep).
Immune function varies seasonally in many mammalian species, including humans, and this variation is likely coordinated and modulated by melatonin signaling (Nelson, 2004). The immunomodulatory role of melatonin was first recognized ∼four decades ago when researchers observed reduced immune capacity following pinealectomy in rats (Csaba & Barath, 1975). The direct action of melatonin on immune function was then tested and described by Maestroni and colleagues (Maestroni et al., 1986), where immunosuppression by propranolol (a beta adrenergic antagonist) and p-chlorophenylalanine (a tryptophan hydroxylase inhibitor) was reversed via melatonin treatment (Maestroni et al., 1986). Since then, MT1 and MT2 receptors have been localized to major immune tissues involved in both innate and adaptive immune responses like the thymus and spleen, and specifically to CD4+ and CD8+ lymphocytes, as well as B cells (Carrillo-Vico et al., 2005). A bidirectional pathway between the immune system and pineal gland exists, with cytokine signaling affecting pinealocytes to transiently inhibit melatonin synthesis and shift melatonin production to circulating macrophages (da Silveira Cruz-Machado et al., 2010; Fernandes et al., 2006; Markus et al., 2013; Markus & Ferreira, 2011). Macrophage derived melatonin acts in a paracrine/autocrine fashion to inhibit nuclear factor κ-light chain enhancer of activated B cells (NF-κB) action to attenuate inflammation. In this way, short-day melatonin levels may facilitate the recovery from immune challenges by buffering the innate inflammatory response. Indeed, short photoperiods attenuate fever, hypothalamic cytokine expression, and ‘sickness behavior’ induced by lipopolysaccharide (LPS) administration in male and female Siberian hamsters (Phodopus sungorus) (Pyter et al., 2005; Wen & Prendergast, 2007). The effect is not limited to a gram-negative bacterial endotoxin (LPS), and short days attenuate responses to gram-positive (muramyl dipeptide) and viral (polyinosinic polycytidylic acid) immune challenges (Baillie & Prendergast, 2008). In male Wistar rats, which do not undergo reproductive responses to changes in photoperiod, short days augment T lymphocyte numbers and attenuate behavioral and cytokine responses to LPS (Prendergast et al., 2007). Therefore, photoperiodic modulation of the immune system can be accomplished independently of seasonal changes in reproductive capacity. Importantly, short day attenuation of sickness responses and cytokine signaling in Siberian hamsters is only accomplished via chronic (but not short term) lengthening of the melatonin signal (Bilbo et al., 2002b; Bilbo & Nelson, 2002).
Broadly, melatonin is immuno-enhancing (Carrillo-Vico et al., 2005), and increased duration of nightly melatonin corresponding with reduced day length is accompanied by increased splenocyte proliferation and IgG production (Demas et al., 1996). However, the actions of melatonin on immune system function depend on the species and ‘arm’ of the immune system analyzed (Martin et al., 2008). For example, short day lengths or chronic melatonin administration via Silastic capsules increases lymphocyte proliferation in deer mice (Peromyscus leucopus), but decreases this measure and antibody production in Siberian hamsters (Bilbo & Nelson, 2002; Drazen et al., 2002; Pawlak et al., 2009; Yellon et al., 1999). Melatonin implants also act on the SCN to reduce sickness behavior in Siberian hamsters (Freeman et al., 2007). In Syrian hamsters (Mesocricetus auratus), melatonin augments humoral and cell-mediated immune responses following dexamethasone-induced stress (Vishwas et al., 2013) and short photoperiods increase IgG responses to ovalbumin in piglets (Lessard et al., 2012). Photoperiodic modulation of immune function may be accomplished not only through alterations in melatonin secretion, but sensitivity to the melatonin signal via changes in receptor expression. For example, MT1 expression in the spleen is decreased by extended light exposure in several species, and is directly related to amount of light and circulating melatonin concentrations (Lahiri et al., 2009; Maestroni, 1993; Shiu et al., 2000; Yadav & Haldar, 2013).
Not all photoperiodic modulation of immune function can be ascribed directly to the actions of melatonin. For instance, short days prevent dimethylbenzathracene (DMBA) induced tumorigenesis (which may be initially suppressed by the immune system (Pardoll, 2003) in deer mice (Peromyscus leucopus); however, treatment of long day animals with exogenous melatonin does not recapitulate the protective effect of short days on tumor growth (Nelson & Blom, 1994). This effect seems to be mediated by photoperiodic changes in prolactin concentrations (see below). Therefore, other neuroendocrine factors that vary seasonally and modulate immune function warrant additional discussion.
2.3 Sex Steroid Hormones
Increased hypothalamic-pituitary-gonadal (HPG) axis activity during puberty initiates adult reproductive physiology and behaviors. Synthesis and secretion of gonadotropin releasing hormone (GnRH) from the hypothalamic preoptic area into the portal blood supply stimulates the anterior lobe of the pituitary to secrete gonadotropins (luteinizing hormone, LH; and follicle stimulating hormone, FSH). In turn, LH and FSH drive increased synthesis and secretion of gonadal steroid hormones. These hormones are regulated by negative feedback mechanisms at the hypothalamic and hypophyseal levels (Bliss et al., 2010; Levine, 2003). In many photoperiodic species, HPG activity and reproductive behaviors will cease during the non-breeding season. Consequently, gonad size (Prendergast et al., 2003) and circulating sex steroid concentrations will vary in different photoperiods (testosterone in Siberian hamsters (Bedrosian et al., 2012) and rams (Casao et al., 2010) and temperate and boreal birds (Dawson et al., 2001); progesterone in sheep (Birch et al., 2003); estradiol in Siberian hamsters: (Salverson et al., 2008)) . In addition to their well-described roles in reproduction, sex steroids also modulate metabolism (Grossmann, 2014; Michalakis et al., 2013) and immune function (Grossman, 1984; Olsen & Kovacs, 1996).
As mentioned, pineal melatonin acts as both a ‘clock and calendar’ to communicate photoperiodic information to the immune system (Reiter, 1993b). Significant interactions among circulating sex steroids, gonadotropins, and melatonin signaling occur to modulate photoperiodic changes in immune function (e.g., (Bentley et al., 1998; Demas et al., 1996)). However, sex steroid hormones exert a largely secondary effect (compared to other humoral mediators) in response to changes in photoperiod, and the extent to which they contribute to immune changes is largely species dependent (Demas & Nelson, 1998a; Drazen et al., 2000).
Investigators initially hypothesized that short days enhance immune responses in part by reducing circulating androgens. However, this hypothesis failed to gain substantial experimental support due to the different immunological effects of estrogens and androgens. Broadly, females have more robust immune responses than males and this phenomenon has been partially attributed to the immune-enhancing functions of estrogens and the immunosuppressant effects of androgens (Casimir et al., 2013; Klein, 2012). Photoperiod-induced gonadal regression should have sex-dependent (i.e., opposite) effects on immune system function. However, several studies have now demonstrated that immunological adaptations in males and females respond similarly to photoperiod with a higher adjustment response in males (Demas & Nelson, 1998a; Weil et al., 2006; Weil et al., 2007).
An alternative hypothesis is that sex steroids alter the response of the immune system to photoperiod. Castration increases the number of lymphocytes, and attenuates weight loss and anorectic response to LPS in male Siberian hamsters housed in both long and short days (Prendergast et al., 2008). Estrogens and androgens enhance lymphocyte proliferation in both sexes housed in long days (Bilbo & Nelson, 2001). However, hormone replacement in gonadectomized deer mice of both sexes does not influence the increase in lymphocyte production in response to short days (Demas & Nelson, 1998a; Demas & Nelson, 1998b). Similarly, no differences in delayed type hypersensitivity (a measure of cell-mediated immunity) occur in castrated Siberian hamsters treated with testosterone (Prendergast et al., 2005). However, in white crowned sparrows, exogenous testosterone supplementation attenuates LPS-induced sickness behavior , whereas castration has no effect (Ashley et al., 2009).
Independent of photoperiod, sex steroids modulate the immune system. In general, females display more pronounced innate and adaptive immune responses than males, allowing for faster clearance of pathogens, but leading to higher vulnerability to inflammatory and autoimmune diseases (Klein, 2012).
Folstad and Karter (1992) highlighted the role of testosterone in increasing the immunocompetence handicap, but also underlined the lack of full understanding of this hypothesis that remains controversial. On the one hand, castration of mature male rodents decreases testosterone production and elevates immunoglobulin levels, thymic weight, and humoral and cell-mediated immune function (Alexander & Stimson, 1988). On the other hand, other studies have documented no relationship between endogenous testosterone concentrations and immune response against parasitic infection (Ganley & Rajan, 2001; Klein et al., 1999). A more recent study suggests that infection increases circulating cortisol and decreases circulating testosterone in rodents. This can be interpreted as physiological response aimed at redistribution of resources under immunological challenge (Lutermann et al., 2012).
Sex steroids exert differential effects on immune function. For example, the first response of the innate immune system is via the recruitment and mobilization of neutrophils and their over-abundance can have adverse effects on healthy tissues. Estrogens can reduce recruitment of neutrophils in humans and experimental animals (Pacifici, 2008; Sheh et al., 2011; Shih et al., 2011). Testosterone inhibits the secretion of interferon-γ (IFN-γ) from natural killer cells increasing pathogen proliferation (Lotter et al., 2013). Estrogen deficiency promotes the production of tumor necrosis factor-α (TNF-α) production from T cells (Pacifici, 2008). In addition to androgens and estrogens, progesterone plays an anti-inflammatory role by suppressing macrophage activity and enhancing T cell activity (Mao et al., 2010; Savita & Rai, 1998).
Sex steroid hormones do not directly affect the relationship between photoperiod and immune system, but play a subtle facilitating role. Apart from photoperiod, the sex steroid hormones are prominent players in the modulation of immune system.
2.4 Other Hormones
Among seasonally breeding rodents (e.g., Siberian hamsters), thyroid hormone signaling plays a central role in reorganization of reproductive physiology in response to short days (Yasuo et al., 2009). Under long day conditions, the conversion of the prohormone, thyroxin (T4), to its receptor-active metabolite triiodothyronine (T3) is accomplished via the increased expression of iodothyronine deiodinase 2 (DIO2). T3 then enhances gonadotropin-releasing hormone (GnRH) signaling to the pituitary from the median eminence of the hypothalamus to mediate reproductive physiology (Nakao et al., 2008; Ono et al., 2008; Prendergast et al., 2013). Short day exposure increases DIO3 expression, which leads to the conversion of T4 to a biologically inactive enantiomer, rT3; and T3 into T2. This effectively eliminates GnRH signaling to inhibit reproductive development (Nakao et al., 2008).
Thyroid hormones play a central role in seasonal variation in reproduction and have immunomodulatory actions (Dorshkind & Horseman, 2000). Thyroid hormone receptors are expressed in many lymphoid tissues (De Vito et al., 2011; Foster et al., 1999; Segal & Ingbar, 1982), and T3 modulates lymphoid cell development and function (Mascanfroni et al., 2008; Mascanfroni et al., 2010). Recently, day length-induced epigenetic modification of the dio3 promoter has been demonstrated to be a mechanism by which short photoperiods alter lymphoid T3-signaling to enhance adaptive immune function (Stevenson et al., 2014). Epigenetic silencing of DIO3 signaling in short day lymphocytes results in the opposite pattern observed to reproductive physiology (Nakao et al., 2008) or in response to exogenous melatonin (Prendergast et al., 2013). Therefore, a reciprocal relationship between dio3 methylation within the hypothalamus and in lymphocytes seems to facilitate seasonal plasticity in reproduction and immune function (Stevenson & Prendergast, 2013). In short days, increases in hypothalamic DIO3 results in reproductive suppression, and epigenetic silencing of dio3 in peripheral lymphocytes leads to simultaneous immune augmentation.
Glucocorticoids play a pivotal role in immune system modulation via hypothalamic-pituitary-adrenal (HPA) axis activation in response to environmental stressors. HPA-axis physiology is modulated by photoperiod as reflected in altered mineralocorticoid receptor (MR), glucocorticoid receptor (GR) gene expression, circulating glucocorticoid concentrations, negative feedback mechanisms, and behavioral response to glucocorticoid administration (Breuner & Wingfield, 2000; Pyter et al., 2007; Ronchi et al., 1998; Walton et al., 2013). MR (high affinity, low capacity) and GR (low affinity, high capacity) are present on most immune cell types, and their complementary signaling properties allows for anti-inflammatory actions in response to both phasic and tonic glucocorticoid elevations, respectively (Armanini et al., 1988; McEwen et al., 1997; Munck et al., 1984). HPA axis responsiveness varies in response to changes in photoperiod in several avian and mammalian species (Astheimer et al., 1995; Breuner & Wingfield, 2000; Reeder & Kramer, 2005; Ronchi et al., 1998; Sapolsky et al., 2000).
The HPA axis plays a prominent negative feedback role in response to immune challenge. Upon HPA activation by inflammatory cytokines (e.g., IL-1β, TNF), glucocorticoids are released and negatively regulate further cytokine production, thereby attenuating and preventing ‘runaway inflammation’. However, upon chronic HPA axis activation, sustained glucocorticoid elevation can lead to a maladaptive suppression of immune responses (McEwen et al., 1997; Sapolsky et al., 2000).
Glucocorticoids can act in a reciprocal fashion to melatonin signals to modulate T-cell mediated immune responses under physiologically stressful conditions (Gupta & Haldar, 2013). The interaction between melatonin and glucocorticoids may underlie differential photoperiodic responses to environmental stress in a tissue specific manner. Several species increase circulating glucocorticoids and alter GR expression in short days (Bilbo et al., 2002a; Pyter et al., 2005; Weil et al., 2006). Indeed, GR expression in the spleen (but not skin) varies seasonally in house sparrows (Passer domesticus), and is increased in the hippocampus of Siberian hamsters following short day exposure (Lattin et al., 2013; Walton et al., 2012). Similar seasonal variation likely exists in other species, and contributes to seasonal plasticity in immune function.
Prolactin, a protein hormone released by the anterior pituitary, has pleiotropic actions on several organ systems, (Goffin et al., 1999) and varies in response to photoperiod in many species (Goldman & Nelson, 1993). Hypophysectomized animals have impaired adaptive and innate immune functions, and either prolactin and/or growth hormone supplementation can restore these functions (Gala, 1991). The large number of functions attributed to prolactin has led to the suggestion that it be re-named “versatilin” or “omnipotin” (Bern & Nicoll, 1968; Weigent, 1996). Specifically, leukocytes express prolactin receptors, and administration of bromocriptine, a drug that blocks prolactin release, impairs immune responses (Gala, 1991). Furthermore, lymphoid cells express prolactin, in an autocrine fashion, to affect proliferation, cytokine secretion and function (Lopez-Rincon et al., 2013).
Short-days typically induce reductions in circulating prolactin concentrations (Auchtung & Dahl, 2004; Goldman & Nelson, 1993). Prolactin is unique in that it is broadly immunoenhancing and consistently increased in long day conditions, and exogenous prolactin administration can promote a long day immune phenotype (Auchtung & Dahl, 2004).
3. Role of Perinatal Photoperiods in Programming Adult Immune Function
In addition to regulating adult phenotypic plasticity, photoperiodic experiences during early life can establish a developmental trajectory with important implications for immune defenses. As noted, small mammals are typically born during the long days of spring and summer (Bronson, 1985; Goldman & Nelson, 1993). However, if these animals are born early in the spring, then they are likely to undergo rapid reproductive development and attempt to mate before the end of their first summer (Gorman, 2001). In contrast, animals born late in the summer are unlikely to have the time necessary to grow, undergo reproductive development, and breed before the onset of winter (Horton, 1984). Late season animals typically maintain a prepubertal phenotype and delay growth and reproductive development until the following spring. These rapid and significant shifts in physiological priorities are mirrored by adjustments in immune physiology (Weil et al., 2006).
The reproductive system appears to measure seasonal time by comparing the ambient photoperiod to the photoperiod that preceded it (Hoffmann et al., 1986). Siberian hamsters and other photoperiodic rodents use a system, termed ‘photoperiod memory’, wherein they compare the ambient photoperiod to one encoded prenatally (Elliott & Goldman, 1989; Stetson et al., 1986). Thus, a reduction in day length will result in gonadal regression to intermediate photoperiods. For instance, hamsters born in long days (15L), then transferred to an intermediate day lengths (13.5L), retard reproductive development (Prendergast et al., 2000). However, hamsters born in short days (12L), then transferred to the same intermediate day length (13.5L) will undergo rapid reproductive development (Prendergast et al., 2000).
The immune system of Siberian hamsters, in contrast, appears to be influenced by the absolute photoperiod, rather than the directionality of change. Hamsters born in long day lengths, then transferred to intermediate day lengths failed to enhance circulating lymphocytes, monocytes, or delayed-type hypersensitivity responses as did hamsters housed in unambiguously short day lengths (Prendergast et al., 2004). Similarly, hamsters housed perinatally in long days, then transferred into short days at weaning, markedly regressed their reproductive system, but failed to undergo the typical short day pattern of delayed-type hypersensitivity responses (Weil et al., 2006).
3.2 The Evolution of Photoperiodic Reproductive-Immune Relationships
The results of studies of perinatal and intermediate photoperiods that immunological and reproductive adjustments to day length could be dissociated have two important implications. First, hamsters can show the short day pattern of gonadal responses simultaneously with long day typical immune responses (Weil et al., 2006; Weil et al., 2007). If enhanced immune responses, in short day lengths, were a byproduct of passive shunting of energy from the reproductive system to the immune system, then this pattern of responses should not occur. Rather, it seems that the investment in immune defenses in short day lengths is an active process that is dissociable from changes in the HPG axis. Importantly, to our knowledge, there are no published examples of animals simultaneously displaying enhanced short day pattern of immune responses and large functional gonads. Whether these two conditions are physiologically impossible to maintain together remains unspecified. One possible experiment would involve housing animals in very short day lengths (6L) and then transferring them to 12L. Theoretically this manipulation should stimulate the gonads to grow but maintain a sufficiently short day length to enhance immune function (Rivkees et al., 1988).
A second, but equally important, conclusion to draw from the dissociation of immune and reproductive responses to day length is that it is possible that the immunological adjustments associated with changing day lengths evolved independently from photoperiodic reproductive regulation. Free-living animals experience day lengths that are constantly, but gradually, changing and the laboratory conditions that are necessary to dissociate the reproductive and immune responses are unlikely to occur (Prendergast et al., 2004). Therefore, in the vast majority of situations the environmental cues responsible for alterations in the reproductive and immunological systems would overlap. However, they have very different formal properties such that the reproductive system attends primarily to the directionality of change in day length where the immune system attends to the absolute day length (Bronson, 1985; Prendergast et al., 2004; Weil et al., 2006).
It seems likely that melatonin regulation of photoperiod-mediated seasonal reproductive function first evolved and that over evolutionary time the immune system evolved to “eavesdrop” on the signals controlling reproduction. An analogy can be drawn to the relationship among sex steroid hormones, gonadal activity, and mating behaviors. Presumably the HPG axis evolved to regulate gonadal function, but over evolutionary time the nervous system co-opted the endocrine signals to synchronize sexual behavior with maximal fertility (Adkins-Regan, 2005; Lange et al., 2002). In the photoperiodism--melatonin circuitry it is possible that a similar process occurred, although this remains conjecture. In any case, the overlapping, but distinct, melatonin signals to which the immune and reproductive systems respond suggest such an arrangement. Finally, although birds are much less dependent on melatonin signaling for control of reproduction the thyroid axis seems to regulate both immune and reproductive physiology and may be another example of the co-opting of one system for the control of another.
4. Future Directions
4. 1 The Gut Microbiota
Increasingly, the role of commensal microbes inhabiting the gut has been revealed to modulate metabolism, inflammation, immune defenses, as well as learning and memory and affective responses (Backhed et al., 2004; Cryan & Dinan, 2012; Round & Mazmanian, 2009). Microorganisms outnumber the number of host cells by nearly two orders of magnitude and chronically colonize mammals (Gordon, 2012; Xu & Gordon, 2003). This symbiotic relationship is critically important to the host as the microbial population can assist in extracting nutrients from otherwise indigestible dietary components (Sonnenburg et al., 2005). Additionally, the microbial population contributes to the defense against pathogenic microbes by sequestration of nutrients, production of antimicrobial compounds, physical fortification of epithelial barriers, and stimulation of host production of secretory IgA (Round & Mazmanian, 2009).
The neuroendocrine system has bidirectional interactions with the gut microbiome. The gut resident microbes can synthesize or alter the production of metabolically (and immunologically) relevant peptides including glucagon such as peptide-1, protein YY, ghrelin, leptin, and several other signaling molecules (Ellis et al., 2008). The interactions among the neuroendocrine system, photoperiodic modulation of physiology, and the gut microbiome warrant discussion here. Siberian hamsters weigh significantly less because of reduced body fat in short compared to long days (Weil et al., 2011). This response likely reflects resistance to leptin signaling in the hypothalamus in long days (Ellis et al., 2008). Leptin knockout mice display significant shifts in gut microbiome constituents (Ley et al., 2005). Because food quality and availability are reduced during the winter at high latitudes, the relative abundance of microbes of the phylum Firmicutes was predicted to increase under short day lengths to aid in extracting increased energy from plant-based diets (Bailey et al., 2010). Previous studies demonstrated that an increase in Firmicutes prevalence is associated with obesity in both humans and nonhuman animals (Ley et al., 2005; Turnbaugh et al., 2006). Bacterial tag-encoded FLX amplicon pyrosequencing of hamsters housed in short or long day lengths indicated that the composition of the Siberian hamster microbiota shifted in response to changing photoperiods. However, the relative abundance of the Firmicutes did not change; rather, hamsters housed in short day lengths reduced numbers of organisms within the phylum Proteobacteria and had fewer organisms from the genus Citrobacter without altering the total numbers of microbes or the overall microbial diversity (Bailey et al., 2010).
One potential explanation for the photoperiodic changes in gut proteobacteria may reflect seasonal variation in diet. In the spring and summer, Siberian hamsters eat a larger proportion of seeds, which are rich in both fats and proteins (Fine & Bartness, 1996). In the laboratory, hamsters increased preference for high fat diets in long day lengths (Fine & Bartness, 1996). High fat feeding increases Proteobacteria and may help in maximizing nutrient extraction from these types of foods (Hildebrandt et al., 2009). Thus, a long day increase in Proteobacteria may reflect a preparatory response to changing diets.
Additionally, it remains possible that changes in the gut microbiota bidirectionally interact with changes in immune defenses across the year; that is the changing priorities in immune defenses may make the gut differentially hospitable to different classes of microbes while the possibility also exists that changes in the gut microbiota drive alterations in immune physiology. Conversely, Citrobacter spp, which are from the Enterobacteriaceae family, are related to intestinal inflammation (Lupp et al., 2007). During experimental intestinal infection, the levels of pathogenic Campylobacter and Salmonella are positively associated with Enterobacteriaceae prevalence; these components of the microbiota provide a conducive environment for pathogenic bacteria (Stecher et al., 2007). Further, Proteobacteria are both pro-inflammatory and increase in response to inflammation suggesting that the short day hamsters limit the proliferation of these microbes in order to reduce the potential for pathological infection and inflammation during the energetically challenging days of winter (Pedron & Sansonetti, 2008).
Conversely, the gut microbiota regulates adult immune defenses in part by regulating mucosal immunity, dendritic cell activation and differentiation and expansion of regulatory T cells and in particular the TH17 population (Round & Mazmanian, 2009). Immune defenses are intimately regulated by these cell types (Martin et al., 2008; Pedron & Sansonetti, 2008). Future studies need to investigate the neuroendocrine signals responsible for changes in gut microbiota and potential immune responses, as well as assess the role of the microbiota in the previously observed photoperiodic changes in immune defenses. Finally, a study of hamsters raised in sterile conditions or otherwise depleted of gut microbiota and then inoculated with the intestinal contents of either long or short day hamsters will provide information regarding the role of these microbes in metabolic and immune changes induced by photoperiod.
4.2. Climate Change and Light Pollution
As temperatures rise worldwide and climate differs from the predictable annual patterns of weather, the relationship between photoperiod and optimal timing of food availability and reproduction are becoming mismatched. Furthermore, photoperiodic neuroendocrine modulation of the immune system evolved over a vast period of time when light was largely restricted to the day (with the exception of moon light and other natural sources at night). However, during the past ∼120 years urbanization and the development of powerful artificial lighting sources have led to pervasive light pollution. For instance, a full moon produces around 0.1–0.3 lux, an overcast night sky is illuminated only at around 0.00003–0.000001 lux whereas street lights produce 5–60 lux depending on the distance and type of light which are sufficient to block nighttime melatonin production (Rich & Longcore, 2006). In urban environments, where multiple light sources are present night time lighting levels may be even higher (Gaston et al., 2012). European blackbirds exposed to light at night at intensities typical for urban environments display significant annual advancements in breeding and molting (Dominoni et al., 2013). Further, photoperiodic bird species typically require prolonged exposure to short photoperiods to reset their annual cycle of breeding (Dawson et al., 2001). In the absence of dark nights a birds did not exhibit annual cycles of testicular development or breeding behavior. Therefore, the consistent and largely noise-free signal that was once available from attending to the daily light-dark cycle is no longer reliable for many free living animals, especially those living in close association with humans.
Immunological responses are altered by light at night. For instance, housing Japanese quail (Coturnix coturnix japonica) in constant light attenuated swelling responses to phytohemaggluttinin and reduced antibody responses to sheep red blood cells (Moore & Siopes, 2000). Nocturnal light exposure reduces natural killer cell responses in rats (Oishi et al., 2006) and reduces both basal and stress-modulated delayed type hypersensitivity responses in Siberian hamsters (Bedrosian et al., 2013a). Additionally, even dim light at night can interfere with the photoperiodic modulation of the immune system such that the generally immune enhancing effects of short days are lost, presumably by inhibiting nighttime melatonin secretion. Siberian hamsters housed in short day lengths increase delayed type hypersensitivity responses relative to long day conspecifics. However, if the dark nights of these photoperiod conditions are replaced with low levels of ambient light (dim light at night, 5-lux), then both the reproductive and immunological adjustments associated with short days are abolished (Aubrecht et al., 2014; Ikeno et al., 2014). Further, whereas acute stress prior to antigenic challenge typically enhances delayed type hypersensitivity responses in both long and short day lengths, exposure to dim light at night abolishes that stress response in hamsters (Aubrecht et al., 2014; Dhabhar & McEwen, 1997; Dhabhar & McEwen, 1999). Therefore, the consequences of nocturnal light pollution are significant for photoperiodic animals because it can prevent the acquisition of the short day phenotype and also directly suppress immune responses. The extent that light pollution is contributing to species extinction requires substantial additional research.
5. Conclusion
Photoperiodic adjustments in immune function are among the most pronounced and important adaptations to the changing seasons that have been observed in free living and laboratory studies. The work of many groups has demonstrated the overarching role of pineal melatonin and many other components of the neuroendocrine system as master regulators of physiological adjustments to the changing seasons. New components in these systems are constantly being discovered and include previously neglected organisms like the gut microbiome. In the modern era, the relationship between the neuroendocrine and immune systems is being obscured by light pollution and climate change. Our challenge now is to understand the way in which animals adapt to changing environmental conditions so that we can begin to understand the physiological and fitness consequences of these new environmental changes. The seasonal and photoperiodic adjustments in the relationship between the neuroendocrine and immune systems is a useful model system for understanding the way organisms adjust to changing conditions, prioritize among competing physiological systems, and increase immune defenses without immunological pathology.
Figure.
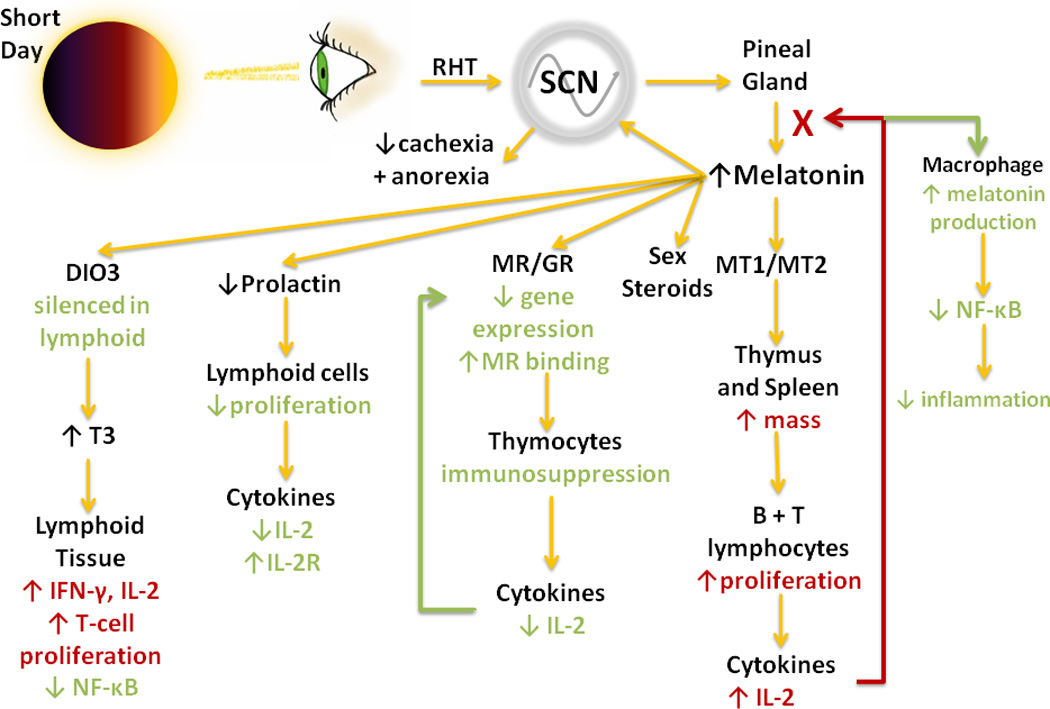
Photic information is transmitted along the retinohypothalamic tract (RHT) by intrinsically photosensitive retinal ganglion cells (iPRGCs) to the suprachiasmatic nucleus (SCN). Photoperiod is transduced from an environmental cue into a neuroendocrine one via the nighttime secretion of pineal melatonin. Short day lengths are represented physiologically by increased duration of melatonin secretion. Melatonin in turn, regulates immune physiology both directly, through MT1/2 receptors on immune tissues, and indirectly via modulation of several neuroendocrine systems. The combined effect results in a system primed for immediate response to immune threats and stunted long term sickness behavior.
RHT, retinohypothalamic tract; SCN: suprachiasmatic nucleus; MT1/2, melatonin receptors 1 and 2; MR, mineralocorticoid receptor; GR, glucocorticoid receptor; DIO3, deiodinase iodothyronine type III; T3, triiodothyronine.
-
--
Animals face different challenges at different times of the year.
-
--
Many species use day length and melatonin to monitor the changing seasons.
-
--
Melatonin drives seasonal changes in the neuroendocrine system.
-
--
Melatonin alters immune function both directly and indirectly via neuroendocrine signaling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E. Hormones and Animal Social Behavior. Princeton, NJ: Princeton University Press; 2005. [Google Scholar]
- Alexander J, Stimson WH. Sex-Hormones and the Course of Parasitic Infection. Parasitol. Today. 1988;4:189–193. [Google Scholar]
- Armanini D, Endres S, Kuhnle U, Weber PC. Parallel determination of mineralocorticoid and glucocorticoid receptors in T- and B-lymphocytes of human spleen. Acta Endocrinol. (Copenh.) 1988;118:479–482. doi: 10.1530/acta.0.1180479. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Hays QR, Bentley GE, Wingfield JC. Testosterone treatment diminishes sickness behavior in male songbirds. Horm. Behav. 2009;56:169–176. doi: 10.1016/j.yhbeh.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Wingfield JC. Sickness Behavior in Vertebrates: Allostasis, Life History Modulation, and Hormonal Regulation. In: Demas GE, Nelson RJ, editors. Ecoimmunology. New York: Oxford University Press; 2012. [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm. Behav. 1995;29:442–457. doi: 10.1006/hbeh.1995.1276. [DOI] [PubMed] [Google Scholar]
- Aubrecht TG, Weil ZM, Nelson RJ. Dim light at night interferes with the development of the short-day phenotype and impairs cell-mediated immunity in Siberian hamsters (Phodopus sungorus) Journal of experimental zoology. Part A, Ecological genetics and physiology. 2014;321:450–456. doi: 10.1002/jez.1877. [DOI] [PubMed] [Google Scholar]
- Auchtung TL, Dahl GE. Prolactin mediates photoperiodic immune enhancement: effects of administration of exogenous prolactin on circulating concentrations, receptor expression, and immune function in steers. Biol. Reprod. 2004;71:1913–1918. doi: 10.1095/biolreprod.104.031005. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, Guillaume JL, Dussaud S, Gbahou F, Yettou K, Liu C, Contreras-Alcantara S, Jockers R, Tosini G. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013;6:ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Walton JC, Dowd SE, Weil ZM, Nelson RJ. Photoperiod modulates gut bacteria composition in male Siberian hamsters (Phodopus sungorus) Brain. Behav. Immun. 2010;24:577–584. doi: 10.1016/j.bbi.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Baillie SR, Prendergast BJ. Photoperiodic regulation of behavioral responses to bacterial and viral mimetics: a test of the winter immunoenhancement hypothesis. J. Biol. Rhythms. 2008;23:81–90. doi: 10.1177/0748730407311518. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Aubrecht TG, Kaugars KE, Weil ZM, Nelson RJ. Artificial light at night alters delayed-type hypersensitivity reaction in response to acute stress in Siberian hamsters. Brain. Behav. Immun. 2013a;34:39–42. doi: 10.1016/j.bbi.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Demas GE, Nelson RJ. Photoperiod-dependent effects of neuronal nitric oxide synthase inhibition on aggression in Siberian hamsters. Horm. Behav. 2012;61:176–180. doi: 10.1016/j.yhbeh.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Herring KL, Walton JC, Fonken LK, Weil ZM, Nelson RJ. Evidence for feedback control of pineal melatonin secretion. Neurosci. Lett. 2013b;542:123–125. doi: 10.1016/j.neulet.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Bentley GE. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microsc. Res. Tech. 2001;53:63–71. doi: 10.1002/jemt.1069. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Demas GE, Nelson RJ, Ball GF. Melatonin, immunity and cost of reproductive state in male European starlings. Proc. Biol. Sci. 1998;265:1191–1195. doi: 10.1098/rspb.1998.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern HA, Nicoll CS. The comparative endocrinology of prolactin. Recent Prog. Horm. Res. 1968;24:681–720. doi: 10.1016/b978-1-4831-9827-9.50019-8. [DOI] [PubMed] [Google Scholar]
- Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc. Natl. Acad. Sci. U. S. A. 2002a;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc. Biol. Sci. 2002b;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Melatonin regulates energy balance and attenuates fever in Siberian hamsters. Endocrinology. 2002;143:2527–2533. doi: 10.1210/endo.143.7.8922. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R207–R213. doi: 10.1152/ajpregu.2001.280.1.R207. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 2010;31:322–340. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC. Rapid behavioral response to corticosterone varies with photoperiod and dose. Horm. Behav. 2000;37:23–30. doi: 10.1006/hbeh.1999.1554. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Bumiller A, Götz F, Rohde W, Dörner G. Effects of repeated injections of interleukin 1beta or lipopolysaccharide on the HPA axis in the newborn rat. Cytokine. 1999;11:225–230. doi: 10.1006/cyto.1999.0423. [DOI] [PubMed] [Google Scholar]
- Carpentieri A, Diaz de Barboza G, Areco V, Peralta Lopez M, Tolosa de Talamoni N. New perspectives in melatonin uses. Pharmacol. Res. 2012;65:437–444. doi: 10.1016/j.phrs.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, Garcia-Maurino S, Reiter RJ, Guerrero JM. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537–9. doi: 10.1096/fj.03-0694fje. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- Casao A, Cebrian I, Asumpcao ME, Perez-Pe R, Abecia JA, Forcada F, Cebrian-Perez JA, Muino-Blanco T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 2010;8:59. doi: 10.1186/1477-7827-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir GJ, Lefevre N, Corazza F, Duchateau J. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol. Sex Dif. 2013;4:16. doi: 10.1186/2042-6410-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celinski K, Konturek SJ, Konturek PC, Brzozowski T, Cichoz-Lach H, Slomka M, Malgorzata P, Bielanski W, Reiter RJ. Melatonin or L-tryptophan accelerates healing of gastroduodenal ulcers in patients treated with omeprazole. J. Pineal Res. 2011;50:389–394. doi: 10.1111/j.1600-079X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011a;17:3888–3898. doi: 10.3748/wjg.v17.i34.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011b;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Csaba G, Barath P. Morphological changes of thymus and the thyroid gland after postnatal extirpation of pineal body. Endocrinol. Exp. 1975;9:59–67. [PubMed] [Google Scholar]
- da Silveira Cruz-Machado S, Carvalho-Sousa CE, Tamura EK, Pinato L, Cecon E, Fernandes PA, de Avellar MC, Ferreira ZS, Markus RP. TLR4 and CD14 receptors expressed in rat pineal gland trigger NFKB pathway. J. Pineal Res. 2010;49:183–192. doi: 10.1111/j.1600-079X.2010.00785.x. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Demas GE, Klein SL, Nelson RJ. Reproductive and immune responses to photoperiod and melatonin are linked in Peromyscus subspecies. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1996;179:819–825. doi: 10.1007/BF00207360. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Photoperiod, ambient temperature, and fo od availability interact to affect reproductive and immune function in adult male deer mice (Peromyscus maniculatus) J. Biol. Rhythms. 1998a;13:253–262. doi: 10.1177/074873098129000093. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus) J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1998b;168:419–426. doi: 10.1007/s003600050161. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain. Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni D, Quetting M, Partecke J. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. Lond. B Biol. Sci. 2013;280 doi: 10.1098/rspb.2012.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr. Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Jasnow AM, Nelson RJ, Demas GE. Exposure to short days, but not short-term melatonin, enhances humoral immunity of male Syrian hamsters (Mesocricetus auratus) J. Pineal Res. 2002;33:118–124. doi: 10.1034/j.1600-079x.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Klein SL, Yellon SM, Nelson RJ. In vitro melatonin treatment enhances splenocyte proliferation in prairie voles. J. Pineal Res. 2000;28:34–40. doi: 10.1034/j.1600-079x.2000.280105.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Goldman BD. Reception of photoperiodic information by fetal Siberian hamsters: role of the mother's pineal gland. J. Exp. Zool. 1989;252:237–244. doi: 10.1002/jez.1402520305. [DOI] [PubMed] [Google Scholar]
- Ellis C, Moar KM, Logie TJ, Ross AW, Morgan PJ, Mercer JG. Diurnal profiles of hypothalamic energy balance gene expression with photoperiod manipulation in the Siberian hamster, Phodopus sungorus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1148–R1153. doi: 10.1152/ajpregu.00825.2007. [DOI] [PubMed] [Google Scholar]
- Fernandes PA, Cecon E, Markus RP, Ferreira ZS. Effect of TNF-alpha on the melatonin synthetic pathway in the rat pineal gland: basis for a 'feedback' of the immune response on circadian timing. J. Pineal Res. 2006;41:344–350. doi: 10.1111/j.1600-079X.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Fine JB, Bartness TJ. Daylength and body mass affect diet self-selection by Siberian hamsters. Physiol. Behav. 1996;59:1039–1050. doi: 10.1016/0031-9384(95)02240-6. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, Bright Males, and the Immunocompetence Handicap. Am. Nat. 1992;139:603–622. [Google Scholar]
- Foster MP, Montecino-Rodriguez E, Dorshkind K. Proliferation of bone marrow pro-B cells is dependent on stimulation by the pituitary/thyroid axis. Immunol. 1999;163:5883–5890. [PubMed] [Google Scholar]
- Freeman DA, Kampf-Lassin A, Galang J, Wen JC, Prendergast BJ. Melatonin acts at the suprachiasmatic nucleus to attenuate behavioral symptoms of infection. Behav. Neurosci. 2007;121:689–697. doi: 10.1037/0735-7044.121.4.689. [DOI] [PubMed] [Google Scholar]
- Gala RR. Prolactin and Growth Hormone in the Regulation of the Immune System. Exp. Biol. Med. 1991;198:513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- Ganley L, Rajan TV. Endogenous testosterone levels do not affect filarial worm burdens in mice. Exp. Parasitol. 2001;98:29–34. doi: 10.1006/expr.2001.4608. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Davies TW, Bennie J, Hopkins J. REVIEW: Reducing the ecological consequences of night-time light pollution: options and developments. J. Appl. Ecol. 2012;49:1256–1266. doi: 10.1111/j.1365-2664.2012.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin V, Binart N, Clement-Lacroix P, Bouchard B, Bole-Feysot C, Edery M, Lucas BK, Touraine P, Pezet A, Maaskant R, Pichard C, Helloco C, Baran N, Favre H, Bernichtein S, Allamando A, Ormandy C, Kelly PA. From the molecular biology of prolactin and its receptor to the lessons learned from knockout mice models. Genet. Anal. 1999;15:189–201. doi: 10.1016/s1050-3862(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian Photoperiodic System: Formal Properties and Neuroendocrine Mechanisms of Photoperiodic Time Measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman BD, Nelson RJ. Melatonin and seasonality in mammals. In: Yu HS, Reiter RJ, editors. Melatonin: Biosynthesis, Physiological Effects, and Clinical Applications. Boca Raton, Fl: CRC Press; 1993. pp. 225–252. [Google Scholar]
- Gomez-Moreno G, Guardia J, Ferrera MJ, Cutando A, Reiter RJ. Melatonin in diseases of the oral cavity. Oral Dis. 2010;16:242–247. doi: 10.1111/j.1601-0825.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- Gordon JI. Honor thy gut symbionts redux. Science. 2012;336:1251–1253. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]
- Gorman MR. A plastic interval timer synchronizes pubertal development of summer- and fall-born hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1613–R1623. doi: 10.1152/ajpregu.2001.281.5.R1613. [DOI] [PubMed] [Google Scholar]
- Grossman CJ. Regulation of the immune system by sex steroids. Endocr. Rev. 1984;5:435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J. Endocrinol. 2014;220:R37–R55. doi: 10.1530/JOE-13-0393. [DOI] [PubMed] [Google Scholar]
- Gupta S, Haldar C. Physiological crosstalk between melatonin and glucocorticoid receptor modulates T-cell mediated immune responses in a wild tropical rodent, Funambulus pennanti. J. Steroid Biochem. Mol. Biol. 2013;134:23–36. doi: 10.1016/j.jsbmb.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Haddad JJ. On the mechanisms and putative pathways involving neuroimmune interactions. Biochem. Biophys. Res. Commun. 2008;370:531–535. doi: 10.1016/j.bbrc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J. Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. Biol. Sci. 2005;272:1039–1046. doi: 10.1098/rspb.2005.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. Lond. B Biol. Sci. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B. Non-vertebrate melatonin. J. Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hazlerigg D. The evolutionary physiology of photoperiodism in vertebrates. Prog. Brain Res. 2012;199:413–422. doi: 10.1016/B978-0-444-59427-3.00023-X. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Wagner GC. Seasonal photoperiodism in vertebrates: from coincidence to amplitude. Trends Endocrinol. Metab. 2006;17:83–91. doi: 10.1016/j.tem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hiebert SM, Green SA, Yellon SM. Daily timed melatonin feedings mimic effects of short days on testis regression and cortisol in circulation in Siberian hamsters. Gen. Comp. Endocrinol. 2006;146:211–216. doi: 10.1016/j.ygcen.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hiebert SM, Thomas EM, Lee TM, Pelz KM, Yellon SM, Zucker I. Photic entrainment of circannual rhythms in golden-mantled ground squirrels: role of the pineal gland. J. Biol. Rhythms. 2000;15:126–134. doi: 10.1177/074873040001500207. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Illnerova H, Vanecek J. Change in duration of the nighttime melatonin peak may be a signal driving photoperiodic responses in the Djungarian hamster (Phodopus sungorus) Neurosci. Lett. 1986;67:68–72. doi: 10.1016/0304-3940(86)90210-7. [DOI] [PubMed] [Google Scholar]
- Horton TH. Growth and reproductive development of male Microtus montanus is affected by the prenatal photoperiod. Biol. Reprod. 1984;31:499–504. doi: 10.1095/biolreprod31.3.499. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang Z, Weng SJ, Sun XH, Yang XL. Neuromodulatory role of melatonin in retinal information processing. Prog. Retin. Eye Res. 2013;32:64–87. doi: 10.1016/j.preteyeres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Weil ZM, Nelson RJ. Dim light at night disrupts the short-day response in Siberian hamsters. Gen. Comp. Endocrinol. 2014;197:56–64. doi: 10.1016/j.ygcen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Iriti M, Varoni EM, Vitalini S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010;49:101–105. doi: 10.1111/j.1600-079X.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. 2001 [Google Scholar]
- Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing KC. The costs of immunity. Acta Zool. Sin. 2004;50:961–969. [Google Scholar]
- Klein SL. Sex differences in prophylaxis and therapeutic treatments for viral diseases. Handb. Exp. Pharmacol. 2012:499–522. doi: 10.1007/978-3-642-30726-3_22. [DOI] [PubMed] [Google Scholar]
- Klein SL, Gamble HR, Nelson RJ. Role of steroid hormones in Trichinella spiralis infection among voles. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1999;277:R1362–R1367. doi: 10.1152/ajpregu.1999.277.5.R1362. [DOI] [PubMed] [Google Scholar]
- Kleszczynski K, Fischer TW. Melatonin and human skin aging. Dermatoendocrinol. 2012;4:245–252. doi: 10.4161/derm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Czesnikiewicz-Guzik M, Kwiecien S, Brzozowski T, Bubenik GA, Pawlik WW. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT) J. Physiol. Pharmacol. 2007;58:381–405. [PubMed] [Google Scholar]
- Lahiri S, Singh P, Singh S, Rasheed N, Palit G, Pant KK. Melatonin protects against experimental reflux esophagitis. J. Pineal Res. 2009;46:207–213. doi: 10.1111/j.1600-079X.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- Lange IG, Hartel A, Meyer HH. Evolution of oestrogen functions in vertebrates. J. Steroid Biochem. Mol. Biol. 2002;83:219–226. doi: 10.1016/s0960-0760(02)00225-x. [DOI] [PubMed] [Google Scholar]
- Lardone PJ, Guerrero JM, Fernandez-Santos JM, Rubio A, Martin-Lacave I, Carrillo-Vico A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res. 2011;51:454–462. doi: 10.1111/j.1600-079X.2011.00909.x. [DOI] [PubMed] [Google Scholar]
- Lattin CR, Waldron-Francis K, Romero LM. Intracellular glucocorticoid receptors in spleen, but not skin, vary seasonally in wild house sparrows (Passer domesticus) Proc. Biol. Sci. 2013;280:20123033. doi: 10.1098/rspb.2012.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard M, Beaudoin F, Menard M, Lachance MP, Laforest JP, Farmer C. Impact of a long photoperiod during lactation on immune status of piglets. J. Anim. Sci. 2012;90:3468–3476. doi: 10.2527/jas.2012-5191. [DOI] [PubMed] [Google Scholar]
- Levine JE. Gonadotropin-releasing hormone (GnRH) In: Helen LH, Anthony WH, editors. Encyclopedia of Hormones. New York: Academic Press; 2003. pp. 157–165. [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln GA, Libre EA, Merriam GR. Long-term reproductive cycles in rams after pinealectomy or superior cervical ganglionectomy. J. Reprod. Fertil. 1989;85:687–704. doi: 10.1530/jrf.0.0850687. [DOI] [PubMed] [Google Scholar]
- Lopez-Rincon G, Pereira-Suarez AL, Del Toro-Arreola S, Sanchez-Hernandez PE, Ochoa-Zarzosa A, Munoz-Valle JF, Estrada-Chavez C. Lipopolysaccharide induces the expression of an autocrine prolactin loop enhancing inflammatory response in monocytes. J. Inflamm. 2013;10:24. doi: 10.1186/1476-9255-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotter H, Helk E, Bernin H, Jacobs T, Prehn C, Adamski J, Gonzalez-Roldan N, Holst O, Tannich E. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNgamma secretion in natural killer T cells. PLoS ONE. 2013;8:e55694. doi: 10.1371/journal.pone.0055694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Lutermann H, Bodenstein C, Bennett NC. Natural Parasite Infection Affects the Tolerance but Not the Response to a Simulated Secondary Parasite Infection. Plos One. 2012;7 doi: 10.1371/journal.pone.0052077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ. The immunoneuroendocrine role of melatonin. J. Pineal Res. 1993;14:1–10. doi: 10.1111/j.1600-079x.1993.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J. Neuroimmunol. 1986;13:19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res. 2010;62:282–287. doi: 10.1016/j.phrs.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- Markus RP, Cecon E, Pires-Lapa MA. Immune-Pineal Axis: Nuc lear Factor kappaB (NF-kB) Mediates the Shift in the Melatonin Source from Pinealocytes to Immune Competent Cells. Int. J. Mol. Sci. 2013;14:10979–10997. doi: 10.3390/ijms140610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus RP, Ferreira ZS. The Immune-Pineal Axis: the Role of Pineal and Extra-Pineal Melatonin in Modulating Inflammation. Advances in Neuroimmune Biology. 2011;1:95–104. [Google Scholar]
- Martin LB, Scheuerlein A, 2nd, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. Biol. Sci. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, 2nd, Nelson RJ. Refining approaches and diversifying directions in ecoimmunology. Integr. Comp. Bio. 2006;46:1030–1039. doi: 10.1093/icb/icl039. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni I, Montesinos Mdel M, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, Masini-Repiso AM, Targovnik HM, Rabinovich GA, Pellizas CG. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22:1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- Mascanfroni ID, Montesinos Mdel M, Alamino VA, Susperreguy S, Nicola JP, Ilarregui JM, Masini-Repiso AM, Rabinovich GA, Pellizas CG. Nuclear factor (NF)-kappaB-dependent thyroid hormone receptor beta1 expression controls dendritic cell function via Akt signaling. J. Biol. Chem. 2010;285:9569–9582. doi: 10.1074/jbc.M109.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Monecke S, Sage-Ciocca D, Wollnik F, Pevet P. Photoperiod can entrain circannual rhythms in pinealectomized European hamsters. J. Biol. Rhythms. 2013;28:278–290. doi: 10.1177/0748730413498561. [DOI] [PubMed] [Google Scholar]
- Moore CB, Siopes TD. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen. Comp. Endocrinol. 2000;119:95–104. doi: 10.1006/gcen.2000.7496. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed. Proc. 1983;42:2783–2789. [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008;136:1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Trainor BC, Nelson RJ. Photoperiod alters macrophage responsiveness, but not expression of Toll-like receptors in Siberian hamsters. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology. 2007;148:354–359. doi: 10.1016/j.cbpa.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Photoperiod-Nonresponsive Morphs - a Possible Variable in Microtine Population-Density Fluctuations. Am. Nat. 1987;130:350–369. [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Blom JM. Photoperiodic effects on tumor development and immune function. J. Biol. Rhythms. 1994;9:233–249. doi: 10.1177/074873049400900305. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Role of melatonin in mediating seasonal energetic and immunologic adaptations. Brain Res. Bull. 1997;44:423–430. doi: 10.1016/s0361-9230(97)00222-0. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Denlinger DL, Somers DE. Photoperiodism: The Biological Calendar. 2010:600. [Google Scholar]
- Oishi K, Shibusawa K, Kakazu H, Kuriyama T, Ohkura N, Machida K. Extended light exposure suppresses nocturnal increases in cytotoxic activity of splenic natural killer cells in rats. Biol. Rhythm. Res. 2006;37:29–35. [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr. Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18238–18242. doi: 10.1073/pnas.0808952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R. Estrogen deficiency, T cells and bone loss. Cell. Immunol. 2008;252:68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010;48:297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- Parham P. The Immune System. 2009:608. [Google Scholar]
- Pawlak J, Golab M, Markowska M, Majewski P, Skwarlo-Sonta K. Photoperiod-related changes in hormonal and immune status of male Siberian hamsters, Phodopus sungorus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;152:299–303. doi: 10.1016/j.cbpa.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Pedron T, Sansonetti P. Commensals, bacterial pathogens and intestinal inflammation: an intriguing menage a trois. Cell Host Microbe. 2008;3:344–347. doi: 10.1016/j.chom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Bodin L, Hanocq E, Malpaux B, Teyssier J, Thimonier J, Chemineau P. Association between expression of reproductive seasonality and alleles of the gene for Mel(1a) receptor in the ewe. Biol. Reprod. 2000;62:1096–1101. doi: 10.1095/biolreprod62.4.1096. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus) Endocrinology. 2010;151:714–721. doi: 10.1210/en.2009-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Baillie SR, Dhabhar FS. Gonadal hormone-dependent and -independent regulation of immune function by photoperiod in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R384–R392. doi: 10.1152/ajpregu.00551.2007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of photoperiod history on immune responses to intermediate day lengths in Siberian hamsters (Phodopus sungorus) J. Neuroimmunol. 2004;149:31–39. doi: 10.1016/j.jneuroim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Short day lengths enhance skin immune responses in gonadectomised Siberian hamsters. J. Neuroendocrinol. 2005;17:18–21. doi: 10.1111/j.1365-2826.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Gorman MR, Zucker I. Establishment and persistence of photoperiodic memory in hamsters. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5586–5591. doi: 10.1073/pnas.100098597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Nelson RJ. Photoperiodic regulation of circulating leukocytes in juvenile Siberian hamsters: mediation by melatonin and testosterone. J. Biol. Rhythms. 2003;18:473–480. doi: 10.1177/0748730403258486. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Kampf-Lassin A, Yee JR, Galang J, McMaster N, Kay LM. Winter day lengths enhance T lymphocyte phenotypes, inhibit cytokine responses, and attenuate behavioral symptoms of infection in laboratory rats. Brain. Behav. Immun. 2007;21:1096–1108. doi: 10.1016/j.bbi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Hormones, Brain and Behavior. Hormones, Brain and Behavior. 2009:507–540. [Google Scholar]
- Prendergast BJ, Pyter LM, Kampf-Lassin A, Patel PN, Stevenson TJ. Rapid induction of hypothalamic iodothyronine deiodinase expression by photoperiod and melatonin in juvenile Siberian hamsters (Phodopus sungorus) Endocrinology. 2013;154:831–841. doi: 10.1210/en.2012-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Adelson JD, Nelson RJ. Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology. 2007;148:3402–3409. doi: 10.1210/en.2006-1432. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Samuelsson AR, Quan N, Nelson RJ. Photoperiod alters hypothalamic cytokine gene expression and sickness responses following immune challenge in female Siberian hamsters (Phodopus sungorus) Neuroscience. 2005;131:779–784. doi: 10.1016/j.neuroscience.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reeder DM, Kramer KM. Stress in free-ranging mammals: Integrating physiology, ecology, and natural history. J. Mammal. 2005;86:225–235. [Google Scholar]
- Reiter RJ. The Melatonin Rhythm - Both a Clock and a Calendar. Experientia. 1993a;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993b;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Rich C, Longcore T. Ecological Consequences of Artificial Night Lighting. Island Press; 2006. [Google Scholar]
- Rivkees SA, Hall DA, Weaver DR, Reppert SM. Djungarian hamsters exhibit reproductive responses to changes in daylength at extreme photoperiods. Endocrinology. 1988;122:2634–2638. doi: 10.1210/endo-122-6-2634. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Spencer RL, Krey LC, McEwen BS. Effects of photoperiod on brain corticosteroid receptors and the stress response in the golden hamster (Mesocricetus auratus) Brain Res. 1998;780:348–351. doi: 10.1016/s0006-8993(97)01303-6. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salverson TJ, McMichael GE, Sury JJ, Shahed A, Young KA. Differential expression of matrix metalloproteinases during stimulated ovarian recrudescence in Siberian hamsters (Phodopus sungorus) Gen. Comp. Endocrinol. 2008;155:749–761. doi: 10.1016/j.ygcen.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Savita Rai U. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;119:199–204. doi: 10.1016/s0742-8413(97)00207-7. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011a;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J. Neurosci. 2011b;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J, Ingbar SH. Specific binding sites for the triiodothyronine in the plasma membrane of rat thymocytes. Correlation with biochemical responses. J. Clin. Invest. 1982;70:919–926. doi: 10.1172/JCI110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheh A, Ge Z, Parry NM, Muthupalani S, Rager JE, Raczynski AR, Mobley MW, McCabe AF, Fry RC, Wang TC, Fox JG. 17beta-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev. Res. 2011;4:1426–1435. doi: 10.1158/1940-6207.CAPR-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HC, Huang MS, Lee CH. Estrogen augments the protection of hypertonic saline treatment from mesenteric ischemia-reperfusion injury. Shock. 2011;35:302–307. doi: 10.1097/SHK.0b013e3181f8b420. [DOI] [PubMed] [Google Scholar]
- Shiu SY, Li L, Siu SW, Xi SC, Fong SW, Pang SF. Biological basis and possible physiological implications of melatonin receptor-mediated signaling in the rat epididymis. Biol. Signals Recept. 2000;9:172–187. doi: 10.1159/000014637. [DOI] [PubMed] [Google Scholar]
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Speakman JR. The physiological costs of reproduction in small mammals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebesteny T, Maronde E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. P ineal Res. 2011;51:17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian-Rhythms in Drinking Behavior and Locomotor Activity of Rats Are Eliminated by Hypothalamic-Lesions. Proc. Natl. Acad. Sci. U. S. A. 1972;69:1583. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson MH, Elliott JA, Goldman BD. Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus) Biol. Reprod. 1986;34:664–669. doi: 10.1095/biolreprod34.4.664. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Onishi KG, Bradley SP, Prendergast BJ. Cell-autonomous iodothyronine deiodinase expression mediates seasonal plasticity in immune function. Brain. Behav. Immun. 2014;36:61–70. doi: 10.1016/j.bbi.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16651–1666. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010;85:607–623. doi: 10.1111/j.1469-185X.2009.00118.x. [DOI] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 1999;406:171–182. [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vishwas DK, Mukherjee A, Haldar C. Melatonin improves humoral and cell-mediated immune responses of male golden hamster following stress induced by dexamethasone. J. Neuroimmunol. 2013;259:17–25. doi: 10.1016/j.jneuroim.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Walton JC, Chen Z, Travers JB, Nelson RJ. Exogenous melatonin reproduces the effects of short day lengths on hippocampal function in male white-footed mice, Peromyscus leucopus. Neuroscience. 2013;248C:403–413. doi: 10.1016/j.neuroscience.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JC, Grier AJ, Weil ZM, Nelson RJ. Photoperiod and stress regulation of corticosteroid receptor, brain-derived neurotrophic factor, and glucose transporter GLUT3 mRNA in the hippocampus of male Siberian hamsters (Phodopus sungorus) Neuroscience. 2012;213:106–111. doi: 10.1016/j.neuroscience.2012.03.043. [DOI] [PubMed] [Google Scholar]
- Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendocrinol. 2011;32:303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Liu C, Reppert SM. Nature's knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol. Endocrinol. 1996;10:1478–1487. doi: 10.1210/mend.10.11.8923472. [DOI] [PubMed] [Google Scholar]
- Weigent DA. Immunoregulatory properties of growth hormone and prolactin. Pharmacol. Ther. 1996;69:237–257. doi: 10.1016/0163-7258(96)00001-0. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Pyter LM, Martin LB, Nelson RJ., 2nd Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1714–R1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Workman JL, Karelina K, Nelson RJ. Short photoperiods alter cannabinoid receptor expression in hypothalamic nuclei related to e nergy balance. Neurosci. Lett. 2011;491:99–103. doi: 10.1016/j.neulet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Workman JL, Nelson RJ. Housing condition alters immunological and reproductive responses to day length in Siberian hamsters (Phodopus sungorus) Horm. Behav. 2007;52:261–266. doi: 10.1016/j.yhbeh.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JC, Prendergast BJ. Photoperiodic regulation of behavioral responsiveness to proinflammatory cytokines. Physiol. Behav. 2007;90:717–725. doi: 10.1016/j.physbeh.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC. Organization of vertebrate annual cycles: implications for control mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Daberkow N, Asche K, Stoltenberg G, Lanksch WR, Volk HD. Brain-IL-1 beta induces local inflammation but systemic anti-inflammatory response through stimulation of both hypothalamic-pituitary-adrenal axis and sympathetic nervous system. Brain Res. 1999;816:563–571. doi: 10.1016/s0006-8993(98)01238-4. [DOI] [PubMed] [Google Scholar]
- Xu J, Gordon JI. Honor thy symbionts. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SK, Haldar C. Reciprocal interaction between melatonin receptors (Mel(1a), Mel(1b), and Mel(1c)) and androgen receptor (AR) expression in immunoregulation of a seasonally breeding bird, Perdicula asiatica: role of photoperiod. J. Photochem. Photobiol. B. 2013;122:52–60. doi: 10.1016/j.jphotobiol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J. Neurosci. 2009;29:2885–2889. doi: 10.1523/JNEUROSCI.0145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Teasley LA, Fagoaga OR, Nguyen HC, Truong HN, Nehlsen-Cannarella L. Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J. Pineal Res. 1999;27:243–248. doi: 10.1111/j.1600-079x.1999.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009;61:383–410. doi: 10.1016/s1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]


