Abstract
Genomics and genetics have invaded all aspects of biology and medicine, opening uncharted territory for scientific exploration. The definition of “gene” itself has become ambiguous, and the central dogma is continuously being revised and expanded. Computational biology and computational medicine are no longer intellectual domains of the chosen few. Next generation sequencing (NGS) technology, together with novel methods of pattern recognition and network analyses, has revolutionized the way we think about fundamental biological mechanisms and cellular pathways. In this review, we discuss NGS-based genome-wide approaches that can provide deeper insights into retinal development, aging and disease pathogenesis. We first focus on gene regulatory networks (GRNs) that govern the differentiation of retinal photoreceptors and modulate adaptive response during aging. Then, we discuss NGS technology in the context of retinal disease and develop a vision for therapies based on network biology. We should emphasize that basic strategies for network construction and analyses can be transported to any tissue or cell type. We believe that specific and uniform guidelines are required for generation of genome, transcriptome and epigenome data to facilitate comparative analysis and integration of multi-dimensional data sets, and for constructing networks underlying complex biological processes. As cellular homeostasis and organismal survival are dependent on gene-gene and gene-environment interactions, we believe that network-based biology will provide the foundation for deciphering disease mechanisms and discovering novel drug targets for retinal neurodegenerative diseases.
Keywords: Systems biology, High throughput genomics, Gene regulatory network, Retinal Degeneration, Macular Degeneration, Photoreceptor, Inherited blindness, Network Medicine, Whole Exome Sequencing, RNA-seq, ChIP-seq, eQTL, Pathway-based drug discovery
1. Introduction
Ramon y Cajal was the first to recognize the retina as ‘a true nervous center, as a peripheral extension of the central nervous system’ (Ramon y Cajal, 1893 La retine des Vertebres; translation taken from Piccolino 1989 Santiago Ramon Y Cajal, the retina and the neuron theory). The retina has thus attained the distinction of being an attractive model to investigate fundamental biology and therapy of the nervous system. The vertebrate retina is composed of six major neuronal cell types that are organized in three cellular layers forming exquisite neuronal circuits for detection of visual information (Figure 1A). Light is captured by photoreceptors; visual signals then undergo enhancement, integration and processing through bipolar, horizontal and amacrine cells and by varied usage of parallel synaptic circuits, before eventually being transmitted via ganglion cells to the brain (Lamb et al., 2007; Masland, 2001). This remarkable complexity makes the retina extremely vulnerable to degeneration caused by genetic defects that eventually lead to vision loss. In fact, a vast majority of incurable blindness is caused by dysfunction or death of retinal photoreceptors (Wright et al., 2010). Thus, knowledge-based design of therapies for blinding retinal degenerative diseases (RDDs) depends on better understanding of pathways that are associated with: (i) normal development of the retinal neurons (specifically photoreceptors) from progenitors and stem cells; (ii) assembly of synaptic circuits; (iii) cellular adaptation and homeostasis; (iv) response to aging and inherited genomic changes; and (v) disease pathogenesis.
Figure 1.
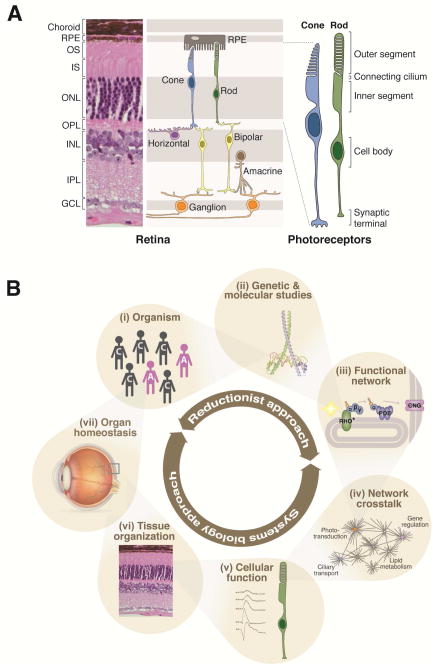
The mammalian retina and systems biology approaches. A, A representative retinal anatomy is shown by hematoxylin and eosin stained cross section of an adult mouse retina (left) and by schematics (center and right). In the mammalian retina, six main neuronal classes are organized into three nuclear layers [outer nuclear layer (ONL), inner nuclear layer (INL) and ganglion cell layer (GCL)] and form synaptic connections in two plexiform layers [outer plexiform layer (OPL) and inner plexiform layer (IPL)]. Cone and rod photoreceptors comprise the outer retina with their cell bodies situated in ONL and their inner and outer segments (IS and OS, respectively) located between ONL and the retinal pigment epithelium (RPE). RPE microvilli ensheath the outer segments, supporting phototransduction and photoreceptor survival. Photoreceptors transfer visual information through bipolar and retinal interneurons to ganglion cells. B, To date, population-based genetic analyses (i) and genetic and molecular studies of known genes (ii) have been major strategies in retinal research. Such studies have long been a major driving force in identifying retinal disease genes and in revealing the function of the disease genes and their functional or structural associates. The “bottom up” approaches have been undertaken to build functional networks (iii) that are critical for retinal function (e.g., phototransduction pathway) by compiling functional/structural relationships among individual molecules. Considering widespread crosstalk between functional and/or regulatory networks (iv), system-wide measurement of various biomolecules is critical in constructing a comprehensive map of complex intermolecular regulatory interactions. Systems biology approach thus complements the traditional reductionist methodologies. The recent advent of next generation sequencing technologies has enabled system-level assessment of various biological processes. Computational analysis of next generation sequencing and other types of high throughput data, ideally by integrating multiple data sets, allows a holistic approach to elucidate cellular function (v) as well as homeostasis of tissues (vi), organs (vii), and organisms (i). RHO, rhodopsin; PDE, phosphodiesterase; CNG, cyclic nucleotide gated channel.
The human genome of almost three billion nucleotides contains the complete instruction for generating over 100 billion neurons and 150 trillion synapses (Pakkenberg et al., 2003; Williams and Herrup, 1988). Despite tremendous cellular heterogeneity and functional complexity, our genome encompasses only approximately 20,000 protein-coding genes (Ezkurdia et al., 2014)(http://www.gencodegenes.org), fewer than some of the “apparently” less-evolved organisms (Carninci et al., 2005; de Laat and Duboule, 2013; Pruitt et al., 2009). However, alternative splicing and use of alternate promoters can produce unique gene expression patterns associated with fate determination and cell-type specific functions (Wang et al., 2008). Furthermore, the non-protein-coding genomic DNA [the so-called “junk DNA” (Balakirev and Ayala, 2003; Brosius and Gould, 1992; Ohno, 1972)] is increasingly being recognized as an important “regulator” of the coding information (Palazzo and Gregory, 2014). Cis-regulatory sequences include the genomic code for binding of transcription complexes that dictate quantitatively precise as well as cell type- and stage-specific gene expression (Levine et al., 2014; Shlyueva et al., 2014). In addition, though the coding transcripts are relatively small in number, a plethora of small and long non-coding RNAs are detected in different cell types and believed to modulate gene expression and homeostasis (Morris and Mattick, 2014; Slack, 2006). Normal gene expression patterns, established by combinatorial action of multiple regulatory modules, can be disrupted in response to changes in microenvironment and/or by inherited genomic variations/mutations, resulting in altered physiology and phenotype (including disease) (Lagha et al., 2012). Thus, comprehensive understanding of integrated gene regulatory networks (GRNs) is critical for deciphering normal development and homeostatic mechanisms as well as pathways leading to disease.
Remarkable advances have been made in elucidating molecules and pathways that control retinal cell fate specification and differentiation (Agathocleous and Harris, 2009; Bassett and Wallace, 2012). Defects in over 200 genes have been associated with inherited monogenic RDDs (RetNet; https://sph.uth.edu/retnet/). We have also begun to dissect the complexities of multifactorial retinal diseases that afflict large segments of the population (Fritsche et al., 2014; Kuo et al., 2014). Impressive gene- and stem cell-based approaches are being developed for treatment of retinal disease; nonetheless, individual research projects have generally centered on single genes or molecules or on a single functional pathway, and therefore the biomedical progress has not been able to keep pace with public expectations (Bull and Martin, 2011; Cuenca et al., 2014; Lindvall and Kokaia, 2010; Rowe-Rendleman et al., 2014). We must recognize that genes/RNAs/proteins are part of complex molecular networks and biochemical pathways and that the disruption of one creates a domino effect leading to multiple changes (including in gene expression patterns) not only within the affected cell(s) but also in neighboring cells and tissues. Therefore, holistic assessment of biological components and of interaction networks constitutes essential tasks in exploring retinal development and disease in the post-genomic era (Barabasi and Oltvai, 2004; Hwang et al., 2012; Kitano, 2002; Schadt, 2009; Vidal et al., 2011; Yu et al., 2004b; Zhou et al., 2014).
The emergence of next generation sequencing (NGS) technology has dramatically broadened the scope in which diverse cellular processes can be interrogated, setting the stage for system-level approaches to comprehend retinal GRNs. In this review, we will describe the current status of genome-wide strategies as applied to the retina and discuss in which direction the field appears to be moving. As the task ahead is daunting yet feasible, we believe that a collaborative consortium-like approach, elegantly demonstrated by genetic studies of age-related macular degeneration (AMD) (Cipriani and International AMD Genomics Consortium, 2014; Fritsche et al., 2013; Fritsche and International AMD Genomics Consortium, 2014), is required to elucidate retinal GRNs that control development, maintain homeostasis and modulate responses to aging, environment and inherited disease-causing variants or mutations. We have therefore taken the liberty to put forward a framework for integrative and comparative analysis of NGS data, with a goal to build comprehensive GRNs pertaining to the retina. Here, we have primarily focused on the photoreceptors because of their association with incurable blinding retinal diseases. Rod photoreceptors, in particular, constitute nearly 80% of retinal neurons in many mammalian species, including mouse and human (Hendrickson et al., 2008; Lamb et al., 2007; Rapaport et al., 2004); thus NGS data sets from retina are especially rich in rod photoreceptor-related information. We envision the photoreceptors as an ideal paradigm for initiating multi-dimensional, system-level studies that can be widely applicable to other cell types, especially in the retina but broadly for the central nervous system.
2. Systems biology approaches
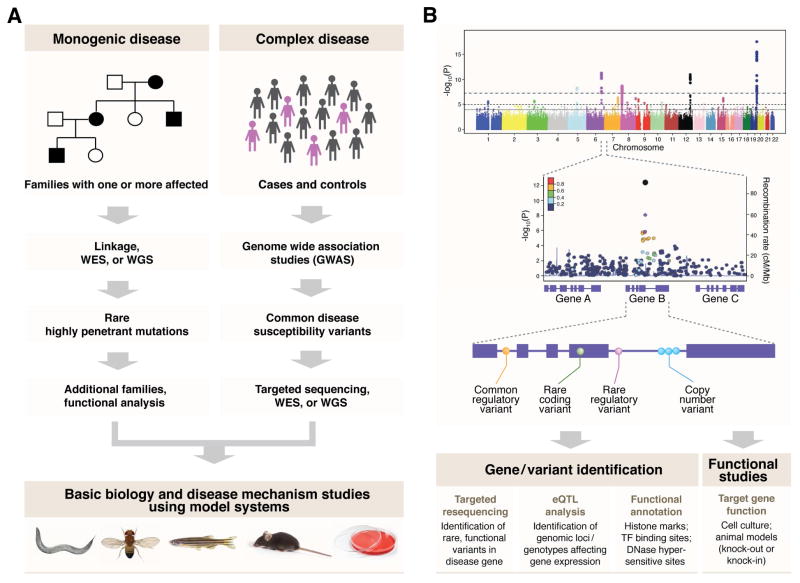
Biomedical research is mostly driven from phenotypic observations and curiosity to understand biological phenomena. For example, forward genetic screening using animal models, such as Drosophila melanogaster (fruit fly), Caenorhabditis elegans and Mus musculus (mouse), have led to identification of many essential genes and their function. Similarly, human genetic studies have focused on identifying genes linked to specific phenotypes or traits, thereby providing significant insights into biological basis of the disease. In addition, biochemical, genetic and molecular biology approaches have further elucidated mechanism(s) of action of specific molecules and cellular components. Though biological pathways can be deciphered by compiling individual molecular functions and binary relationships, current approaches are not optimal in mapping complex regulatory interactions among discrete constituents. Systems biology takes a bird’s eye view of cellular function with a goal of delineating molecular interactions and crosstalk among the pathways (Ideker et al., 2001; Ryan et al., 2013; Sorger, 2005), complementing the reductionist approach (Figure 1B). Although the concept of “systems biology” is not new (Trewavas, 2006), it has had only limited success because of unavailability of extensive data sets.
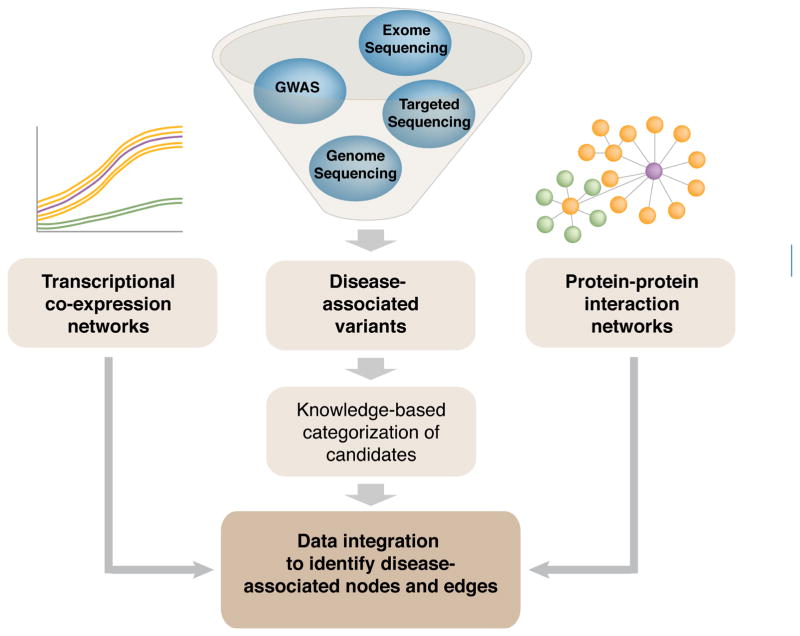
Recent advent of NGS and computational methodology has permitted biologists to generate system-wide data sets, leading to rapid advances in this field. Three major components can be assigned to systems biology, especially when we discuss GRNs (Figure 2A):
Figure 2.
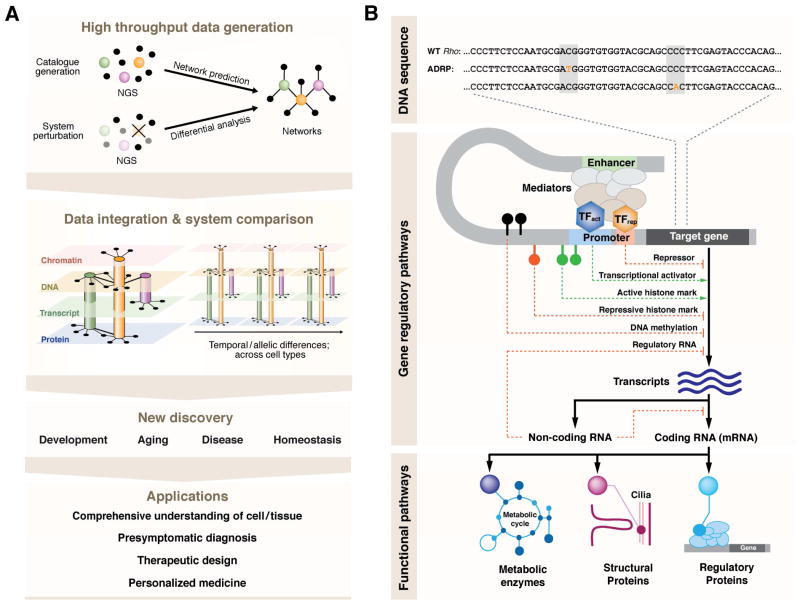
Strategies and aims of system-wide, multi-dimensional data analysis. A, Networks of a tissue or a cell type of interest can be inferred from high throughput data analysis. Next generation sequencing (NGS) allows cataloguing cellular constituents at a steady state and functional interactions when combined with system perturbation and differential analysis. Molecular interactions are not confined to only one molecular type such as DNA, transcripts, chromatin marks or proteins. Thus, multi-dimensional data integration further refines the networks. In addition, comparative analysis is critical as discrete cells are subjected to temporal changes (i.e. development and aging) as well as interactions with neighboring cells and the microenvironment, which evoke physiological modulation of the tissue and eventually of the organism. These holistic approaches will lead to new discoveries of the biological systems and offer broad application. B, Cellular function is regulated at multiple levels. The DNA sequence contains the instructions of protein coding and gene regulation, and diverse gene regulatory mechanisms ensure expression of a unique set of components highly specialized for each cell identity. Intrinsic and/or exogenous damage to any level can lead to deleterious effects on function and survival of the system. TFact, transcription activator; TFrep, transcription repressor.
Prediction of regulatory networks via generation of various high throughput data sets and by computational analysis
Extension and/or refinement of the networks by superimposing measurements made at multiple levels of cellular constituents such as DNA, transcribed sequences, chromatin state, proteins, and metabolites.
Assessment of system’s response to time, risk factors or interaction with its microenvironment (e.g., neighboring cells/tissues)
A comprehensive system-level understanding of a cell/tissue/organism requires integrated analysis of all intracellular molecular interactions and pathways, including data sets from proteomic and metabolomic studies. However, high throughput data from such investigations is not readily obtainable for most tissues including the retina (or other ocular cell types). We therefore limit our discussion to genetic and epigenetic control networks that can be measured by NGS.
2.1. High throughput data generation
NGS is a versatile technology that can be coupled with an endless list of classical assay strategies (Table 1), enabling genome-wide measurements of DNA sequence variations as well as components of GRNs at both transcriptional and epigenetic levels. High throughput genome-wide data generation requires a careful study design, which can be summarized in two complementary strategies (Figure 2A). In the first approach, the output is a nearly complete catalog of components of a given class of biomolecules with relevant quantitative information. For example, GRNs associated with rod photoreceptors can be constructed by integrating a comprehensive and quantitative catalog of mRNA transcripts (using RNA-seq), target genes for key transcription factors (using chromatin immunoprecipitation-sequencing, termed ChIP-seq), global profiles of epigenome [histone modifications using ChIP-seq, and DNA methylation using different NGS-based methods such as reduced representation bisulfite sequencing (RRBS)], and other regulatory molecules (such as miRNA, using small RNA-seq). In this review, we will not discuss NGS-based methods (summarized in Table 1), which have been subjects of excellent reviews recently (Furey, 2012; Metzker, 2010; Park, 2009; Telese et al., 2013; Wang et al., 2009). With these data, regulatory networks can be inferred based on co-expression and prior knowledge of interactions though additional experiments are needed for validation. In the second strategy, a ‘seed network’ is employed for the experimental design. A known molecular interaction or functional hub is altered experimentally, and the consequences of system perturbation are interrogated genome-wide. For example, gene knock-out studies followed by mRNA or epigenomic profiles would greatly assist in deciphering the associated GRN. While the former approach charts all the possible physical or regulatory interactions, the latter refines the networks by directly examining functional relationships.
Table 1.
High throughput techniques used in system-level data generation at multiple levels of biological pathways
| Method | Information | Strategy | Reference | |
|---|---|---|---|---|
| Genome | WGS (whole genome sequencing) | Genetic variation in the entire genome | High throughput sequencing of the complete genome | |
| WES (whole exome sequencing) | Genetic variations in the exome | Exome capture followed by high throughput sequencing. | (Ng et al., 2009) | |
| Repli-seq (replication sequencing) | Temporal mapping of DNA replication | BrdU labeling of newly replicated DNA and FACS-mediated fractionation of cell populations according to cell cycle phases followed by high throughput sequencing. | (Hansen et al., 2010) | |
| ChIP-seq (chromatin immunoprecipitation sequencing), ChIP-exo, or DamID | Genome-wide protein binding sites | Genome-wide profiling of occupancy by a protein of interest. A specific antibody is used to isolate protein-bound chromatin areas (ChIP-seq), and the resolution of the assay can be further enhanced by employing exonuclease digestion of protein-bound DNA ends (ChIP-exo). In cases that the specific ChIP quality antibody is not available, the protein of interest that is fused to E. Coli DNA adenine methyltransferase (Dam) and therefore methylates DNA adjacent to the binding sites can be used instead. | (Barski et al., 2007), (Rhee and Pugh, 2011) | |
| Transcriptome | RNA-seq | Gene expression profile | Genome-wide profiling of transcripts. Typically, polyadenylated RNAs are enriched for subsequent cDNA synthesis and high throughput sequencing. | (Mortazavi et al., 2008) |
| small RNA-seq | Regulatory RNA profile | Genome-wide profiling of small RNA species by high throughput sequencing with modified cDNA synthesis protocols. | (Morin et al., 2008) | |
| Deep CAGE (cap analysis of gene expression) | Transcription start sites | Capturing cDNA tags corresponding 5′ ends of transcripts by cap trapping system followed by high throughput sequencing. | (de Hoon and Hayashizaki, 2008) | |
| RNA-PET (RNA paired end tag) sequencing | 5′ and 3′ ends of transcripts | Profiling of 5′ and 3′ end tags of transcripts. This method is especially powerful in detection of fusion transcripts. | (Ruan and Ruan, 2012) | |
| GRO-seq (global run-on sequencing) | Nascent transcripts | Global mapping of transcriptionally active polymerase density by genome-wide profiling of nascent RNA. | (Core et al., 2008) | |
| Ribo-seq (ribosome profiling) | Transcripts engaged with translation | Profiling of ribosome-protected mRNA fragments by deep sequencing. | (Ingolia et al., 2009) | |
| CLIP-seq (Cross-linking immunoprecipitation sequencing) or RIP-seq (RNA immunoprecipitation sequencing) | Transcripts bound by a protein of interest | High throughput sequencing of cDNA made from RNA pulled down with antibody against a protein of interest. RNA-protein complexes are cross-liked with UV in CLIP-seq. In RIP-seq, no cross-linking is necessary although formaldehyde-mediated cross-linking can be included. | (Yeo et al., 2009) (Zhao et al., 2010) | |
| Epigenome & chromatin conformation | Histone modification ChIP-seq | Genome-wide map of histone modification | ChIP-seq can be also applied for genome-wide profiling of histone modifications. For histones, ChIP can be also performed without cross-linking of proteins to DNA (native ChIP). | (Barski et al., 2007) |
| MeDIP-seq (methylated DNA immunoprecipitation sequencing), WGBS (whole genome bisulfite sequencing) or RRBS (reduced representation bisulfite sequencing) | Genome-wide map of DNA methylation | Genome-wide mapping of methylated cytosine (5mC) of DNA. Methylated DNA can be detected by various methods such as immunoprecipitation using 5mC-specific antibody (MeDIP-seq), MBD2b/MBD3L1 protein complexes with high affinity to 5mC (MIRA-seq) and sodium bisulfite conversion of unmethylated C to U (WGBS or RRBS). | (Berman et al., 2012; Gu et al., 2011; Lister et al., 2009; Weber et al., 2005) | |
| hMeDIP-seq (hydroxymethylated DNA immunoprecipitation sequencing), TAB-seq (Tet-assisted bisulfite sequencing) | Genome-wide map of DNA hydroxymethylation | Genome-wide mapping of hydroxymethylated cytosine (5hmC) of DNA. 5hmC is either isolated by immunoprecipitation (hMeDIP-seq) or by glucosylation-mediated protection of 5hmC sites from the subsequent Tet enzyme assisted conversion of 5hmC or MspI digestion (TAB-seq and RRHP-seq, respectively). | (Jin et al., 2011; Song et al., 2011a; Wu et al., 2011) | |
| MNase-seq (micrococcal nuclease sequencing) | Nucleosome positioning | MNase digestion of chromatin followed by high throughput sequencing. MNase preferentially cuts at the linker DNA between nucleosomes. | (Henikoff et al., 2011) | |
| 4C-seq (circular chromosome conformation capture sequencing) or HiC-seq | Chromatin-chromatin interaction | Variations of chromatin conformation capture (3C) assays to detect intra- or interchromosomal interaction genome-wide. 4C-seq detects chromatin areas interacting with one genomic locus of interest in genome-wide manner, whereas HiC-seq captures all detectable chromatin-to-chromatin interactions. | (Lieberma n-Aiden et al., 2009; Rao et al., 2014; Splinter et al., 2012) | |
| ChIA-PET (chromatin interaction analysis by paired-end tag sequencing) | Chromatin-chromatin interaction mediated by a protein of interest | Chromatin immunoprecipitation and ligation of the adjacent DNA ends followed by high throughput sequencing. | (Zhang et al., 2012) | |
| DNase-seq (DNase I hypersensitivity sequencing), FAIRE-seq (formaldehyde assisted isolation of regulatory sequences), or ATAC-seq (assay for transposase-accessible chromatin sequencing) | Open chromatin | Genome-wide mapping of open chromatin. Open chromatin can be selectively isolated by the following characteristics: high sensitivity to DNase I digestion (DNase-seq), segregation in aqueous phase upon phenol/chloroform extraction (FAIRE-seq), or preferential transposon integration. | (Crawford et al., 2006) (Giresi et al., 2007) |
2.1.1. Gene expression profiling
Transcriptome analysis is central to comprehensive understanding of complex biological systems such as photoreceptors (Swaroop and Zack, 2002). In RNA-seq assays, total RNA isolated from a cell type or a tissue of interest is converted to cDNA and deep-sequenced to obtain a comprehensive catalogue of transcripts (Mortazavi et al., 2008). Although mRNA-seq is most commonly conducted, genome-wide profiling of regulatory RNAs is also possible and provides an additional layer of information regarding gene regulation (Morin et al., 2008). RNA-seq offers many advantages over hybridization-based expression profiling (such as microarrays), permitting greater sensitivity and dynamic range in transcript detection (Zhao et al., 2014) and more accurate identification of alternatively spliced transcripts (Shen et al., 2014). As detection and quantification of transcripts is not limited to the current annotation, RNA-seq analysis enables discovery of novel genes and of new transcripts from annotated genes (Wang et al., 2009). Finally, RNA-seq can be performed even at the level of a single cell (Shapiro et al., 2013). With increasing accessibility and affordability, RNA-seq analysis is fast becoming a routine procedure replacing microarrays.
2.1.2. Transcriptional and epigenetic regulation
Gene expression is controlled by combinatorial action of diverse regulatory programs (Figure 2B). Interaction of transcription factors with specific cis-regulatory motifs is one of the primary mechanisms underlying temporal and cell type-specific gene regulation. ChIP, a widely used procedure to study transcription factor occupancy in vivo (Gilmour and Lis, 1985; Weinmann and Farnham, 2002), can now be applied in conjunction with tiling array (ChIP-on-chip) (Ren et al., 2000) or NGS (ChIP-seq) (Barski et al., 2007) to examine genome-wide binding of a specific protein. NGS-based methods (‘-seq’ assays) are now being used to delineate global profiles of chromatin states (such as histone modification, DNA methylation, nucleosome positioning and chromatin accessibility) and to identify novel fundamental mechanisms underlying gene transcription (see Table 1).
2.1.3. DNA sequence variation
The human genome includes millions of sequence variations, primarily in the non-coding regions; of these, tens of thousands may be unique to an individual (Schaibley et al., 2013), and many can even be linked to disease phenotype. Linkage analysis has traditionally been successful in identifying the disease variant/gene in monogenic RDDs, and genome-wide association studies (GWAS) have identified variants associated with complex traits. More recently, profiling of genetic variants using NGS methods, such as whole exome sequencing (WES) and targeted or whole genome sequencing (WGS), have transformed human genetic research. The identified variants/mutations can provide valuable insights into normal biology as well as elucidate mechanism(s) of disease pathogenesis. For instance, gene ontology analysis of 142 genes implicated in photoreceptor degeneration has identified a handful of biological pathways including those associated with cilia biogenesis, lipid metabolism, and phototransduction (Wright et al., 2010). Similarly, genetic variants associated with susceptibility to AMD have revealed the involvement of complement regulation, cholesterol transport and extracellular matrix remodeling in disease pathogenesis (Fritsche et al., 2014).
2.2. Data integration
Once high throughput data is collected, computational tools are employed to predict and construct GRNs. Although system-level measurements are made at discrete levels such as transcriptome, DNA-protein interaction, epigenome and genome, intermolecular interactions that form a network are generally not confined to a single category. Thus, the experimental data pertaining to distinct aspects of the network must be analyzed simultaneously and assimilated to identify new patterns of a biological system, which single data sets fail to detect.
Generation of networks by integrating multiple data sets necessitates that data are acquired under conditions of minimal variability; e.g., from a single cell type since cellular heterogeneity in the retina would create high transcriptional noise affecting the generation of a viable and verifiable GRN across development and disease states. Ideally, transcriptional and regulatory data for network formation should be obtained from a specific cell type at distinct stages of differentiation, aging or disease. Efforts have been made to isolate individual neuronal cells of the retina for genome-wide analysis. Such attempts, however, have been few and primarily limited to gene expression profiling (Akimoto et al., 2006; Siegert et al., 2012; Trimarchi et al., 2007). Table 2 lists public databases containing high throughput data relevant to studies of the retina.
Table 2.
Public databases containing clinical or high throughput retina data sets
| Database | URL | Description | Reference |
|---|---|---|---|
| Allen Brain Atlas | http://www.brain-map.org/ | Public resources integrating comprehensive gene expression and anatomical data from developing or adult mouse and human brain | (Hawrylycz et al., 2012; Lein et al., 2007) |
| BrainCloud | http://braincloud.jhmi.edu/plots/ | Database of temporal gene expression dynamics in the human prefrontal cortex across the lifespan | (Colantuoni et al., 2011) |
| EyeSAGE | http://neibank.nei.nih.gov/EyeSAGE/index.shtml | Resource for retina, RPE and trabecular meshwork transcriptomes | (Bowes Rickman et al., 2006) |
| Gene Expression Omnibus (GEO) | http://ncbi.nlm.nih.gov/geo | Public data repository of functional genomics data supporting MIAME-compliant data submissions | |
| Gene Expression Profile Database | http://www.fmi.ch/roska.data/index.php | Microarray data across diverse adult mouse retinal cell types | (Siegert et al., 2012) |
| High resolution fundus image database | https://www5.cs.fau.de/research/data/fundus-images/ | Database of fundus images of healthy eyes and eyes with diabetic retinopathy or glaucoma | |
| Human Retinal Transcriptome | http://oculargenomics.meei.harvard.edu/index.php/ret-trans | The human retina transcriptome data generated from three normal human retinas using RNA-seq | (Farkas et al., 2013) |
| mirNEYE | http://mirneye.tigem.it | Expression atlas of 221 miRNAs in the developing and adult wild type mouse eye | |
| Mouse Retina SAGE library | http://cepko.med.harvard.edu | Database of gene expression profiling of developing and adult mouse retina and a few other non-ocular tissues. In situ hybridization data are also available for select genes. | (Blackshaw et al., 2001; Blackshaw et al., 2004) |
| NEIbank | http://neibank.nei.nih.gov/index.shtml | Database of assembled EST data from ocular tissues of various organisms | |
| Retina Central (WEBER) | http://www.retinacentral.org/ | Database of genes experimentally shown to be expressed in the retina/the retinal pigment epithelium. | (Schulz et al., 2004) |
| Retina International | http://www.retina-international.org/sci-news/databases/mutation-database/ | Database of retinal diseases, mutations, animal models and disease-associated proteins | |
| Retinal Express | http://odin.mdacc.tmc.edu/RetinalExpress/ | E14.5 mouse retina cDNA/EST database | |
| RetinoBase | http://alnitak.u-strasbg.fr/RetinoBase/ | Microarray database generated from all publicly available retina-related gene expression profiles | (Kalathur et al., 2008) |
| RetNet (Retinal information network) | https://sph.uth.edu/retnet/ | Database of genes and loci causing inherited retinal diseases | |
| RP Gene Expression Atlas | http://rpexp.tigem.it/ | Collection of in situ hybridization data for retinitis pigmentosa genes in mouse and human retina | |
| STARE (Structured analysis of retina) | http://www.ces.clemson.edu/~ahoover/stare/ | Database of retinal images of various clinical manifestations | (Hoover and Goldbaum, 2003; Hoover et al., 1998) |
2.3. System comparison
Biological systems are highly dynamic and undergo progressive transition over time (Hwang et al., 2012). During development, intrinsic and extrinsic factors sequentially restrict cell fates and specify morphology and function most suited for a given cellular identity. Cumulative adaptive responses to internal and/or environmental challenges over a lifespan also impact spatio-temporal architecture of a system. Therefore, comparisons along time series data sets represent valuable strategies to assess system dynamics. Additionally, continuous fluctuations imposed by microenvironment and/or intrinsic genetic variations may induce changes beyond the cell tolerance level and manifest as a disease. The use of animal models for retinal traits or diseases provides a powerful tool to evaluate direct influence of genetic mutation(s) or experimental stress on networks as relatively little variation is expected in congenic strains raised under uniform conditions. Another important factor in system dynamics is variability among cell types exhibiting functional interaction such as between photoreceptors and retinal pigment epithelium (RPE); for example, defects in RPE are associated with photoreceptor dysfunction or death (Strauss, 2005). Even cells of the same neuronal type may display high variability based on temporal context and spatial organization (Trimarchi et al., 2007).
Change in system architecture can be ascertained from human population genetics using control individuals and patients with a specific disease/trait (e.g., AMD). Such genetic association studies exploit naturally occurring differences that cannot necessarily be generated by experimental means. However, it should be noted that the strength of data sets depends on the size of the cohort and penetrance of a specific variant/mutation. Elucidation of multifactorial complex biological phenomena, such as aging and pathogenesis of complex disease, can especially benefit from comparative system-level analysis using large NGS-based genomic data.
In the following sections, we will elaborate on system-level analysis of rod photoreceptors in three disciplines: development (section 3), aging (section 4) and degenerative disease (section 5).
3. System-level analysis of retinal photoreceptor development
Early attempts for system-wide assessment of photoreceptor development included gene expression profiling of the retina using expressed sequence tags (ESTs), serial analysis of gene expression (SAGE) and microarrays (Figure 3A) (Akimoto et al., 2006; Blackshaw et al., 2001; Gieser and Swaroop, 1992; Livesey et al., 2000; Sharon et al., 2002; Yoshida et al., 2004; Yu et al., 2003b). NGS-based methods have dramatically expedited the pace of expression profiling and permitted the application of gene regulation assays to genome-wide scale (Figure 3B). In addition to examining the whole retina, we can now develop quantitatively precise expression profiles of individual cell types and obtain global data on transcription factor binding and epigenomic marks. Multi-dimensional data integration should therefore enable us to identify novel patterns in GRNs that control functional architecture of individual retinal cells, such as photoreceptors.
Figure 3.
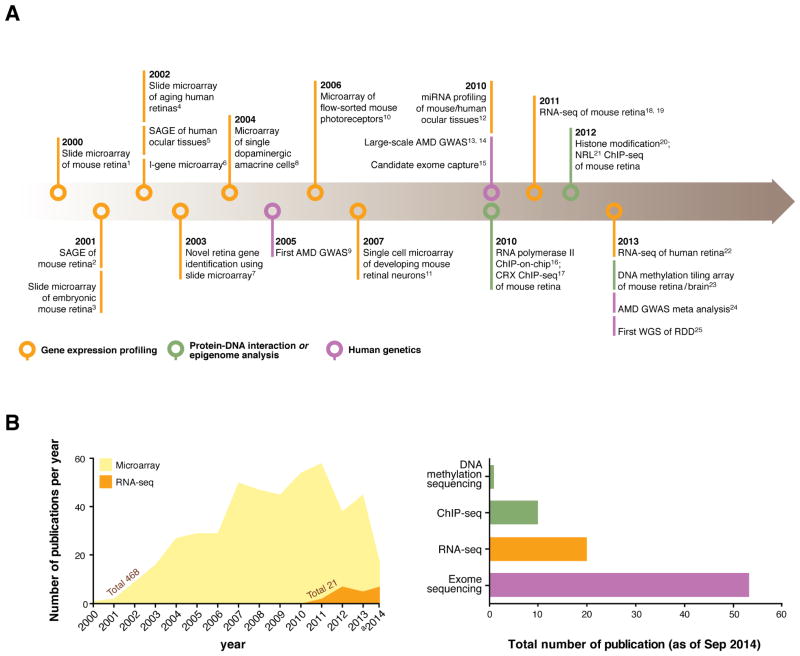
Timeline of genome-wide studies of the retina biology and disease pathogenesis. The advent of genome-scale profiling technologies has been a critical step for systems biology approaches. A, From the pioneering high throughput transcript analysis, such as the initial application of microarray and serial analysis of gene expression (SAGE) to whole genome sequencing, an ever-growing number of genome-wide studies have advanced our knowledge about healthy retina and disease pathogenesis. Highlights of such innovative genome-scale studies were selected and presented chronologically. B, For more than a decade, microarray has been a widely used methodology of choice for gene expression profiling, yielding a substantial number of publications each year. RNA-seq, deep sequencing of cDNA using NGS technology, is becoming more accessible and affordable and thus expected to be applied more widely. In addition to transcriptome analysis, NGS is applicable to a variety of other conventional research techniques and has already generated numerous data sets surveying whole exomes for genetic variation (whole exome sequencing), transcription factor targetome (ChIP-seq) and epigenome (ChIP-seq for histone modifications and various DNA methylome sequencing methodologies). 1(Livesey et al., 2000), 2(Blackshaw et al., 2001), 3(Mu et al., 2001), 4(Yoshida et al., 2002), 5(Sharon et al., 2002), 6(Farjo et al., 2002), 7(Chowers et al., 2003b), 8(Gustincich et al., 2004), 9(Klein et al., 2005), 10(Akimoto et al., 2006), 11(Trimarchi et al., 2007), 12(Arora et al., 2010; Hackler et al., 2010; Karali et al., 2010, Wang, 2010 #304), 13(Chen et al., 2010b), 14(Neale et al., 2010), 15(Otto et al., 2010), 16(Tummala et al., 2010), 17(Corbo et al., 2010), 18(Grant et al., 2011), 19(Brooks et al., 2011; Mustafi et al., 2011), 20(Popova et al., 2012), 21(Hao et al., 2012), 22(Farkas et al., 2013), 23(Oliver et al., 2013b), 24(Fritsche et al., 2013), 25(Nishiguchi et al., 2013), a number of publications until September 2014.
In the developing vertebrate retina, rod and cone photoreceptors differentiate from common pools of retinal progenitor cells with distinct temporal profiles. While cone generation ceases prenatally in rodents, rod birth spans a long time window from embryonic day (E)12 to postnatal day (P)10 with peak at P0-P2 (Carter-Dawson and LaVail, 1979; Rapaport et al., 2004). Photoreceptor identity is largely dictated by an intrinsic transcriptional program (Swaroop et al., 2010) that involves a number of transcription factors, including cone-rod homeobox protein CRX (Chen et al., 1997; Furukawa et al., 1997), neural retina leucine zipper protein NRL (Mears et al., 2001; Swaroop et al., 1992) and thyroid hormone receptor THRB (also called TRβ2) (Ng et al., 2001). CRX plays an essential role in photoreceptor development (Furukawa et al., 1999) by controlling the expression of both rod and cone genes (Corbo et al., 2010; Hennig et al., 2008). NRL and THRB, however, determine the genesis of rods (Mears et al., 2001) and medium wavelength sensitive (M) cones (Ng et al., 2011), respectively, from photoreceptor precursors that seem to be otherwise specified by “default” as short wavelength sensitive (S) cones (Swaroop et al., 2010). Other essential regulators of photoreceptor development include OTX2 (Nishida et al., 2003; Roger et al., 2014), RORB (Jia et al., 2009; Srinivas et al., 2006) and NR2E3 (Chen et al., 2005; Cheng et al., 2006; Cheng et al., 2011; Haider et al., 2000; Oh et al., 2008; Peng et al., 2005). These key transcriptional regulators constitute central nodes (“hubs”) in the GRN that controls photoreceptor development.
3.1. Cell type-specific approaches for generating GRN
Although few studies have been performed using purified photoreceptor cells, rod photoreceptor-specific information can be predicted by genome-wide data generated from the predominantly rod-containing mouse or human retina. Considering that NRL and CRX expression are largely photoreceptor-specific, ChIP-seq data for these two key transcription factors would broadly reflect photoreceptor biology. The same is not true for transcriptome and epigenome data obtained from the whole retina. Isolation of photoreceptors is necessary to acquire true photoreceptor-specific information. Cell type-specific approaches are even more critical for developmental studies since the proportion of photoreceptors in mouse retina continuously increases until P10, making it difficult to discern true cellular changes in gene expression or epigenetic marks. The first example demonstrating the importance of cell type-specific studies comes from microarray-based expression profiling of flow-sorted rod photoreceptors from Nrlp-GFP mice (Akimoto et al., 2006). The resulting rod-specific transcriptome data effectively detected many low level transcripts that had not been observed in the whole retina transcriptome data. Although many transgenic mouse lines expressing fluorescence reporters in specific retinal cell types have been described and are being used for transcriptome analysis (Siegert et al., 2012), identification of new cell type-specific markers (Koso et al., 2009) would greatly facilitate NGS studies of retinal neuronal subtypes.
An array of subtypes, each with unique morphology and function, can be recognized within major classes of retinal neurons (Masland, 2012). Single cell gene expression profiling has demonstrated a high degree of heterogeneity among individual retinal cells (Trimarchi et al., 2008). A better understanding of biological events thus requires delineation of GRNs specific not only at the level of individual subtypes but perhaps even at a single cell level. Due to limitations in obtaining sufficient material, conventional methods need to be miniaturized to gain enough sensitivity for single cells or cell types. However, technical hurdles including sample loss, degradation and contamination can have more pronounced impact on quality and robustness of data generated from single cells or small quantities versus large pools of cells. In addition, many rounds of PCR amplification may generate increased noise and inaccurate quantification. Nevertheless, cell type-specific or even single cell level analyses are necessary to extract gene regulatory information relevant to the cell of interest. Single cell collection methods based on micro-fluidic systems (Shalek et al., 2014), high-resolution imaging (Lubeck and Cai, 2012), and better protocols for constructing NGS libraries from small amount of starting RNA (Brooks et al., 2012; Tang et al., 2009; Tariq et al., 2011) have made it possible to attempt more robust expression profiling of individual retinal neurons. However, a major challenge remains for the generation of epigenomic and other NGS data sets with small number of cells. Our laboratory and others are currently developing such protocols (Adli et al., 2010; Guo et al., 2013; Smallwood et al., 2014).
The importance of using a purified single cell type for system-level analysis cannot be over-emphasized since biological processes such as development and aging have broad and concurrent impact on multiple cell types in a tissue and on the organism as a whole. Deciphering photoreceptor GRN from the whole retina studies would be complicated by transcriptional noise and differences in epigenome and regulatory molecules among different cell types. This is especially true for dissecting changes in GRNs during the pathogenesis of photoreceptor degeneration. As photoreceptor-specific data are not available for all NGS-based assays, we also cover studies from the whole retina in the discussion below.
3.2. Construction of photoreceptor GRN
Gene expression requires context-specific interaction among cis-regulatory DNA elements, basal transcriptional machinery and transcription regulatory proteins. In addition, transcriptional activity is under the influence of chromatin architecture as well as regulatory RNAs. Thus, basic elements needed to generate a rod photoreceptor GRN include:
Global mRNA profiling by RNA-seq (section 3.2.1)
Identification of target genes for cell specific transcription factors by ChIP-seq (sections 3.2.2 and 3.2.3)
Chromatin state such as histone modifications by ChIP-seq, DNA methylation profiling, and chromatin accessibility by DNase I hypersensitivity sequencing (section 3.2.4)
Regulatory RNA profiling (section 3.2.5)
Transcriptional profiles are also needed at multiple stages of rod development in order to build a developmental GRN. Gene expression profiling can be used in conjunction with gain- or loss-of function mutations of relevant “hub” transcription factors to further validate GRNs. For example, known hubs in the photoreceptor network such as NRL and CRX can be perturbed (e.g., in mouse mutants) before genome-wide gene expression analysis. The genes showing altered expression in the absence of a regulatory factor would represent direct or indirect (i.e., through secondary regulatory nodes) transcriptional targets. Additionally, GRN can also be inferred from co-expression data. Groups of genes that share similar expression signatures in response to experimental manipulation and/or during development would likely represent common gene regulatory pathways. Although such information is only suggestive, co-expression data allow for the identification of novel testable regulatory patterns.
3.2.1. Expression profiling
Global profiling of gene expression in the developing and mature retina was first accomplished by Affymetrix™ microarrays (Dorrell et al., 2004; Yoshida et al., 2004). Expression profiles have also been generated from flow-sorted rod photoreceptors (Akimoto et al., 2006; Parapuram et al., 2010) and from single retinal cells (Ma et al., 2013; Roesch et al., 2008; Trimarchi et al., 2007; Xue et al., 2011). However, NGS-based RNA-seq profiling has yielded more quantitatively precise information on mRNA transcripts in the mammalian retina (Brooks et al., 2011; Farkas et al., 2013; Gamsiz et al., 2012; Kandpal et al., 2012; Kozhevnikova et al., 2013; Roger et al., 2014). More recently, we have produced extensive RNA-seq-based temporal expression profiles of purified rod photoreceptors that reveal novel insights in cellular pathways (Figure 4A) (J-W Kim, H-J Yang and A Swaroop, manuscript in preparation) as well as additional modes of gene regulation, such as alternative splicing (Figure 4B).
Figure 4.
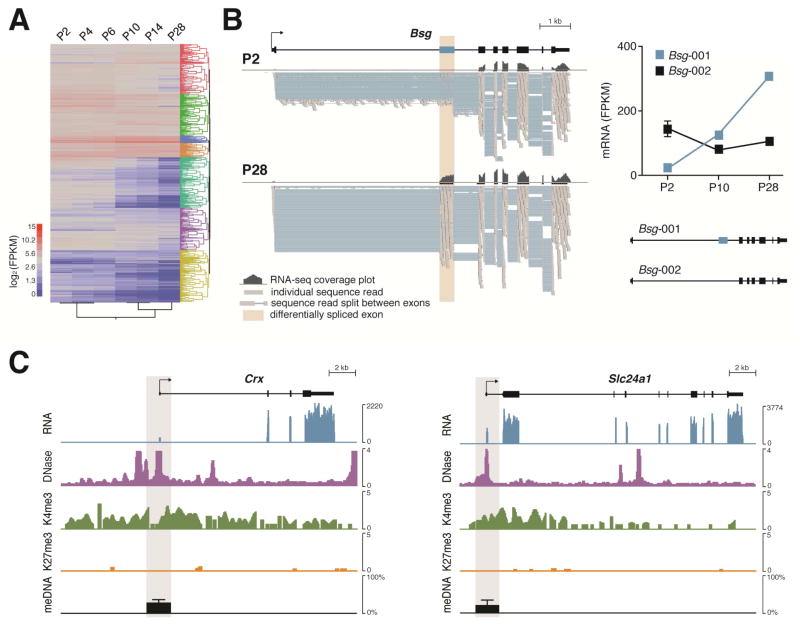
Cell-type specific system-level analysis. A, Heat map of time course RNA-seq data generated from isolated, developing (P2 to P14) and mature (P28) mouse rod photoreceptors. Dynamic gene regulation during rod photoreceptor maturation and clusters of genes with similar temporal expression patterns are apparent. Heat map was generated based on log2FPKM, and individual co-expression clusters were highlighted with different colors in the dendogram. FPKM, fragments per kilobase of exon per million reads. B, Transcript-level analysis of RNA-seq data enables detection of complex gene regulatory programs such as distinct splicing events during development. Alternative splicing of Bsg (basigin) gene during rod photoreceptor development is shown as an example. Coverage plots (dark grey histogram) and read alignments (grey blocks indicating individual sequence reads with thin blue horizontal lines connecting portions of sequence reads that are split between exons) show differential inclusion of the second exon (brown shade) in P2 and P28 rod photoreceptors. Expression level of each splice variant during rod development is plotted and shown on the right. C, System-level profiling of diverse chromatin signatures, including chromatin accessibility [DNase-seq (DNase I hypersensitivity sequencing)], active and repressive promoter histone modifications (H3K4me3 and H3K27me3, respectively; mini-ChIP-seq) and DNA methylation (bisulfite sequencing). Application of genome-wide analysis of chromatin states used to be limited to in vitro samples and pooled tissues of heterogeneous cell types. Bisulfite sequencing and ChIP-seq have now been miniaturized for flow-sorted single type of neurons, which are available in small amounts. Shown are active gene expression and hallmark of active chromatin state of two select photoreceptor-specific genes, Crx and Slc24a1, in P28 flow-sorted rod photoreceptors. Scale on y-axis of RNA, DNase, K4me3 and K27me3 tracks indicates RPM (reads per million reads). Average percent 5-methylcytosine (meDNA) within promoter (±1 kb from the transcription start site, highlighted with a grey shade) was plotted as a bar graph.
3.2.1.1. Perturbation studies
For elucidating GRNs, it is valuable to generate gene profiles after disrupting one of the key regulatory nodes. This strategy was first applied to Crx−/− mice (Blackshaw et al., 2001; Hennig et al., 2008; Livesey et al., 2000). CRX is essential for both cone and rod development (Furukawa et al., 1999); however, its role in cell fate determination is debatable because of overlapping functions with another homeodomain protein OTX2, which regulates the expression of CRX as well as NRL (Nishida et al., 2003; Omori et al., 2011; Roger et al., 2014; Terrell et al., 2012). A majority of genes with altered expression in Crx−/− mice showed photoreceptor enriched expression, and functional categorization of differentially expressed genes has revealed an array of CRX-regulated cellular functions, including those involved in phototransduction, metabolism, signal transduction and cytoskeletal components.
Another extensively studied gene regulatory program is the NRL-centered transcriptional network. The first observation that NRL is the master switch for generating rod cell fate came from studies of Nrl−/− mice as their retina completely lacks rod photoreceptors, with concurrent enhancement of S cone function (Mears et al., 2001). In concordance, ectopic expression of NRL in photoreceptor precursors or in early-born S-cones resulted in formation of functional rods (Oh et al., 2007). Gene profiling of Nrl−/− retina revealed little or no expression of rod-specific genes, including those associated with rod phototransduction, and a concomitant increase in the expression of S cone photopigment (encoded by Opn1sw) and other cone genes (Brooks et al., 2011; Yoshida et al., 2004; Yu et al., 2004a). Global expression analysis of purified photoreceptors from Nrl−/− retina has validated the essential role of NRL in activating the expression of rod genes and suppressing cone genes (Akimoto et al., 2006).
NR2E3, an orphan nuclear receptor, constitutes another important hub in photoreceptor GRN and is a direct transcriptional target of NRL (Bumsted O’Brien et al., 2004; Oh et al., 2008). Mutations in NR2E3 cause enhanced S cone syndrome in humans (Haider et al., 2000; Sharon et al., 2003; Wright et al., 2004), similar to the retinal phenotype exhibited by Nrl−/− mice (Mears et al., 2001). Retinal and rod gene expression profiling of rd7 mutant mice, in which Nr2e3 function is ablated, further validates NR2E3 function primarily as a suppressor of cone genes and as a co-activator (with NRL and CRX) of rod genes (Chen et al., 2005; Cheng et al., 2006; Cheng et al., 2011; Cheng et al., 2004; Corbo and Cepko, 2005; Haider et al., 2009; Oh et al., 2008; Peng et al., 2005).
3.2.1.2. Co-expression network analysis
Target genes of the a transcriptional regulator likely display similar expression patterns and may participate in common or related cellular functions (Yu et al., 2003a). Clusters of co-expressed genes can thus be used to model gene regulatory events. The inferred GRNs enable the detection of putative protein-DNA and protein-protein interactions without requirement of prior knowledge. Mathematical tools have been developed to predict GRNs from gene expression profile data, and putative regulatory relationships can then be validated experimentally (Hecker et al., 2009). Co-expression network inference thus represents a powerful analytical tool for expression profiling data, complementing the perturbation studies discussed above. As activity of a given transcriptional regulator is confined only to the same cell as its target, only the information obtained from a single cell or cell type provides accurate prediction without analytical noise caused by sample heterogeneity. Transcriptome analysis of flow-sorted rod photoreceptors from wild type and Nrl−/− retina has revealed multiple clusters of co-expressed genes, which likely represent diverse regulatory events (Akimoto et al., 2006). Co-expression analysis of newer and deeper RNA-seq data sets should provide valuable insights, including those relevant for identification of disease genes and pathways (see also section 5.4).
3.2.2. Transcription targets (“targetome”) of NRL and CRX
Differential gene expression analysis does not distinguish direct transcriptional regulation from secondary regulatory events. ChIP-seq analysis, on the other hand, assays direct physical interactions between a transcription factor and the cognate DNA elements. Recent studies have superimposed transcriptome and transcription factor targetome data to identify biologically relevant target genes of CRX (Corbo et al., 2010) and NRL (Hao et al., 2012). ChIP-seq analysis of mouse retina demonstrated CRX binding to 67% of mis-regulated genes in Crx−/− retina, likely representing direct CRX targets (Corbo et al., 2010). Similarly, only a subgroup (15%) of genes differentially expressed in Nrl−/− retina exhibited NRL ChIP-seq peaks and thus are direct transcriptional targets of NRL (Hao et al., 2012).
One of the challenges in deciphering targetome data is assigning transcription factor binding to relevant genes. A majority of NRL- and CRX-bound genomic DNA regions are in close proximity of or within the genes. However, binding of regulatory factors far away from genes is not uncommon and may exert transcriptional control by chromatin looping. Recently, high throughput reporter gene assays have successfully validated the enhancer activity of over 1,000 CRX interacting genomic loci (White et al., 2013). Similar large-scale enhancer assays and chromatin state mapping are expected to confirm the functional significance of distant ChIP-seq peaks. Integrated analysis with gene expression data and long distance chromatin association profiling [by chromatin conformation capture (3C) assay (Peng and Chen, 2011) and its NGS applications such as 4C-seq and HiC-seq] (see Table 1) will further facilitate the identification of novel transcription targets of key photoreceptor transcription factors.
ChIP-seq analyses have identified thousands of genomic loci (sequence regions) that are occupied by NRL or CRX in vivo, and both transcription factors bind to common enhancer regions that specify photoreceptor genes (Hao et al., 2012). In embryonic stem cells (ESCs), master transcriptional regulators are reported to form super-enhancers, atypical long enhancer clusters that are associated with unusually high level of mediators as well as enhancer-associated epigenetic marks (Whyte et al., 2013). Subsequent studies have demonstrated the importance of super-enhancers in many differentiated cell types (Hnisz et al., 2013) and their implications in developmental and neurological disease (Lee and Young, 2013). Further investigations are necessary to evaluate whether NRL- and CRX-bound enhancer elements exhibit characteristics of super-enhancers and whether variants in these elements are associated with retinal disease.
3.2.3. Dynamicity and combinatorial action of transcription factors
Most of the transcription factor targetome analyses have so far been conducted at a single time point, revealing only a snapshot of gene regulation. The function of transcriptional regulators, however, is highly dynamic (Davidson and Levine, 2008; Hwang et al., 2012). Transcriptional complexes are under continuous modulation by availability of co-factors and by chemical modifications at the level of protein, DNA and/or chromatin (Berger, 2007; Voss and Hager, 2014). Thus, the regulatory outcome can vary depending on cellular context such as developmental stage, cell identity and fitness. Comparative transcriptome analysis of the retina and of flow-sorted rod photoreceptors across developmental stages has revealed a striking variation in expression kinetics among photoreceptor genes controlled by NRL (Akimoto et al., 2006; Yoshida et al., 2004; Yu et al., 2004a). Nrl expression is initiated immediately after the final mitosis of photoreceptor precursors, leading to expression of a large number of target genes including Nr2e3. Notably, a number of NRL target genes, including visual pigment rhodopsin (encoded by Rho), exhibit substantial delay in expression during rod photoreceptor development.
Although underlying factors for differential onset of expression of NRL target genes are not completely understood, analyses of multiple data sets suggest additional control mechanisms. One of the possible scenarios can be inferred by linking NRL targetome data with gene expression profiles and with previously identified retinal disease loci (Hao et al., 2012). NRL target genes with putative functional relevance can be selected based on the assumption that such genes are likely associated with rod function and cause retinal degeneration when mutated. Such analyses have identified histone demethylase KDM5B as a secondary regulatory node, and Kdm5b knock-down partially phenocopies the rod-to-cone transition observed in Nrl−/− retina (Hao et al., 2012). KDM5B is one of the histone demethylases implicated in gene repression through demethylation of H3 lysine 4 trimethylation (H3K4me3), a histone mark associated with transcriptional activation. Thus, it can be hypothesized that NRL function is mediated through multiple as yet unidentified secondary nodes (including epigenetic factors) and that other regulatory mechanisms may define precise temporal control of gene expression. Functional interaction between NRL-centered transcriptional network(s) and the epigenetic program will be further elaborated in the following section 3.2.4.
Another important feature of GRNs is combinatorial actions of transcriptional regulators (Davidson and Levine, 2008). Numerous pre-genomic approaches have reported cooperative functions among photoreceptor transcription factors. Interaction between NRL and CRX exerts synergistic effects on transcriptional activation of rod-specific genes (Mitton et al., 2000; Pittler et al., 2004; Yoshida et al., 2004). As part of the same complex, NR2E3 exhibits dual function by inducing rod and suppressing cone gene expression (Oh et al., 2008; Webber et al., 2008), thereby constituting a critical secondary transcriptional regulatory node (Chen et al., 2005; Cheng et al., 2006; Cheng et al., 2004; Haider et al., 2000; Peng et al., 2005). Compiling system-wide surveys of putative target genes for these three regulators has revealed significant overlaps in their targets (Hao et al., 2012; Qian et al., 2005; Yu et al., 2006), suggesting that combinatorial functions among transcription factors exert tight regulation of photoreceptor development and homeostasis. Salient features of NRL transcriptional regulatory network have been assembled (Hao et al., 2012), and further computational analyses of existing and new data sets are expected to provide a comprehensive map of rod photoreceptor GRNs (Hwang et al., 2012; Wan et al., 2013).
Combinatorial action of regulatory factors is highly context-dependent and cell type-specific, and the composition of transcriptional complexes is expected to influence the selectivity of downstream target genes. For instance, although CRX expression is common to both rods and cones, transcriptional regulatory outcomes show a striking difference. CRX cooperates with RORB to induce S cone photopigment (Opn1sw) expression in cone precursors (Srinivas et al., 2006), whereas, in the presence of NRL and NR2E3, CRX enhances rod-specific gene expression in rod photoreceptors (Hao et al., 2012). ChIP-seq analysis has shown that OTX2-bound cis-regulatory elements are quite different in RPE and neural retina (Samuel et al., 2014). Only retina-specific OTX2 target sites, but not RPE-specific ones, show redundancy with CRX occupancy, further demonstrating the importance of combinatorial transcriptional programs in cell fate specification.
3.2.4. Chromatin state and gene regulation
DNA within a cell is packaged into DNA-protein complexes (termed chromatin), which constitute a dynamic structure. DNA itself and the histones, which are primary protein components in the chromatin, are subjected to chemical alterations that can influence transcriptional activity. Regulation of chromatin state is an important mechanism for modulating biological states, including lineage restriction and cell fate specification during development (Hirabayashi and Gotoh, 2010; Meshorer and Misteli, 2006; Sasaki and Matsui, 2008; Wan et al., 2013). Studies of epigenetic marks such as DNA methylation and posttranslational modifications of histones in the retina have revealed cell type- and stage-specific chromatin states, recapitulating context-dependent differential gene regulation.
3.2.4.1. DNA methylation
DNA methylation at gene promoters has been negatively associated with gene expression and is considered a key mechanism for maintenance of chromatin state (Suzuki and Bird, 2008). Integration of global DNA methylation profiles with gene expression is critical for deciphering the overall structure of a GRN. System-level analysis of DNA methylome has implicated differential 5-methylcytosine in altering gene expression during photoreceptor degeneration in rd1 retina (Farinelli et al., 2014) as well as in tissue-specific splicing in the retina and the brain (Wan et al., 2013). More restricted approaches examining selected genes further support the association of DNA methylation with photoreceptor gene regulation. Bisulfite sequencing of targeted genomic areas from WERI and Y9 human retinoblastoma cell lines and laser-capture micro-dissected mouse photoreceptors and non-photoreceptor cell types has revealed photoreceptor-specific cytosine hypomethylation within opsin genes such as Rho, Opn1sw and Opn1mw (Merbs et al., 2012), demonstrating cell type-specific patterns of DNA methylation. Conditional knock-out of DNA methyltransferase 1 (Dnmt1) shows lack of outer segments despite nearly normal expression of phototransduction and cilia genes in photoreceptors (Nasonkin et al., 2013), suggesting a broad impact of DNA methylation on morphological specification.
Contrary to rapidly growing number of gene expression profiling studies, only few genome-wide DNA methylome profiles have been generated for the retina (Figure 3B). New highly sensitive detection assays for 5-methylcytosine and 5-hydroxymethylcytosine (see Table 1)(Oliver et al., 2013b; Smallwood et al., 2014) and with single cell-type resolution (Powell et al., 2013) are expected to facilitate such investigations at the level of a single retinal neuron. We have recently completed the generation of DNA methylome for flow-sorted rod photoreceptors and are examining its relationship to RNA-seq and histone modification data sets (Figure 4C) (H-J Yang, J-W Kim and A Swaroop, manuscript in preparation). Further technical improvements should therefore unravel system-wide interactions between DNA methylation and cell type-specific dynamics of gene expression.
3.2.4.2. Histone modifications
More than 60 amino acid residues on different histones undergo diverse posttranslational modifications including acetylation and methylation (Kouzarides, 2007; Zentner and Henikoff, 2013). While histone acetylation is almost strictly associated with active gene expression, methylation represents more complex modes in gene regulation. Transcriptional activity is shown to depend on the degree of methylation – mono-, di-, and trimethylation, the specific modified amino acid residue and associated DNA elements (e.g., promoter, gene body, enhancers). Among multitudes of reported histone methylations, H3K4 and H3K27 methylation are closely correlated with transcription status and thus constitute the most commonly examined histone modifications. Promoters of active and silent genes are enriched with H3K4me2/me3 and H3K27me3, respectively, while active and repressive enhancers are demarcated with H3K4me1/H3K27ac and H3K4me1/H3K27me3, respectively. Dynamic regulation of histone methylation patterns in rod or cone photoreceptors is expected to have significant impact on photoreceptor GRNs. ChIP-seq is fast becoming a routine procedure for genome-wide profiling of histone modifications. Histone signature, however, is highly heterogeneous across distinct cell types (ENCODE Project Consortium, 2012); thus, cell type-specific histone modification profiling is necessary to obtain useful information on the chromatin state. Purified cells do not easily meet the requirement of sample size for reported epigenome profiling assays as yet.
The whole retina histone ChIP-seq performed with appropriate control tissue such as rd1 mutant retina, a degenerative mouse model that lacks photoreceptors, has offered significant photoreceptor-specific epigenomic information as rod photoreceptors account for a vast majority of retinal neurons in mature retina (Popova et al., 2012). In this study, genome-wide signatures of H3K4me2 and H3K27me3 in the mouse retina were mapped and compared with transcriptome at various developmental stages. Distinct clusters of chromatin modification dynamics were identified among rod-specific genes (Popova et al., 2012). Surprisingly, rod genes showed varied H3K4me2 signature. While some rod genes have a detectable level of H3K4me2 as early as E17.5 and at all subsequent developmental stages, another group of rod genes including phototransduction genes shows delayed de novo accumulation of H3K4me2 from P7 to P15. Concordantly, phototransduction genes gain full chromatin accessibility only after P10 as revealed by DNase I hypersensitivity sequencing (DNase-seq) of the mouse retina (ENCODE, www.mouseencode.org). ChIP experiments have demonstrated a limited interaction of NRL with cognate DNA binding site in Rho at P2 (Hao et al., 2011), suggesting that inactive chromatin state at Rho and other phototransduction genes may interfere with NRL binding to its target genes in newborn rods. These results, however, should be interpreted with caution as the detected change in histone marks, chromatin accessibility and NRL recruitment may simply reflect a dramatic increase in the proportion of rod photoreceptors in the retina from P2 to P28. Assays using purified rod photoreceptors offer an ideal alternative. Our initial analysis of histone ChIP-seq data from purified rod photoreceptors has revealed promising insights into rod-specific histone signature (Figure 4C) (H-J Yang et al., manuscript in preparation).
3.2.5. miRNA and other transcribed sequences
Non-coding RNAs (ncRNAs) constitute as much as 80% of the human transcriptome (Rosenbloom et al., 2013) and contribute additional complexity to GRNs (reviewed in (Mortimer et al., 2014). Small ncRNAs (<200 bp) include microRNAs (miRNAs) of 18-24 nucleotides. Long ncRNAs (lncRNAs) are over 200 bp in length but can be up to hundreds of kb. Each of the reported 2,000-3,000 miRNAs (miRBASE Release 21, June 2014) can regulate hundreds of target genes, and each mRNA can be targeted by several miRNAs, fine tuning gene expression of an estimated 30–80% of the human genome (John et al., 2004; Lim et al., 2005; Lu and Clark, 2012). Tens of thousands of lncRNAs are expressed at low levels in a tissue-restricted manner and believed to regulate the expression of related genes associated with most biological processes in a given cell (Li and Chang, 2014).
Although photoreceptor-specific profiling of ncRNA species is yet to be conducted, dynamic regulation of specific ncRNAs has been reported during retinal development and disease. In silico, RT-PCR and in situ hybridization studies have revealed spatio-temporal expression of miRNAs in the developing and mature retina (Arora et al., 2010; Deo et al., 2006; Hackler et al., 2010; Karali et al., 2010; Ryan et al., 2006; Xu, 2009). In addition, miRNAs exhibit altered expression during circadian cycle (Krol et al., 2010; Xu et al., 2007), cell survival (Damiani et al., 2008; Lumayag et al., 2013; Sundermeier et al., 2014), and in disease conditions (Loscher et al., 2008; Shen et al., 2008). The effect of miRNAs at the intersection of several GRN is exemplified by the “miR-96, miR-182 and miR-183” cluster, which contributes to circadian regulation of gene expression in the retina and has a more general role in neurosensory cell-specific GRNs (Xu et al., 2007).
Eighteen lncRNAs are highly conserved in mammalian eyes; of these, fourteen are expressed in the macular region and others in rest of the retina (Mustafi et al., 2013). Furthermore, lncRNAs are shown to play a role in retinal development (Rapicavoli and Blackshaw, 2009); e.g., in regulation of cell cycle (Vax2os1), modulation of transcription factor activity (Six3OS), and specification of cell fate (RNCR2) (Meola et al., 2012; Rapicavoli et al., 2011), highlighting their potential involvement in GRNs. As transcriptome databases expand, functional studies of existing and newly-identified small and lncRNAs will provide novel insights into their role in fine-tuning GRNs.
3.2.6. Proteome analysis
Transcriptome analysis does not accurately reflect protein expression in a cell or tissue since protein content is also regulated by translational and post-translational events and reflects the equilibrium between synthesis, stability and degradation. Complex protein-protein interactions add another level of complexity to how cellular physiology and homeostasis are controlled. Protein profiles of the retina (Barnhill et al., 2010; Finnegan et al., 2008) have been generated using mass spectrometry, validating the presence of a multitude of retinal proteins as previously predicted by microarray results. Studies have also reported photoreceptor-enriched proteome acquired from planar cryosections of the photoreceptor layer (McKay et al., 2004) or rod outer segment disk purification (Elliott et al., 2008; Kwok et al., 2008; Panfoli et al., 2008). However, global proteomic methodologies are more qualitative than quantitative and not suitable for the analyses that require high detection sensitivity and comprehensiveness as in genomics approaches. Various micro-scale protein detection techniques are being developed for protein analysis. Quantification of protein expression using nanopore technology (Wei et al., 2012b), single protein molecule counting on a two-dimensional surface (Tessler et al., 2009), and single cell western blotting (Hughes et al., 2014) are among promising new technologies, which can potentially be adapted for increased throughput.
A targeted proteomic approach has now begun to provide insights into photoreceptor gene regulation. A recent study focused on NRL-containing transcriptional complexes and identified the transcription-splicing protein, NonO, and other binding partners of NRL as important regulators of rod gene expression (Yadav et al., 2014). We should note that translation of mRNA into protein has been successfully monitored indirectly through ribosomal profiling (Ribo-seq) (Ingolia et al., 2009). Application of newly evolving proteomic approaches would also be valuable to complement the gene expression profiling and epigenome data. More targeted or global proteomic analysis is expected to completely define photoreceptor GRNs.
4. System-level analysis of retinal aging
Aging in higher organisms reflects the interplay of genetic factors with the environment and influence of stochastic events (Figure 5), requiring investigation(s) of interaction networks underlying biological processes rather than focus on a single gene or protein (Kriete et al., 2006). Although aging does not cause a clinical disease per se, phenotypes of aging can resemble disease phenotypes. Conversely, aging-associated changes increase vulnerability and instability of the system, making it more susceptible to further damage and to the effects of genetic mutations. Thus, aging is a leading risk factor for several common late-onset diseases. In this section, we explore genome-wide studies of aging as a time-dependent event relevant for eye function and pathology, with focus on the retina.
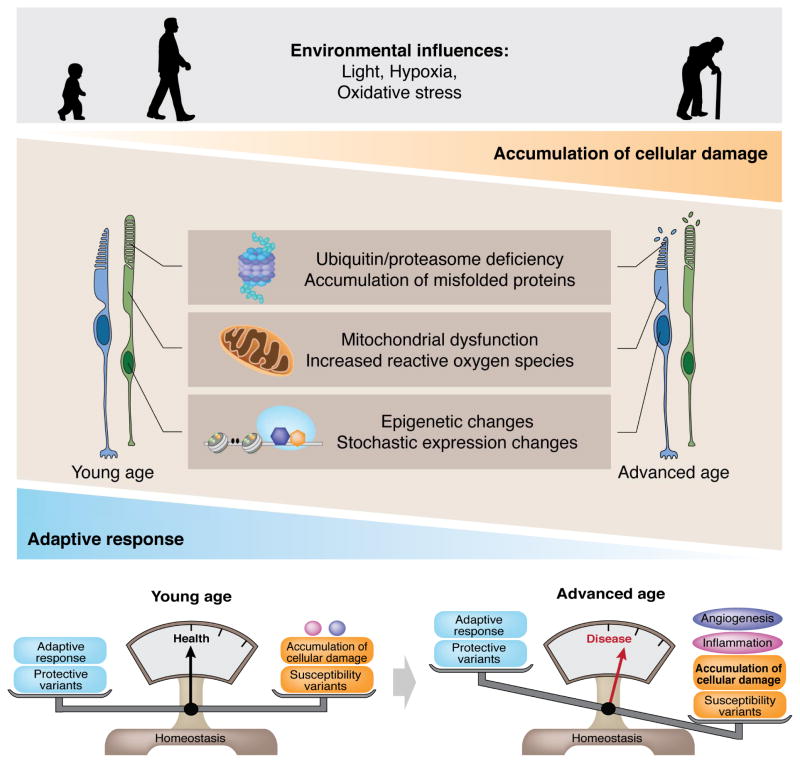
Figure 5.
Interface between aging and disease. The environment influences photoreceptor homeostasis throughout life. The cell adaptive response helps maintain a balanced homeostasis. As the adaptive response becomes insufficient to overcome “insults” to the system, damage accumulates in aging photoreceptors. Major metabolic failure is observed for the ubiquitin-proteasome system and mitochondria. Epigenetic changes and stochastic changes in gene expression combined with the presence of susceptibility variants, eventually tilt the equilibrium towards the disease state. At which point, inflammation and angiogenesis become pathologic, further aggravating disease manifestations.
Each component of the eye contributes differently to overall decline in visual function with aging. Progressive deterioration in retinal anatomy (Curcio, 2001; Curcio et al., 1993; Gao and Hollyfield, 1992) and psychophysical parameters (Birch and Anderson, 1992; Bonnel et al., 2003; Freund et al., 2011; Shinomori and Werner, 2012) is observed with advanced age. Structural and functional changes have also been reported in photoreceptors (Curcio, 2001; Gresh et al., 2003; Kolesnikov et al., 2010; Parapuram et al., 2010; Samuel et al., 2011).
4.1 Expression profiling of aging retina
Systems biology of the aging retina starts with gene expression profiling to detect chronological events that alter cellular homeostasis (Table 3). Early studies were performed using SAGE (Sharon et al., 2002) or microarray platforms (Cai et al., 2012; Chowers et al., 2003b; Yoshida et al., 2002) on post-mortem human retinas, which have inherent variability associated with individual genetic variations, pre- and post-mortem conditions, and quality of RNA. Little to no concordance among studies was observed at the gene level, due to differences in samples, platforms and analyses. However, when genes are considered in the context of ontologies and gene networks, characteristic patterns begin to emerge in the aging retina, suggesting a general metabolic slow down (Cai et al., 2012; Chowers et al., 2003b; Yoshida et al., 2002) and decrease in expression of phototransduction genes (Chowers et al., 2003b; Sharon et al., 2002). Genes associated with stress response and inflammation are generally up-regulated (Cai et al., 2012; Chowers et al., 2003b; Yoshida et al., 2002), thus highlighting manifestations of retinal aging that are shared with the brain (Lee et al., 2000). Notably, there appears to be a trend towards increased expression in older age of extracellular matrix genes, with relevance to the ontogenesis of AMD (Chowers et al., 2003b) and differential aging mechanisms in the macula compared to the peripheral retina (Cai et al., 2012).
Table 3.
Gene expression profiling studies of aging ocular tissues
| Organism | Tissue/cell type | Platform | Number of genes | Age range | Reference |
|---|---|---|---|---|---|
| Human | Neural retina/macula and periphery | SAGE | 320,998 tags | 44–88 yr | (Sharon et al., 2002) |
| Human | Neural retina | Microarray, Micromax | 2,400 probes | 13–72 yr | (Yoshida et al., 2002) |
| Human | Neural retina | Microarray, Custom | 10,034 genes | 29–90 yr | (Chowers et al., 2003a) |
| Human | Neural retina/macula and periphery | Microarray, Affymetrix Human Genome U133 plus 2 | 54,600 gene probes | 18–79 yr | (Cai et al., 2012) |
| Mouse | Neural retina | Microarray, Agilent Technologies, Whole Mouse Genome Oligo Microarrays 4x44K | 43,379 gene features | 3–20 mo | (Chen et al., 2010a) |
| Mouse - senescence | Neural retina | Microarray, Affymetrix MG_U74Av2 GeneChip | 36,000 full-length genes and EST clusters | 3–21 mo | (Carter et al., 2005) |
| Mouse | Rod photoreceptors (flow-sorted Nrlp-EGFP retina) | Microarray, Affymetrix Mouse Exon 1.0ST Array GeneChip | >28,000 coding, >7,000 non-coding transcripts | 1.5–12 mo | (Parapuram et al., 2010) |
| Mouse | Microglia (flow-sorted, CD11b-immunopositive cells) | Microarray, Affymetrix Mouse Exon 1.0ST Array GeneChip | >28,000 coding, >7,000 non-coding transcripts | 3–24 mo | (Ma et al., 2013) |
| Rat - senescence | Neural retina | RNA-Seq, Illumina GA IIx | 15,300 identified transcriptsa | 3–18 mo | (Kozhevniko va et al., 2013) |
Approximate number of transcripts detected in at least three samples with FPKM>1
Some of the variability reported by the human studies can be overcome by molecular studies of aging in mouse. Comparison of two senescence-accelerated mouse prone strains (SAMP8 and SAMP10) to the senescence-accelerated mouse resistant strain (SAMR1) and the commonly inbred C57Bl/6J strain using Affymetrix™ oligonucleotide microarrays (Table 3) has highlighted the strong genetic background effect on the transcriptional response to aging (Carter et al., 2005). Expression of genes involved in inflammation and response to injury/stress is higher in aging mouse retina (Chen et al., 2010a), in concordance with the studies on human aging and with SAMR mice.
A limitation of gene expression data in the aging retina is that it represents average changes in six neuronal and two glial cell types, which contribute differently to tissue homeostasis. While these data may provide insights into the behavior of the system at the tissue level, expression profiling of the aging mouse rod photoreceptors (Parapuram et al., 2010) and of microglia (Ma et al., 2013) has expanded our understanding at the cellular level. Notably, genes associated with inflammatory signaling and stress response pathways constitute predominant hubs in network analysis of photoreceptor aging data (Parapuram et al., 2010). Furthermore, expression of genes involved in oxidative phosphorylation is reduced in aging photoreceptors (J. Barb, N. Gotoh, J-W Kim, T. Cogliati and A. Swaroop, manuscript in preparation), highlighting an age-related pathway that was not detected by whole retina studies but is a common feature of aging (de Magalhaes et al., 2009). Finally, angiogenesis and retinoid/lipid metabolism (regulated through signaling from the retinoic acid receptor) have emerged as unique pathways to rod photoreceptor aging (Parapuram et al., 2010). Focusing on photoreceptors as a single cell type, the question is whether gene expression changes observed in aging are intrinsic or an adaptive response to modification of retinal microenvironment, and whether these changes are protective, permissive or causative of the functional decline (Parapuram et al., 2010). Transcriptome analysis of isolated aging microglia shows changes in genes involved in cell metabolism, shape and motility, and in neurotrophic signaling, which could compromise the supporting role of microglia in the retina (Ma et al., 2013). Furthermore, expression changes in genes associated with microglia activation and inflammatory signaling suggest a contribution to the general retinal neuro-inflammatory process (von Bernhardi et al., 2010; Xie et al., 2003). As in case of rod photoreceptors, expression changes also affect genes involved with lipid metabolism and angiogenesis. The latter underscores the complexity of angiogenic events in AMD, in which signaling from multiple cell types, including RPE, photoreceptors and microglia, may converge to determine pathological changes, further demonstrating the importance of approaching retinal aging from a systems biology perspective.
From existing expression data, it is reasonable to assume that aging involves small changes in several genes that contribute to common pathways rather than major changes in individual genes. It becomes therefore critical to detect even small expression shifts with good statistical confidence. RNA-seq using NGS, with much higher reproducibility and greater dynamic range compared to microarray, is the ideal platform for more in depth molecular studies of retinal aging. RNA-seq has been employed in expression studies of human and mouse retina, however, not in relation to aging (Brooks et al., 2012; Farkas et al., 2013; Gamsiz et al., 2012; Li et al., 2014). One RNA-seq study so far published in the aging retina investigates the senescence-accelerated OXYS rats compared to Wistar controls (Kozhevnikova et al., 2013). Because of the profound pathological phenotype observed in the OXYS rats, it is difficult to distinguish aging from overt strain-dependent disease. However, differential expression of genes between 3 and 18 month old Wistar rats shows significant down-regulation of genes associated with extracellular matrix, response to nutrient levels and aminoacyl-tRNA biosynthesis, together with up-regulation of genes encoding negative regulators of transcription and components of synaptic transmission pathways. Immune response genes appear in both up-regulated and down-regulated gene ontologies, suggesting potentially impaired immune function in the aging rat retina, similar to the findings by microarrays in mouse and human.
4.2. Limitations and potential of aging studies in the retina
The scenario emerging from gene expression analyses of the aging retina is one of a tissue that is metabolically slowing down, activates stress and signaling pathways, and presents a complex immune and inflammatory activity. Because of the nature of aging, data collection from the same individual over time would be ideal but it is not a viable option for the retina. For human studies, a random selection of subjects from different age groups (post-mortem donors) suffers from large individual variations that can only be overcome if enough replicates can be collected and if experimental variables are minimally altered. This underscores the importance of strict collection and transportation protocols that minimize the time from death to enucleation and processing. Eliminating technical variability becomes even more critical if one wants to take an unbiased approach and use principal component analysis (PCA) to partition on the first component of the samples between young and old.
RNA-seq has revealed the expression of novel transcripts and genes in the retina (Farkas et al., 2013; Gamsiz et al., 2012; Li et al., 2014) suggesting the need to extend this analysis to aging individual cell types in mouse and, where possible, in human. In depth analysis of RNA-seq data will allow the identification and study of emerging modulators of aging such as regulatory ncRNAs described in section 3.2.5 (reviewed in (Montano and Long, 2011)). Reproducibility of RNA-seq will also facilitate compilation of different studies to increase sample number and statistical significance. Finally, comprehensive cross-comparisons of existing databases (Table 2) may provide deeper insight than that achieved by individual analyses.
5. Systems biology of retinal degeneration
Retinal degenerative diseases (RDDs) are genetically heterogeneous, ranging from monogenic forms such as retinitis pigmentosa to complex disorders such as AMD. Since the first linkage analysis that identified a genomic locus associated with X-linked retinitis pigmentosa (Bhattacharya et al., 1984), we have witnessed a remarkable progress in elucidating the genetic basis of retinal diseases ((Swaroop and Sieving, 2013); RetNet, http://sph.uth.edu/retnet/). Photoreceptor cell death remains central to many RDDs (Wright et al., 2010). Functional analyses of the identified disease genes through cell-based and animal models have enhanced our understanding of their normal function in ocular development and homeostasis. However, elucidation of underlying mechanism(s) has not kept pace with disease gene identification.
The advent of NGS is now transforming, at an unprecedented rate, the way we study genetic basis of human disease. Holistic approaches to RDDs have become possible through a combination of NGS-based strategies including disease-associated variant identification, transcriptome analysis, and high throughput search for biomarkers (Figure 6). We can learn about regulatory networks, disease-relevant pathways and their crosstalk, which can be invaluable for translational and personalized medicine. In this section, we discuss the following areas, which will provide the necessary impetus:
Figure 6.
Integration of multi-level data sets for system-level understanding. Simultaneous analysis of genomics, transcriptomics and proteomics data can provide a comprehensive multidimensional view of the system and can help identify pathways and networks that are specific to development and disease. This can lead to better understanding of the biology as well as to identification of disease associated nodes, which can be potential drug targets (e.g., hub depicted as a purple node in the example network).
Whole exome and whole genome sequencing (WES and WGS) of patients with monogenic RDDs for better genotype-phenotype correlation and search for genetic modifiers
Fine mapping and functional dissection of associated loci
Genetics of RDDs beyond genetic variants
5.1. NGS applications for disease variant/mutation discovery
NGS methods have expedited the process of cataloging human genetic variation. While precise correlation of variants associated with disease phenotypes is still challenging, NGS approaches, such as WES, WGS and targeted sequencing, have become preferred methods for disease gene discovery (Figure 7A). A number of novel genes have been identified through WES in small Mendelian RDD families and in sporadic cases that were not suitable for customary linkage studies (Ratnapriya and Swaroop, 2013).
Figure 7.
General recommendations for genetic analysis of monogenic and complex diseases using next-generation approaches. A. Families with a monogenic (i.e., Mendelian) disease, even those with a small number of individuals, can be analyzed by whole exome or genome sequencing. In case of identification of mutation(s) in a novel gene, additional validation is required, including genetic analysis of more families/patients as well as functional analysis using animal models to understand the role in disease causation. For complex diseases, genome wide association study is performed on a large number of samples including patients and population-matched controls, to identify common susceptibility variants associated with the disease. Targeted sequencing, whole exome or genome sequencing can be applied to identify the causal variant at the susceptibility locus. Finally, multiple molecular genetic and cellular assays as well as studies in animal models are required to identify functional variants underlying trait association. WES, whole exome sequencing; WGS, whole genome sequencing, GWAS, genome wide association study. B. Schematic representation of follow-up on a GWAS hit to identify the causal gene/variant. Causal variants could be common regulatory variants, rare coding variants or copy number variants. Targeted sequencing around the associated locus can help identify the causal variant. Generally, more than one candidate gene is found at an associated locus, and eQTL analysis in disease relevant tissues can help identify the specific genotypes affecting gene expression within the causal gene. Additional functional annotation, including histone modifications, transcription factor binding sites and DNase I hypersensitivity sites can also help in identifying the causal gene. Ultimately, a high throughput functional assay in animal and/or cell-based models is highly recommended to understand the role of the gene in disease pathophysiology. cM/Mb, centimorgan (cM) per megabase pair (Mb); eQTL, expression quantitative trait locus, TF, transcription factor.
Progress in dissecting genetic architecture of complex RDDs has been relatively slow since these multifactorial diseases are more common in the population and often do not exhibit familial segregation. Furthermore, non-genetic factors, such as environment, can contribute to disease onset and severity. We have come a long way since the initial association of CFH variant with AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Jakobsdottir et al., 2005; Zareparsi et al., 2005a). A total of 19 AMD susceptibility loci have been detected so far, including 7 new loci identified in the meta-analysis of 17,000 cases and 60,000 controls (Fritsche et al., 2013). These discoveries have provided clues about the underlying disease processes and have implicated complement cascade, high-density lipoprotein cholesterol and extracellular matrix pathways in AMD pathogenesis (Fritsche et al., 2014; Priya et al., 2012). However, associated locus variants are usually not causal and often reside outside the gene region. Thus, more than one gene at each associated locus can be potential disease gene. The nature of causal variants/genes at these loci remains debatable and needs further explorations as the causal variants could be an associated variant, another common variant, a rare variant or even a structural variant (Figure 7B).
Identification of rare variant(s) in a specific gene at the associated locus can provide functional clues. For AMD, a rare penetrant mutation, R1210C was first identified at the CFH locus (Raychaudhuri et al., 2011). Subsequently, rare variants in several complement pathway genes have been associated with AMD through targeted and whole genome sequencing (Helgason et al., 2013; Seddon et al., 2013; van de Ven et al., 2013; Yu et al., 2014; Zhan et al., 2013). However, the search for rare variants in RDDs has been limited to known loci and sequencing of the candidate association regions. Whole exome and whole genome sequencing studies in large patient cohorts may facilitate cataloging of rare and common causal variants, identification of novel disease genes and assist in biological investigations. However, rare variant association study design requires a large sample size since, as predicted, not many individuals will harbor a rare variant. Genetic refinement of associated signals with improved imputation methods can further help in identifying potential causal variants. Recently, a new SNP array (“exome-chip”) was developed to explore the role of common as well as rare variants in complex traits (Huyghe et al., 2013). Sixteen additional AMD loci have now been identified by exome-chip analysis and imputation of variants in approximately 26,000 cases and 22,000 controls (Cipriani and International AMD Genomics Consortium., 2014; Fritsche and International AMD Genomics Consortium., 2014)(International AMD Genomics Consortium, manuscript in preparation). Notably, only a small number of rare variants were identified in this study, and whole genome sequencing might be necessary for evaluating the role of rare variants in AMD.
5.2. Less-recognized complexities associated with genetic variations and human retinal diseases
Human genome harbors a large number of genetic variations, and NGS has made it possible to systematically catalog genome-wide sequence variants. However, methods for distinguishing a neutral variant from the causal one are not yet fully established. The current criteria include nature of change, allele frequency and nucleotide conservation across species. However, these criteria do not seem sufficient as we are learning more about the architecture of variants in the human genome. A single human genome can harbor thousands of variants (10,000–11,000 non-synonymous and 10,000–12,000 synonymous variations)(Genomes Project et al., 2012), with as many as 300 loss-of-function alleles having little or no clinical consequence (Kukurba et al., 2014; MacArthur et al., 2012). Incorrect assignment of variants can have immediate implication for diagnosis, therapeutic advice and research direction. The recommendations for identifying causal genes/variants associated with specific phenotypes through NGS studies have been reviewed recently (Chakravarti et al., 2013; MacArthur et al., 2014; Samocha et al., 2014). Here, we revisit some of the issues that have implications for retinal genetics.
5.2.1. Are mutations rare genetic variations?
Mutations causing monogenic diseases are believed to be rare, and often a blanket minor allele frequency cut-off is applied to filter out disease variants. Such an approach can miss cases where disease variants are common and/or likely modify the disease phenotype (Ebermann et al., 2010; Khanna et al., 2009; Louie et al., 2010; Venturini et al., 2012). Notably, carrier frequency of the null mutations responsible for RDDs is unusually high in general population (Nishiguchi and Rivolta, 2012). A vast majority of known genetic variants associated with complex diseases (such as AMD) are common and yet some may have functional relevance to pathology (such as Y402H in CFH). As investigations of genetic variation underlying complex traits continue to mature, we are seeing examples of rare and common regulatory as well as copy number variants associated with disease. Thus, no general analysis pipeline should be applied to identify the variants of interest, and one must customize based on genetic architecture of the disease under study.
5.2.2. Do disease-causing mutations have to be conserved?
Computational prediction tools incorporate evolutionary conservation across species as a criterion when assigning causality to variants. While a majority of disease-related variants follow this pattern, less conserved residues, silent variants or changes in the non-coding region have also been implicated in disease (Cartegni et al., 2002; Sauna and Kimchi-Sarfaty, 2011). Furthermore, the effect of a variant is likely to be influenced by size, polarity, codon-usage, neighboring amino-acid residues and location with respect to the overall protein conformation. Prediction programs fail to account for many of these important parameters. Thus, filtering out any “potentially interesting” variant based on prediction criteria should not be a common practice. Development of more sophisticated analytical tools is therefore necessary for better predictive power.
5.2.3. Are monogenic diseases caused by defects in only one gene?
Until recently, a majority of disease genes for monogenic traits have been identified through traditional linkage approach, where loci that segregate with the disease phenotype are screened for mutation(s) in candidate genes. Once a segregating disease mutation is identified, the researchers usually no longer search for mutations/variations. However, extensive genetic and even phenotypic heterogeneity observed in RDDs has been an obstacle to diagnosis and counseling. Different RDDs also share overlapping clinical findings; thus, mutations in many different genes may result in similar phenotypes and/or mutations in one gene can lead to distinct phenotypes. In Mendelian diseases, inheritance of a genetic defect implies that one will get the disease. However, many individuals carrying a mutation may show no clinical phenotype (incomplete penetrance) or variable expressivity. As every individual carries thousands of unique variants, one can envisage compensatory variations in interacting genes (epistasis) that can alter the clinical phenotype(s) (Lehner et al., 2006). Thus, in addition to the “primary” gene defect, many patients may harbor alleles that can modify the disease phenotype (modifier alleles). For example, a common allele in the RPGRIP1L gene, associated with Joubert and Meckel syndromes (Arts et al., 2007; Delous et al., 2007), is shown to contribute to retinal degeneration phenotype in individuals with ciliopathies caused by mutations in other genes (Khanna et al., 2009). In other instances, mutations in more than one gene might be necessary to observe a disease phenotype (e.g., “digenic” RP caused by ROM1 and RDS alleles) (Kajiwara et al., 1994). NGS offers an unbiased approach for unveiling genetic modifiers of retinal degeneration across the genome and can help in refining the genetic definition of phenotypic variations in RDD patients.
The spectrum of potentially disease-causing variants makes the correlation of the variant with the phenotype challenging. Given that causative variants could be rare, common, regulatory, intronic, silent or compensatory, establishing the role of a gene or a variant in disease pathogenesis is not straightforward. Genes that are identified through genetic studies should be referred as “candidates” until their association with the disease phenotype is validated by additional evidence including functional data. Further support can emerge from enrichment of genetic variants (gene-level evidence) in patients/families compared with controls. Disruption of the gene in a model system can, in some instances, reproduce the relevant human phenotype. In addition, one should be able to rescue the phenotype with the wild type allele but not with mutant allele(s), thereby mimicking the “Koch’s postulates” (Chakravarti et al., 2013). While mouse (or zebrafish) models do not always recapitulate the human phenotype, such studies could provide disease mechanisms and opportunities for evaluation of treatments (Veleri et al., 2015). Alternative models using patient-derived human induced pluripotent stem (iPS) cells also appear promising.
5.2.4. Missing heritability of complex traits
In addition to common and rare genetic variants, other components including copy number variation, epigenetic signature and gene-environment interaction contribute to complex disease phenotype. While many studies have looked at copy number variations in candidate genes with limited success and replication (Cantsilieris and White, 2013; Liu et al., 2012), genome-wide surveys have not been useful in identifying such variations associated with major risk of AMD (Fritsche et al., 2013; Meyer et al., 2011; Neale et al., 2010; Sobrin et al., 2012).
Epigenetics has gained immense interest because of its role in human diseases such as cancer (Feinberg et al., 2006). Disease-related changes include stochastic modifications in epigenetic marks as well as local gene-specific alterations (Pujadas and Feinberg, 2012). Beyond cancer, epigenetic changes have been widely associated with human diseases involving chromosomal abnormalities and intellectual disability (Brookes and Shi, 2014). Retinal diseases have also been explored for possible involvement of epigenetic alterations, and genes such as IL17RC (Wei et al., 2012a) and Apo J (Suuronen et al., 2007) have been suggested to contribute to the pathogenesis of AMD. However, such studies require further validation (Oliver et al., 2013a). More extensive analysis of the epigenome must be performed using normal or disease retina (or target cell types).
Complex diseases, such as AMD, exhibit tremendous phenotypic heterogeneity, and multiple classification and grading systems have been put forward (Bird et al., 1995; Ferris et al., 2005; Ferris et al., 2013). However, much of the genetic analysis of AMD has been performed on cohorts with advanced stage of disease, with phenotypes broadly classified as geographic atrophy and choroidal neovascularization. While an ARMS2 risk variant is reportedly more strongly associated to neovascularization compared to geographic atrophy (Chen et al., 2010b; Sobrin et al., 2011), it has been difficult to identify genes and pathways associated with distinct clinical subtypes (Fritsche et al., 2014). The recently completed exome-chip analysis of the large case-control cohort may provide additional insights on subtype specific genetic variants. Whole genome sequencing of 6,000 AMD cases and controls is currently in progress (Kwong, 2014) and should be valuable for deciphering susceptibility and protective alleles for AMD subtypes.
5.3. Integration of transcriptome with genetic data
Though heritable mutations are present in every cell of an individual, disease manifestation is often tissue or cell specific because of differences in spatio-temporal expression or function of the responsible gene(s). Thus, it would be important to correlate genetic variations to gene expression in the retina (or photoreceptors) to obtain insights into the regulatory network/pathways perturbed in RDDs. NGS methods allow high throughput analysis of genotypes as well as provide an opportunity to sequence all species of RNA, including the splice variants and relatively less studied miRNAs and lncRNAs.
A majority of variants associated with complex diseases often reside outside the coding region of a gene or even in non-transcribed regions. It is assumed that such variants would have subtle effects on gene expression or function. The expression quantitative trait locus (eQTL) analysis combines gene expression data with genotype information and can assist in identifying risk alleles (Cheung et al., 2003; Lappalainen et al., 2013). Expression profiling of disease-relevant tissue or cells in case-control samples may reveal functional variants with biological relevance to the phenotype. eQTLs are primarily cis-acting, suggesting a common mechanism at many associated loci (Edwards et al., 2013). Identification of target genes modulated by cis-regulatory risk variants will allow better understanding of disease network(s) and/or mechanism(s) through which they act. In fact, ENCODE project has demonstrated that a large proportion of associated GWAS SNPs appear to map to functional regulatory elements (Schaub et al., 2012). As noted earlier, gene expression patterns are cell type- and tissue-specific, and eQTL analyses are more useful in cells/tissue types relevant to the human disease of interest. The current databases do not include eQTL information on the retina or other ocular cell types owing to limitations in obtaining human tissues.
GWAS studies have yielded a large number of new genetic loci associated with AMD susceptibility. However, several genes that seem to have biological relevance to AMD pathology have not been validated even in large GWAS meta-analyses, in part because of false positive results or phenotypic overlap together with etiological heterogeneity; these include ABCA4 (Allikmets, 2000), TLR4 (Zareparsi et al., 2005b), TLR3 (Kleinman et al., 2008; Yang et al., 2008), CX3CR1 (Tuo et al., 2004), DICER (Kaneko et al., 2011), and AHR (Choudhary et al., 2014; Kim et al., 2014). Another explanation could be that the effect size of these variants is small, and much larger sample size is needed to achieve genome-wide significance. We should also note that the biological significance of many genes/variants identified by GWAS is not yet clear. The genetic variants identified through different approaches may have subtle yet meaningful influence on specific pathways and networks that when disrupted lead to AMD pathology. We recently suggested that cumulative impact of few strong or multiple weaker alleles must reach a threshold of disruption for producing a clinically identifiable phenotype (Fritsche et al., 2014). An integrated approach of spatial and temporal mapping of disease genes and variants onto the expression data generated from disease-relevant tissues would be necessary to generate disease-specific networks. Distinct candidates and pathways may converge in networks of co-expressed genes in disease-relevant cell/tissue at a specific time. Such disease networks can help in identifying novel regulatory nodes and therapeutic targets.
5.4. Approaches for elucidating disease networks
Identifying disease-associated genetic variants is an important piece of the puzzle in understanding pathogenic mechanism(s). Rather than examining one gene (and its function) at a given time, our goal must be to understand the “disease” itself and elucidate complex interplay among multiple factors (genes, epigenetic changes, and interacting environment). Initially, one can investigate all genes associated with a retinal disease phenotype (such as Leber Congenital Amaurosis or AMD) to deduce relevant biological pathways and then integrate genes and pathways into networks. For examples, 19 AMD loci, published so far, have implicated complement, extracellular matrix and lipid metabolism pathways (Fritsche et al., 2014; Priya et al., 2012). Other studies on GWAS loci have hinted towards localization of cis-regulatory variation at enhancer elements (Pennacchio et al., 2013). Integrating high throughput functional annotations, such as histone marks, transcription factor binding sites and DNase I hypersensitive sites (DHSs), relevant to AMD cell types can help in prioritization of non-coding variants at associated loci for subsequent functional evaluation. Combining this information with the genetic data can provide novel insights into disease mechanisms; for example, exome sequencing in small families can identify large number of rare and novel potential disease variants, and ChIP-seq data can allow filtering of genetic variants, as shown in case of MAK (Ozgul et al., 2011) where CRX Chip-seq data was used to rank the candidate genes. Combined systems level analyses of multiple datasets have already begun to reveal novel insights in disease mechanisms and targets for drug discovery (Cohen et al., 2006; Dadu and Ballantyne, 2014; Sahebkar, 2014).
Rare variants or common loci with small effect often require large sample size to reach genome-wide statistical significance. However, there could be alternate ways to extract meaningful information about the disease process. For example, exome sequencing of families where the disease is clustered can identify rare segregating variants that can impact susceptibility. Enrichment analysis of candidate variants can either link to existing disease-associated pathways or reveal novel insights into the disease process (Ratnapriya, 2014). Alternatively, pathogenic mechanisms associated with a specific phenotype within the complex disease (such as AMD) can also be derived from delineating the biology of Mendelian RDDs that include overlapping clinical features (such as Stargardt disease) (Ratnapriya et al., 2014).
6. Next generation diagnosis and drug development
The promise of next generation genomics to medicine is exciting and unprecedented; yet, we have a long way before incorporating NGS into routine clinical practice. Meanwhile, NGS has created new opportunities for faster genetic diagnosis that can help in improved disease management. Given the allelic heterogeneity of RDDs, the current sequencing methods are not very efficient. Decreasing cost of sequencing and approval from U.S. Food and Drug Administration allowing NGS methods for clinical diagnosis constitute steps in the rapid realization of personalized medicine. NGS-based screening methods are now being utilized for RDDs as well (Booij et al., 2011; Bowne et al., 2011; Neveling et al., 2012; Song et al., 2011b). The National Ophthalmic Disease Genotyping and Phenotyping Network (eyeGene), a CLIA certified community resource designed to bring together patients, providers, molecular testing laboratories and researchers, is incorporating NGS tools for investigating disease causing mutations (Blain et al., 2013).
Another major application of NGS is in the area of pharmacogenomics. Patients with the same disease often respond differently to drug efficacy and safety because of unique genetic variations they inherit. Identification of large number of susceptibility alleles has propelled the field of pharmacogenomics to a potential future in personalized medicine. In AMD, the effect of GWAS susceptibility loci, mainly CFH and ARMS2 variants on treatment outcome of Avastin (bevacizumab) and Lucentis (ranibizumab), has received a lot of attention, though such studies have not yielded consistent results (Hagstrom et al., 2013; Kanoff and Miller, 2013). Small sample size, incorporation of only few disease markers, and short term-follow up represent some of the challenges. More comprehensive, large-scale prospective studies are necessary to establish definitive associations between genotypes and drug response/efficacy.
Relevance of NGS datasets to development of translational strategies has been limited so far because of inadequate understanding of underlying biology of the disease. GWAS hits are rarely direct drug targets (Teslovich et al., 2010). Limited predictive value of the disease models and inability to accurately estimate the efficacy, toxicity and long-term side effects have made discovery of new drugs very expensive and time consuming (Plenge et al., 2013). NGS technology has remarkable promise for genomic medicine, impacting both predictive and preventive medicine. Few studies have already shown novel means for drug discovery using genetic data and bioinformatics tools, such as functional annotation, cis-acting eQTLs and pathway analyses (Okada et al., 2014). We need to better define and map interactome networks with its components (nodes) and interactions (edges) (Vidal et al., 2011). These biological networks can be integrated with known drug and disease information to build drug-target networks (Yildirim et al., 2007). As NGS technology becomes widely available, pharmacological interventions will have personalized focus on disease treatment. Identification of clinically important variants, together with transcriptome and epigenetic profiling, should permit dissection of human disease genome that is amenable to pharmacological interventions.
7. Challenges and further considerations
Here, we have examined new developments in photoreceptor biology stemming from NGS technologies. Despite much progress, challenges remain; these include development of effective bioinformatics tools for multi-layer data integration, standardization of procedures to facilitate collaborative works, data sharing within the research community and application of novel findings from system-level analysis to develop new hypothesis.
7.1. Multi-layer data integration
Most high throughput data generation and analysis have so far been conducted at one or two layers of biological information. For instance, transcriptome analysis has been the most dominant form of genome-wide information, and other types of data such as transcription factor targetome or epigenome profiling have been individually compared to transcriptome data but not to one another. With increasing amount of studies examining various layers of biological pathways, including regulatory RNAs, epigenome and proteome, the resulting higher order integrative analysis is expected to further refine the current knowledge of molecular networks. To this end, applying robust computational analysis of the data sets is critical. The large-scale ENCODE (encyclopedia of DNA elements) consortium probably provides the best model. Upon completion of the human genome project, ENCODE was launched to investigate functional elements of human and mouse genome (http://encodeproject.org). By employing more than two-dozen assays, a large number of data sets have been generated from 147 distinct cell types or tissues (ENCODE Project Consortium, 2012). Several powerful software tools were developed with a goal of effective data integration across heterogeneous data sets (http://encodeproject.org/ENCODE/encodeTools.html). Additional open source bioinformatics tools are freely available.
7.2. Standardization of procedures and quality control
Given the significant amount of expertise, workloads, and funding required for high throughput data collection, the practice of comprehensive and multi-dimensional data analysis demands collaboration among various disciplines. Independently generated data sets will often have to be compiled together for integrative analysis. The vision research community, therefore, needs standardized procedures for experimentation and data analysis. Detailed instructions for high throughput assay protocols and quality control are also provided as a part of the ENCODE project (ENCODE Project Consortium, 2011), and all the upcoming data generation should refer to those. The retina, however, consists of highly specialized sets of neurons and supporting cells; thus, additional guidelines specific to the vision field must be implemented. Directions and relevant resources for retinal sample preparation should be established. As retina is rich with tissue-specific transcript isoforms, genome annotations tailored for the retina should be established for standardized data analysis.
7.3. Data sharing
A fundamental consideration for systems approaches is to facilitate data sharing. High throughput studies are unique in that the generated data can be re-analyzed to discover new patterns and to test new hypotheses distinct from those posed in the original study. Therefore, after the initial analysis and publication, high throughput data sets should be readily accessible for any research group to view, verify and reanalyze. More than 200 retina-related data series can be retrieved from public databases, such as Gene Expression Omnibus (GEO), which archive genome-wide information generated by microarray and NGS technologies. Microarray data deposited to GEO follows, for the most part, the guidelines from Minimum Information About a Microarray Experiment (MIAME) (Brazma et al., 2001) put forward by the microarray gene expression data (MGED) society. However, standardized directions specific for NGS data have not been implemented yet, nor the strict measures for quality control are in place. We have recently undertaken the establishment of a web-based database for retinal NGS data repository with the following goals:
Implementation of uniform guidelines for sample collection, assay procedures and data analysis
Ensure the quality of the deposited data and encourage data sharing in a timely manner
Consolidation of high throughput data sets related to retina (or other ocular tissues) commonly affected in retinal neurodegenerative diseases
Provide structured and comprehensive annotation of each data set
Our archive of high throughput data sets should provide a critical resource to facilitate higher-order integrated data analysis and/or novel discovery through re-analysis.
7.4. From in silico analysis to the bench
The observations made from system-wide analyses, in most instances, are descriptive and hypothesis-generating rather than hypothesis-testing. Wet bench research is needed to verify and further develop the knowledge obtained from systems level computational analyses. Networks and/or patterns implicated in retinal development, aging or disease must be tested by traditional in vivo and in vitro model systems. The three-dimensional retinal cultures derived from human or mouse stem cells (Eiraku et al., 2011; Nakano et al., 2012) provide a useful model for human retina in the context of evaluating the role of microenvironment and genetic pathways. Patient-specific induced pluripotent stem cells provide hope for the development of personalized medicine.
8. The future of systems biology approaches: biology of the organism as a whole
In this review, we have primarily focused on GRNs that maintain photoreceptor function and on RDDs; nonetheless, we do realize that a comprehensive systems biology strategy would also incorporate population-wide genetic variations, protein interaction networks and metabolomics. In general, we believe that reproducible topologies in the networks with principal regulators would constitute the hubs of sub-networks. It is not difficult to imagine that perturbation of such primary hubs (e.g., key regulators, rate-limiting substrates and enzymes) likely imposes more pronounced impact on photoreceptor health than the defect in locally confined nodes with fewer interactions (e.g., those with limited physiological reach) (Figure 8). Targeting “key” hubs would therefore represent an attractive strategy for drug discovery.
Figure 8.
Model of multi-dimensional network of photoreceptors. Networks consist of nodes and edges, representing cellular components (genes, transcripts, proteins, etc.) and their interactions, respectively. The nodes with the greatest number of interactions constitute primary hubs. Secondary hubs themselves are the interacting partners of primary hubs and simultaneously connect with many other nodes, thereby partially mediating the function of the primary hubs. Local sub-networks are also prevalent. Perturbation of primary or secondary hubs of the network likely poses detrimental impact to the system, while perturbation of a more locally confined network or a node with few interacting partners may not exert substantial effect on the system integrity. Identification of commonly affected networks in diverse retinal diseases will allow the better drug design.
One of the major bottlenecks in therapeutic development has been the overwhelming number of disease-associated nodes (i.e., disease genes) due to heterogeneity of RDDs. As suggested earlier (Swaroop et al., 2010; Yu et al., 2004b), it is conceivable that distinct mutations resulting in a broad spectrum of retinal diseases converge onto a manageable number of “key” hubs (with overlapping disease-associated pathways/networks) prior to photoreceptor death. Thus, one may not need to target individual disease causing genes to rescue photoreceptor integrity. Identification of common retinal disease-related networks and associated hubs may now be within reach for discovering better drug targets.
We have come a long way in defining the biology of individual genes. Nonetheless, we now must provide an appropriate cellular context to individual genes and their functional products. We have already started to examine genetic, transcriptional and epigenetic architectures and await integrated analysis to incorporate proteome and metabolome data. Networks constructed from multi-dimensional data analysis will allow us to simulate functional and physiological outcome of photoreceptors in response to alteration in the microenvironment and variations in the genetic makeup. Human genomes are highly polymorphic, and even monozygotic twins may carry genetic differences (Bruder et al., 2008). Notably, individuals are estimated to carry over 2,500 functionally relevant genetic variations (Genomes Project et al., 2012). Such variability is what makes each of us unique and would be expected to influence the network structure. Genetic polymorphism, together with aging and environmental stress, may have profound impact on susceptibility to diseases and drug response. The holistic approaches thus have the key to accurate diagnosis and personalized medicine.
In this review, we have primarily discussed system-level analysis of the whole cell (rather than focusing on a gene or a pathway in isolation). We recognize that cells in higher organisms do not function independently but constitute higher order structures that are morphologically and functionally distinct (e.g., retina, lens, cerebellum), which in turn are in constant communication with structures farther away (e.g., via systemic circulation or neuronal connectivity). Thus, aberrant function of a gene in a specific cell may have wide-ranging impact on other tissues and the whole organism. A true system-level analysis will eventually incorporate both limited and long-range effects on GRNs and cell function. The retinal research has an exciting future, as we are closer to better predictive and treatment strategies for every individual.
Acknowledgments
We are grateful to Vijender Chaitanker for designing one of the figures and thank N-NRL colleagues, especially Alexis Boleda, Matthew Brooks, Linn Gieser, Koray Kaya, and Soo-Young Kim for assistance and discussions. We thank Belinda Seto for comments on the manuscript. This research is supported by Intramural Research program (ZO1-EY000450; ZO1-EY000473, ZO1-EY000475) of the National Eye Institute, National Institutes of Health.
List of abbreviations
- AMD
Age-related macular degeneration
- ChIP
Chromatin immunoprecipitation
- ChIP-seq
Chromatin immunoprecipitation-sequencing
- CLIA
Clinical laboratory improvement amendments
- eQTL
Expression quantitative trait locus
- GA
Geographic atrophy
- GRN
Gene regulatory network
- GWAS
Genome-wide association study
- H3K4me2(3)
Histone H3 lysine 4 di(tri)methylation
- H3K27me3
Histone H3 lysine 27 trimethylation
- M cone
Medium wavelength sensitive cone
- MIAME
Minimum information about a microarray experiment
- NGS
Next generation sequencing
- ONL
Outer nuclear layer
- PCA
Principal component analysis
- RDD
Retinal degenerative disease
- RNA-seq
RNA-sequencing
- RPE
Retinal pigment epithelium
- S cone
Short wave length sensitive cone
- SNP
Single nucleotide polymorphism
- WES
Whole exome sequencing
- WGS
Whole genome sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adli M, Zhu J, Bernstein BE. Genome-wide chromatin maps derived from limited numbers of hematopoietic progenitors. Nat Methods. 2010;7:615–618. doi: 10.1038/nmeth.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet. 2000;67:487–491. doi: 10.1086/303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Guduric-Fuchs J, Harwood L, Dellett M, Cogliati T, Simpson DA. Prediction of microRNAs affecting mRNA expression during retinal development. BMC Dev Biol. 2010;10:1. doi: 10.1186/1471-213X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Balakirev ES, Ayala FJ. Pseudogenes: are they “junk” or functional DNA? Annu Rev Genet. 2003;37:123–151. doi: 10.1146/annurev.genet.37.040103.103949. [DOI] [PubMed] [Google Scholar]
- Barabasi AL, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Barnhill AE, Hecker LA, Kohutyuk O, Buss JE, Honavar VG, Greenlee HW. Characterization of the retinal proteome during rod photoreceptor genesis. BMC Res Notes. 2010;3:25. doi: 10.1186/1756-0500-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35:565–573. doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Berman BP, Weisenberger DJ, Aman JF, Hinoue T, Ramjan Z, Liu Y, Noushmehr H, Lange CP, van Dijk CM, Tollenaar RA, Van Den Berg D, Laird PW. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat Genet. 2012;44:40–46. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SS, Wright AF, Clayton JF, Price WH, Phillips CI, McKeown CM, Jay M, Bird AC, Pearson PL, Southern EM, et al. Close genetic linkage between X-linked retinitis pigmentosa and a restriction fragment length polymorphism identified by recombinant DNA probe L1.28. Nature. 1984;309:253–255. doi: 10.1038/309253a0. [DOI] [PubMed] [Google Scholar]
- Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–1576. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Fraioli RE, Furukawa T, Cepko CL. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain D, Goetz KE, Ayyagari R, Tumminia SJ. eyeGENE(R): a vision community resource facilitating patient care and paving the path for research through molecular diagnostic testing. Clin Genet. 2013;84:190–197. doi: 10.1111/cge.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel S, Mohand-Said S, Sahel JA. The aging of the retina. Exp Gerontol. 2003;38:825–831. doi: 10.1016/s0531-5565(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Booij JC, Bakker A, Kulumbetova J, Moutaoukil Y, Smeets B, Verheij J, Kroes HY, Klaver CC, van Schooneveld M, Bergen AA, Florijn RJ. Simultaneous mutation detection in 90 retinal disease genes in multiple patients using a custom-designed 300-kb retinal resequencing chip. Ophthalmology. 2011;118:160–167. e161–163. doi: 10.1016/j.ophtha.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Bowes Rickman C, Ebright JN, Zavodni ZJ, Yu L, Wang T, Daiger SP, Wistow G, Boon K, Hauser MA. Defining the human macula transcriptome and candidate retinal disease genes using EyeSAGE. Invest Ophthalmol Vis Sci. 2006;47:2305–2316. doi: 10.1167/iovs.05-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne SJ, Sullivan LS, Koboldt DC, Ding L, Fulton R, Abbott RM, Sodergren EJ, Birch DG, Wheaton DH, Heckenlively JR, Liu Q, Pierce EA, Weinstock GM, Daiger SP. Identification of disease-causing mutations in autosomal dominant retinitis pigmentosa (adRP) using next-generation DNA sequencing. Invest Ophthalmol Vis Sci. 2011;52:494–503. doi: 10.1167/iovs.10-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- Brookes E, Shi Y. Diverse Epigenetic Mechanisms of Human Disease. Annu Rev Genet. 2014 doi: 10.1146/annurev-genet-120213-092518. [DOI] [PubMed] [Google Scholar]
- Brooks MJ, Rajasimha HK, Roger JE, Swaroop A. Next-generation sequencing facilitates quantitative analysis of wild-type and Nrl(−/−) retinal transcriptomes. Mol Vis. 2011;17:3034–3054. [PMC free article] [PubMed] [Google Scholar]
- Brooks MJ, Rajasimha HK, Swaroop A. Retinal transcriptome profiling by directional next-generation sequencing using 100 ng of total RNA. Methods Mol Biol. 2012;884:319–334. doi: 10.1007/978-1-61779-848-1_23. [DOI] [PubMed] [Google Scholar]
- Brosius J, Gould SJ. On “genomenclature”: a comprehensive (and respectful) taxonomy for pseudogenes and other “junk DNA”. Proc Natl Acad Sci U S A. 1992;89:10706–10710. doi: 10.1073/pnas.89.22.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder CE, Piotrowski A, Gijsbers AA, Andersson R, Erickson S, Diaz de Stahl T, Menzel U, Sandgren J, von Tell D, Poplawski A, Crowley M, Crasto C, Partridge EC, Tiwari H, Allison DB, Komorowski J, van Ommen GJ, Boomsma DI, Pedersen NL, den Dunnen JT, Wirdefeldt K, Dumanski JP. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am J Hum Genet. 2008;82:763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Martin KR. Concise review: toward stem cell-based therapies for retinal neurodegenerative diseases. Stem Cells. 2011;29:1170–1175. doi: 10.1002/stem.676. [DOI] [PubMed] [Google Scholar]
- Bumsted O’Brien KM, Cheng H, Jiang Y, Schulte D, Swaroop A, Hendrickson AE. Expression of photoreceptor-specific nuclear receptor NR2E3 in rod photoreceptors of fetal human retina. Invest Ophthalmol Vis Sci. 2004;45:2807–2812. doi: 10.1167/iovs.03-1317. [DOI] [PubMed] [Google Scholar]
- Cai H, Fields MA, Hoshino R, Priore LV. Effects of aging and anatomic location on gene expression in human retina. Front Aging Neurosci. 2012;4:8. doi: 10.3389/fnagi.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantsilieris S, White SJ. Correlating multiallelic copy number polymorphisms with disease susceptibility. Hum Mutat. 2013;34:1–13. doi: 10.1002/humu.22172. [DOI] [PubMed] [Google Scholar]
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schonbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y FANTOM Consortium; RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- Cartegni L, Chew SL, Krainer AR. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- Carter TA, Greenhall JA, Yoshida S, Fuchs S, Helton R, Swaroop A, Lockhart DJ, Barlow C. Mechanisms of aging in senescence-accelerated mice. Genome Biol. 2005;6:R48. doi: 10.1186/gb-2005-6-6-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Clark AG, Mootha VK. Distilling pathophysiology from complex disease genetics. Cell. 2013;155:21–26. doi: 10.1016/j.cell.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci. 2010a;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris F, III, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010b;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Aleman TS, Cideciyan AV, Khanna R, Jacobson SG, Swaroop A. In vivo function of the orphan nuclear receptor NR2E3 in establishing photoreceptor identity during mammalian retinal development. Hum Mol Genet. 2006;15:2588–2602. doi: 10.1093/hmg/ddl185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khan NW, Roger JE, Swaroop A. Excess cones in the retinal degeneration rd7 mouse, caused by the loss of function of orphan nuclear receptor Nr2e3, originate from early-born photoreceptor precursors. Hum Mol Genet. 2011;20:4102–4115. doi: 10.1093/hmg/ddr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Cheung VG, Conlin LK, Weber TM, Arcaro M, Jen KY, Morley M, Spielman RS. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet. 2003;33:422–425. doi: 10.1038/ng1094. [DOI] [PubMed] [Google Scholar]
- Choudhary M, Kazmin D, Hu P, Thomas RS, McDonnell DP, Malek G. Aryl hydrocarbon receptor knockout exacerbates choroidal neovascularization via multiple pathogenic pathways. J Pathol. 2014 doi: 10.1002/path.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers I, Gunatilaka TL, Farkas RH, Qian J, Hackam AS, Duh E, Kageyama M, Wang C, Vora A, Campochiaro PA, Zack DJ. Identification of novel genes preferentially expressed in the retina using a custom human retina cDNA microarray. Invest Ophthalmol Vis Sci. 2003a;44:3732–3741. doi: 10.1167/iovs.02-1080. [DOI] [PubMed] [Google Scholar]
- Chowers I, Liu D, Farkas RH, Gunatilaka TL, Hackam AS, Bernstein SL, Campochiaro PA, Parmigiani G, Zack DJ. Gene expression variation in the adult human retina. Hum Mol Genet. 2003b;12:2881–2893. doi: 10.1093/hmg/ddg326. [DOI] [PubMed] [Google Scholar]
- Cipriani V International AMD Genomics Consortium. Unravelling the complex genetics of age-related macular degeneration. 64th Annual Meeting of The American Society of Human Genetics. Personal communication.2014. [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, Kleinman JE. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9:e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genet. 2005;1:e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo JC, Lawrence KA, Karlstetter M, Myers CA, Abdelaziz M, Dirkes W, Weigelt K, Seifert M, Benes V, Fritsche LG, Weber BH, Langmann T. CRX ChIP-seq reveals the cis-regulatory architecture of mouse photoreceptors. Genome research. 2010;20:1512–1525. doi: 10.1101/gr.109405.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GE, Holt IE, Whittle J, Webb BD, Tai D, Davis S, Margulies EH, Chen Y, Bernat JA, Ginsburg D, Zhou D, Luo S, Vasicek TJ, Daly MJ, Wolfsberg TG, Collins FS. Genome-wide mapping of DNase hypersensitive sites using massively parallel signature sequencing (MPSS) Genome Res. 2006;16:123–131. doi: 10.1101/gr.4074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43C:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond) 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–575. doi: 10.1038/nrcardio.2014.84. [DOI] [PubMed] [Google Scholar]
- Damiani D, Alexander JJ, O’Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Levine MS. Properties of developmental gene regulatory networks. Proc Natl Acad Sci U S A. 2008;105:20063–20066. doi: 10.1073/pnas.0806007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon M, Hayashizaki Y. Deep cap analysis gene expression (CAGE): genome-wide identification of promoters, quantification of their expression, and network inference. Biotechniques. 2008;44:627–628. 630, 632. doi: 10.2144/000112802. [DOI] [PubMed] [Google Scholar]
- de Laat W, Duboule D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature. 2013;502:499–506. doi: 10.1038/nature12753. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Weber C, Friedlander M. Global gene expression analysis of the developing postnatal mouse retina. Invest Ophthalmol Vis Sci. 2004;45:1009–1019. doi: 10.1167/iovs.03-0806. [DOI] [PubMed] [Google Scholar]
- Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schafer E, Roux AF, Dafinger C, Bernd A, Zrenner E, Claustres M, Blanco B, Nurnberg G, Nurnberg P, Ruland R, Westerfield M, Benzing T, Bolz HJ. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest. 2010;120:1812–1823. doi: 10.1172/JCI39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93:779–797. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Nash ZA, Takemori N, Fliesler SJ, McClellan ME, Naash MI. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J Neurochem. 2008;104:336–352. doi: 10.1111/j.1471-4159.2007.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML. Multiple evidence strands suggest that there may be as few as 19 000 human protein-coding genes. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli P, Perera A, Arango-Gonzalez B, Trifunovic D, Wagner M, Carell T, Biel M, Zrenner E, Michalakis S, Paquet-Durand F, Ekstrom PA. DNA methylation and differential gene regulation in photoreceptor cell death. Cell Death Dis. 2014;5:e1558. doi: 10.1038/cddis.2014.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Yu J, Othman MI, Yoshida S, Sheth S, Glaser T, Baehr W, Swaroop A. Mouse eye gene microarrays for investigating ocular development and disease. Vision Res. 2002;42:463–470. doi: 10.1016/s0042-6989(01)00219-x. [DOI] [PubMed] [Google Scholar]
- Farkas MH, Grant GR, White JA, Sousa ME, Consugar MB, Pierce EA. Transcriptome analyses of the human retina identify unprecedented transcript diversity and 3.5 Mb of novel transcribed sequence via significant alternative splicing and novel genes. BMC Genomics. 2013;14:486. doi: 10.1186/1471-2164-14-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, Milton RC, Bressler SB, Klein R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan S, Robson JL, Wylie M, Healy A, Stitt AW, Curry WJ. Protein expression profiling during chick retinal maturation: a proteomics-based approach. Proteome Sci. 2008;6:34. doi: 10.1186/1477-5956-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund PR, Watson J, Gilmour GS, Gaillard F, Sauve Y. Differential changes in retina function with normal aging in humans. Doc Ophthalmol. 2011;122:177–190. doi: 10.1007/s10633-011-9273-2. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP, Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, CNK, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR AMD Gene Consortium. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. 439e431–432. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG International AMD Genomics Consortium. The role of rare TIMP3 mutations in Age-Related Macular Degeneration. Personal communication.64th Annual Meeting of The American Society of Human Genetics; 2014. [Google Scholar]
- Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-Related Macular Degeneration: Genetics and Biology Coming Together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey TS. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet. 2012;13:840–852. doi: 10.1038/nrg3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gamsiz ED, Ouyang Q, Schmidt M, Nagpal S, Morrow EM. Genome-wide transcriptome analysis in murine neural retina using high-throughput RNA sequencing. Genomics. 2012;99:44–51. doi: 10.1016/j.ygeno.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 1000 Genomes Project Consortium. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieser L, Swaroop A. Expressed sequence tags and chromosomal localization of cDNA clones from a subtracted retinal pigment epithelium library. Genomics. 1992;13:873–876. doi: 10.1016/0888-7543(92)90173-p. [DOI] [PubMed] [Google Scholar]
- Gilmour DS, Lis JT. In vivo interactions of RNA polymerase II with genes of Drosophila melanogaster. Mol Cell Biol. 1985;5:2009–2018. doi: 10.1128/mcb.5.8.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17:877–885. doi: 10.1101/gr.5533506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, Stoeckert CJ, Hogenesch JB, Pierce EA. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM) Bioinformatics. 2011;27:2518–2528. doi: 10.1093/bioinformatics/btr427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresh J, Goletz PW, Crouch RK, Rohrer B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/s0952523803202108. [DOI] [PubMed] [Google Scholar]
- Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc. 2011;6:468–481. doi: 10.1038/nprot.2010.190. [DOI] [PubMed] [Google Scholar]
- Guo H, Zhu P, Wu X, Li X, Wen L, Tang F. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013;23:2126–2135. doi: 10.1101/gr.161679.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, Bono H, LeMieux J, Walsh P, Carninci P, Hayashizaki Y, Okazaki Y, Raviola E. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci U S A. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler L, Jr, Wan J, Swaroop A, Qian J, Zack DJ. MicroRNA profile of the developing mouse retina. Invest Ophthalmol Vis Sci. 2010;51:1823–1831. doi: 10.1167/iovs.09-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Callanan DG, Kim IK, Klein ML, Maguire MG, Martin DF Comparison of AMDTTRG. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2013;120:593–599. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- Haider NB, Mollema N, Gaule M, Yuan Y, Sachs AJ, Nystuen AM, Naggert JK, Nishina PM. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res. 2009;89:365–372. doi: 10.1016/j.exer.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Thomas S, Sandstrom R, Canfield TK, Thurman RE, Weaver M, Dorschner MO, Gartler SM, Stamatoyannopoulos JA. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc Natl Acad Sci U S A. 2010;107:139–144. doi: 10.1073/pnas.0912402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Kim DS, Klocke B, Johnson KR, Cui K, Gotoh N, Zang C, Gregorski J, Gieser L, Peng W, Fann Y, Seifert M, Zhao K, Swaroop A. Transcriptional regulation of rod photoreceptor homeostasis revealed by in vivo NRL targetome analysis. PLoS Genet. 2012;8:e1002649. doi: 10.1371/journal.pgen.1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Tummala P, Guzman E, Mali RS, Gregorski J, Swaroop A, Mitton KP. The transcription factor neural retina leucine zipper (NRL) controls photoreceptor-specific expression of myocyte enhancer factor Mef2c from an alternative promoter. J Biol Chem. 2011;286:34893–34902. doi: 10.1074/jbc.M111.271072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Lambeck S, Toepfer S, van Someren E, Guthke R. Gene regulatory network inference: data integration in dynamic models-a review. Biosystems. 2009;96:86–103. doi: 10.1016/j.biosystems.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Helgason H, Sulem P, Duvvari MR, Luo H, Thorleifsson G, Stefansson H, Jonsdottir I, Masson G, Gudbjartsson DF, Walters GB, Magnusson OT, Kong A, Rafnar T, Kiemeney LA, Schoenmaker-Koller FE, Zhao L, Boon CJ, Song Y, Fauser S, Pei M, Ristau T, Patel S, Liakopoulos S, van de Ven JP, Hoyng CB, Ferreyra H, Duan Y, Bernstein PS, Geirsdottir A, Helgadottir G, Stefansson E, den Hollander AI, Zhang K, Jonasson F, Sigurdsson H, Thorsteinsdottir U, Stefansson K. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat Genet. 2013;45:1371–1374. doi: 10.1038/ng.2740. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Bumsted-O’Brien K, Natoli R, Ramamurthy V, Possin D, Provis J. Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res. 2008;87:415–426. doi: 10.1016/j.exer.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff JG, Belsky JA, Krassovsky K, MacAlpine DM, Henikoff S. Epigenome characterization at single base-pair resolution. Proc Natl Acad Sci U S A. 2011;108:18318–18323. doi: 10.1073/pnas.1110731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig AK, Peng GH, Chen S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 2008;1192:114–133. doi: 10.1016/j.brainres.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci. 2010;11:377–388. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover A, Goldbaum M. Locating the optic nerve in a retinal image using the fuzzy convergence of the blood vessels. IEEE Trans Med Imaging. 2003;22:951–958. doi: 10.1109/TMI.2003.815900. [DOI] [PubMed] [Google Scholar]
- Hoover A, Kouznetsova V, Goldbaum M. Locating blood vessels in retinal images by piece-wise threshold probing of a matched filter response. Proc AMIA Symp. 1998:931–935. [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Spelke DP, Xu Z, Kang CC, Schaffer DV, Herr AE. Single-cell western blotting. Nat Methods. 2014;11:749–755. doi: 10.1038/nmeth.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe JR, Jackson AU, Fogarty MP, Buchkovich ML, Stancakova A, Stringham HM, Sim X, Yang L, Fuchsberger C, Cederberg H, Chines PS, Teslovich TM, Romm JM, Ling H, McMullen I, Ingersoll R, Pugh EW, Doheny KF, Neale BM, Daly MJ, Kuusisto J, Scott LJ, Kang HM, Collins FS, Abecasis GR, Watanabe RM, Boehnke M, Laakso M, Mohlke KL. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45:197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Hackler L, Jr, Wu G, Ji H, Zack DJ, Qian J. Dynamics of regulatory networks in the developing mouse retina. PLoS One. 2012;7:e46521. doi: 10.1371/journal.pone.0046521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev Genomics Hum Genet. 2001;2:343–372. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. American journal of human genetics. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Oh EC, Ng L, Srinivas M, Brooks M, Swaroop A, Forrest D. Retinoid-related orphan nuclear receptor RORbeta is an early-acting factor in rod photoreceptor development. Proc Natl Acad Sci U S A. 2009;106:17534–17539. doi: 10.1073/pnas.0902425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res. 2011;39:5015–5024. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kalathur RK, Gagniere N, Berthommier G, Poidevin L, Raffelsberger W, Ripp R, Leveillard T, Poch O. RETINOBASE: a web database, data mining and analysis platform for gene expression data on retina. BMC Genomics. 2008;9:208. doi: 10.1186/1471-2164-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandpal RP, Rajasimha HK, Brooks MJ, Nellissery J, Wan J, Qian J, Kern TS, Swaroop A. Transcriptome analysis using next generation sequencing reveals molecular signatures of diabetic retinopathy and efficacy of candidate drugs. Mol Vis. 2012;18:1123–1146. [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee DK, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, Zhang Q, Grossniklaus HE, Provis JM, Madigan MC, Milam AH, Justice NL, Albuquerque RJ, Blandford AD, Bogdanovich S, Hirano Y, Witta J, Fuchs E, Littman DR, Ambati BK, Rudin CM, Chong MM, Provost P, Kugel JF, Goodrich JA, Dunaief JL, Baffi JZ, Ambati J. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoff J, Miller J. Pharmacogenetics of the Treatment Response of Age-Related Macular Degeneration with Ranibizumab and Bevacizumab. Semin Ophthalmol. 2013 doi: 10.3109/08820538.2013.825292. [DOI] [PubMed]
- Karali M, Peluso I, Gennarino VA, Bilio M, Verde R, Lago G, Dolle P, Banfi S. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics. 2010;11:715. doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, MacDonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attie-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Yang HJ, Chang YS, Kim JW, Brooks M, Chew EY, Wong WT, Fariss R, Rachel R, Cogliati T, Qian H, Swaroop A. Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Invest Ophthalmol Vis Sci. 2014;55:6031–6040. doi: 10.1167/iovs.14-15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Fan J, Crouch RK, Kefalov VJ. Age-related deterioration of rod vision in mice. J Neurosci. 2010;30:11222–11231. doi: 10.1523/JNEUROSCI.4239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koso H, Minami C, Tabata Y, Inoue M, Sasaki E, Satoh S, Watanabe S. CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest Ophthalmol Vis Sci. 2009;50:5411–5418. doi: 10.1167/iovs.08-3246. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kozhevnikova OS, Korbolina EE, Ershov NI, Kolosova NG. Rat retinal transcriptome: effects of aging and AMD-like retinopathy. Cell Cycle. 2013;12:1745–1761. doi: 10.4161/cc.24825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriete A, Sokhansanj BA, Coppock DL, West GB. Systems approaches to the networks of aging. Ageing Res Rev. 2006;5:434–448. doi: 10.1016/j.arr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schubeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Kukurba KR, Zhang R, Li X, Smith KS, Knowles DA, How Tan M, Piskol R, Lek M, Snyder M, Macarthur DG, Li JB, Montgomery SB. Allelic expression of deleterious protein-coding variants across human tissues. PLoS Genet. 2014;10:e1004304. doi: 10.1371/journal.pgen.1004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok MC, Holopainen JM, Molday LL, Foster LJ, Molday RS. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteomics. 2008;7:1053–1066. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong AZX, Fritsche LG, Bragg-Gresham J, Branham KE, Othman M, Boleda A, Gieser L, Ratnapriya R, Stambolian D, Chew EY, Swaroop A, Abecasis G. Whole-Genome Sequencing Study of ~6,000 Samples for Age-related Macular Degeneration 2014 [Google Scholar]
- Lagha M, Bothma JP, Levine M. Mechanisms of transcriptional precision in animal development. Trends Genet. 2012;28:409–416. doi: 10.1016/j.tig.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen T, Sammeth M, Friedlander MR, t Hoen PA, Monlong J, Rivas MA, Gonzalez-Porta M, Kurbatova N, Griebel T, Ferreira PG, Barann M, Wieland T, Greger L, van Iterson M, Almlof J, Ribeca P, Pulyakhina I, Esser D, Giger T, Tikhonov A, Sultan M, Bertier G, MacArthur DG, Lek M, Lizano E, Buermans HP, Padioleau I, Schwarzmayr T, Karlberg O, Ongen H, Kilpinen H, Beltran S, Gut M, Kahlem K, Amstislavskiy V, Stegle O, Pirinen M, Montgomery SB, Donnelly P, McCarthy MI, Flicek P, Strom TM, Geuvadis C, Lehrach H, Schreiber S, Sudbrak R, Carracedo A, Antonarakis SE, Hasler R, Syvanen AC, van Ommen GJ, Brazma A, Meitinger T, Rosenstiel P, Guigo R, Gut IG, Estivill X, Dermitzakis ET. Transcriptome and genome sequencing uncovers functional variation in humans. Nature. 2013;501:506–511. doi: 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–1251. doi: 10.1016/j.cell.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B, Crombie C, Tischler J, Fortunato A, Fraser AG. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nature genetics. 2006;38:896–903. doi: 10.1038/ng1844. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levine M, Cattoglio C, Tjian R. Looping back to leap forward: transcription enters a new era. Cell. 2014;157:13–25. doi: 10.1016/j.cell.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chang HY. Physiological roles of long noncoding RNAs: insight from knockout mice. Trends Cell Biol. 2014;24:594–602. doi: 10.1016/j.tcb.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jia C, Kazmierkiewicz KL, Bowman AS, Tian L, Liu Y, Gupta NA, Gudiseva HV, Yee SS, Kim M, Dentchev T, Kimble JA, Parker JS, Messinger JD, Hakonarson H, Curcio CA, Stambolian D. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum Mol Genet. 2014;23:4001–4014. doi: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MM, Chan CC, Tuo J. Genetic mechanisms and age-related macular degeneration: common variants, rare variants, copy number variations, epigenetics, and mitochondrial genetics. Hum Genomics. 2012;6:13. doi: 10.1186/1479-7364-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Furukawa T, Steffen MA, Church GM, Cepko CL. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Curr Biol. 2000;10:301–310. doi: 10.1016/s0960-9822(00)00379-1. [DOI] [PubMed] [Google Scholar]
- Loscher CJ, Hokamp K, Wilson JH, Li T, Humphries P, Farrar GJ, Palfi A. A common microRNA signature in mouse models of retinal degeneration. Exp Eye Res. 2008;87:529–534. doi: 10.1016/j.exer.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Grone HJ, Lopez I, Gudiseva HV, O’Toole JF, Vallespin E, Ayyagari R, Ayuso C, Cremers FP, den Hollander AI, Koenekoop RK, Dallapiccola B, Ghiggeri GM, Hildebrandt F, Valente EM, Williams DS, Gleeson JG. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22:1243–1254. doi: 10.1101/gr.132514.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck E, Cai L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat Methods. 2012;9:743–748. doi: 10.1038/nmeth.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumayag S, Haldin CE, Corbett NJ, Wahlin KJ, Cowan C, Turturro S, Larsen PE, Kovacs B, Witmer PD, Valle D, Zack DJ, Nicholson DA, Xu S. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci U S A. 2013;110:E507–516. doi: 10.1073/pnas.1212655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Cojocaru R, Gotoh N, Gieser L, Villasmil R, Cogliati T, Swaroop A, Wong WT. Gene expression changes in aging retinal microglia: relationship to microglial support functions and regulation of activation. Neurobiol Aging. 2013;34:2310–2321. doi: 10.1016/j.neurobiolaging.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, Albers CA, Zhang ZD, Conrad DF, Lunter G, Zheng H, Ayub Q, DePristo MA, Banks E, Hu M, Handsaker RE, Rosenfeld JA, Fromer M, Jin M, Mu XJ, Khurana E, Ye K, Kay M, Saunders GI, Suner MM, Hunt T, Barnes IH, Amid C, Carvalho-Silva DR, Bignell AH, Snow C, Yngvadottir B, Bumpstead S, Cooper DN, Xue Y, Romero IG, Wang J, Li Y, Gibbs RA, McCarroll SA, Dermitzakis ET, Pritchard JK, Barrett JC, Harrow J, Hurles ME, Gerstein MB, Tyler-Smith C Genomes Project C. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay GJ, Campbell L, Oliver M, Brockbank S, Simpson DA, Curry WJ. Preparation of planar retinal specimens: verification by histology, mRNA profiling, and proteome analysis. Mol Vis. 2004;10:240–247. [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Meola N, Pizzo M, Alfano G, Surace EM, Banfi S. The long noncoding RNA Vax2os1 controls the cell cycle progression of photoreceptor progenitors in the mouse retina. RNA. 2012;18:111–123. doi: 10.1261/rna.029454.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merbs SL, Khan MA, Hackler L, Jr, Oliver VF, Wan J, Qian J, Zack DJ. Cell-specific DNA methylation patterns of retina-specific genes. PloS one. 2012;7:e32602. doi: 10.1371/journal.pone.0032602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Meyer KJ, Davis LK, Schindler EI, Beck JS, Rudd DS, Grundstad AJ, Scheetz TE, Braun TA, Fingert JH, Alward WL, Kwon YH, Folk JC, Russell SR, Wassink TH, Stone EM, Sheffield VC. Genome-wide analysis of copy number variants in age-related macular degeneration. Hum Genet. 2011;129:91–100. doi: 10.1007/s00439-010-0904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton KP, Swain PK, Chen S, Xu S, Zack DJ, Swaroop A. The leucine zipper of NRL interacts with the CRX homeodomain. A possible mechanism of transcriptional synergy in rhodopsin regulation. J Biol Chem. 2000;275:29794–29799. doi: 10.1074/jbc.M003658200. [DOI] [PubMed] [Google Scholar]
- Montano M, Long K. RNA surveillance-an emerging role for RNA regulatory networks in aging. Ageing Res Rev. 2011;10:216–224. doi: 10.1016/j.arr.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, O’Connor MD, Griffith M, Kuchenbauer F, Delaney A, Prabhu AL, Zhao Y, McDonald H, Zeng T, Hirst M, Eaves CJ, Marra MA. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18:610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Mortimer SA, Kidwell MA, Doudna JA. Insights into RNA structure and function from genome-wide studies. Nat Rev Genet. 2014;15:469–479. doi: 10.1038/nrg3681. [DOI] [PubMed] [Google Scholar]
- Mu X, Zhao S, Pershad R, Hsieh TF, Scarpa A, Wang SW, White RA, Beremand PD, Thomas TL, Gan L, Klein WH. Gene expression in the developing mouse retina by EST sequencing and microarray analysis. Nucleic Acids Res. 2001;29:4983–4993. doi: 10.1093/nar/29.24.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Bai X, Maeda T, Sears JE, Khalil AM, Palczewski K. Evolutionarily conserved long intergenic non-coding RNAs in the eye. Hum Mol Genet. 2013;22:2992–3002. doi: 10.1093/hmg/ddt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Genoud C, Okano K, Cideciyan AV, Sumaroka A, Roman AJ, Jacobson SG, Engel A, Adams MD, Palczewski K. Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 2011;25:3157–3176. doi: 10.1096/fj.11-186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nasonkin IO, Merbs SL, Lazo K, Oliver VF, Brooks M, Patel K, Enke RA, Nellissery J, Jamrich M, Le YZ, Bharti K, Fariss RN, Rachel RA, Zack DJ, Rodriguez-Boulan EJ, Swaroop A. Conditional knockdown of DNA methyltransferase 1 reveals a key role of retinal pigment epithelium integrity in photoreceptor outer segment morphogenesis. Development. 2013;140:1330–1341. doi: 10.1242/dev.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Jr, Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling K, Collin RW, Gilissen C, van Huet RA, Visser L, Kwint MP, Gijsen SJ, Zonneveld MN, Wieskamp N, de Ligt J, Siemiatkowska AM, Hoefsloot LH, Buckley MF, Kellner U, Branham KE, den Hollander AI, Hoischen A, Hoyng C, Klevering BJ, van den Born LI, Veltman JA, Cremers FP, Scheffer H. Next-generation genetic testing for retinitis pigmentosa. Hum Mutat. 2012;33:963–972. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, Forrest D. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J Neurosci. 2011;31:11118–11125. doi: 10.1523/JNEUROSCI.1709-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, Shaffer T, Wong M, Bhattacharjee A, Eichler EE, Bamshad M, Nickerson DA, Shendure J. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Nishiguchi KM, Tearle RG, Liu YP, Oh EC, Miyake N, Benaglio P, Harper S, Koskiniemi-Kuendig H, Venturini G, Sharon D, Koenekoop RK, Nakamura M, Kondo M, Ueno S, Yasuma TR, Beckmann JS, Ikegawa S, Matsumoto N, Terasaki H, Berson EL, Katsanis N, Rivolta C. Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proc Natl Acad Sci U S A. 2013;110:16139–16144. doi: 10.1073/pnas.1308243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Cheng H, Hao H, Jia L, Khan NW, Swaroop A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008;1236:16–29. doi: 10.1016/j.brainres.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci U S A. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. So much “junk” DNA in our genome. Brookhaven Symp Biol. 1972;23:366–370. [PubMed] [Google Scholar]
- Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, Graham RR, Manoharan A, Ortmann W, Bhangale T, Denny JC, Carroll RJ, Eyler AE, Greenberg JD, Kremer JM, Pappas DA, Jiang L, Yin J, Ye L, Su DF, Yang J, Xie G, Keystone E, Westra HJ, Esko T, Metspalu A, Zhou X, Gupta N, Mirel D, Stahl EA, Diogo D, Cui J, Liao K, Guo MH, Myouzen K, Kawaguchi T, Coenen MJ, van Riel PL, van de Laar MA, Guchelaar HJ, Huizinga TW, Dieude P, Mariette X, Bridges SL, Jr, Zhernakova A, Toes RE, Tak PP, Miceli-Richard C, Bang SY, Lee HS, Martin J, Gonzalez-Gay MA, Rodriguez-Rodriguez L, Rantapaa-Dahlqvist S, Arlestig L, Choi HK, Kamatani Y, Galan P, Lathrop M, Eyre S, Bowes J, Barton A, de Vries N, Moreland LW, Criswell LA, Karlson EW, Taniguchi A, Yamada R, Kubo M, Liu JS, Bae SC, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Raychaudhuri S, Stranger BE, De Jager PL, Franke L, Visscher PM, Brown MA, Yamanaka H, Mimori T, Takahashi A, Xu H, Behrens TW, Siminovitch KA, Momohara S, Matsuda F, Yamamoto K, Plenge RM RACI consortium; GARNET consortium. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver VF, Franchina M, Jaffe AE, Branham KE, Othman M, Heckenlively JR, Swaroop A, Campochiaro B, Vote BJ, Craig JE, Saffery R, Mackey DA, Qian J, Zack DJ, Hewitt AW, Merbs SL. Hypomethylation of the IL17RC promoter in peripheral blood leukocytes is not a hallmark of age-related macular degeneration. Cell Rep. 2013a;5:1527–1535. doi: 10.1016/j.celrep.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver VF, Wan J, Agarwal S, Zack DJ, Qian J, Merbs SL. A novel methyl-binding domain protein enrichment method for identifying genome-wide tissue-specific DNA methylation from nanogram DNA samples. Epigenetics Chromatin. 2013b;6:17. doi: 10.1186/1756-8935-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Katoh K, Sato S, Muranishi Y, Chaya T, Onishi A, Minami T, Fujikado T, Furukawa T. Analysis of transcriptional regulatory pathways of photoreceptor genes by expression profiling of the Otx2-deficient retina. PLoS One. 2011;6:e19685. doi: 10.1371/journal.pone.0019685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermuller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nurnberg G, Nurnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgul RK, Siemiatkowska AM, Yucel D, Myers CA, Collin RW, Zonneveld MN, Beryozkin A, Banin E, Hoyng CB, van den Born LI, Bose R, Shen W, Sharon D, Cremers FP, Klevering BJ, den Hollander AI, Corbo JC European Retinal Disease C. Exome sequencing and cis-regulatory mapping identify mutations in MAK, a gene encoding a regulator of ciliary length, as a cause of retinitis pigmentosa. Am J Hum Genet. 2011;89:253–264. doi: 10.1016/j.ajhg.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Gregory TR. The case for junk DNA. PLoS Genet. 2014;10:e1004351. doi: 10.1371/journal.pgen.1004351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfoli I, Musante L, Bachi A, Ravera S, Calzia D, Cattaneo A, Bruschi M, Bianchini P, Diaspro A, Morelli A, Pepe IM, Tacchetti C, Candiano G. Proteomic analysis of the retinal rod outer segment disks. J Proteome Res. 2008;7:2654–2669. doi: 10.1021/pr7006939. [DOI] [PubMed] [Google Scholar]
- Parapuram SK, Cojocaru RI, Chang JR, Khanna R, Brooks M, Othman M, Zareparsi S, Khan NW, Gotoh N, Cogliati T, Swaroop A. Distinct signature of altered homeostasis in aging rod photoreceptors: implications for retinal diseases. PLoS One. 2010;5:e13885. doi: 10.1371/journal.pone.0013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–764. doi: 10.1093/hmg/ddi070. [DOI] [PubMed] [Google Scholar]
- Peng GH, Chen S. Active opsin loci adopt intrachromosomal loops that depend on the photoreceptor transcription factor network. Proc Natl Acad Sci U S A. 2011;108:17821–17826. doi: 10.1073/pnas.1109209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio LA, Bickmore W, Dean A, Nobrega MA, Bejerano G. Enhancers: five essential questions. Nat Rev Genet. 2013;14:288–295. doi: 10.1038/nrg3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler SJ, Zhang Y, Chen S, Mears AJ, Zack DJ, Ren Z, Swain PK, Yao S, Swaroop A, White JB. Functional analysis of the rod photoreceptor cGMP phosphodiesterase alpha-subunit gene promoter: Nrl and Crx are required for full transcriptional activity. J Biol Chem. 2004;279:19800–19807. doi: 10.1074/jbc.M401864200. [DOI] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- Popova EY, Xu X, DeWan AT, Salzberg AC, Berg A, Hoh J, Zhang SS, Barnstable CJ. Stage and gene specific signatures defined by histones H3K4me2 and H3K27me3 accompany mammalian retina maturation in vivo. PLoS One. 2012;7:e46867. doi: 10.1371/journal.pone.0046867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc Natl Acad Sci U S A. 2013;110:19814–19819. doi: 10.1073/pnas.1312009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya RR, Chew EY, Swaroop A. Genetic studies of age-related macular degeneration: lessons, challenges, and opportunities for disease management. Ophthalmology. 2012;119:2526–2536. doi: 10.1016/j.ophtha.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt KD, Harrow J, Harte RA, Wallin C, Diekhans M, Maglott DR, Searle S, Farrell CM, Loveland JE, Ruef BJ, Hart E, Suner MM, Landrum MJ, Aken B, Ayling S, Baertsch R, Fernandez-Banet J, Cherry JL, Curwen V, Dicuccio M, Kellis M, Lee J, Lin MF, Schuster M, Shkeda A, Amid C, Brown G, Dukhanina O, Frankish A, Hart J, Maidak BL, Mudge J, Murphy MR, Murphy T, Rajan J, Rajput B, Riddick LD, Snow C, Steward C, Webb D, Weber JA, Wilming L, Wu W, Birney E, Haussler D, Hubbard T, Ostell J, Durbin R, Lipman D. The consensus coding sequence (CCDS) project: Identifying a common protein-coding gene set for the human and mouse genomes. Genome Res. 2009;19:1316–1323. doi: 10.1101/gr.080531.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas E, Feinberg AP. Regulated noise in the epigenetic landscape of development and disease. Cell. 2012;148:1123–1131. doi: 10.1016/j.cell.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Esumi N, Chen Y, Wang Q, Chowers I, Zack DJ. Identification of regulatory targets of tissue-specific transcription factors: application to retina-specific gene regulation. Nucleic Acids Res. 2005;33:3479–3491. doi: 10.1093/nar/gki658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Rapicavoli NA, Blackshaw S. New meaning in the message: noncoding RNAs and their role in retinal development. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2103–2114. doi: 10.1002/dvdy.21844. [DOI] [PubMed] [Google Scholar]
- Rapicavoli NA, Poth EM, Zhu H, Blackshaw S. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural development. 2011;6:32. doi: 10.1186/1749-8104-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapriya R, Swaroop A. Genetic architecture of retinal and macular degenerative diseases: the promise and challenges of next-generation sequencing. Genome Med. 2013;5:84. doi: 10.1186/gm488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapriya R, Zhan X, Fariss RN, Branham KE, Zipprer D, Chakarova CF, Sergeev YV, Campos MM, Othman M, Friedman JS, Maminishkis A, Waseem NH, Brooks M, Rajasimha HK, Edwards AO, Lotery A, Klein BE, Truitt BJ, Li B, Schaumberg DA, Morgan DJ, Morrison MA, Souied E, Tsironi EE, Grassmann F, Fishman GA, Silvestri G, Scholl HP, Kim IK, Ramke J, Tuo J, Merriam JE, Merriam JC, Park KH, Olson LM, Farrer LA, Johnson MP, Peachey NS, Lathrop M, Baron RV, Igo RP, Jr, Klein R, Hagstrom SA, Kamatani Y, Martin TM, Jiang Y, Conley Y, Sahel JA, Zack DJ, Chan CC, Pericak-Vance MA, Jacobson SG, Gorin MB, Klein ML, Allikmets R, Iyengar SK, Weber BH, Haines JL, Leveillard T, Deangelis MM, Stambolian D, Weeks DE, Bhattacharya SS, Chew EY, Heckenlively JR, Abecasis GR, Swaroop A. Rare and common variants in extracellular matrix gene Fibrillin 2 (FBN2) are associated with macular degeneration. Hum Mol Genet. 2014;23:5827–5837. doi: 10.1093/hmg/ddu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapriya RPS, Mutsuddi M, Branham K, Othman M, Heckenlively J, Swaroop A. Exome sequencing in extended families with age-related macular degeneration reveals enrichment of genes involved in extracellular matrix pathway. Personal communication.64th Annual Meeting of The American Society of Human Genetics; 2014. [Google Scholar]
- Raychaudhuri S, Iartchouk O, Chin K, Tan PL, Tai AK, Ripke S, Gowrisankar S, Vemuri S, Montgomery K, Yu Y, Reynolds R, Zack DJ, Campochiaro B, Campochiaro P, Katsanis N, Daly MJ, Seddon JM. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43:1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, Zeitlinger J, Schreiber J, Hannett N, Kanin E, Volkert TL, Wilson CJ, Bell SP, Young RA. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–2309. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell. 2011;147:1408–1419. doi: 10.1016/j.cell.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger JE, Hiriyanna A, Gotoh N, Hao H, Cheng DF, Ratnapriya R, Kautzmann MA, Chang B, Swaroop A. OTX2 loss causes rod differentiation defect in CRX-associated congenital blindness. J Clin Invest. 2014;124:631–643. doi: 10.1172/JCI72722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, Wong MC, Maddren M, Fang R, Heitner SG, Lee BT, Barber GP, Harte RA, Diekhans M, Long JC, Wilder SP, Zweig AS, Karolchik D, Kuhn RM, Haussler D, Kent WJ. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41:D56–63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF. Drug and gene delivery to the back of the eye: from bench to bedside. Invest Ophthalmol Vis Sci. 2014;55:2714–2730. doi: 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Ruan Y. Genome wide full-length transcript analysis using 5′ and 3′ paired-end-tag next generation sequencing (RNA-PET) Methods Mol Biol. 2012;809:535–562. doi: 10.1007/978-1-61779-376-9_35. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Cimermancic P, Szpiech ZA, Sali A, Hernandez RD, Krogan NJ. High-resolution network biology: connecting sequence with function. Nat Rev Genet. 2013;14:865–879. doi: 10.1038/nrg3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- Sahebkar A. Beyond anti-PCSK9 therapies: the potential role of resistin inhibitors. Nat Rev Cardiol. 2014;11:12. doi: 10.1038/nrcardio.2013.139-c1. [DOI] [PubMed] [Google Scholar]
- Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM, Kosmicki JA, Rehnstrom K, Mallick S, Kirby A, Wall DP, MacArthur DG, Gabriel SB, DePristo M, Purcell SM, Palotie A, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Gibbs RA, Schellenberg GD, Sutcliffe JS, Devlin B, Roeder K, Neale BM, Daly MJ. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014 doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel A, Housset M, Fant B, Lamonerie T. Otx2 ChIP-seq reveals unique and redundant functions in the mature mouse retina. PLoS One. 2014;9:e89110. doi: 10.1371/journal.pone.0089110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011;31:16033–16044. doi: 10.1523/JNEUROSCI.3580-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- Schaibley VM, Zawistowski M, Wegmann D, Ehm MG, Nelson MR, St Jean PL, Abecasis GR, Novembre J, Zollner S, Li JZ. The influence of genomic context on mutation patterns in the human genome inferred from rare variants. Genome Res. 2013;23:1974–1984. doi: 10.1101/gr.154971.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub MA, Boyle AP, Kundaje A, Batzoglou S, Snyder M. Linking disease associations with regulatory information in the human genome. Genome Res. 2012;22:1748–1759. doi: 10.1101/gr.136127.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz HL, Goetz T, Kaschkoetoe J, Weber BH. The Retinome - defining a reference transcriptome of the adult mammalian retina/retinal pigment epithelium. BMC Genomics. 2004;5:50. doi: 10.1186/1471-2164-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, Goldstein JI, Triebwasser M, Anderson HE, Zerbib J, Kavanagh D, Souied E, Katsanis N, Daly MJ, Atkinson JP, Raychaudhuri S. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013 doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Shuga J, Trombetta JJ, Gennert D, Lu D, Chen P, Gertner RS, Gaublomme JT, Yosef N, Schwartz S, Fowler B, Weaver S, Wang J, Wang X, Ding R, Raychowdhury R, Friedman N, Hacohen N, Park H, May AP, Regev A. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature. 2014;509:363–369. doi: 10.1038/nature13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- Sharon D, Blackshaw S, Cepko CL, Dryja TP. Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE) Proc Natl Acad Sci U S A. 2002;99:315–320. doi: 10.1073/pnas.012582799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003;121:1316–1323. doi: 10.1001/archopht.121.9.1316. [DOI] [PubMed] [Google Scholar]
- Shen J, Yang X, Xie B, Chen Y, Swaim M, Hackett SF, Campochiaro PA. MicroRNAs regulate ocular neovascularization. Mol Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A. 2014;111:E5593–5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomori K, Werner JS. Aging of human short-wave cone pathways. Proc Natl Acad Sci U S A. 2012;109:13422–13427. doi: 10.1073/pnas.1119770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le YZ, Fehling HJ, Gaidatzis D, Stadler MB, Roska B. Transcriptional code and disease map for adult retinal cell types. Nat Neurosci. 2012;15:487–495. S481–482. doi: 10.1038/nn.3032. [DOI] [PubMed] [Google Scholar]
- Slack FJ. Regulatory RNAs and the demise of ‘junk’ DNA. Genome Biol. 2006;7:328. doi: 10.1186/gb-2006-7-9-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SA, Lee HJ, Angermueller C, Krueger F, Saadeh H, Peat J, Andrews SR, Stegle O, Reik W, Kelsey G. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11:817–820. doi: 10.1038/nmeth.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrin L, Reynolds R, Yu Y, Fagerness J, Leveziel N, Bernstein PS, Souied EH, Daly MJ, Seddon JM. ARMS2/HTRA1 locus can confer differential susceptibility to the advanced subtypes of age-related macular degeneration. Am J Ophthalmol. 2011;151:345–352. e343. doi: 10.1016/j.ajo.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrin L, Ripke S, Yu Y, Fagerness J, Bhangale TR, Tan PL, Souied EH, Buitendijk GH, Merriam JE, Richardson AJ, Raychaudhuri S, Reynolds R, Chin KA, Lee AY, Leveziel N, Zack DJ, Campochiaro P, Smith RT, Barile GR, Hogg RE, Chakravarthy U, Behrens TW, Uitterlinden AG, van Duijn CM, Vingerling JR, Brantley MA, Jr, Baird PN, Klaver CC, Allikmets R, Katsanis N, Graham RR, Ioannidis JP, Daly MJ, Seddon JM. Heritability and genome-wide association study to assess genetic differences between advanced age-related macular degeneration subtypes. Ophthalmology. 2012;119:1874–1885. doi: 10.1016/j.ophtha.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011a;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Smaoui N, Ayyagari R, Stiles D, Benhamed S, MacDonald IM, Daiger SP, Tumminia SJ, Hejtmancik F, Wang X. High-throughput retina-array for screening 93 genes involved in inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 2011b;52:9053–9060. doi: 10.1167/iovs.11-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger PK. A reductionist’s systems biology: opinion. Curr Opin Cell Biol. 2005;17:9–11. doi: 10.1016/j.ceb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Splinter E, de Wit E, van de Werken HJ, Klous P, de Laat W. Determining long-range chromatin interactions for selected genomic sites using 4C-seq technology: from fixation to computation. Methods. 2012;58:221–230. doi: 10.1016/j.ymeth.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Ng L, Liu H, Jia L, Forrest D. Activation of the blue opsin gene in cone photoreceptor development by retinoid-related orphan receptor beta. Mol Endocrinol. 2006;20:1728–1741. doi: 10.1210/me.2005-0505. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Sundermeier TR, Zhang N, Vinberg F, Mustafi D, Kohno H, Golczak M, Bai X, Maeda A, Kefalov VJ, Palczewski K. DICER1 is essential for survival of postmitotic rod photoreceptor cells in mice. FASEB J. 2014;28:3780–3791. doi: 10.1096/fj.14-254292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suuronen T, Nuutinen T, Ryhanen T, Kaarniranta K, Salminen A. Epigenetic regulation of clusterin/apolipoprotein J expression in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2007;357:397–401. doi: 10.1016/j.bbrc.2007.03.135. [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nature reviews Genetics. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Sieving PA. The golden era of ocular disease gene discovery: race to the finish. Clin Genet. 2013;84:99–101. doi: 10.1111/cge.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Xu JZ, Pawar H, Jackson A, Skolnick C, Agarwal N. A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc Natl Acad Sci U S A. 1992;89:266–270. doi: 10.1073/pnas.89.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Zack DJ. Transcriptome analysis of the retina. Genome Biol. 2002;3:REVIEWS1022. doi: 10.1186/gb-2002-3-8-reviews1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Tariq MA, Kim HJ, Jejelowo O, Pourmand N. Whole-transcriptome RNAseq analysis from minute amount of total RNA. Nucleic Acids Res. 2011;39:e120. doi: 10.1093/nar/gkr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telese F, Gamliel A, Skowronska-Krawczyk D, Garcia-Bassets I, Rosenfeld MG. “Seq-ing” insights into the epigenetics of neuronal gene regulation. Neuron. 2013;77:606–623. doi: 10.1016/j.neuron.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell D, Xie B, Workman M, Mahato S, Zelhof A, Gebelein B, Cook T. OTX2 and CRX rescue overlapping and photoreceptor-specific functions in the Drosophila eye. Dev Dyn. 2012;241:215–228. doi: 10.1002/dvdy.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA, Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessler LA, Reifenberger JG, Mitra RD. Protein quantification in complex mixtures by solid phase single-molecule counting. Anal Chem. 2009;81:7141–7148. doi: 10.1021/ac901068x. [DOI] [PubMed] [Google Scholar]
- Trewavas A. A brief history of systems biology. “Every object that biology studies is a system of systems.” Francois Jacob (1974) Plant Cell. 2006;18:2420–2430. doi: 10.1105/tpc.106.042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Roska B, Billings N, Sun B, Bartch B, Cepko CL. Molecular heterogeneity of developing retinal ganglion and amacrine cells revealed through single cell gene expression profiling. J Comp Neurol. 2007;502:1047–1065. doi: 10.1002/cne.21368. [DOI] [PubMed] [Google Scholar]
- Tummala P, Mali RS, Guzman E, Zhang X, Mitton KP. Temporal ChIP-on-Chip of RNA-Polymerase-II to detect novel gene activation events during photoreceptor maturation. Mol Vis. 2010;16:252–271. [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven JP, Nilsson SC, Tan PL, Buitendijk GH, Ristau T, Mohlin FC, Nabuurs SB, Schoenmaker-Koller FE, Smailhodzic D, Campochiaro PA, Zack DJ, Duvvari MR, Bakker B, Paun CC, Boon CJ, Uitterlinden AG, Liakopoulos S, Klevering BJ, Fauser S, Daha MR, Katsanis N, Klaver CC, Blom AM, Hoyng CB, den Hollander AI. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet. 2013;45:813–817. doi: 10.1038/ng.2640. [DOI] [PubMed] [Google Scholar]
- Veleri S, Lazar CH, Chang B, Swaroop A. Insights into the biology and therapy of human retinal neurodegeneration using mose models. Dis Model Mech. 2015 doi: 10.1242/dmm.017913. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini G, Rose AM, Shah AZ, Bhattacharya SS, Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8:e1003040. doi: 10.1371/journal.pgen.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Cusick ME, Barabasi AL. Interactome networks and human disease. Cell. 2011;144:986–998. doi: 10.1016/j.cell.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15:69–81. doi: 10.1038/nrg3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Oliver VF, Zhu H, Zack DJ, Qian J, Merbs SL. Integrative analysis of tissue-specific methylation and alternative splicing identifies conserved transcription factor binding motifs. Nucleic Acids Res. 2013;41:8503–8514. doi: 10.1093/nar/gkt652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Hodor P, Thut CJ, Vogt TF, Zhang T, Holder DJ, Petrukhin K. Dual role of Nr2e3 in photoreceptor development and maintenance. Exp Eye Res. 2008;87:35–48. doi: 10.1016/j.exer.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schubeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- Wei L, Liu B, Tuo J, Shen D, Chen P, Li Z, Liu X, Ni J, Dagur P, Sen HN, Jawad S, Ling D, Park S, Chakrabarty S, Meyerle C, Agron E, Ferris FL, 3rd, Chew EY, McCoy JP, Blum E, Francis PJ, Klein ML, Guymer RH, Baird PN, Chan CC, Nussenblatt RB. Hypomethylation of the IL17RC promoter associates with age-related macular degeneration. Cell Rep. 2012a;2:1151–1158. doi: 10.1016/j.celrep.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Gatterdam V, Wieneke R, Tampe R, Rant U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat Nanotechnol. 2012b;7:257–263. doi: 10.1038/nnano.2012.24. [DOI] [PubMed] [Google Scholar]
- Weinmann AS, Farnham PJ. Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods. 2002;26:37–47. doi: 10.1016/S1046-2023(02)00006-3. [DOI] [PubMed] [Google Scholar]
- White MA, Myers CA, Corbo JC, Cohen BA. Massively parallel in vivo enhancer assay reveals that highly local features determine the cis-regulatory function of ChIP-seq peaks. Proc Natl Acad Sci U S A. 2013;110:11952–11957. doi: 10.1073/pnas.1307449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- Wright AF, Reddick AC, Schwartz SB, Ferguson JS, Aleman TS, Kellner U, Jurklies B, Schuster A, Zrenner E, Wissinger B, Lennon A, Shu X, Cideciyan AV, Stone EM, Jacobson SG, Swaroop A. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum Mutat. 2004;24:439. doi: 10.1002/humu.9285. [DOI] [PubMed] [Google Scholar]
- Wu H, D’Alessio AC, Ito S, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25:679–684. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- Xue W, Cojocaru RI, Dudley VJ, Brooks M, Swaroop A, Sarthy VP. Ciliary neurotrophic factor induces genes associated with inflammation and gliosis in the retina: a gene profiling study of flow-sorted, Muller cells. PLoS One. 2011;6:e20326. doi: 10.1371/journal.pone.0020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SP, Hao H, Yang HJ, Kautzmann MA, Brooks M, Nellissery J, Klocke B, Seifert M, Swaroop A. The transcription-splicing protein NonO/p54nrb and three NonO-interacting proteins bind to distal enhancer region and augment rhodopsin expression. Hum Mol Genet. 2014;23:2132–2144. doi: 10.1093/hmg/ddt609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Stratton C, Francis PJ, Kleinman ME, Tan PL, Gibbs D, Tong Z, Chen H, Constantine R, Yang X, Chen Y, Zeng J, Davey L, Ma X, Hau VS, Wang C, Harmon J, Buehler J, Pearson E, Patel S, Kaminoh Y, Watkins S, Luo L, Zabriskie NA, Bernstein PS, Cho W, Schwager A, Hinton DR, Klein ML, Hamon SC, Simmons E, Yu B, Campochiaro B, Sunness JS, Campochiaro P, Jorde L, Parmigiani G, Zack DJ, Katsanis N, Ambati J, Zhang K. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359:1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GW, Coufal NG, Liang TY, Peng GE, Fu XD, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nat Struct Mol Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M. Drug-target network. Nat Biotechnol. 2007;25:1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Mears AJ, Friedman JS, Carter T, He S, Oh E, Jing Y, Farjo R, Fleury G, Barlow C, Hero AO, Swaroop A. Expression profiling of the developing and mature Nrl−/− mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum Mol Genet. 2004;13:1487–1503. doi: 10.1093/hmg/ddh160. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yashar BM, Hiriyanna S, Swaroop A. Microarray analysis of gene expression in the aging human retina. Invest Ophthalmol Vis Sci. 2002;43:2554–2560. [PubMed] [Google Scholar]
- Yu H, Luscombe NM, Qian J, Gerstein M. Genomic analysis of gene expression relationships in transcriptional regulatory networks. Trends Genet. 2003a;19:422–427. doi: 10.1016/S0168-9525(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Yu J, Farjo R, MacNee SP, Baehr W, Stambolian DE, Swaroop A. Annotation and analysis of 10,000 expressed sequence tags from developing mouse eye and adult retina. Genome Biol. 2003b;4:R65. doi: 10.1186/gb-2003-4-10-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, He S, Friedman JS, Akimoto M, Ghosh D, Mears AJ, Hicks D, Swaroop A. Altered expression of genes of the Bmp/Smad and Wnt/calcium signaling pathways in the cone-only Nrl−/− mouse retina, revealed by gene profiling using custom cDNA microarrays. J Biol Chem. 2004a;279:42211–42220. doi: 10.1074/jbc.M408223200. [DOI] [PubMed] [Google Scholar]
- Yu J, Mears AJ, Yoshida S, Farjo R, Carter TA, Ghosh D, Hero A, Barlow C, Swaroop A. From disease genes to cellular pathways: a progress report. Novartis Found Symp. 2004b;255:147–160. doi: 10.1002/0470092645.ch11. discussion 160–144, 177–148. [DOI] [PubMed] [Google Scholar]
- Yu X, Lin J, Zack DJ, Qian J. Computational analysis of tissue-specific combinatorial gene regulation: predicting interaction between transcription factors in human tissues. Nucleic Acids Res. 2006;34:4925–4936. doi: 10.1093/nar/gkl595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Triebwasser MP, Wong EK, Schramm EC, Thomas B, Reynolds R, Mardis ER, Atkinson JP, Daly M, Raychaudhuri S, Kavanagh D, Seddon JM. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014;23:5283–5293. doi: 10.1093/hmg/ddu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005a;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, Petty HR, Richards JE, Abecasis GR, Elner VM, Swaroop A. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005b;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20:259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- Zhan X, Larson DE, Wang C, Koboldt DC, Sergeev YV, Fulton RS, Fulton LL, Fronick CC, Branham KE, Bragg-Gresham J, Jun G, Hu Y, Kang HM, Liu D, Othman M, Brooks M, Ratnapriya R, Boleda A, Grassmann F, von Strachwitz C, Olson LM, Buitendijk GH, Hofman A, van Duijn CM, Cipriani V, Moore AT, Shahid H, Jiang Y, Conley YP, Morgan DJ, Kim IK, Johnson MP, Cantsilieris S, Richardson AJ, Guymer RH, Luo H, Ouyang H, Licht C, Pluthero FG, Zhang MM, Zhang K, Baird PN, Blangero J, Klein ML, Farrer LA, DeAngelis MM, Weeks DE, Gorin MB, Yates JR, Klaver CC, Pericak-Vance MA, Haines JL, Weber BH, Wilson RK, Heckenlively JR, Chew EY, Stambolian D, Mardis ER, Swaroop A, Abecasis GR. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet. 2013;45:1375–1379. doi: 10.1038/ng.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Poh HM, Peh SQ, Sia YY, Li G, Mulawadi FH, Goh Y, Fullwood MJ, Sung WK, Ruan X, Ruan Y. ChIA-PET analysis of transcriptional chromatin interactions. Methods. 2012;58:289–299. doi: 10.1016/j.ymeth.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, Song JJ, Kingston RE, Borowsky M, Lee JT. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One. 2014;9:e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Menche J, Barabasi AL, Sharma A. Human symptoms-disease network. Nat Commun. 2014;5:4212. doi: 10.1038/ncomms5212. [DOI] [PubMed] [Google Scholar]