Abstract
Xanthophyll carotenoids zeaxanthin and lutein play a special role in the prevention and treatment of visual diseases. These carotenoids are not produced by the human body and must be consumed in the diet. On the other hand, extremely low water solubility of these carotenoids and their instability restrict their practical application as components of food or medicinal formulations. Preparation of supramolecular complexes of zeaxanthin and lutein with glycyrrhizic acid, its disodium salt and the natural polysaccharide arabinogalactan allows one to minimize the aforementioned disadvantages when carotenoids are used in food processing as well as for production of therapeutic formulations with enhanced solubility and stability. In the present study, the formation of supramolecular complexes was investigated by NMR relaxation, surface plasmon resonance (SPR) and optical absorption techniques. The complexes increase carotenoid solubility more than 1000-fold. The kinetics of carotenoid decay in reactions with ozone molecules, hydroperoxyl radicals and metal ions were measured in water and organic solutions, and significant increases in oxidation stability of lutein and zeaxanthin in arabinogalactan and glycyrrhizin complexes were detected.
Keywords: carotenoids, lutein, zeaxanthin, water-soluble complexes, arabinogalactan, glycyrrhizic acid, ozone, oxygen radicals, oxidation, NMR relaxation, surface plasmon resonance
Introduction
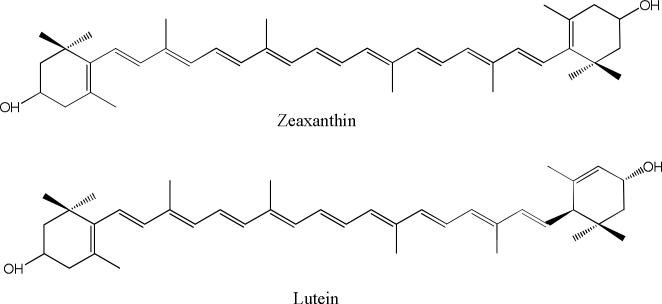
Xanthophylls are the special group of carotenoids which contain oxygen functionalities at one or both ends of the molecule, providing them with unique biological, chemical and physical properties. In particular, these hydroxyl groups are responsible for the xanthophylls’ abilities to orient within cell membranes in ways other carotenoids cannot [1-4]. The present study is devoted to two representatives of xanthophyll carotenoids, lutein and zeaxanthin (Figure 1), which play a special role in the prevention and treatment of visual diseases. These carotenoids are not produced by the human body and must be consumed in the diet.
Fig. 1.
Structural formulas of zeaxanthin and lutein.
Lutein and zeaxanthin selectively accumulate at an extremely high concentration in the macula of the primate eye retina through the action of specific high-affinity binding proteins [5], potentially slowing the onset of age-related macular degeneration [6, 7], and they have been recently added to the list of beneficial nutrients provided by leafy greens. It is suggested that lutein and zeaxanthin play a role in blue light filtration and antioxidant function [8, 9]. The higher-energy, blue wavelengths of visible light are 100 times more effective at inducing free radical formation in the cells of the retina than the lower-energy, red wavelengths of visible light [10]. Reacting as antioxidants with free radicals and reactive oxygen species, macular carotenoids protect the retina against peroxidation and photo-damage [11-15].
The important feature of xanthophyll carotenoids is their ability to form J- and H-type of self-assembled complexes in aqueous media and even in lipid membranes [16-20]. Molecular self-assembly in biological systems attracts considerable attention, since it is important for the functioning of living organisms. It has been shown that lutein and zeaxanthin form aggregates when dissolved in hydrated polar solvents and that this aggregation is characterized by dramatic changes in their absorption spectra and photo-physical properties [16, 18, 20]. The H-aggregates, in which the molecules are tightly stacked with the conjugated chains oriented more or less parallel to each other, show a large blue shift of the absorption spectrum and loss of vibrational structure of the S2 excited state. The blue shift of the absorption spectrum is explained in terms of excitonic interaction between the closely packed carotenoid chromophores [18-20]. The second aggregation type, characterized by a red shift of the absorption spectrum where the resolution of vibrational bands is preserved, is attributed to J-type aggregation, in which there is a more head-to-tail organization of the conjugated chains. The self-assembly of xanthophylls leads to new photo-physical properties which can have an impact on various applications, particularly in relation to solar energy conversion [21]. One photo-physical mechanism that generally requires the proximity of two chromophores is singlet fission. In this mechanism, a chromophore is photoexcited to its singlet excited state and subsequently partitions its energy over two neighboring chromophores that both remain in triplet excited states. The chromophore of zeaxanthin is favorable for the production of triplet excited states via fission, and a high yield of triplet excited states via singlet fission was found for its aggregate [22]. On the other hand, only very restricted data exist on the influence of aggregation on the chemical properties of carotenoids. Recently it was demonstrated by optical and EPR spin trapping techniques that aggregation of xanthophyll carotenoids results in an increase in their photostability in aqueous solution, but it significantly reduces their antioxidant ability [16].
The practical use of lutein, zeaxanthin and other carotenoids is restricted by their insolubility in water and their poor chemical stability. A successful approach to improve the effectiveness and safety of poorly soluble drugs is to increase their solubility and dissolution rate by the formation of “host-guest” complexes with synthetic or natural water-soluble polymers or oligomers [23-34]. Most of the earlier attempts to increase the solubility of carotenoids were based on the preparation of cyclodextrin inclusion complexes [23, 35-40]; however, cyclodextrin complexes demonstrate low solubility and fast aggregation in aqueous solution.
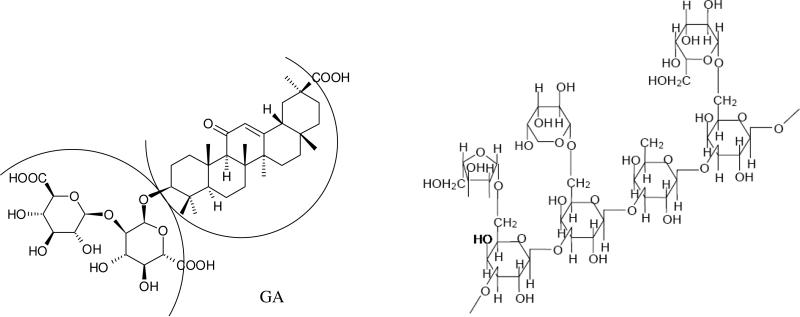
Recently, we have described the synthesis of novel carotenoid complexes with unique physicochemical properties [16, 24-28]. In these studies we used two “host” molecules derived from plants: the triterpene glycoside glycyrrhizic acid (GA), a natural compound extracted from the licorice root [41, 42], and arabinogalactan (AG), a natural water soluble polysaccharide extracted from Siberian larch [43-45].
Glycyrrhizic acid (Figure 2) self-associates into dimers containing a hydrophobic and hydrophilic component in a donut like shape [25, 46]. The polyene chains of carotenoids can reside within the hydrophobic area while allowing the hydrophilic terminal rings to stick out on each end. As a result, complexation with GA does not reduce scavenging ability of carotenoids with low oxidation potential towards oxygen radicals, and it may even enhance this ability considerably for carotenoids with high oxidation ability (canthaxanthin, for example) [26]. Arabinogalactan is a highly branched polysaccharide polymer composed of galactose and arabinose fragments in a 6:1 ratio. It has previously been reported to function as a complexing agent to make carotenoids water dispersible with increased photostability [27] and photocatalytic activity [28]. The unique properties of GA and AG complexes were demonstrated for two natural carotenoids: β-carotene and canthaxanthin [23-28].
Fig. 2.
Structural formulas of glycyrrhizic acid (GA) arabinogalactan (fragment).
The influence of arabinogalactan and glycyrrhizin on the reactivity of lutein and zeaxanthin and their H-aggregates in some practically important processes, such as prevention of photo-degradation and improvement of antioxidant activity, was investigated in our previous study [16]. A strong influence of complexation on the reactivity of carotenoid monomers and aggregates was found. In particular, an increase of photostability of both monomers and aggregates in aqueous solutions was detected [16]. The increase of scavenging ability towards peroxyl radicals was also detected by EPR and explained by the decreasing aggregation rate in the presence of GA and AG [16].
The present study is devoted to the investigation of oxidation stability of lutein and zeaxanthin in solution and in their inclusion complexes with AG, GA and its disodium salt (sGA). The kinetics of carotenoid decay in reactions with ozone molecules, hydroperoxyl radicals and metal ions was measured in water and organic solutions. We then compared the behavior of the complexes prepared by using two different approaches, namely, liquid-phase synthesis and solid-state mechanochemical association. The latter approach permits solid-state preparation of complexes of insoluble drugs in one technological step without use of any organic solvents [27, 31-33]. In addition, complex formation with sGA was demonstrated by NMR relaxation methods, and complexation with arabinogalactan was analyzed by surface plasmon resonance (SPR) techniques [47].
Experimental
Lutein (>90%, the rest amount is zeaxanthin) was provided by Kemin Health (Des Moines, Iowa), and zeaxanthin (>95%) came from Kalsec (Kalamazoo, Michigan). As the “host” molecules we used arabinogalactan from Siberian larch wood [44], glycyrrhizic acid from Ural licorice root [48] and disodium salt of glycyrrhizic acid (CFS, 98%) from Shaanxi Sciphar Biotechnology Co., Ltd (Xi'an, China). Amine-coupling reagents (N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), and 1 M sodium ethanolamine hydrochloride, pH 8.5) were purchased from Biacore AB (Uppsala, Sweden). All other chemicals used were of the highest purity available and were purchased from Sigma Chemicals (Saint Louis, Missouri) unless otherwise stated.
Preparation of water-soluble complexes by the mechanochemical method is described in detail in our previous papers [27, 31-33].
Optical spectra and decay kinetics of carotenoids were recorded using an SF-2000 spectrophotometer (Spectrum, Saint-Petersburg, Russia) in the spectral range from 190 to 1100 nm in a 1 cm quartz cuvette.
1H NMR spectra in solutions were recorded on a Bruker DPX-200 NMR spectrometer (200 MHz) in CD3OD and D2O. Measurement of the phase relaxation times T2 was performed using the standard Carr-Purcell-Meiboom-Gill sequence (CPMG) of the type P1(90°) – (τ – P2(180°)n – registration, where τ = 0.6 ms is a fixed time delay, and the n value was varied from 0 to 4028.
A SensiQ Pioneer (SensiQ Technologies Inc, Oklahoma) surface plasmon resonance biosensor was used in this study. The arabinogalactan was immobilized on XantecPolycarboxylate HC200 chips (XanTecbioanalytics, GmbH) using aldehyde-coupling chemistry. Briefly, saccharide containing ligands on carboxyl functionalized sensor chip surfaces were immobilized using oxidized cis-diols. 1 mM NaIO4 was used to oxidize OH groups and was further purified on a Sephadex G-25 column using 5 mM sodium acetate buffer pH 4. Samples were stored at −20°C until use. The sensor surface was activated by injecting a freshly prepared mixture of NHS and 2-(N-morpholino)-ethane sulphonic acid pH 5 containing 100 mM EDC. Carbohydrazide (5 mM) solution was injected to incorporate the hydrazide functionality on the sensor chip surface. Excess reactive groups were quenched by injecting ethanolamine (1 M) for 7-10 minutes. The oxidized arabinogalactan (1 mg/ml) was injected on the functionalized surface for 10 minutes. Cyanoborohydride(100 mM) solution in 100 mM acetate buffer pH 5 was injected to stabilize the covalent linkage. Zeaxanthin stocks were prepared in 100% DMSO. It was then diluted into 5 % working solution in phosphate buffered saline (PBS) with 0.01% Tween-20, which was used as a running buffer. Serial double dilutions of zeaxanthin stocks were prepared in running buffer. The samples were injected at 10 μl/minute flow-rate for 5 minutes over the arabinogalactan immobilized surface and the reference surface. Buffer blanks were injected for double-referencing purposes. Regeneration was carried out using 50 % DMSO solution in PBS buffer.
Results and Discussion
Preparation of water soluble supramolecular complexes of carotenoids
It is well known that carotenoids are highly hydrophobic, air- and light-sensitive compounds. Earlier there were several attempts to prepare water soluble complexes of carotenoids using water-oil emulsion or cyclodextrin solutions [24, 37-40]. Cyclodextrins are widely used in pharmacology to increase the solubility of drugs [35, 36]; however, in the case of carotenoids, these complexes exist only as large aggregates with average size more than 100 nm [37, 38]. The solutions of all carotenoid-cyclodextrin complexes show a considerable change in color as compared to carotenoid solutions in organic solvents [24]. For example, the aqueous solution of the β-carotene-CD complex is an intense opalescent pink-orange, the CD complex of 7’-apo-7’,7’-dicyano-β-carotene is black, whereas its unaggregated MeOH and CH2Cl2 solutions are violet.
In this study, we present examples of water soluble composites of the carotenoids lutein and zeaxanthin. The method of complex preparation used in the present work was a mechanochemical treatment of the solid mixture of carotenoid crystals with arabinogalactan or glycyrrhizic acid disodium salt powders. Typical mechanochemical reactions are those activated by co-grinding or milling of powder materials. These reactions are usually carried out either manually, in an agate mortar, or electro-mechanically, as in ball milling. In all these cases, the crystal lattice is destroyed and reformed through recrystallization. Co-grinding of solid materials results in penetration of carotenoid molecules into the “host” macromolecule without use of any organic solvents. Complex formation was monitored by X-ray diffraction phase analysis and differential scanning calorimetry (DSC) techniques. In the initial mixture of compounds before treatment, one can see the characteristic peaks of the crystal structure of the carotenoid, which disappear after mechanochemical treatment [27]. We suggest that the absence of a crystal structure in this case is due to molecular penetration of the carotenoid into the polymer matrix (in the case of arabinogalactan). We interpret this to mean that complex formation occurs in the solid state but not during further solubilization. We assume that deep penetration of carotenoid into the arabinogalactan matrix does not occur when we try to prepare the complex by addition of a carotenoid solution to an arabinogalactan solution. This was confirmed by the significant increase in solubility of the complex prepared mechanochemically as compared with traditional solvent-mediated methods. The important advantage of this method over solvent-mediated methods is also the possibility of complex preparation without using any toxic organic solvents.
For evidence of binding between “guest” and “host” molecules in solution, various methods can be applied. For poorly soluble molecules like carotenoids, the increase in their solubility can be considered as a test for complex formation [49, 50]. In the present study, the solubility of mechanochemically prepared complexes was measured by HPLC after paper filtration (Filters decalcified “Blue Ribbon”, “ECOHIM” Saint-Petersburg, Russia). The results are presented in Table 1. We suppose that the real water solubility of non-complexed lutein and zeaxanthin may be even lower than shown in the Table 1 due to incomplete filtration of carotenoid microcrystals. As one can see from the data of Table 1, the increase of carotenoid solubility in AG and GA complexes is approximately 1000-fold.
Table 1.
The solubility of mechanochemically prepared lutein and zeaxanthin complexes with AG and GA disodium and ammonium salts in water (in mg/l), measured by HPLC after paper filtration.
| Sample | Free | AG | GA-NH3 | GA-Na2 |
|---|---|---|---|---|
| Lutein 1:20 | 0.01* | 18 | 15 | 10 |
| Lutein 1:40 | 10 | |||
| Zeaxanthin 1:20 | 0.01* | 21 | 9 | 2.8 |
| Zeaxanthin 1:40 | 8 |
This approximate value may be an upper limit due to penetration of small carotenoid crystals through paper filter.
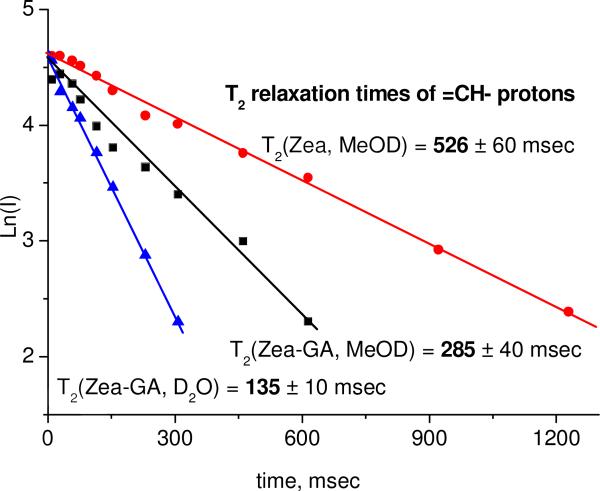
Another approach to prove molecular complexation is the detection of a change of the spectroscopic properties of the “guest” molecule in the presence of the “host”. In this study, we have applied NMR relaxation techniques to detect the binding of zeaxanthin with the disodium salt of GA in methanol and water solutions. The T2 relaxation time is very sensitive to intermolecular interactions and diffusion mobility of molecules. Proton relaxation time of the molecule inside the complex is significantly reduced because diffusive and rotational mobilities are slow [51]. A change of T2 is usually considered as proof of complex formation [29, 30, 52]. Relaxation times T2 were measured for the CH protons at the double bond. Figure 3 shows the echo decay kinetics of zeaxanthin CH protons in the CPMG experiment (see Experimental section) in pure form in deuterated methanol and in the complex with sGA (5 mM) in deuterated methanol and D2O.
Fig. 3.
The echo decay kinetics of zeaxanthin CH protons in CPMG experiment in pure form in deuterated methanol and in the complex with sGA (5 mM) in deuterated methanol and D2O.
Relaxation time of CH protons of free carotenoid in methanol is 560 ± 60 msec. T2 is slowed down to 285 ± 40 msec after addition of 5 mM of disodium salt of GA to this solution, due to complex formation. In the water environment, glycyrrhizic acid can form micelles; therefore the further decrease of relaxation time to 135 ± 10 msec can be explained by incorporation of carotenoid inside the micelle.
SPR investigation of carotenoid binding to arabinogalactan
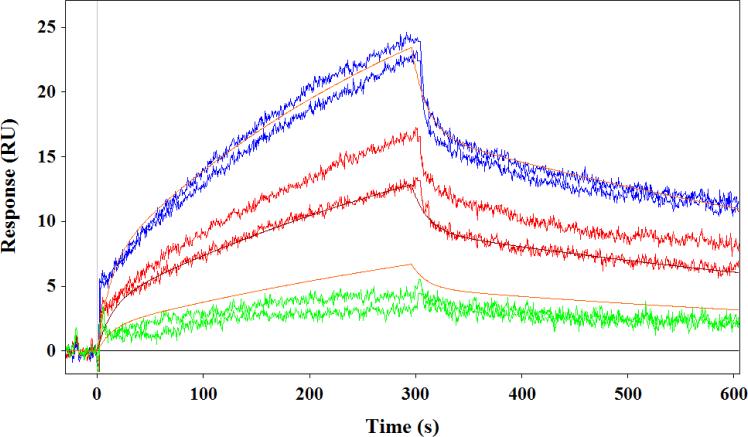
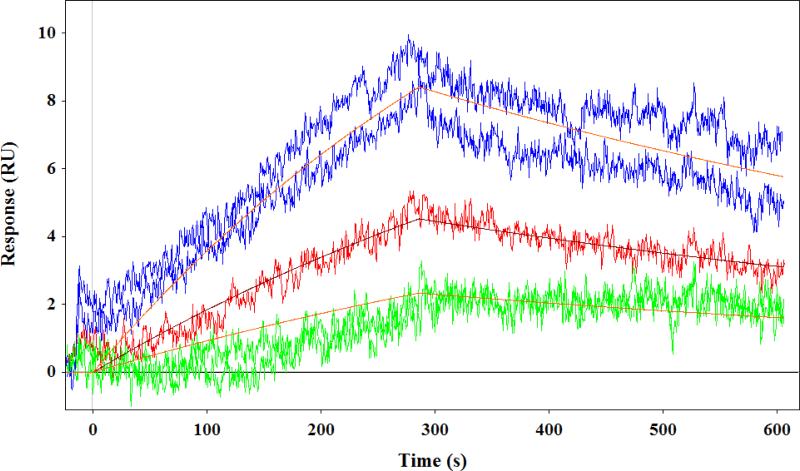
Arabinogalactan-zeaxanthin binding was investigated using SPR technique [47]. The SPR studies revealed that the arabinogalactan could bind zeaxanthin. We compared the binding affinities of Russian arabinogalactan prepared from Siberian larch (Siberian-arabinogalactan) and American arabinogalactan obtained from Sigma Chemicals (Sigma-arabinogalactan). Figure 4 displays the sensorgram obtained from the interaction of the two arabinogalactan compounds and zeaxanthin. As shown in Table 2, the arabinogalactan-zeaxanthin interaction was fitted using a kinetic model with QdatTM analysis software (Biologic Software, Australia) to extract kinetic parameters of binding. One can see from Figure 4 that the Siberian-arabinogalactan has bimodal association and dissociation kinetics. We consider the presence at least two binding sites with different binding abilities (see Table 2). The Siberian-arabinogalactan had a strong affinity toward zeaxanthin with a KD(1) of 2.2 μM and KD(2) of 14.2 μM, whereas Sigma-arabinogalactan is 10 times weaker than the Siberian version. Unlike protein-carotenoid interactions, this binding interaction depends on the contact time, as it is driven by the diffusion of zeaxanthin molecules into the arabinogalactan matrix. We therefore used similar conditions for all comparisons.
Fig. 4.
SPR sensorgram showing interaction of Siberian arabinogalactan (top) and Sigma arabinogalactan (bottom) with zeaxanthin.
Table 2.
Kinetic parameters of zeaxanthin-arabinogalactan binding extracted from SPR experiment. KD - equilibrium dissociation constant; ka _ association constant; kd - dissociation constant.
| ka, M−1s−1 | kd, s−1 | Kd, μM | |
|---|---|---|---|
| Siberian arabinogalactan (Site-1) | 6.4±0.3 ×102 | 0.00140± 0.0003 | 2.2 ±0.1 |
| Siberian arabinogalactan (Site-2) | 4.9±0.7 ×103 | 0.070± 0.003 | 14 ± 2 |
| Sigma arabinogalactan | 48 ± 3 | 0.00118 ± 0.0002 | 25 ± 2 |
*Errors represent the residual from the model fit.
The possible reason of the difference in the properties of Siberian and Sigma arabanogalactans might be the different internal structure of these branched polymers which results in different pore size.
Investigations on the oxidation stability of carotenoids and their inclusion complexes
The influence of complexation on the oxidation stability of carotenoids was investigated using model reactions with ozone molecules, hydroperoxyl radicals and metal ions. Ozone molecules are very reactive oxygen species which are able to break double bonds of carotenoids with high efficiency [53]. The reaction occurs via addition of ozone to the double bond with formation of an ozonide, followed by breakage of the double bond with formation of a number of oxygenated products with reduced conjugated chains [54]:
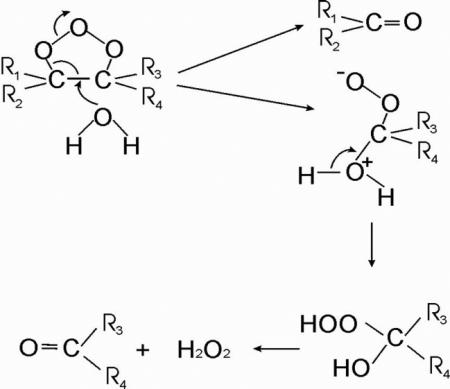
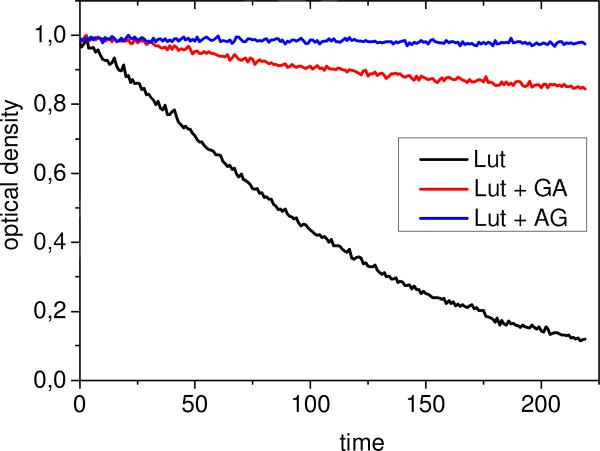
In the present study, the decay kinetics of lutein and zeaxanthin in reaction with ozone were monitored by optical absorption techniques. The reaction was carried out in 75% water-ethanol solution for 5 minutes at 380 nm with continuous ozone bubbling. For free carotenoids, fast decay of the absorption signal was observed; however, the solutions of AG, GA and sGA complexes show significant reduction of the oxidation rate (see Figure 5 as an example). The fitting of experimental decay kinetics was made using exponential approximation assuming constant ozone concentration during experiment.
Fig. 5.
Kinetics of lutein concentration decay in 75% water-ethanol solution during oxidation by ozone in free form and in the presence of AG, 0.1 mM, and GA, 1 mM.
The exponential fitting of the decay kinetics shown in Figure 5 results in the following values of decay time: for free lutein: = 150 ± 20 sec; for lutein with GA: = 1290 ± 30 sec; and no decay was observed for lutein-AG complex at this concentration. Table 3 summarizes the results for all systems under study.
Table 3.
Decay times (in sec) of carotenoids zeaxanthin and lutein in reactions with ozone molecules in 75% water-ethanol solution.
| Free carotenoid | 1 mM GA | 1 mM sGA | 0.05 mM AG | |
|---|---|---|---|---|
| Lut + O3 | 150 ± 10 | 1290 ± 20 | 1310 ± 20 | ∞ |
| Zea + O3 | 200 ± 20 | 1100 ± 20 | 1140 ± 10 | 2150 ± 50 |
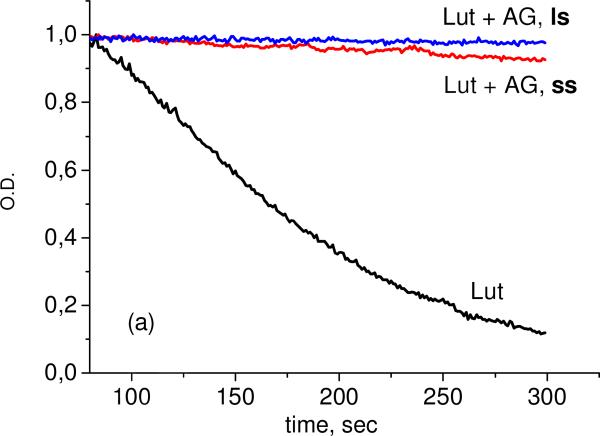
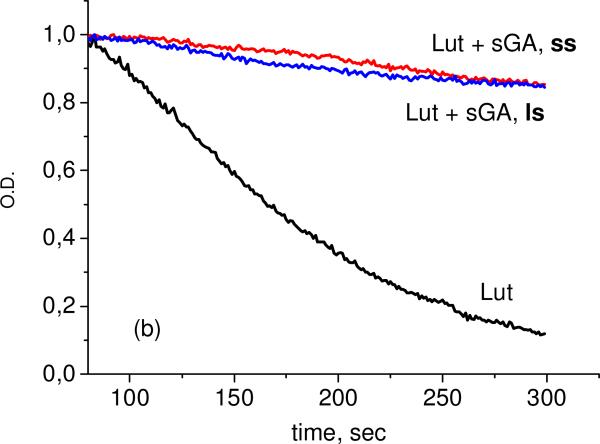
As one can see from these results, significant reduction of oxidation rate was detected for all systems under study. The most stable are the complexes with arabinogalactan. We did not observe significant differences between complexes prepared by different methods (solid state (ss) or liquid state (ls), Figure 6).
Fig. 6.
Kinetics of lutein concentration decay in 75% water-ethanol solution during oxidation by ozone in free form and in the presence of AG, 0.05 mM (a), and sGA, 1 mM (b), prepared by “liquid state, ls” and “mechanochemical, ss” methods.
We assume that this is due to establishment of equilibrium between complex and free carotenoid. The very strong inhibition of the reaction in AG complexes points to the deep penetration of carotenoid molecules into the polymer matrix of arabinogalactan. The stability of GA and sGA complexes depends on the concentration of “host” molecules. Significant stabilization was detected for concentrations near 1 mM when GA exists mainly in the micellar form [30]. Earlier the similar effect was observed for canthaxanthin-GA complex [55].
The important processes which influence the stability of carotenoids in solutions are the reactions of carotenoids with metal ions (Cu2+ or Fe3+) and free radicals, mainly with peroxyl radicals produced in Fenton-like reactions or in the reaction of C-centered radicals with oxygen. The mechanisms of these reactions were investigated in detail earlier [56-59]:
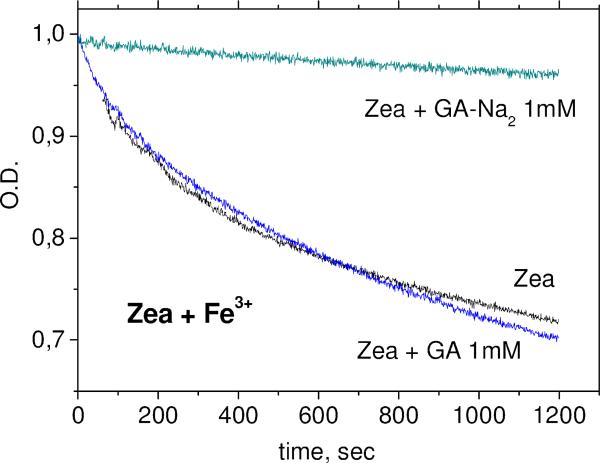
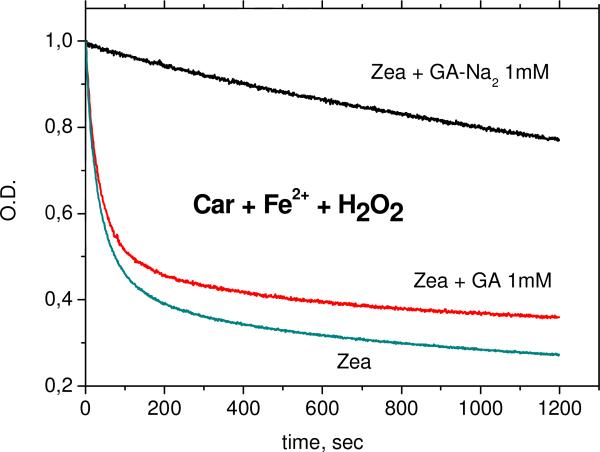
The kinetics of carotenoid decay in the reactions with Fe3+ ions and •OOH radicals (in Fenton reaction with excess of H2O2) were measured in DMSO solution for glycyrrhizic acid (GA) complexes and glycyrrhizic acid disodium salt (sGA) complexes and in 50% aqueous DMSO solution for arabinogalactan complexes. Figures 7 and 8 show the decay kinetics for zeaxanthin in the absence and in the presence of 1 mM GA or sGA.
Fig. 7.
Kinetics of zeaxanthin concentration decay in DMSO solution during oxidation by Fe3+ ions in the absence and in the presence of 1 mM of GA and sGA.
Fig. 8.
Kinetics of zeaxanthin concentration decay in DMSO solution during oxidation by •OOH radicals (Fenton reaction with excess of H2O2) in the absence and in the presence of 1 mM of GA and sGA. [H2O2] = 125 mM, [Fe2+] = 1 mM.
Similar effects were detected for lutein complexes and zeaxanthin-AG complexes (Table 4).
Table 4.
Decay times (in sec) of carotenoids zeaxanthin and lutein in reactions with Fe3+ ions and ·OOH radicals. Experimental conditions as described in Figures 7 and 8.
| Free carotenoid | 1 mM GA | 1 mM sGA | 0.05 mM AG* | |
|---|---|---|---|---|
| Lut + Fe3+ | 570 ± 10 | 590 ± 20 | 6600 ± 50 | - |
| Lut + Fe2+ + H2O2 | 90 ± 10 | 100 ± 10 | 4560 ± 60 | - |
| Zea + Fe3+ | 510 ± 10 | 630 ± 10 | 10200 ± 170 | ∞ |
| Zea + Fe2+ + H2O2 | 65 ± 5 | 70 ± 5 | 9050 ± 50 | 3780 ± 30 |
In 50% DMSO.
One can see from Figures 5-6 and Table 4 that sGA but not GA protects xanthophyll carotenoids from oxidation by Fe3+ ions and peroxyl radicals. The absence of effect of GA on antioxidant activity of zeaxanthin in DMSO solution was detected also by EPR spin trapping techniques [26]. On the other hand, the increase of antioxidant activity of zeaxanthin in the presence of GA in aqueous solution detected by the same technique was explained by the influence of GA on the aggregation rate of xanthophyll carotenoids [16].
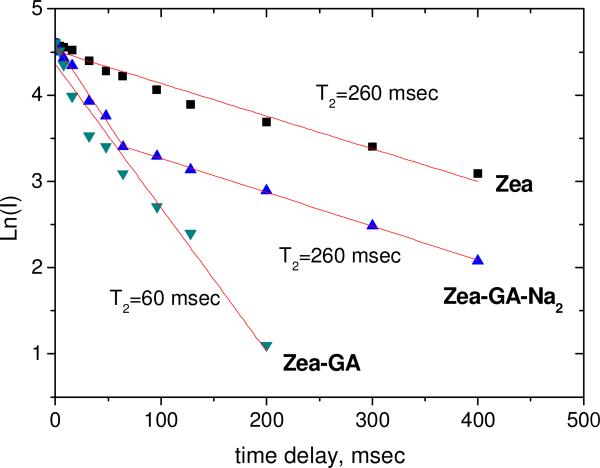
To check the binding of GA molecules with zeaxanthin in DMSO solution, we have applied NMR relaxation methods as describer earlier (Figure 9).
Fig. 9.
Decay kinetics (in logarithmic scale) of the NMR signal of zeaxanthin 4-H protons in T2 relaxation experiment in DMSO. [Zea] = 2 mM, [GA] = 5 mM.
One can see from these measurements that GA has strong binding with zeaxanthin in DMSO solution. As was suggested earlier, the interaction of carotenoids with •OOH radicals occurs mainly by hydrogen abstraction from the most acidic 4-H position of the cyclohexene ring [16]. We can assume from our results that the polyene chains of carotenoids can reside within the hydrophobic area of GA self-associates, while allowing the hydrophilic terminal rings to stick out on each end. As a result, complexation with GA does not reduce scavenging ability of these carotenoids towards oxygen radicals. We can assume also that the presence of positively charged Na+ ions in sGA-carotenoid complexes reduces the access of Fe3+ and Fe2+ ions to the carotenoids. This can result in the increased carotenoid stability detected in the present study.
Conclusion
Non-covalent binding of the carotenoids lutein and zeaxanthin with the polysaccharide arabinogalactan and the oligosaccharide glycyrrhizic acid (or its disodium salt) results in formation of water soluble aggregates with unique physicochemical properties. The most important physical properties are enhanced water solubility and decreased self-aggregation of the xanthophyll carotenoids. This significantly changes the optical and photophysical properties of these carotenoids.
The increased chemical stability of xanthophyll carotenoids is also important finding of this study. A significant increase in oxidation stability of lutein and zeaxanthin in arabinogalactan, glycyrrhizic acid (GA) complexes and glycyrrhizic acid disodium salt (sGA) has been detected. It was found that arabinogalactan has the strongest binding with these carotenoids, as well as the most significant influence on carotenoid stability in aqueous solutions. From our results, we can conclude that carotenoids penetrate into the arabinogalactan matrix completely, providing defense from interactions with oxidizing species, such as metal ions, enzymes or reactive oxygen species. On the other hand, the advantage of glycyrrhizic acid complexes is their stability in both aqueous and non-aqueous environments. Taking into account the important role of these carotenoids in eye and skin health, glycyrrhizic acid and arabinogalactan can be considered as potential delivery systems which can provide enhanced stability and solubility of xanthophyll carotenoids.
Highlights.
- Arabinogalactan and glycyrrhizic acid improve the carotenoids’ solubility in water.
- Arabinogalactan complexes of lutein and zeaxanthin exhibit enhanced oxidation stability.
- Arabinogalactan complexes show very slow release of lutein and zeaxanthin in water.
- The disodium salt of GA protects carotenoids from oxidation by metal ions.
Acknowledgements
This work was supported by grant RUC-7067-NO-12 from the U.S. Civilian Research & Development Foundation (CRDF Global) with funding from the United States Department of State, by NIH grant EY-11600, by unrestricted departmental funds from Research to Prevent Blindness, and by research project of Russian Academy of Science V.48.1.8. The development of drug delivery systems based on mechanochemically synthesized supramolecular systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krinsky NI, Mathews-Roth MM, Taylor RF. Carotenoids: Chemistry and Biology. Plenum. Press; 1989. [Google Scholar]
- 2.Goulinet S, Chapman MJ. Arterioscl. Throm. Vas. 1997;17:786–796. doi: 10.1161/01.atv.17.4.786. [DOI] [PubMed] [Google Scholar]
- 3.Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Arch. Biochem. Biophys. 1999;371:301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- 4.Sujak A, Okulski W, Gruszecki WI. BBA-Biomembranes. 2000;1509:255–263. doi: 10.1016/s0005-2736(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 5.Li BX, Vachali P, Bernstein PS. Photochem. Photobiol. Sci. 2010;9:1418–1425. doi: 10.1039/c0pp00126k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale CR, Hall NF, Phillips DIW, Martyn CN. Invest. Ophth. Vis. Sci. 2003;44:2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 7.Krinsky NI, Mayne ST, Sies H. Carotenoids in Health and Disease. Taylor & Francis; 2004. [Google Scholar]
- 8.Kirschfeld K. P. Roy. Soc. B - Biol. Sci. 1982;216:71–85. doi: 10.1098/rspb.1982.0061. [DOI] [PubMed] [Google Scholar]
- 9.Martin HD, Ruck C, Schmidt M, Sell S, Beutner S, Mayer B, Walsh R. Pure Appl. Chem. 1999;71:2253–2262. [Google Scholar]
- 10.Ham WT, Mueller HA, Sliney DH. Nature. 1976;260:153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- 11.Krinsky NI. Free Rad. Bio. Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen K, Skibsted LH. Z. Lebensm. Unters. For. 1993;196:423–429. doi: 10.1007/BF01190806. [DOI] [PubMed] [Google Scholar]
- 13.Edge R, McGarvey DJ, Truscott TG. J. Photoch. Photobio. B. 1997;41:189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 14.Woodall AA, Lee SWM, Weesie RJ, Jackson MJ, Britton G. BBA-Gen. Subjects. 1997;1336:33–42. doi: 10.1016/s0304-4165(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 15.El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ. Arch. Biochem. Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Polyakov NE, Magyar A, Kispert LD. J. Phys. Chem. B. 2013;117:10173–10182. doi: 10.1021/jp4062708. [DOI] [PubMed] [Google Scholar]
- 17.Mosquera MIM, Galán MJ, Méndez DH. Pigments in Food Technology: Proceedings of 1st International Congress Pft. 1999 [Google Scholar]
- 18.Ruban AV, Horton P, Young AJ. J. Photochem. Photobiol. B. 1993;21:229–234. [Google Scholar]
- 19.Billsten HH, Sundstrom V, Polivka T. J. Phys. Chem. A. 2005;109:1521–1529. doi: 10.1021/jp044847j. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Berg CJ, Hsu CC, Merrill BA, Tauber MJ. J. Phys. Chem. B. 2012;116:10617–10630. doi: 10.1021/jp3069514. [DOI] [PubMed] [Google Scholar]
- 21.McHale JL. J. Phys. Chem. Lett. 2012;3:587–597. doi: 10.1021/jz3000678. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Tauber MJ. J. Am. Chem. Soc. 2010;132:13988–13991. doi: 10.1021/ja102851m. [DOI] [PubMed] [Google Scholar]
- 23.Polyakov NE, Leshina TV. The Open Conf. Proc. J. 2011;2:64–72. [Google Scholar]
- 24.Polyakov NE, Leshina TV, Konovalova TA, Hand EO, Kispert LD. Free Rad. Biol. Med. 2004;36:872–880. doi: 10.1016/j.freeradbiomed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Polyakov NE, Leshina TV, Salakhutdinov NF, Kispert LD. J. Phys. Chem. B. 2006;110:6991–6998. doi: 10.1021/jp056038l. [DOI] [PubMed] [Google Scholar]
- 26.Polyakov NE, Leshina TV, Salakhutdinov NF, Konovalova TA, Kispert LD. Free Rad. Biol. Med. 2006;40:1804–1809. doi: 10.1016/j.freeradbiomed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Polyakov NE, Leshina TV, Meteleva ES, Dushkin AV, Konovalova TA, Kispert LD. J. Phys. Chem. B. 2009;113:275–282. doi: 10.1021/jp805531q. [DOI] [PubMed] [Google Scholar]
- 28.Polyakov NE, Leshina TV, Meteleva ES, Dushkin AV, Konovalova TA, Kispert LD. J. Phys. Chem. B. 2010;114:14200–14204. doi: 10.1021/jp908578j. [DOI] [PubMed] [Google Scholar]
- 29.Polyakov NE, Khan VK, Taraban MB, Leshina TV. J. Phys. Chem. B. 2008;112:4435–4440. doi: 10.1021/jp076850j. [DOI] [PubMed] [Google Scholar]
- 30.Kornievskaya VS, Kruppa AI, Polyakov NE, Leshina TV. J. Phys. Chem. B. 2007;111:11447–11452. doi: 10.1021/jp0739770. [DOI] [PubMed] [Google Scholar]
- 31.Dushkin AV. Mechanochemical synthesis of organic compounds and rapidly soluble materials. In: Sopicka-Lizer M, editor. High-energy ball milling. Mechanochemical processing of nanopowders. Woodhead Publishing Limited; 2010. pp. 249–273. [Google Scholar]
- 32.Dushkin AV, Chistyachenko Yu. S., Tolstikova TG, Khvostov MV, Polyakov NE, Lyakhov NZ, Tolstikov GA. Dokl. Biochem. Biophys. 2013;451:180–182. doi: 10.1134/S1607672913040030. [DOI] [PubMed] [Google Scholar]
- 33.Dushkin AV, Meteleva ES, Tolstikova TG, Tolstikov GA, Polyakov NE, Medvedeva EN, Neverova NA, Babkin VA. Russ. Chem. Bull. 2008;6:1299–1307. [Google Scholar]
- 34.Tachaprutinun A, Udomsup T, Luadthong C, Wanichwecharungruang S. Int. J. Pharm. 2009;374:119–124. doi: 10.1016/j.ijpharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Loftsson T, Brewster ME. J. Pharm. Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 36.Szejtli J. Cyclodextrin Technology. 1988. Vol. 1. Springer; [Google Scholar]
- 37.Mele A, Mendichi R, Selva A. Carbohyd. Res. 1998;310:261–267. [Google Scholar]
- 38.Mele A, Mendichi R, Selva A, Molnár P, Tóth G. Carbohyd. Res. 2002;337:1129–1136. doi: 10.1016/s0008-6215(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 39.Szente L, Mikuni K, Hashimoto H, Szejtli J. J. Inclus. Phenom. Mol. 1998;32:81–89. [Google Scholar]
- 40.Lancrajan I, Diehl HA, Socaciu C, Engelke M, Zorn-Kruppa M. Chem. Phys. Lipids. 2001;112:1–10. doi: 10.1016/s0009-3084(01)00138-4. [DOI] [PubMed] [Google Scholar]
- 41.Mazza GO, Oomah BD. Herbs Botanicals and Teas. Technomic Publishing Company, Incorporated; 2000. [Google Scholar]
- 42.Tolstikov GA, Baltina LA, Shults EE, Pokrovsky AG. Bioorg. Khim. 1997;23:691–709. [PubMed] [Google Scholar]
- 43.Odonmazig P, Ebringerova A, Machova E, Alföldi J. Carbohyd. Res. 1994;252:317–324. doi: 10.1016/0008-6215(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 44.Medvedev EN, Vavkin VA, Ostroukhova LA. Chem. Nat. Comp. (in Russian) 2003;1:27–37. [Google Scholar]
- 45.D'Adamo P. J. Naturopath. Med. 1996;6:32–39. [Google Scholar]
- 46.Borisenko SN, Lekar AV, Milov AA, Vetrova EV, Borisenko NI. Chemistry of plant materials. 2013;2:85–92. (in Russian, DOI: 10.14258/jcprm.1321085) [Google Scholar]
- 47.Vachali P, Li B, Nelson K, Bernstein PS. Arch. Biochem. Biophys. 2012;519:32–37. doi: 10.1016/j.abb.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondratenko R, Baltina L, Mustafina S, Makarova N, Nasyrov KM, Tolstikov G. Pharm. Chem. J. 2001;35:101–104. [Google Scholar]
- 49.Nicolescu C, Aram C, Nedelcu A, Monciu C-M. Farmacia. 2010;58:620–628. [Google Scholar]
- 50.Higuchi T, Connors KA. Adv. Anal. Chem. Instr. 1965;4:117–212. [Google Scholar]
- 51.Poole CP, Farrah HA. Academic Press. 1971:392. [Google Scholar]
- 52.Chistyachenko Yu. S., Dushkin AV, Polyakov NE, Khvostov MV, Tolstikova TG, Tolstikov GA, Lyakhov NZ. Drug Delivery, Early Online. 2014:1–8. doi: 10.3109/10717544.2014.884655. doi: 10.3109/10717544.2014.884655. [DOI] [PubMed] [Google Scholar]
- 53.Henry LK, Puspitasari-Nienaber NL, Jaren-Galan M, van Breemen RB, Catignani GL, Schwartz SJ, Agric J. Food Chem. 2000;48:5008–5013. doi: 10.1021/jf000503o. [DOI] [PubMed] [Google Scholar]
- 54.Bailey PS. Chem. Rev. 1958;58:925. [Google Scholar]
- 55.Gluschenko OY, Polyakov NE, Leshina TV. Appl. Magn. Res. 2011;41:283–294. [Google Scholar]
- 56.Polyakov NE, Kruppa AI, Leshina TV, Konovalova TA, Kispert LD. Free Rad. Biol. Med. 2001;31:43–52. doi: 10.1016/s0891-5849(01)00547-0. [DOI] [PubMed] [Google Scholar]
- 57.Polyakov NE, Leshina TV, Konovalova TA, Kispert LD. Free Rad. Biol. Med. 2001;31:398–404. doi: 10.1016/s0891-5849(01)00598-6. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Benito JF. J. Phys. Chem. A. 2004;108:4853–4858. [Google Scholar]
- 59.Barbusinski K. Ecol. Chem. Eng. S. 2009;16:347–358. [Google Scholar]