SUMMARY
Background
The relationships between primary sclerosing cholangitis (PSC) and the environment are largely unknown.
Aims
To validate associations reported in previous studies and to identify novel environmental exposures among PSC patients.
Methods
We performed a multicenter, case–control analysis utilising self-administered questionnaires. Responses between cases (n = 1000) and controls (n = 663) were compared using multivariable logistic regression adjusted for age and gender. The model was further stratified based on inflammatory bowel disease (IBD) status (with IBD n = 741 without IBD n = 259).
Results
Smoking was associated with PSC only when IBD was present (OR, 0.5; 95% CI 0.4–0.7) but not among those PSC patients without IBD (OR, 0.9; 95% CI 0.7–1.2). Compared to controls, women with PSC (irrespective of the presence of IBD) were less likely to have received hormone replacement therapy (HRT; OR, 0.5; 95% CI 0.4–0.7) and were more likely to have recurrent urinary tract infections (OR, 1.6; 95% CI 1.2–2.3). PSC patients regardless of gender or IBD status were less likely to eat fish (OR, 0.4; 95% CI 0.3–0.6) and grilled/barbecued meat (OR, 0.8; 95% CI 0.7–0.9). In contrast, PSC patients with and without IBD were more likely to consume steak/burgers that were more well done (OR, 1.3; 95% CI 1.2–1.5).
Conclusions
IBD (rather than PSC) is associated with smoking. Women with PSC are more likely to have recurrent urinary tract infections and less likely to receive HRT. Dietary intake and methods of food preparation differ in PSC patients when compared to controls.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterised by inflammation of the intra and or extrahepatic bile ducts.1, 2 Population based cohorts from Europe and North America report that 68–73% of PSC patients have concurrent inflammatory bowel disease (IBD), typically ulcerative colitis (UC).3, 4 Although the pathogenesis of PSC remains ill-defined, it may develop through immune-mediated mechanisms triggered by complex gene-environment interactions in susceptible individuals.1
There is a growing body of evidence, which suggests environmental exposures may play a role in the pathogenesis of immune-mediated conditions. For example, an increased incidence of IBD has been observed in industrialised nations and migrants who leave ‘developing’ areas and become established in industrialised regions.5–8 Dietary factors such as fast food intake, sweets, fats and animal protein have been associated with IBD, which adds credence to the hypothesis that environmental risk factors and a western lifestyle may contribute to the pathogenesis of IBD.6 In turn, dietary practices can affect the composition of the human microbiome and metabolome and it has been postulated that alterations in the intestinal microbiome may play a key role in the pathogenesis in IBD and PSC.9–11
Despite advances in our knowledge of exposures associated with other immune-mediated conditions, our understanding of non-genetic risk factors associated with PSC and concurrent IBD (PSC-IBD) and PSC without IBD (PSC-woIBD) is limited. Four studies have reported a negative association with smoking and PSC.12–15 However, a negative association between smoking and UC without PSC has also been reported16 and it is possible that the presence of IBD among PSC patients may act as a confounder when examining disease-environment associations related to PSC (rather than IBD). Tonsillectomy and hormone-based contraceptives were also reported to have a negative association with PSC.14, 15 While appendectomy has been reported to be less common among patients with UC, an association between having an appendectomy and PSC was not observed in a previous report.14 Although two studies independently suggested a negative association with coffee and PSC, data concerning other dietary habits is unknown.15, 17
A comprehensive assessment of environmental factors associated with PSC is important. Identification of such exposures would enhance our understanding of the disease pathogenesis, lay the foundation for future studies to delineate important gene-environment interactions between select genes and environmental exposures, contribute to future therapies and guide preventative measures in at risk populations. However, many of the reports that have examined exposures associated with PSC have been limited to a small subset of environmental factors, localised to a single centre or had a small sample size, which makes adjusting for the presence of IBD challenging. Therefore, we performed a large, prospective multicenter study with two key aims: (i) identify novel exposures associated with PSC among those with and without IBD; (ii) validate or refute environmental exposures that could potentially be associated with PSC (regardless of IBD status) that have been described in previous studies.
METHODS
Subjects
Patients with PSC were prospectively identified and recruited to participate in the present study from 2004 to 2013. The PSC patients were seen and recruited from a consortium of eight academic medical centres in the USA and Canada, which comprise a large study group, entitled: PSC Resource of Genetic Risk, Environment and Synergy Studies (PROGRESS). In addition, a subset of PSC patients were self-referred (i.e. not seen as a patient and recruited through one of the eight centres) and contacted the lead study centre to participate in the study (Figure S1). Patients were diagnosed with PSC by standard methods and the diagnosis was confirmed by review of the medical record.18 However, 33 self-referred patients had a self-reported history of PSC but did not have medical records available to confirm the diagnosis. These patients were included in the primary analysis and later excluded in a sensitivity analysis (below).
Control patients were recruited from the Mayo Clinic Division of General Internal Medicine during visits for preventative health care. Like the cases, the controls resided from areas across North America (71% of controls and 90% of cases lived outside of Minnesota). Controls with a history of liver disease were excluded. This study was approved by the institutional review board for each participating centre and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Data collection and study instrument
Cases and controls were directly given or mailed (in a prepaid envelope) a paper questionnaire at the time of enrolment. If a patient did not return the questionnaire after 2 months a second form was mailed. The 37-page survey consisted of 70 primary questions and 300 secondary questions and was developed by the Mayo Clinic Survey Center. The survey collected detailed, self-reported information concerning patient demographics, education, medical/surgical history and environmental exposures including dietary habits. The dietary questions concerning food frequency were based on a subset of the previously validated Block 1995 Revision of the Health Habits and History Questionnaire.19–21 Female patients were asked about their obstetric and gynecological history, history of urinary tract infections (UTIs), use of hormone replacement therapy (HRT) and hormone-based contraceptives. A self-diagnosis and reporting of UTIs has previously been shown to be reliable and accurate.22 We reviewed the electronic medical histories of a subset of female patients (n = 166) who had regular primary care visits at the lead study site to determine the accuracy of self-reported HRT use and history of recurrent UTIs in our questionnaire as compared to the medical record. Indeed, there was near perfect agreement between the survey answers for HRT use (99%) and recurrent UTIs (98%) and the medical record. In addition, a questionnaire-based assessment of IBD status among PSC patients has been examined and has been shown to have a near perfect agreement with patient medical records in a previous study.15 Moreover, several of the items included in the questionnaire form were similar to those in other questionnaire-based studies among patients with cholestatic liver disease published previously.14, 15, 23 Subjects were asked about their exposure history at the time of the questionnaire and not asked to recall if an exposure occurred before being diagnosed with PSC with the exception of smoking.
Statistical analysis
For the unadjusted results, categorical data are reported as percentages and compared using the χ2 test. Continuous data are reported as medians and interquartile ranges and compared using the Wilcoxon rank sum test. Patients with missing data were not included in the analysis for that particular missing covariate. Because of age and gender differences between the groups, logistic regression analysis was used to compare cases and controls adjusting for age and gender. In addition to displaying results using odds ratios (OR) and 95% confidence intervals (CI), descriptive statistics for the control data were adjusted to have the same age and gender distribution of the cases. Consequently, the number of subjects is not reported.
Patient responses that differed significantly between cases and controls or those exposures examined in prior studies underwent a secondary analysis where the IBD patients in the control group were excluded and PSC patients were stratified into two subgroups (self-reported history of PSC-IBD and PSC-woIBD) and compared to controls using a generalised logistic model adjusting for age and gender. This was done to mitigate the potential confounding effect of IBD when assessing the role of environmental factors associated with PSC. Finally, the consistency of the results from the stratified analysis was assessed by five sensitivity analyses which excluded the following patients: age less than 21 years (n = 67), self-reported diagnosis of PSC (n = 33), history of a liver transplant (n = 230), a subset of patients where the IBD status could not be verified due to a lack of medical records available for the lead study centre to corroborate the diagnosis of IBD (n = 147) and patients with Crohn’s disease or indeterminate colitis (confirmed by chart review) or their IBD status could not be verified (n = 486; i.e. included only those cases with confirmed UC). Variables that achieved statistical significance in the models for both PSC subgroups and remained significant in the sensitivity analyses were considered to be associated with PSC. All tests were 2-sided and a P < 0.05 was considered significant. The analysis was performed using sas 9.3 (SAS Institute; Cary, NC, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Subjects included & demographical features
One-thousand six hundred and sixty-three subjects were included. The response rate was 74% (1000/1354) for cases and 86% (663/774) for controls. Cases who responded were more likely to be older (55.6 years vs. 50.8 years, P < 0.001), Caucasian (95% vs. 90%, P = 0.04) and have been seen at the primary study site (69% vs. 63%, P = 0.04). The sites of enrolment for the cases are shown in Figure S1. PSC patients were younger, had a lower body mass index (BMI), and were less likely to be Caucasian and female when compared to controls (Table 1).
Table 1.
Demographical features among cases and controls*
| Controls (n = 663) | Cases (PSC) (n = 1000) | P-value | PSC-IBD (n = 741) | PSC-woIBD (n = 259) | P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 61.0 (19.3–86.1) | 50.4 (9.9–82.9) | <0.001 | 49.2 (34.6, 60.1) | 54.4 (42.1, 63.8) | <0.001 |
| Female | 488 (74%) | 381 (38%) | <0.001 | 271 (37%) | 110 (42%) | 0.09 |
| Caucasian | 655 (99%) | 947 (95%) | 0.05 | 711 (96%) | 236 (91%) | 0.003 |
| BMI (kg/m2) | 27.0 (15.9–53.7) | 25.1 (15.9–60.0) | <0.001 | 24.8 (22.6, 28.0) | 25.4 (23.0, 28.4) | 0.10 |
| Education | 0.70 | 0.82 | ||||
| High School or less | 124 (19%) | 209 (20%) | 149 (20%) | 60 (23%) | ||
| College/Vocational School | 328 (50%) | 470 (46%) | 343 (46%) | 127 (49%) | ||
| Beyond College | 206 (31%) | 317 (33%) | 247 (33%) | 70 (27%) |
BMI, body mass index; PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; PSC-IBD, PSC with IBD; PSC-woIBD, PSC without IBD.
Continuous variables are median (interquartile range)
Unadjusted
Comorbidities in subjects
After adjusting for age and gender, controls were more likely to report their health as ‘excellent,’ ‘very good’ or ‘good’ when compared to PSC patients (91% vs. 71%, P < 0.001). IBD was reported among nine controls (UC n = 4; Crohn’s disease n = 4; indeterminate colitis n = 1) and 741 cases (UC n = 538; Crohn’s disease n = 110; indeterminate colitis n = 93). Among the 853 (85%) patients where their IBD status could be verified based on medical record review, 795 (93%) had records which were consistent with the patients IBD status reported on the questionnaire. In addition, we were able to verify through the medical record that 230 patients with PSC and UC were diagnosed with either condition at least 5 years apart. A diagnosis of UC occurred at least 5 years after the diagnosis of PSC in 28 (12%) patients while a diagnosis of PSC occurred at least 5 years after the diagnosis of UC in 202 (88%) individuals. There were no differences among their demographical features, smoking history or other exposure history between the PSC first and UC first groups (data not shown). Compared to controls, coeliac disease was more common among PSC patients (1% vs. 2%, P = 0.01). However, the prevalence of other immune-mediated conditions was similar between cases and controls (Table S1).
The medical and surgical history of cases stratified by the self-reported IBD status is shown in Table 2. Those with PSC-IBD were more likely to be diagnosed with PSC at a younger age (35.0 years vs. 45.0 years, P < 0.001) and have a longer duration of PSC (7.4 years vs. 5.6 years, P < 0.01) but were less likely to have a self-reported diagnosis of autoimmune hepatitis (5% vs. 8%, p<0.03) when compared to those with PSC-woIBD. Colon cancer was more common among those with PSC-IBD vs. PSC-woIBD (8% vs. 1%, Table 2). However, the prevalence of colon cancer among PSC-woIBD was the same for controls (P = 0.70). The severity of liver disease based on a self-reported history of hepatic malignancy or a prior liver transplant was similar between the two PSC groups (Table 2).
Table 2.
Medical and surgical history among cases by IBD status
| PSC-IBD (n = 741) | PSC-woIBD (n = 259) | P-value* | |
|---|---|---|---|
| Age at PSC diagnosis (years) | 35.0 (25.0–45.0) | 45.0 (35.0–55.0) | <0.001 |
| PSC duration (years) | 7.4 (2.8–13.4) | 5.6 (1.7–11.1) | <0.01 |
| Liver transplantation | 24% | 21% | 0.35 |
| Hepatic malignancy† | 5% | 5% | 0.69 |
| Autoimmune hepatitis | 5% | 8% | 0.03 |
| IBD type | |||
| Crohn’s disease | 15% | – | – |
| Ulcerative colitis | 73% | – | |
| Indeterminate colitis | 13% | – | |
| IBD age diagnosis (years) | 25.0 (15.0–45.0) | – | – |
| IBD duration (years) | 12.9 (5.9–26.6) | – | – |
| Colon cancer | 8% | 1% | <0.001 |
| Colectomy | 31% | 1% | <0.001 |
PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease.
Age- and sex-adjusted P-values.
Hepatic malignancy includes self-reported history of cholangiocarcinoma or ‘liver cancer’ (cholangiocarcinoma n = 21; ‘liver cancer’ n = 36).
Assessment of exposures and events examined in prior studies: smoking, tonsillectomy, appendectomy, hormone-based contraception
The proportion of current smokers was similar between cases and controls (Table 3). In addition, exposure to second hand smoke or chewing tobacco was similar between PSC patients and controls (Table S1). In contrast, 37% of controls reported a smoking history (>100 cigarettes during a lifetime) compared to 25% of patients with PSC (P < 0.001) (Table 3). The median age of smoking onset was 18 years for both patients and controls. However, controls had a greater personal smoking exposure (pack-years): 10.5 (4.5–21.8) vs. 5.6 (2.3–15.0), P = 0.05 (Table 3). Following stratification, the negative association between smoking history and PSC was observed only among those with PSC-IBD patients and not in the PSC-woIBD group (Table 4). Similarly, those with PSC-IBD had fewer pack-years before PSC was diagnosed when compared to the total pack-year exposure among controls (PSC-IBD: OR, 0.9; 95% CI 0.7–0.9). However, the duration of smoking before a diagnosis of PSC did not differ between PSC-woIBD patients and the total pack-year exposure for controls (PSC-woIBD: OR, 0.9; 95% CI 0.8–1.1).
Table 3.
Exposures (nondietary) and events reported by cases and controls*
| Controls (n = 663) | Cases (PSC) (n = 1000) | P-value | |
|---|---|---|---|
| Exposures and events examined previously | |||
| Smoking (ever)† | 37% | 25% | <0.001 |
| Currently smoke cigarettes | 8% | 7% | 0.38 |
| Pack-years (total) | 10.5 (4.5–21.8) | 5.6 (2.3–15.0) | 0.05 |
| Appendectomy | 18% | 25% | <0.001 |
| Tonsillectomy (ever) | 35% | 30% | 0.23 |
| Tonsillectomy ≤19 years | 30% | 28% | 0.90 |
| Hormone-based contraception use (ever)‡ | 84% | 74% | 0.15 |
| Age started hormone-based contraception (years)‡ | 20.0 (18.0–22.0) | 20.0 (18.0–23.0) | 0.89 |
| Novel exposures‡ | |||
| HRT Use (ever) | 40% | 28% | <0.001 |
| Age started HRT (years) | 47.0 (43.0–48.0) | 48.0 (42.0–50.0) | 0.42 |
| Number of years using HRT | |||
| <1 year | 18% | 16% | 0.47 |
| 1–5 years | 36% | 32% | |
| 6–10 years | 21% | 26% | |
| ≥11 years | 25% | 26% | |
| Recurrent UTIs | 20% | 27% | <0.01 |
| Frequency of UTIs | |||
| Once every 2 years | 43% | 34% | 0.69 |
| Once every years | 21% | 25% | |
| ≥2 times per years | 36% | 40% | |
PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; HRT, hormone replacement therapy; UTIs, urinary tract infections.
Control values are age- and gender-adjusted to the distribution of the cases. Therefore, the number of subjects is not reported. The P-value is derived from the logistic regression model adjusting for age and gender.
Ever smoking defined as a history of smoking >100 cigarettes over lifetime.
Among women only (controls n = 488; cases n = 381).
Table 4.
Selected nondietary exposures and events stratified based on IBD status*
| OR (95% CI), P-value | |||
|---|---|---|---|
| PSC vs. controls | PSC-IBD vs. controls | PSC-woIBD vs. controls | |
| Cigarettes smoking | |||
| Ever smoked >100 cigarettes | 0.6 (0.5–0.8), <0.001 | 0.5 (0.4–0.7), <0.001 | 0.9 (0.7–1.2), 0.52 |
| Pack-years (total, per 10 years) | 0.9 (0.8–1.1), 0.053 | 0.8 (0.7–0.95), 0.012 | 1.0 (0.8–1.1), 0.64 |
| Surgical history | |||
| Appendectomy | 1.6 (1.2–2.0), 0.001 | 1.8 (1.3–2.3), <0.001 | 1.1 (0.8–1.6), 0.52 |
| Tonsillectomy | 0.9 (0.7–1.1), 0.23 | 0.8 (0.6–1.1), 0.13 | 1.0 (0.7–1.3), 0.86 |
| Tonsillectomy ≤19 years | 1.0 (0.8–1.3), 0.91 | 0.9 (0.7–1.2), 0.67 | 1.1 (0.8–1.5), 0.57 |
| Female specific covariates | |||
| Hormone-based contraception use (ever) | 0.8 (0.5–1.1), 0.15 | 0.7 (0.5–1.1), 0.09 | 0.90 (0.5–1.5), 0.65 |
| HRT use (ever) | 0.5 (0.4–0.7), <0.001 | 0.6 (0.4–0.8), <0.01 | 0.5 (0.3–0.8), <0.01 |
| Recurrent UTIs | 1.6 (1.2–2.3), <0.01 | 1.5 (1.01–2.2), 0.047 | 2.0 (1.2–3.2), <0.01 |
IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; PSC-IBD, PSC with IBD; PSC-woIBD, PSC without IBD; OR, odds ratio; CI, confidence interval; HRT, hormone replacement therapy; UTIs, urinary tract infections.
Adjusted for age and gender. Controls with inflammatory bowel disease were excluded in the stratified analysis.
Tonsillectomy was not associated with PSC (Tables 3 and 4). In contrast, controls were more likely to have undergone an appendectomy when compared to cases (Table 3). However, in the stratified analysis, appendectomy was associated with PSC-IBD but not PSC-woIBD when compared to controls (PSC-IBD: OR, 1.8; 95% CI 1.3–2.3; PSC-woIBD: OR, 1.1; 95% CI 0.8–1.6; Table 4).
Among the women enrolled in the study, the proportion of PSC patients and controls who reported a history (current or previous) of hormone-based contraception use was similar (Table 3) and there was no association with hormone-based contraception and PSC in the stratified analysis (Table 4). Akin to a prior study, our questionnaire did not distinguish between the various types of hormone-based contraceptives.15
The above observations concerning smoking, tonsillectomy, appendectomy and hormone-based contraceptives remained consistent in the sensitivity analyses and none of these variables were associated with both PSC subgroups, even after excluding patients with Crohn’s disease and indeterminate colitis from the PSC-IBD subgroup (Tables S2–S6).
Novel exposures among women
Compared to female controls, women with PSC were more likely to report recurrent UTIs (Table 3) and this positive association persisted regardless of the presence of IBD (PSC-IBD: OR, 1.5; 95% CI 1.01–2.2; PSC-woIBD: OR, 2.0; 95% CI 1.2–3.2) (Table 4). However, there was no association observed between the frequency of recurrent UTIs and PSC (Table 3).
Women with PSC were less likely to have received HRT when compared to female controls (Table 3) and this negative association continued after stratifying cases based on the presence of IBD (PSC-IBD: OR, 0.6; 95% CI 0.4–0.8; PSC-woIBD: OR, 0.5; 95% CI 0.3–0.8) (Table 4). In contrast, we did not detect a relationship between duration of HRT use and PSC (Table 3).
In the sensitivity analyses, both recurrent UTIs and HRT therapy continued to be associated with PSC, regardless of IBD status (Tables S2–S6).
Dietary habits and methods of food preparation
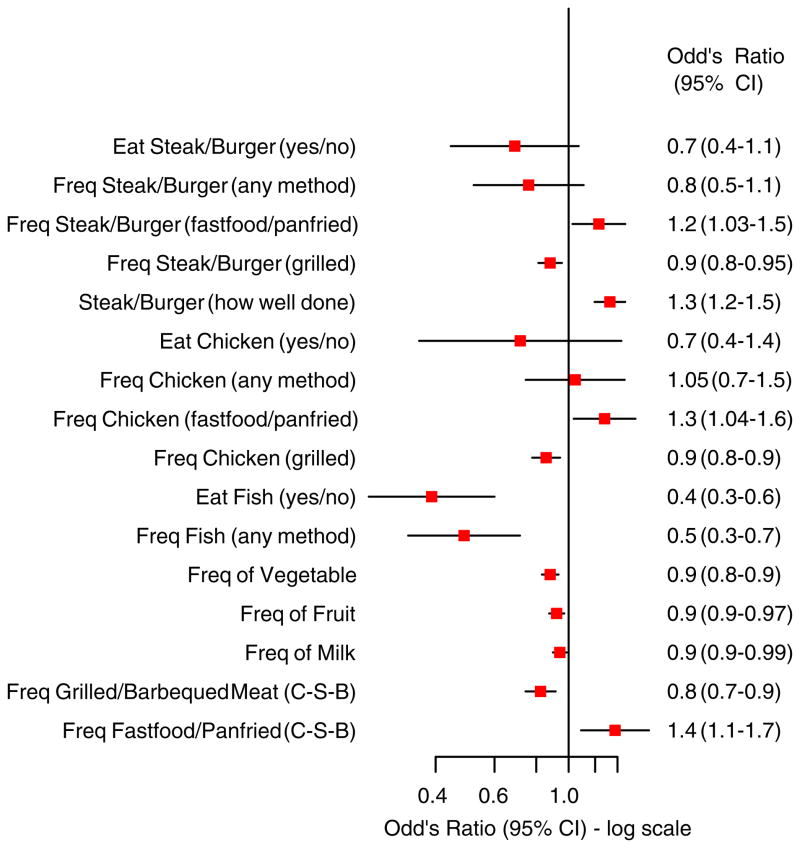
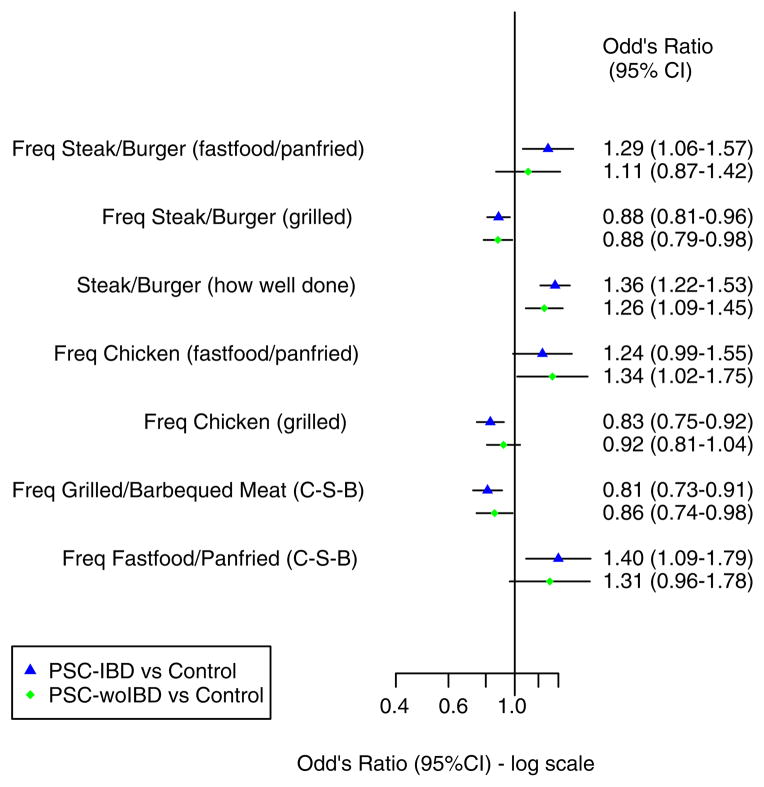
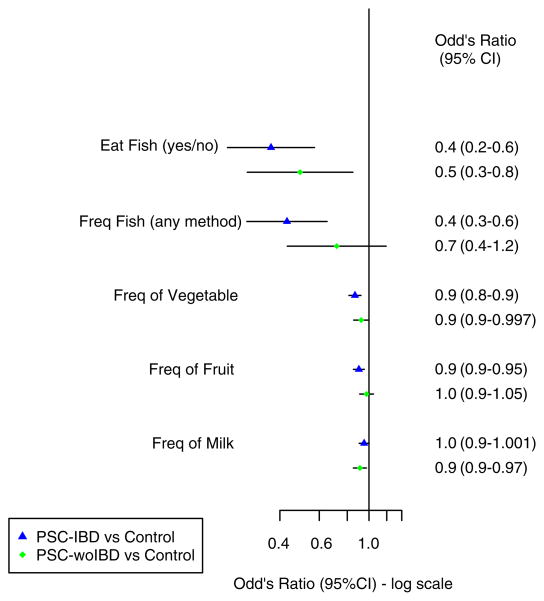
Several dietary habits and methods of food preparation were found to have an association with PSC prior to stratification (Figure 1). However, after PSC patients were stratified based on their IBD status the frequency of the following food intake had either a positive or negative association among those with IBD but not PSC-woIBD: steak/burger (fast food or pan fried), chicken (grilled), fast food or pan-fried food (chicken-steak-burgers), fish and fruit (Figures 2 and 3).
Figure 1.
Dietary habits and methods of food preparation in cases compared to controls (unstratified). CI, confidence interval; Freq, frequency; C-S-B, chicken-steak-burger.
Figure 2.
Dietary habits and methods of food preparation in cases stratified based on inflammatory bowel disease status compared to controls (Part 1). CI, confidence interval; Freq, frequency; C-S-B, chicken-steak-burger; PSC-IBD, primary sclerosing cholangitis with inflammatory bowel disease; PSC-woIBD, primary sclerosing cholangitis without inflammatory bowel disease.
Figure 3.
Dietary habits and methods of food preparation in cases stratified based on inflammatory bowel disease status compared to controls (Part 2). CI, confidence interval; Freq, frequency; PSC-IBD, primary sclerosing cholangitis with inflammatory bowel disease; PSC-woIBD, primary sclerosing cholangitis without inflammatory bowel disease.
In contrast, several types of food and methods of preparation were more common in controls compared to both PSC-IBD and PSC-woIBD groups (Figures 2 and 3). Indeed, there was a negative association with PSC and consumption of grilled steak or burgers and a composite variable which included grilled or barbecued meat (PSC-IBD: OR, 0.8; 95% CI 0.7–0.9; PSC-woIBD: OR, 0.9; 95% CI 0.7–0.98; Figure 2). Similarly, PSC patients were less likely to eat fish (yes vs. no) than controls (PSC-IBD: OR, 0.4; 95% CI 0.2–0.6; PSC-woIBD: OR, 0.5; 95% CI 0.3–0.8). However, our ability to detect a difference in the frequency of fish intake among those without IBD may have been limited by our smaller sample size in that subgroup (Figure 3). Vegetables appeared to be consumed less frequently in PSC patients when compared to controls (PSC-IBD: OR, 0.9; 95% CI 0.8–0.9; PSC-woIBD: OR, 0.9; 95% CI 0.9–0.997). However, statistical significance of this observation did not persist in the PSC-woIBD subgroup when we excluded patients whose IBD status could not be verified (PSC-IBD: OR, 0.9; 95% CI 0.8–0.9; PSC-woIBD: OR, 0.9; 95% CI 0.9–1.01; Table S5).
In addition to potential protective dietary associations, certain methods of food preparation had a positive association with PSC following stratification (Figure 2). For example, cooking steak or burgers so they are more well done was associated with PSC (PSC-IBD: OR, 1.4; 95% CI 1.2–1.5; PSC-woIBD: OR, 1.3; 95% CI 1.1–1.4) despite a negative association with grilled steak or burgers. While the point estimates and confidence intervals suggest that the frequency of fast food and pan-fried food consumption may also be associated with PSC, this did not reach statistical significance which could be secondary to our limited power to detect differences in each subgroup (Figure 2).
The negative association between PSC and grilled and barbecued meat, and fish intake persisted in the sensitivity analyses (Tables S2–S6). Similarly, the positive association between cooking red meat so it is more well done also remained consistent in the sensitivity analyses (Tables S2–S6).
DISCUSSION
To date, this study represents the largest cohort of PSC patients assembled to examine novel and previously explored environmental exposures and events associated with PSC. The size of the cohort, which represents about 3.4% of the predicted number of PSC patients in the USA,4 enabled us to examine the relationships of exposures among PSC patients with and without IBD when compared to controls. This strategy is important because IBD is a common comorbid condition among PSC patients and may act as confounder, when trying to elucidate potential risk factors associated with PSC rather than IBD.
In contrast to prior studies, our findings suggest that the relationship between smoking and PSC may be driven by the presence of IBD.16 Indeed, a prior history of smoking and the total duration of smoking were lower among those cases with IBD when compared to controls, but this was not observed among PSC-woIBD patients. This observation persisted when we excluded those with Crohn’s disease or indeterminate colitis (Table S6). The largest prior study (also a questionnaire-based study), which examined smoking and PSC included a total of 240 cases (57 without IBD) and found that a history of smoking was more prevalent in controls when compared to cases (43% vs. 20%) and the proportion of PSC patients with a history of smoking was similar among those with and without IBD (PSC-IBD n = 19% vs. PSC-woIBD n = 22%).15 Our current study also found a similar difference between controls and all PSC patients (37% vs. 25%). However, despite having nearly five times as many PSC patients without IBD, we were unable to detect an association with PSC and smoking when IBD was absent. Similarly, we were unable to validate an association between PSC and either a tonsillectomy or hormone-based contraceptive use, which has been previously described.14, 15 Lastly, our results reaffirmed a prior observation that an appendectomy is not associated with PSC.14
We detected two exposures associated with PSC among women. Our results suggest women with PSC are less likely to receive HRT. Indeed, PSC is more common in men and this observation raises the question if increased oestrogen could play a role in the gender differences observed among PSC patients.1 Oestrogen has been shown to have anti-inflammatory properties via the down regulation of chemoattractant proteins and vascular cell adhesion molecules.24, 25 Furthermore, HRT has been shown to have a protective effect on IBD disease activity in a dose-dependent manner.26 In contrast to the negative association between PSC and HRT, women with PSC were more likely to have recurrent UTIs when compared to controls. UTIs have been associated with other immune-mediated liver diseases such as primary biliary cirrhosis.27 This is postulated to occur through molecular mimicry between antigens in the microorganism and endogenous host proteins.27 Future studies should examine the relationship between oestrogen and recurrent UTIs and PSC.
Apart from coffee intake, the role of diet and PSC has been largely unexplored.15, 17 In this study, PSC patients were less likely to consume fish. We also observed a negative association between grilled or barbecued meat (chicken, steak or burger) and a positive association with how well done steak or burgers were cooked and PSC. Yet, the frequency of chicken, steak or burger consumption was not associated with PSC unless the method of food preparation was taken into account. Furthermore, there appeared to be a relationship between an increased frequency of fast food or pan-fried food intake and decrease frequency of vegetable consumption and PSC (Figure 2), but this did not reach statistical significance in both subgroups in all of the subsequent analyses (Tables S2–S6).
Differences in dietary habits may be relevant to the pathogenesis of PSC. A variety of immune-mediated conditions including IBD have been associated the so-called ‘western diet’ which is enriched in fats, sugars and processed foods and low in vegetables.28 Dietary choices can impact the microbiome, metabolome, cellular barriers and the immune system.29–31 It is plausible that such changes could create favourable conditions for the development of PSC in susceptible individuals. Indeed, the microbiome, disruption of cellular barriers and upregulation of the immune system have been postulated to play a role in the pathogenesis of PSC.1, 10 Patients with PSC were less likely to consume fish. Similar to our findings, a diet high in fish has been associated with a decreased risk of immune-mediated conditions such as rheumatoid arthritis.32, 33 High concentrations of n−3 fatty acids can be found in some fish.34 Low intake of n−3 fatty acids has been associated with an upregulation of a variety of immune functions.35 In contrast, n−3 fatty acids have anti-inflammatory properties through a variety of mechanisms.36 Docosahexaenoic acid, an n−3 fatty acid, appears to attenuate cholestatic liver injury in animal models following bile duct ligation.37 Furthermore, docosahexaenoic acid has been shown to decrease serum alkaline phosphatase levels in patients with PSC and a reduction in serum alkaline phosphatase level has been linked with improved outcomes.38, 39 However, n−3 fatty acid concentrations can vary between fish species and cooking techniques.34 Therefore, the relationship between fish and PSC may be more complex and other factors (fish type, origin, cooking method and host influences) may also contribute to this association. In addition to fish consumption, our findings suggest the methods of food preparation may be important in PSC. Such variations in the cooking processes can impact the microbiome differently.40 In addition, cooking steaks or burgers so they are more well done was associated with PSC and this cooking practice has been shown to increase the presence of dietary advanced glycoxidation end products (AGEs).41, 42 Receptors for AGE (RAGE) can be found in a variety of locations, but they appear to be strongly expressed in cholangiocytes when compared to other cell populations in the liver.43 Stimulation of RAGE can lead to production of proinflammatory cytokines, vascular adhesion molecules and increase vascular permeability.44 In animal models, a diet high in AGE can lead to hepatic inflammation without steatosis.45 Furthermore, AGE has also been shown to stimulate RAGE gene expression, exacerbate oxidative stress and increase hepatic fibrosis.46 While these potential pathogenic mechanisms are largely speculative, future studies are needed to examine the complex relationships between the diet, microbiome, metabolome, immune system and the host genome among PSC patients.
Our study has several limitations. First, our survey may be subject to recall bias and we are unable to assess the timing of each exposure relative to the onset of PSC. To overcome this limitation, cases would need to be captured prospectively before the diagnosis or at the time of the PSC diagnosis. However, this would be very challenging since PSC is a rare disease with a subclinical disease course. Second, our study design does not enable us to demonstrate causality and we cannot conclude that altering ones exposure of one or more of the associations presented herein could either prevent PSC or alter the disease course. Third, we were unable to recruit a sufficient number of young and middle-aged men as controls. This prevented us from directly matching cases and controls based on their age and gender. To mitigate this imbalance, we performed logistic regression analysis adjusting for age and gender when comparing exposures between cases and controls.
In conclusion, our results have expanded the pool of potential risk and protective factors which may be associated with PSC. Our data demonstrate that smoking is only associated with PSC when IBD is present, which suggest this observation is secondary to IBD rather than PSC. Among women with PSC, HRT use is less common whereas recurrent UTIs occur more frequently when compared to controls. In addition, fish, grilled or barbecue meat consumption was more common in controls. In contrast, PSC patients were more likely to consume red meat that was more well done. These findings have the potential to lay the foundations for future studies, which seek to examine the complex interplay between genes and environmental exposures in PSC.
Supplementary Material
Acknowledgments
Declaration of funding interests: The project was supported by the following NIH grants: DK 080670, DK 84960 and M01RR000065.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Site of case recruitment.
Table S1. Comorbidities, chewing tobacco & second hand smoke exposure reported among cases and controls.
Table S2. Sensitivity analysis excluding those younger than 21 years.
Table S3. Sensitivity analysis excluding self-reported diagnosis of primary sclerosing cholangitis.
Table S4. Sensitivity analysis excluding patients with liver transplant at time of questionnaire.
Table S5. Sensitivity analysis excluding patients when self-reported inflammatory bowel disease status could not be corroborated by medical record review.
Table S6. Sensitivity analysis including only those cases with confirmed ulcerative colitis diagnosis.
This article was accepted for publication after full peer-review.
AUTHORSHIP
Guarantor of the article: Konstantinos N. Lazaridis.
Author contributions: Konstantinos N. Lazaridis: study concept and design; data collection; interpretation of data; critical revision of the manuscript. John E. Eaton: study concept and design; data collection; interpretation of data; drafting of the manuscript; critical revision of the manuscript. Brian D. Juran: study concept and design; data collection; interpretation of data; critical revision of the manuscript. Elizabeth J. Atkinson, Xiao Xie, Mariza de Andrade: study design; analysis and interpretation of data; critical revision of the manuscript. Erik M. Schlicht: data collection; critical revision of the manuscript. Craig S. Lammert: interpretation of data; critical revision of the manuscript. Velimir A. Luketic, Joseph A. Odin, Ayman A. Koteish, Kris V. Kowdley, Kapil B. Chopra, Naga P. Chalasani and Gideon M. Hirschfield: data collection; interpretation of data; critical revision of the manuscript. Transcript profile: None. Writing assistance: Not utilised.
All authors approved the final version of the manuscript.
Declaration of personal interests: None
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39:282–301. doi: 10.1111/apt.12581. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–55. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 4.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–9. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Thia KT, Loftus EV, Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–82. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–49. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 7.Probert CS, Jayanthi V, Pinder D, Wicks AC, Mayberry JF. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut. 1992;33:687–93. doi: 10.1136/gut.33.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Munoz JE. Emigration to western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566–9. doi: 10.1016/j.crohns.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–72. doi: 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel diseases: current status and the future ahead. Gastroenterology. 2014;146:1489–99. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int. 2013;2013:389537. doi: 10.1155/2013/389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftus EV, Jr, Sandborn WJ, Tremaine WJ, et al. Primary sclerosing cholangitis is associated with nonsmoking: a case-control study. Gastroenterology. 1996;110:1496–502. doi: 10.1053/gast.1996.v110.pm8613055. [DOI] [PubMed] [Google Scholar]
- 13.van Erpecum KJ, Smits SJ, van de Meeberg PC, et al. Risk of primary sclerosing cholangitis is associated with nonsmoking behavior. Gastroenterology. 1996;110:1503–6. doi: 10.1053/gast.1996.v110.pm8613056. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell SA, Thyssen M, Orchard TR, Jewell DP, Fleming KA, Chapman RW. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: a case control study. Gut. 2002;51:567–73. doi: 10.1136/gut.51.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen IM, Tengesdal G, Lie BA, Boberg KM, Karlsen TH, Hov JR. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;12:1019–28. doi: 10.1016/j.cgh.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–71. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 17.Lammert C, Juran BD, Schlicht E, et al. Reduced coffee consumption among individuals with primary sclerosing cholangitis but not primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1562–8. doi: 10.1016/j.cgh.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–6. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 22.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lammert C, Nguyen DL, Juran BD, et al. Questionnaire based assessment of risk factors for primary biliary cirrhosis. Dig Liver Dis. 2013;45:589–94. doi: 10.1016/j.dld.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson BO. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm Res. 2007;56:269–73. doi: 10.1007/s00011-007-6198-z. [DOI] [PubMed] [Google Scholar]
- 25.Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–6. doi: 10.1111/j.1572-0241.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 27.Bogdanos DP, Baum H, Vergani D, Burroughs AK. The role of E. coli infection in the pathogenesis of primary biliary cirrhosis. Dis Markers. 2010;29:301–11. doi: 10.3233/DMA-2010-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salonen A, de Vos WM. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol. 2014;5:239–62. doi: 10.1146/annurev-food-030212-182554. [DOI] [PubMed] [Google Scholar]
- 30.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–7. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosell M, Wesley AM, Rydin K, Klareskog L, Alfredsson L. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology. 2009;20:896–901. doi: 10.1097/EDE.0b013e3181b5f0ce. [DOI] [PubMed] [Google Scholar]
- 33.Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-term intake of dietary long-chain n−3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis. 2014;73:1949–53. doi: 10.1136/annrheumdis-2013-203338. [DOI] [PubMed] [Google Scholar]
- 34.Scherr C, Figueiredo VN, Moura FA, Sposito AC. Not simply a matter of fish intake. Curr Vasc Pharmacol. 2014 doi: 10.2174/1570161112666141002120744. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n−6 essentiality and the balance between n−6 and n−3. Lipids. 2003;38:323–41. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC. n−3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72:326–36. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 37.Chen WY, Lin SY, Pan HC, et al. Beneficial effect of docosahexaenoic acid on cholestatic liver injury in rats. J Nutr Biochem. 2012;23:252–64. doi: 10.1016/j.jnutbio.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Martin CR, Blanco PG, Keach JC, et al. The safety and efficacy of oral docosahexaenoic acid supplementation for the treatment of primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2012;35:255–65. doi: 10.1111/j.1365-2036.2011.04926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–301. doi: 10.1111/apt.12979. [DOI] [PubMed] [Google Scholar]
- 40.Shen Q, Chen YA, Tuohy KM. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16:572–7. doi: 10.1016/j.anaerobe.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–6. e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–91. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 43.Butscheid M, Hauptvogel P, Fritz P, Klotz U, Alscher DM. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J Clin Pathol. 2007;60:415–8. doi: 10.1136/jcp.2005.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalousova M, Zima T, Tesar V, Dusilova-Sulkova S, Skrha J. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res. 2005;579:37–46. doi: 10.1016/j.mrfmmm.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Patel R, Baker SS, Liu W, et al. Effect of dietary advanced glycation end products on mouse liver. PLoS ONE. 2012;7:e35143. doi: 10.1371/journal.pone.0035143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodwin M, Herath C, Jia Z, et al. Advanced glycation end products augment experimental hepatic fibrosis. J Gastroenterol Hepatol. 2013;28:369–76. doi: 10.1111/jgh.12042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.