Abstract
GABAB receptors assemble from GABAB1 and GABAB2 subunits. GABAB2 additionally associates with auxiliary KCTD subunits (named after their K+ channel tetramerization-domain). GABAB receptors couple to heterotrimeric G–proteins and activate inwardly-rectifying K+ channels through the βγ subunits released from the G-protein. Receptor-activated K+ currents desensitize in the sustained presence of agonist to avoid excessive effects on neuronal activity. Desensitization of K+ currents integrates distinct mechanistic underpinnings. GABAB receptor activity reduces protein kinase-A activity, which reduces phosphorylation of serine-892 in GABAB2 and promotes receptor degradation. This form of desensitization operates on the time scale of several minutes to hours. A faster form of desensitization is induced by the auxiliary subunit KCTD12, which interferes with channel activation by binding to the G-protein βγ subunits. Here we show that the two mechanisms of desensitization influence each other. Serine-892 phosphorylation in heterologous cells rearranges KCTD12 at the receptor and slows KCTD12-induced desensitization. Likewise, protein kinase-A activation in hippocampal neurons slows fast desensitization of GABAB receptor-activated K+ currents while protein kinase-A inhibition accelerates fast desensitization. Protein kinase-A fails to regulate fast desensitization in KCTD12 knock-out mice or knock-in mice with a serine-892 to alanine mutation, thus demonstrating that serine-892 phosphorylation regulates KCTD12-induced desensitization in vivo. Fast current desensitization is accelerated in hippocampal neurons carrying the serine-892 to alanine mutation, showing that tonic serine-892 phosphorylation normally limits KCTD12-induced desensitization. Tonic serine-892 phosphorylation is in turn promoted by assembly of receptors with KCTD12. This cross-regulation of serine-892 phosphorylation and KCTD12 activity sharpens the response during repeated receptor activation.
Keywords: GABA-B, G-protein coupled receptor, GPCR, PKA, Kir3
1. Introduction
GABAB receptors are the G-protein coupled receptors (GPCRs) for GABA, the main inhibitory neurotransmitter in the central nervous system [1]. GABAB receptors are obligate heteromers composed of GABAB1 (GB1) and GABAB2 (GB2) subunits. Two GB1 isoforms exist, GB1a and GB1b, which regulate axonal versus dendritic distribution of the receptors [2]. In the heteromer GB1 binds GABA while GB2 facilitates surface trafficking and binds the G-protein [3–6]. GABAB receptors are expressed by most neurons in the brain and regulate neuronal excitability by modulating the activity of G protein-gated inwardly rectifying K+ channels (GIRK or Kir3 channels), voltage-gated Ca2+ channels and adenylyl cyclase [1,7,8]. To avoid prolonged effects on neuronal activity in the continuous presence of GABA, GABAB receptors exhibit a time-dependent decrease in receptor response, a phenomenon referred to as homologous desensitization [9–12]. GABAB receptor activity induces homologous desensitization through the inhibition of adenylate cyclase, which in turn decreases the cAMP-dependent activity of protein kinase-A (PKA). Since PKA phosphorylation of serine-892 (S892) in the C-terminal domain of GB2 increases receptor stability at the plasma membrane, reduced PKA activity facilitates receptor degradation and consequently desensitization of the receptor response [13,14]. This phosphorylation-dependent mechanism regulates desensitization on the time scale of several minutes to hours. In addition, faster mechanisms induce homologous desensitization of the receptor response within seconds of agonist application. These mechanisms operate at the G-protein rather than at the receptor. The “Regulator of G-protein Signaling” (RGS) protein 4 induces desensitization by accelerating the rate of GTP hydrolysis at the Gα subunit of the activated G-protein [15–17]. Fast desensitization is additionally induced by the KCTD proteins 12 and 12b [18,19]. The KCTDs are auxiliary receptor subunits that constitutively associate with the C-terminal domain of GB2, in which mutation of Y902 to alanine (Y902A) completely abolishes association [18,20,21]. KCTD12 induces GABAB receptor-activated K+ current desensitization by binding to the βγ subunits of the activated G-protein, which interferes with activation of the effector K+ channel [19]. Of note, this post-receptor mechanism of desensitization is rendered receptor-specific through the exclusive association of native KCTD12 protein with GABAB receptors [19].
Since KCTD12 binds to GB2 in proximity of the PKA phosphorylation-site S892 (Fig. 1A), phosphorylation of S892 may influence the ternary receptor/KCTD12/G-protein complex and vice versa, KCTD12 may influence S892 phosphorylation. We therefore addressed whether PKA regulates KCTD12-induced K+ current desensitization. We found that PKA phosphorylation of S892 reduces KCTD12-induced K+ current desensitization in heterologous cells and cultured hippocampal neurons. Mechanistically, S892 phosphorylation induces a conformational change in the GABAB receptor/KCTD12 complex that slows KCTD12-induced desensitization. Reciprocally, the binding of KCTD12 promotes phosphorylation of S892, consistent with previous data showing that KCTD12 stabilizes receptors at the cell surface [21].
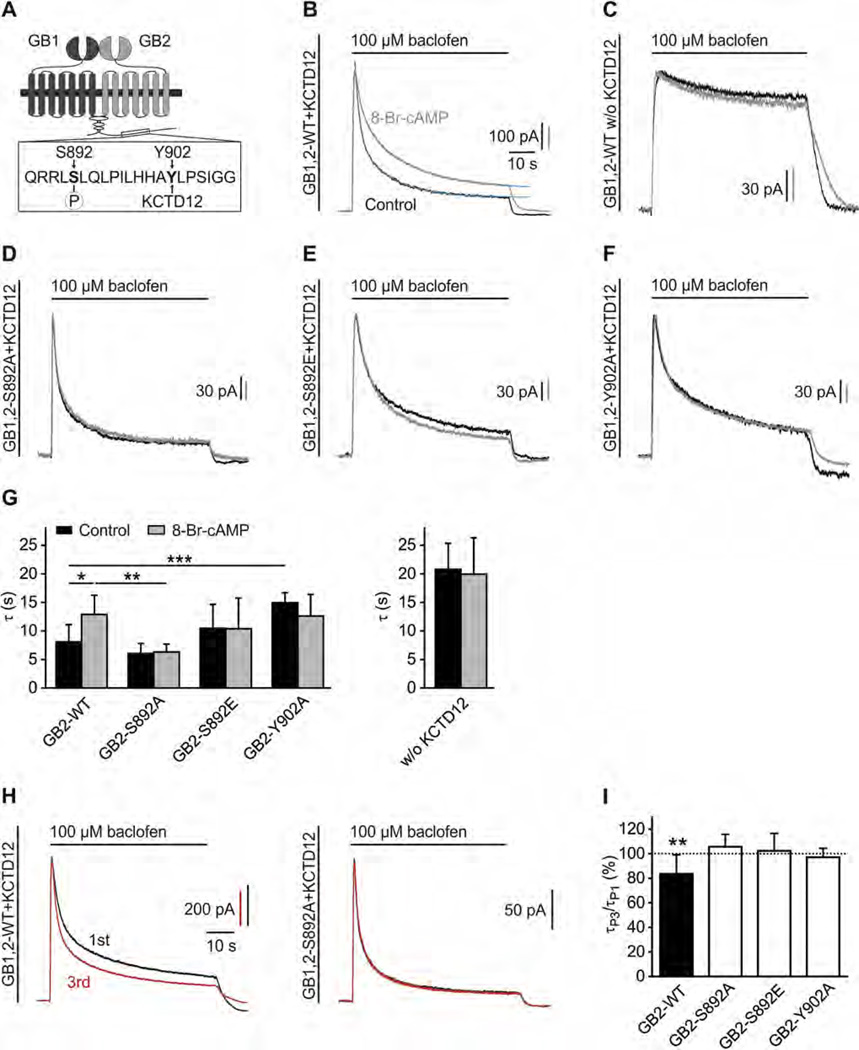
Fig. 1.
S892 phosphorylation slows KCTD12-induced desensitization of baclofen-activated K+ currents. (A) Scheme highlighting the close proximity of the PKA phosphorylation-site S892 and the KCTD12 binding-site Y902 in the C-terminal domain of GB2. (B) Representative traces of GIRK currents activated by baclofen (100 µM) and recorded at −50 mV from CHO-K1 cells expressing GB2-WT, GB1b, GIRK1/2 and KCTD12. Cells were pre-incubated without (Control, black trace) or with 8-Br-cAMP(1 mM, 30 min; grey trace). The desensitization time constants τ1 and τ2 were derived from double-exponential fits (blue trace) to the decay phase of K+ currents during baclofen application. (C) Representative traces as in (B) from CHO-K1 cells lacking KCTD12. (D–F) Representative traces as in (B) from CHO-K1 cells expressing the GB2 mutants S892A (D), S892E (E) or Y902A (F). (G) Bar graph summarizing the time constant τ (amplitude-weighted mean time constant calculated from τ1 and τ2) of baclofen-induced K+ current desensitization. Data are means ± SD, n = 5–16. *,p < 0.05; **,p < 0.01; ***,p < 0.001; Sidak’s multiple comparison test. (H) Repeated activation of GIRK currents in CHO-K1 cells expressing WT GABAB receptors (GB1,2-WT) together with KCTD12 results in a sharpening of the current response (left; 1st response black, 3rd response red). No sharpening of the current response upon repeated activation is observed with mutated GABAB receptors (GB1,2-S892A) that cannot be phosphorylated at S892 (right). Baclofen applications were for 60-s in intervals of 5 min. (I) Bar graph summarizing experiments as shown in (H). In experiments with GB2-WT, the τ of the 3rd response (τP3) was significantly reduced compared to the τ of the 1st response (τP1). No change in the desensitization of the current response upon repeated activation is seen (i) when S892 cannot be phosphorylated (S892A), (ii) with the phospho-mimetic S892E and (iii) when KCTD12 cannot bind to GB2 (Y902A). Data are means ± SD, n = 7–12. **, p < 0.01; t test.
2. Material and methods
2.1. Cell lines and cultures of hippocampal neurons
HEK293T and CHO-K1 cells were maintained in DMEM (Life Technologies, CA, US) supplemented with 10% FBS (GE Healthcare, Buckinghamshire, UK) at 37 °C with 5% CO2. CHO-K1 cells stably expressing GB1 and GB2 subunits were cultured as described [22]. Cultured hippocampal neurons were prepared from wild-type (WT), KCTD12−/− [19,23] or GB2-S892A mice on embryonic day 16.5 as described [24].
2.2. Drugs
To activate GABAB receptors, baclofen (Cat.No. 417) was used at a final concentration of 100 µM. To activate PKA, 8-Br-cAMP (Cat.No. 1140) and forskolin (Cat.No. 1099) were used at a final concentration of 1 mM and 20 µM, respectively. To inhibit PKA, H89 (Cat.No. 2910) and protein kinase inhibitor-(14–22)-amide (PKI, Cat.No. 2546) were used at a final concentration of 2 µM or 10 µM, and 10 µM, respectively. All reagents were purchased from Tocris Bioscience, UK.
2.3. Generation of GB2-S892A knock-in mice
The gene targeting construct contained a neomycin resistance cassette (pRay-2; Genbank accession number U63120) flanked by two arms of Balb/c GB2 genomic DNA. The S892A mutation in exon 19 was introduced into the 3’ arm along with a diagnostic silent NheI restriction site. The targeting construct was electroporated into Balb/c embryonic stem cells. Neomycin resistant colonies were screened for homologous recombination by PCR, and positive clones confirmed by Southern blot. Positive ES cells were microinjected into C57BL/6 blastocysts. Chimeric males were crossed with female Balb/c mice to generate inbred Balb/c mice heterozygous for the S892A allele. The neomycin cassette in the S892A allele was then excised by mating heterozygous mice with Balb/c Cre-deleter mice [25]. Whole brain membranes were probed with the primary antibodies guinea-pig anti-GB2 (AB5394, 1:2000, Millipore, Darmstadt, Germany), rabbit anti-GB2-pS892 [13] (1:2000), rabbit anti-GB1 [26] (1:3000), mouse anti-β-III-tubulin (T8660, 1:1000, Sigma–Aldrich, MO, US). All animal experiments were subjected to institutional review and approved by the Veterinary Office of Basel-Stadt.
2.4. Molecular biology
The plasmids encoding HA-GB2, HA-GB2-Y902A, Myc-GB1b, Flag-GB1a, pRK6-GB1a-YFP, pcDNA3.1-GIRK1/2, Myc-KCTD12, pIRES-EGFP-KCTD12, Rluc-KCTD12 and Rluc-Gβ1 were described earlier [19,21,27,28]. The HA-GB2-S892A and HA-GB2-S892E mutations were generated by replacing S892 with alanine or glutamate, respectively. Lipofectamine 2000 (Life Technologies, CA, US) was used for transient transfections. For electrophysiology we co-expressed green fluorescent protein (GFP) or pIRES-EGFP-KCTD12. The amount of DNA in the transfections was kept constant by supplementing with pCI plasmid DNA (Promega, WI, US).
2.5. Electrophysiology
CHO-K1 cells and cultured hippocampal neurons were plated onto Thermanox™ plastic coverslips (Thermo Scientific, MA, US) or poly-l-lysine (P9155, Sigma–Aldrich, MO, US) coated glass cover-slips, respectively. Experiments with CHO-K1 cells and cultured hippocampal neurons were performed at room temperature 2–3 days or 1–2 weeks (DIV14–21) after transfection, respectively. Cells were continuously superfused with an extracellular solution (ECS) composed of (in mM): 145 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 25 glucose; pH 7.3, 323 mosm. Neurons were superfused with ECS supplemented with (in µM): 10 DNQX, 0.5 TTX, 100 picrotoxin, 5 D-CPP. Patch pipettes had resistances between 3 and 4 MΩ when filled with intracellular solution (ICS) composed of (in mM): 107.5 potassium gluconate, 32.5 KCl, 10 HEPES, 5 EGTA, 4 MgATP, 0.6 NaGTP, 10 Tris-phosphocreatine; pH 7.2, 295 mosm. To activate PKA the ICS was supplemented with 250 µM 8-Br-cAMP. Series resistance (<5 MΩ) was compensated by 80%. GABAB responses were evoked by fast application of baclofen and recorded with an Axopatch 200B patch-clamp amplifier; filtering and sampling frequencies were set to 1 kHz and 5 kHz, respectively [29]. Desensitization time constants were derived from bi-exponential fits to the decay phase of GIRK currents during agonist application. Curve fitting and data analysis were done with pClamp10 software (Molecular Devices, CA, US). Data are given as mean ± SD. Pharmacological treatments were carried out after DIV15 (neurons) or 48 h after transfection (CHO-K1 cells). Before recording, hippocampal neurons or CHO-K1 cells were pre-incubated for 30 min with 8-Br-cAMP, forskolin, PKI and H89 in the ECS.
2.6. Co-immunoprecipitation experiments and drug treatments
Forty-eight hours after transfection, HEK293T cells were starved for 4 h in DMEM w/o FCS prior to incubation for 30 min with 8-Br-cAMP. Cells were harvested, washed once with ice-cold PBS, and subsequently lysed in NETN buffer (100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 20 mM Tris/HCl, pH 7.4) supplemented with complete EDTA-free protease inhibitor mixture (Hoffmann-La Roche, Basel, Switzerland) and phosphatase inhibitors 2 and 3 (P5726 and P0044, Sigma–Aldrich, MO, US). After rotation for 10 min at 4°C, the lysates were cleared by centrifugation at 1000 × g for 10 min at 4 °C. Lysates were directly used for Western blot analysis or immunoprecipitated with anti-Rluc antibody (MAB4410, Millipore, Darmstadt, Germany) coupled to Dyna-beads® Protein-G (10004D, Life Technologies, CA, US). Lysates and immunoprecipitates were resolved using standard SDS-PAGE and probed with the primary antibodies guinea-pig anti-GB2 (AB5394, 1:2000, Millipore, Darmstadt, Germany) or guinea-pig anti-KCTD12 [23] (1:1000) and peroxidase-coupled secondary antibodies donkey anti-guinea pig (A7289, 1:10,000, Sigma–Aldrich, MO, US). The chemiluminescence detection kit (Thermo Scientific, MA, US) was used for visualization. The band intensity on Western blots was quantified from non-saturated images using a Fusion FX Chemiluminescence System (Vilber Lourmat, Witec AG, Lucerne, Switzerland). GB2 protein sometimes runs as two bands on SDS–PAGE, depending on the acrylamide concentration of the gel, the voltage applied across the gel and the electrophoresis time.
Drug treatments of HEK293T cells and cultured hippocampal neurons were performed 48 h after transfection or at DIV14, respectively. HEK293T cells were incubated with 8-Br-cAMP for 30 min. Cultured hippocampal neurons were incubated with H89 for 2 h. Lysates were probed with the primary antibodies guinea-pig anti-GB2 (AB5394, 1:2000, Millipore, Darmstadt, Germany) or rabbit anti-GB2-pS892 [13] (1:750) and peroxidase-coupled secondary antibodies donkey anti-guinea pig (A7289, 1:10,000, Sigma–Aldrich, MO, US) and donkey anti-rabbit (NA9340, 1:10,000, GE Healthcare, Buckinghamshire, UK). β-III-Tubulin (T8660, 1:10,000, Sigma–Aldrich, MO, US; peroxidase-coupled secondary antibody sheep anti-mouse, NA931, 1:10,000, GE Healthcare, Buckinghamshire, UK) was used to control for loading and to normalize GB2 and phosphorylated GB2 protein levels in neurons.
2.7. BRET measurements
BRET measurements were performed as described [19,21]. CHO-K1 cells stably expressing GB1 and GB2 subunits and HEK293T cells were transiently transfected with plasmids encoding Rluc and YFP fusion proteins and distributed into 96-well microplates (Greiner Bio-One, Kremsmünster, Austria; coated with poly-l-ornithine hydrobromide; P3655, Sigma–Aldrich, MO, US) at a density of 50,000 and 100,000 cells/well, respectively. Twenty-four hours after transfection, cells were starved for 4 h in DMEM w/ o FCS. After washing, coelenterazine h (5 µM, NanoLight Technologies, AZ, US) was added with or without 8-Br-cAMP for 30 min. Luminescence and fluorescence signals were detected sequentially using an Infinite® F500 Microplate Reader (Tecan, Männedorf, Switzerland). Baclofen was added 5 min prior measurement. Each data point represents a technical quadruplicate.
2.8. Data analysis
All data are presented as mean ± SD. Statistical significance was assessed using unpaired Student’s t test, one-way ANOVA followed by Dunnett’s multiple comparison test or two-way ANOVA followed by Sidak’s multiple comparison test, using Prism 5.04 software (GraphPad, CA, US). BRET data were analyzed and fitted using a “one-site specific binding” equation (GraphPad, CA, US).
3. Results
3.1. S892 phosphorylation slows KCTD12-induced K+ current desensitization
To investigate the effects of S892 phosphorylation on KCTD12-induced desensitization we performed whole-cell patch-clamp recordings from CHO-K1 cells expressing KCTD12, GIRK channels (GIRK1/2) and GB1b together with GB2-WT or the GB2 mutants S892A (phospho-deficient), S892E (phospho-mimetic) and Y902A (deficient in KCTD12-binding). Applications of the GABAB receptor agonist baclofen for 60 s elicited K+ currents with much more pronounced desensitization in the presence (91.0 ±3.4%; n = 14; Fig. 1B) than in the absence of KCTD12 (26.0 ± 7.5%; n = 10; Fig. 1C), as reported earlier [18]. Approximation of the time-course of the KCTD12-induced desensitization by a double exponential function yielded time constants of 1.6 ± 0.4 s (τ1; relative amplitude 47.5 ±15.3%) and 12.9 ± 2.1 s(τ2; Fig. 1B) [18,19]. Activation of PKA by pre-incubation of cells with 8-Br-cAMP for 30 min significantly slowed both components of desensitization (τ1= 3.7 ± 1.8 s, relative amplitude 44.2 ± 14.5%; τ2 = 20.8 ± 2.2 s; p < 0.01 and p < 0.001; t test; mean time constant τ shown in Fig. 1G). In contrast, 8-Br-cAMP did not significantly influence GABAB-activated K+ currents in the absence of KCTD12 showing that PKA selectively modulates KCTD12-induced fast desensitization (Fig. 1C and G). Inhibition of PKA using H89 accelerated desensitization, suggestive of tonic PKA activity in CHO-K1 cells ( τ1 = 1.1 ±0.3 s, relative amplitude 47.8 ±19.5%; τ2 = 7.2 ± 1.2 s, p < 0.05 and p < 0.01; t test). 8-Br-cAMP did not influence desensitization when expressing GB2-S892A or GB2-S892E instead of GB2-WT (Fig. 1D, E and G) thus implicating S892 in PKA modulation. Fast desensitization was not influenced by PKA when expressing GB2-Y902A (Fig. 1F, G). Of note, fast desensitization was already significantly slowed (increased τ) with GB2-Y902A compared to GB2-WT in the absence of 8-Br-cAMP (Fig. 1G). This confirms that assembly of receptors with KCTD12 is required for PKA regulation of fast desensitization.
Inhibition of PKA accelerates fast desensitization, suggestive of basal S892 phosphorylation. We therefore investigated whether repetitive activation of GABAB receptors, which reduces PKA-dependent phosphorylation of S892 [13], accelerates the KCTD12-induced desensitization. We recorded K+ currents in response to repeated baclofen application for 60 s in 5-min intervals. In experiments with GB2-WT, but not with GB2-S892A, GB2-S892E or GB2-Y902A, desensitization of the 3rd K+ current response was significantly faster by 16.4 ± 15.5% compared to the first response (Fig. 1H, I). In summary, the results show that KCTD12-induced desensitization of GABAB-activated K+ currents is regulated by receptor activity and PKA phosphorylation of S892. Repeated activation of GABAB receptors results in a sharpening of the receptor response due to a receptor activity-dependent reduction in S892 phosphorylation.
3.2. S892 phosphorylation rearranges the receptor/KCTD12 complex
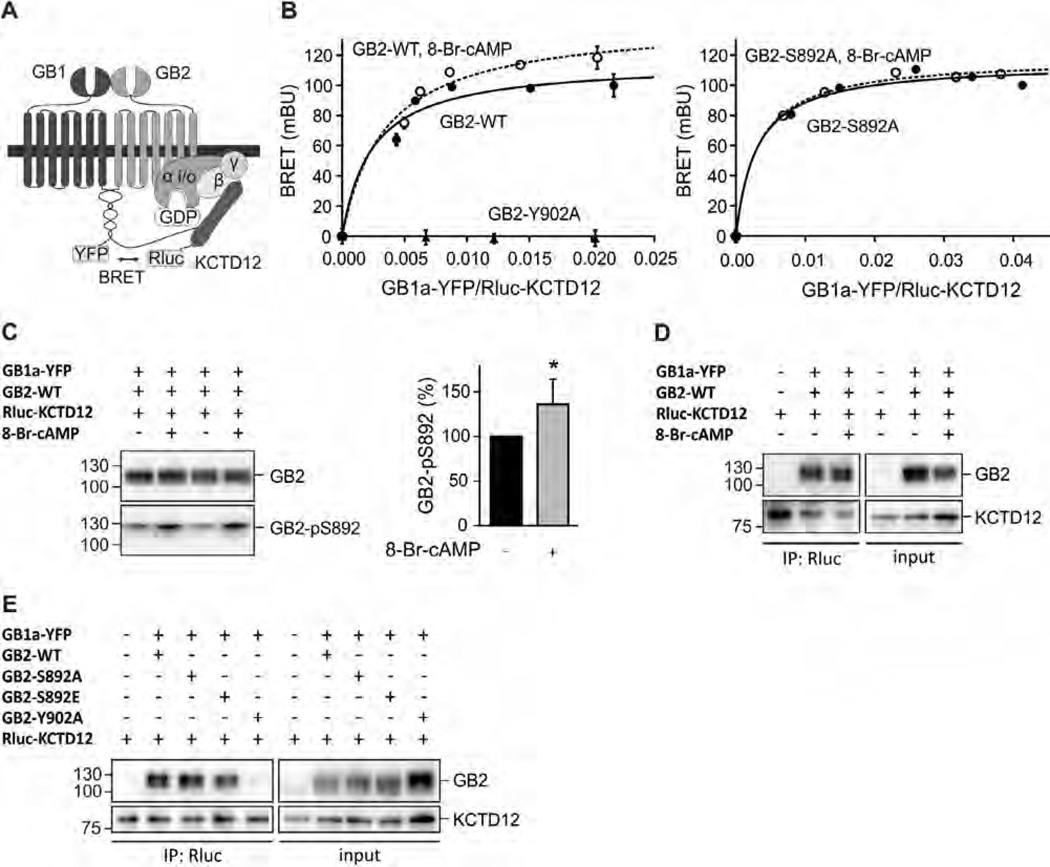
It is conceivable that dissociation of KCTD12 from the receptor underlies the slowing of desensitization in response to PKA phosphorylation of S892. To test this hypothesis, we used a BRET assay monitoring the interaction of KCTD12 with GABAB receptors in living cells [21]. Binding of the donor fusion protein Rluc-KCTD12 to GB2 allows for specific BRET to the acceptor fusion protein GB1a-YFP (Fig. 2A). Expression of increasing amounts of GB1a-YFP with a fixed amount of Rluc-KCTD12 and GB2-WT resulted in hyperbolic BRET donor saturation curves, consistent with a specific interaction between KCTD12 and GABAB receptors (Fig. 2B, left). As a control, very low non-specific linear BRET was observed between Rluc-KCTD12 and GB1a-YFP in experiments with GB2-Y902A. When GB2-expressing cells were pre-incubated with 8-Br-cAMP for 30 min, we still observed a specific BRET donor saturation curve. This demonstrates that KCTD12 does not dissociate from the receptor upon receptor phosphorylation. However, compared to BRET experiments with untreated cells, the donor saturation curves in response to PKA activation were shifted towards higher maximal BRET (BRETmax) values (Fig. 2B, left, and Table 1). Given that similar amounts of BRET acceptor were required to reach 50% of the BRETmax signal (BRET50), the increase in BRETmax most likely reflects a conformational rearrangement in the GABAB receptor/KCTD12 complex and not a change in the relative affinity of the proteins for one another or increased levels of GABAB receptors at the plasma membrane. Western blot analysis demonstrated that 8-Br-cAMP treatment significantly increased phosphorylation of S892 without altering total GB2 expression levels (Fig. 2C). We next acquired BRET donor saturation curves for Rluc-KCTD12 and GB1a-YFP in cells expressing GB2-S892A. As expected, we obtained hyperbolic BRET donor saturation curves demonstrating a specific interaction between KCTD12 and GABAB receptors assembled with GB2-S892A (Fig. 2B, right). However, 8-Br-cAMP did not alter the BRETmax and BRET50 values showing that PKA rearranges the receptor/KCTD12 complex through phosphorylation of S892 (Fig. 2B, right, and Table 1).
Fig. 2.
S892 phosphorylation rearranges the receptor/KCTD12 complex. (A) Scheme of the BRET Rluc-KCTD12 donor and GB1a-YFP acceptor pair. (B) BRET donor saturation curves from HEK293T cells expressing fixed amounts of GB2-WT, GB2-Y902A (both left) or GB2-S892A (right) together with Rluc-KCTD12 and increasing amounts of GB1a-YFP. Cells were pre-incubated with 8-Br-cAMP(1 mM, 30 min; open circles) to activate PKA. BRET is expressed as milli BRET units (mBU) determined as net BRET × 1000. Data points are means ± SD of technical quadruplicates of a representative experiment, n = 4–7. (C) Western blot of lysates of HEK293T cells expressing GB1a-YFP, GB2-WT and Rluc-KCTD12 in the presence and absence of 8-Br-cAMP(1 mM, 30 min pre-incubation). Activation of PKA does not affect the total amount of GB2 but increases phosphorylation at S892 (GB2-pS892). GB2 runs as two bands on SDS–PAGE, likely due to differences in posttranslational modification. The higher molecular weight band appears to be more heavily phosphorylated at S892. Blots are representative of six independent experiments. Bar graph summarizing the amount of S892 phosphorylation normalized to the amount of GB2 on the same blot (GB2-pS892 in %). Data are means ± SD, n = 6. *, p < 0.05; t test. (D) Co-immunoprecipitation of GABAB receptors with KCTD12 from HEK293T cells expressing GB1a-YFP, GB2-WT and Rluc-KCTD1 2 in the presence and absence of 8-Br-cAMP(1 mM, 30 min pre-incubation). Equivalent amounts of GABAB receptors were co-precipitated. Blots are representative of five independent experiments. (E) Co-immunoprecipitation of GABAB receptors with KCTD12 from HEK293T cells expressing GB1a-YFP, GB2 (WT, S892A, S892E or Y902A) and Rluc-KCTD12. Y902A does not bind KCTD12 and was used as a negative control. Blots are representative of three independent experiments.
Table 1.
Parameters from BRET saturation curves with Rluc-KCTD12 and GB1a-YFP. BRET donor saturation assays were performed in HEK293T cells transfected with fixed amounts of GB2-WT or GB2-S892A, Rluc-KCTD12 (donor) and increasing amounts of GB1a-YFP (acceptor) with and without activation of PKAby pre-incubation with 8-Br-cAMP for 30 min. The BRETmax is the maximal BRET signal obtained for a given donor/acceptor pair and the BRET50 corresponds to the amount of acceptor needed to obtain 50% of the BRETmax. Data are presented as the mean ± SD of the indicated number of independent experiments and were derived from data as presented in Fig. 2.
| GB2 constructs | BRETmax | SD | BRET50 | SD | N |
|---|---|---|---|---|---|
| GB2-WT | 119.0 | 9.2 | 0.0037 | 0.0014 | 7 |
| GB2-WT, 8-Br-cAMP | 132.1* | 12.4 | 0.0042 | 0.0013 | 7 |
| GB2-S892A | 112.8 | 3.6 | 0.0034 | 0.0008 | 4 |
| GB2-S892A, 8-Br-cAMP | 111.8 | 4.7 | 0.0033 | 0.0004 | 4 |
p<0.05; compared to GB2-WT; t test.
The finding that KCTD12 remains associated with GABAB receptors when activating PKA was further confirmed in co-immunoprecipitation experiments showing that similar amounts of GB2 are associated with KCTD12 in the presence and absence of 8-Br-cAMP (8-Br-cAMP: 102.8 ± 15.9%, p > 0.05 normalized to without 8-Br-cAMP; t test; n = 5; Fig. 2D). Co-immunoprecipitation experiments with receptors assembled with GB2-S892A or GB2-S892E confirmed that phosphorylation of S892 in GB2 is neither required nor prohibitive for binding of KCTD12 (Fig. 2E).
3.3. PKA does not impair dynamic binding of KCTD12 to the G-proteins
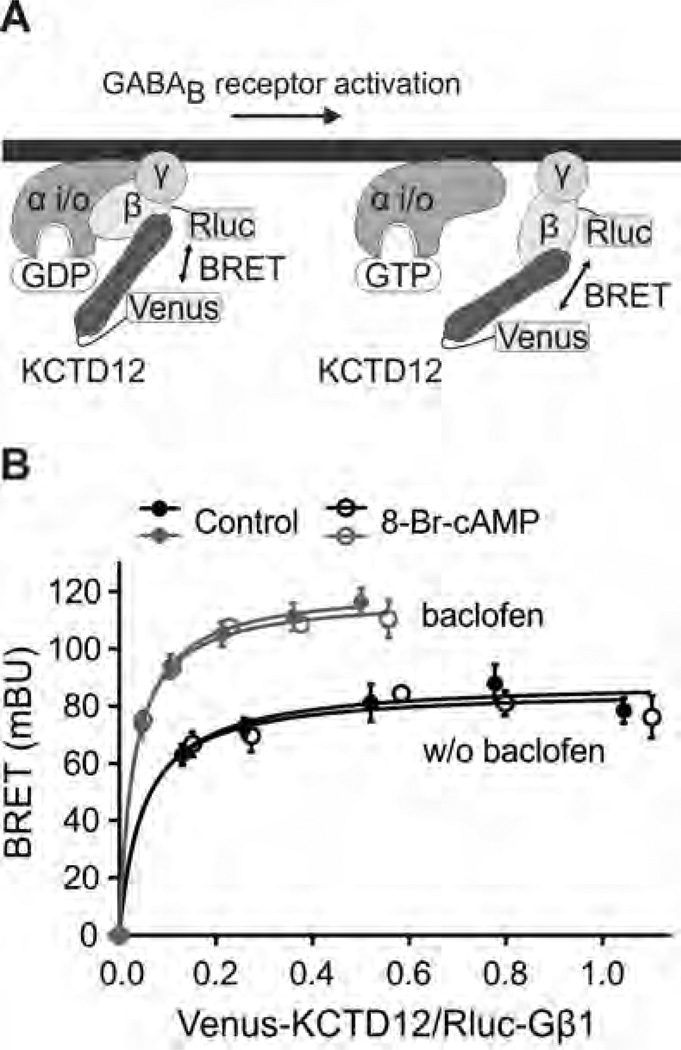
Since PKA induces a conformational rearrangement in the receptor/KCTD12 complex, we next investigated whether PKA also influences the interaction of KCTD12 with the G-protein [19]. This can be monitored by BRET between Rluc-Gβ1 and Venus-KCTD12 in transfected CHO-K1 cells stably expressing GB1 and GB2 subunits (Fig. 3A). In agreement with KCTD12 dynamically interacting with the G-protein [19], the BRET donor saturation curves were shifted towards higher BRETmax values following activation of GABAB receptors with baclofen (Fig. 3B and Table 2). This agrees with an activity-dependent conformational rearrangement of KCTD12 at the G-protein reported earlier [19]. However, 8-Br-cAMP did not alter the BRET donor saturation curve between Rluc-Gβ1 and Venus-KCTD12 irrespective of whether GABAB receptors were activated with baclofen or not (Fig. 3B and Table 2). These data therefore demonstrate that activation of PKA does not alter the interaction between KCTD12 and the G-protein in a measurable way.
Fig. 3.
Binding of KCTD12 to the G-protein βγ subunits is modulated by receptor activation but not by PKA activation. (A) Scheme of the BRET Rluc-Gβ1 donor and Venus-KCTD12 acceptor pair. (B) BRET donor saturation curves from CHO-K1 cells stably expressing GB1 and GB2 and transiently expressing fixed amounts of Rluc-Gβ1 and Gγ2 together with increasing amounts of Venus-KCTD12. Following receptor activation with baclofen (100 µM, 5 min pre-incubation; grey traces) the BRET curves were shifted towards higher BRETmax values. Activation of PKA with 8-Br-cAMP (1 mM, 30 min pre-incubation; open circles) had no effect on the BRET curves. BRET is expressed as milli BRET units (mBU) determined as net BRET × 1000. Data points are means ± SD of technical quadruplicates of a representative experiment, n = 3.
Table 2.
Parameters from BRET saturation curves with Rluc-Gβ1 and Venus-KCTD12. BRET donor saturation assays were performed in CHO-K1 cells stably expressing GABAB receptors and transiently expressing fixed amounts of Rluc-Gβ1 (donor) and Gγ2 and increasing amounts of Venus-KCTD12 (acceptor). To activate GABAB receptors, cells were pre-incubated with baclofen for 5 min. To activate PKA, cells were pre-incubated with 8-Br-cAMP for 30 min. The BRETmax is the maximal BRET signal obtained for a given donor/acceptor pair and the BRET50 corresponds to the amount of acceptor needed to obtain 50% of the BRETmax. Data are presented as the mean ± SD of the indicated number of independent experiments and were derived from data as presented in Fig. 3.
| GABAB receptor activation | BRETmax | SD | BRET50 | SD | N |
|---|---|---|---|---|---|
| Control, no baclofen | 97.9 | 11.4 | 0.0490 | 0.0321 | 3 |
| 8-Br-cAMP, no baclofen | 94.3 | 7.1 | 0.0517 | 0.0338 | 3 |
| Control, baclofen | 131.1* | 21.9 | 0.0372 | 0.0125 | 3 |
| 8-Br-cAMP, baclofen | 130.0* | 21.9 | 0.0459 | 0.0264 | 3 |
p<0.05; compared to control, no baclofen; compared to 8-Br-cAMP, no baclofen; t test.
3.4. S892 phosphorylation slows KCTD12-induced K+ current desensitization in cultured hippocampal neurons
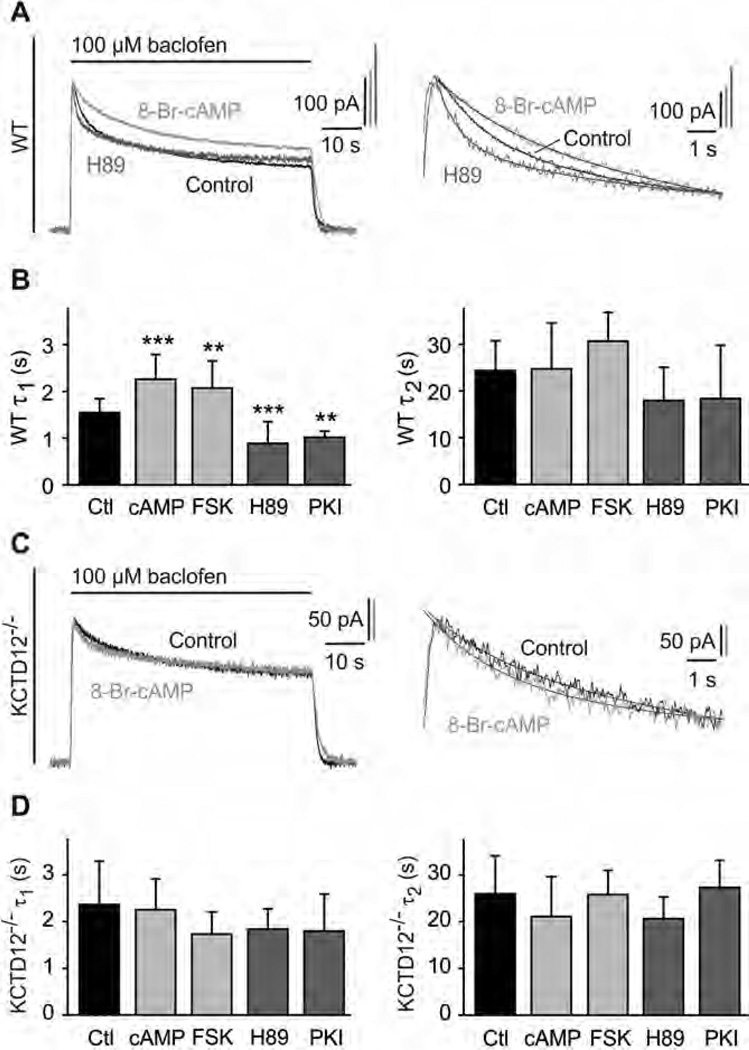
We next investigated whether PKA modulates the desensitization of GABAB-activated K+ currents in cultured hippocampal neurons, which are known to express KCTD12 [18,19,23]. With WT neurons, K+ currents elicited by 60-s long applications of baclofen showed a steady-state desensitization of 53.3 ±9.3% (n = 41, Fig. 4A). The time course of desensitization was approximated by a double exponential function with time constants of 1.5 ± 0.3 s (τ1) and 24.4 ± 6.4 s (τ2) (Fig. 4B), values that are similar as in earlier experiments [18,19]. Activation of PKA with 8-Br-cAMP or forskolin significantly increased the fast component τ1 of the desensitization, while inhibition of PKA with H89 or PKI had the opposite effect (Fig. 4B). Neither activation nor inhibition of PKA had a significant effect on the slow component τ2 of the desensitization (Fig. 4B). The relative contribution of the fast and slow components (τ1 and τ2, respectively) to the desensitization did not change with any of the treatments (τ1 of control: 46.5 ±10.0%; cAMP: 36.3 ±15.4%; FSK: 40.3 ±17.8%; H89: 55.7 ± 12.2%; PKI: 47.7 ± 7.7%; p>0.05; Dun-nett’s multiple comparison test). These results reveal that PKA activity specifically influences the fast component of baclofen-induced K+ current desensitization in WT neurons.
Fig. 4.
PKA activation slows fast desensitization of baclofen-activated K+ currents in cultured hippocampal neurons of WT but not of KCTD12−/− mice. (A) Representative baclofen-activated K+ currents recorded at −50 mV from WT hippocampal neurons. PKA activity was modulated by pre-incubation for 30 min with 8-Br-cAMP (1 mM; bright grey trace) or H89 (2 µM; dark grey trace). Controls represent recordings from untreated neurons (black trace). The desensitization time constants τ1 and τ2 were derived from double-exponential fits to the decay phase of K+ currents during baclofen application (enlarged on the right). (B) Bar graph summarizing the time constants τ1 and τ2 of baclofen-induced K+ current desensitization for the indicated treatments. Data are means ± SD, n = 7–35. **, p < 0.01; ***, p < 0.001; Dunnett’s multiple comparison test, compared to Ctl. Ctl, control; FSK, forskolin; cAMP, 8-Br-cAMP. (C) Representative baclofen-activated K+ current responses from hippocampal KCTD12−/− neurons pre-incubated with 8-Br-cAMP (grey trace) or untreated KCTD12−/− neurons (control, black trace) as in (A). Traces were fitted as in (A). (D) Bar graph summarizing the time constants τ1 and τ2 of baclofen-induced K+ current desensitization for the indicated treatments. Data are means ± SD, n = 5–17, Dunnett’s multiple comparison test, compared to Ctl.
In cultured hippocampal neurons of KCTD12−/− mice [19] τ1 but not τ2 was significantly increased compared to WT mice (τ1 of WT: 1.5 ± 0.3 s; KCTD12−/−: 2.4 ± 0.9 s; p < 0.001; τ2 of WT: 24.4 ± 6.4 s; KCTD12−/−: 26.0 ±8.1 s; p > 0.05; t test; compare Fig. 4B and D). Neither activation nor inhibition of PKA had a significant effect on the desensitization of baclofen-induced K+ currents in KCTD12−/−neurons (Fig. 4D). These data support that the PKA-modulated fast component of K+ current desensitization in neurons depends on KCTD12.
3.5. Lack of S892 phosphorylation in S892A knock-in mice accelerates fast desensitization of K+ currents and prevents regulation by PKA
PKA inhibition with H89 or PKI accelerates fast desensitization of K+ currents in WT hippocampal neurons. Endogenous PKA activity must therefore provide a high level of basal S892 phosphorylation. Indeed, Western blot analysis of hippocampal neurons revealed that GB2 is highly phosphorylated at S892 (Fig. 5), which is reduced by inhibition of PKA with H89. We next addressed whether phosphorylation of S892 in GB2 is essential for PKA effects on baclofen-induced K+ current desensitization in neurons. We generated S892A knock-in mice carrying a S892 to alanine mutation in the GB2 gene using standard gene targeting techniques (Fig. 6A and B). S892A mice display no overt behavioral abnormalities. Western blot analysis revealed similar levels of GB1a, GB1b and GB2 protein in S892A and WT brain extracts (Fig. 6C). Recordings of baclofen-induced K+ currents from cultured hippocampal neurons revealed that the desensitization was significantly faster in S892A compared to WT neurons (Fig. 6D and E). Activation of PKA with 8-Br-cAMP did not significantly increase τ1 of the desensitization in S892A neurons, in contrast to control WT neurons (Fig. 6E). The τ2 of the desensitization was similar in both genotypes and did not change upon activation of PKA with 8-Br-cAMP (Fig. 6E). The lack of PKA effect in S892A neurons indicates that S892 phosphorylation is mandatory for PKA-mediated attenuation of fast desensitization. In summary, our results show that basal PKA-mediated phosphorylation of S892 slows KCTD12-induced desensitization in neurons.
Fig. 5.
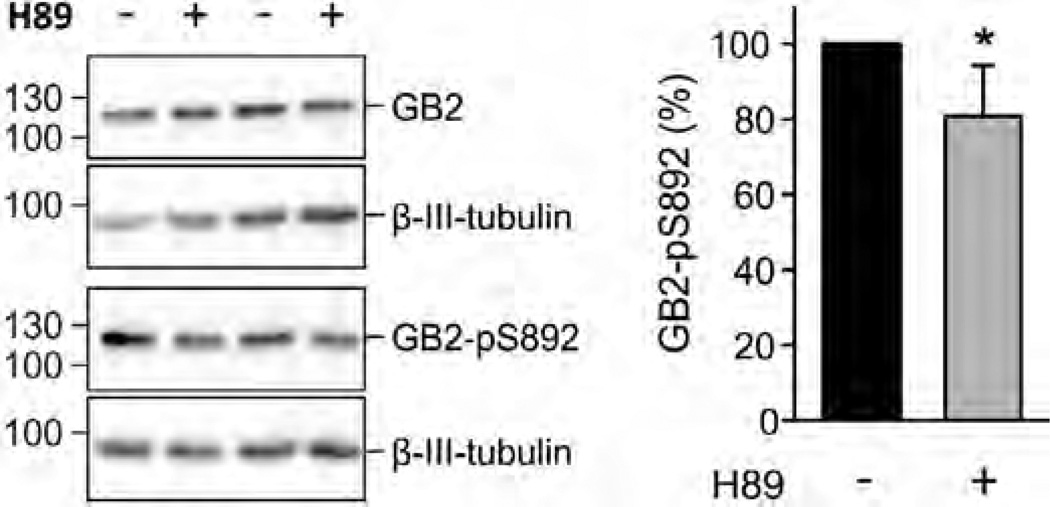
Basal phosphorylation of S892 is decreased by inhibiting PKA in hippocampal neurons of WT mice. Western blot of cultured neurons in the presence and absence of H89 (10 µM, 2 h pre-incubation; left). Inhibition of PKA does not affect the amount of GB2 protein but decreases the amount of S892 phosphorylation (GB2-pS892). β-III-Tubulin was used as a loading control. Bar graph summarizing the amount of phosphorylated S892 (GB2-pS892 in %; right). The total amount of GB2 and GB2-pS892 were normalized to β-III-tubulin on the same blot. Data are means ± SD, n = 6. *, p < 0.05; t test.
Fig. 6.
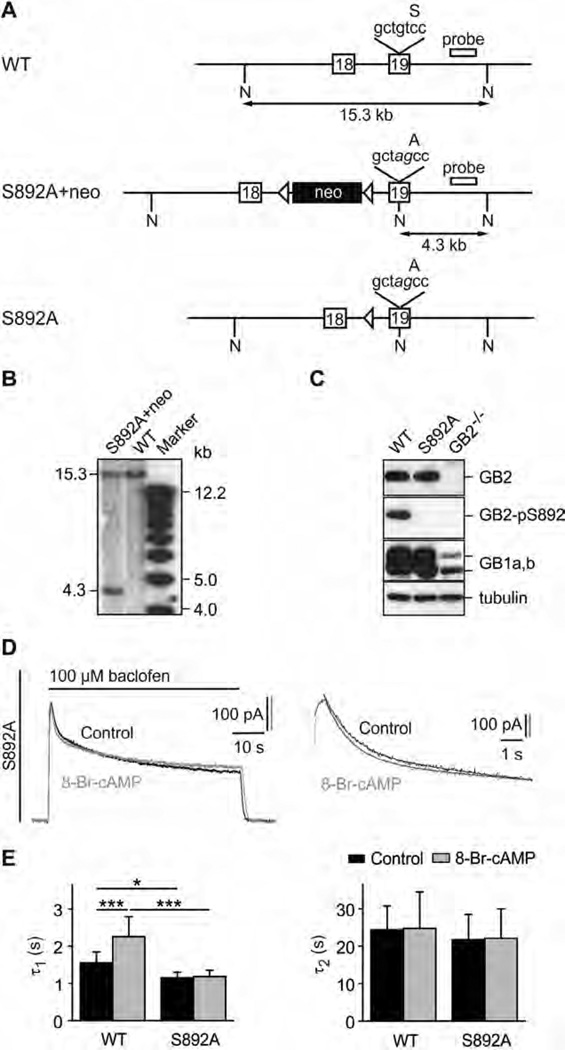
Lack of S892 phosphorylation in S892A knock-in mice accelerates fast desensitization of GABAB-activated K+ currents. (A) WT and mutated GB2 alleles. The S892 to alanine mutation (tcc → gcc) and a silent diagnostic NheI restriction site (gctagc) were introduced into exon 19 using homologous recombination in Balb/c embryonic stem (ES) cells. Mutated nucleotides are shown in italic. A neomycin marker (neo) flanked by loxP sites (arrowheads) was used for selection of ES cells. Correctly targeted ES cells (S892A + neo allele) were injected into C57BL/6 blastocysts. A founder mouse was crossed with a Balb/c mouse expressing Cre-recombinase to excise the neomycin cassette, leaving one loxP site behind (S892A allele). The hybridization probe used in the Southern blot in (B) is indicated. A, alanine; N, NheI restriction sites; S, serine. (B) Southern blot of NheI cut genomic DNA from correctly targeted ES cells. The probe labels a 15.3 kb fragment for the WT allele and a 4.3 kb fragment for the S892A + neo allele. (C) Western blot analysis of brain extracts showing that S892A mice express normal levels of GB2, GB1a and GB1b proteins. S892 was phosphorylated in brain extracts of WT but not S892A mice as shown with an antibody specific for phosphorylated S892 (GB2-pS892). Brain extracts of GB2-deficient mice (GB2−/−) [38] confirm the specificity of the GB2 and GB2-pS892 antibodies. β-III-Tubulin was used as a loading control. (D) Representative GABAB-activated K+ currents recorded at −50 mV in response to baclofen application (100 µM) from cultured hippocampal neurons of S892A mice. PKA was activated by pre-incubation for 30 min with 8-Br-cAMP (1 mM; grey trace). Controls represent recordings from untreated neurons (black trace). The desensitization time constants τ1 and τ2 were derived from double-exponential fits to the decay phase of K+ currents during baclofen application (enlarged on the right). (E) Bar graph summarizing the time constants τ1 and τ2 of baclofen-induced K+ current desensitization in WT and S892A neurons. Data are means ± SD, n = 5–7. *,p < 0.05; ***, p < 0.001; Sidak’s multiple comparison test.
3.6. Assembly of KCTD12 with GABAB receptors increases S892 phosphorylation
We additionally investigated whether assembly of GABAB receptors with KCTD12 influences S892 phosphorylation. We found that co-expression of KCTD12 with GABAB receptors in HEK293T cells increases phosphorylation of S892 in WT but not in GB2-Y902A receptors (Fig. 7). These data reveal that binding of KCTD12 and phosphorylation of S892 influence each other.
Fig. 7.
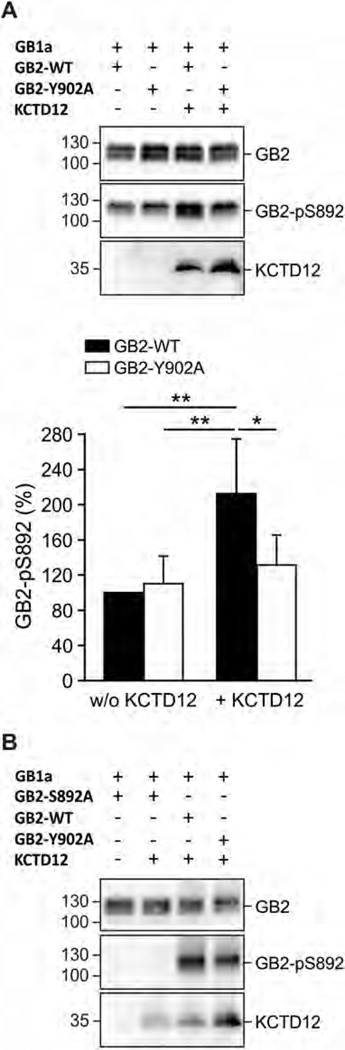
Binding of KCTD12 to GABAB receptors increases S892 phosphorylation. (A) Lysates of HEK293T cells expressing GB1a and either GB2-WT or GB2-Y902A in the presence and absence of KCTD12 (up). S892 phosphorylation (GB2-pS892) is increased in the presence of KCTD12 for GB2-WT, but not for GB2-Y902A. Blots are representative of five independent experiments. Bar graph summarizing the amount of phosphorylated S892 normalized to GB2 protein on the same blot (GB2-pS892 in %; bottom). Data are means ± SDn = 5. *, p < 0.05; **, p < 0.01; Sidak’s multiple comparison test. (B) Western blot of lysates from transfected HEK293T cells expressing the indicated proteins. Phosphorylation of S892 (GB2-pS892) is observed with GB2-WT and GB2-Y902A but not with GB2-S892A, showing that the pS892 antibody specifically detects phosphorylation of S892. Note increased phosphorylation of S892 in the presence of KCTD12 with WT receptors (GB2-WT) compared to mutant receptors that cannot bind KCTD12 (GB2-Y902A). Blots are representative of three independent experiments.
4. Discussion
4.1. Slow and fast mechanisms of homologous desensitization of GABAB receptor responses influence each other
A slow form of homologous desensitization of GABAB receptor responses relies on the activity-dependent decrease of S892 phosphorylation in GB2 by PKA [13,14]. Here we show that phosphorylation of S892 by PKA slows KCTD12-induced desensitization, a faster form of desensitization that is induced at the G-protein rather than at the receptor. We observed a high basal level of S892 phosphorylation in hippocampal neurons, consistent with earlier reports [13,30]. Accordingly, KCTD12-induced current desensitization is significantly attenuated in WT hippocampal neurons compared to GB2-S892A neurons. This may to some extent explain the moderate desensitization of GABAB-activated K+ currents in hippocampal neurons, despite a high level of KCTD12 expression [19]. Conversely, KCTD12 promotes S892 phosphorylation, which may explain why KCTD12 stabilizes receptors at the cell surface and increases the magnitude of GABAB receptor signaling [21]. These results indicate that the mechanism of fast and slow desensitization influence each other.
4.2. Interplay of slow and fast desensitization mechanisms
KCTD12 binds to GB2 in close proximity of the PKA phosphorylation-site S892. In principle, phosphorylation of S892 could facilitate unbinding of KCTD12 from the receptor, which would explain the slowing of fast desensitization. However, BRET analysis and co-immunoprecipitation experiments show that neither PKA activity nor the phospho-mimetic mutant GB2-S892E unbind KCTD12 from the receptor. This agrees with earlier findings showing that KCTD12 remains associated with the receptor during receptor activity [21]. However, BRET analysis revealed that PKA-mediated S892 phosphorylation induces a conformational change in the receptor/KCTD12 complex without measurably altering the interaction between KCTD12 and the G-protein. This suggests that S892 phosphorylation does not stabilize a receptor/KCTD12 conformation that prevents binding of KCTD12 to Gβγ [19]. Since GB2 and KCTD12 both bind to the G-protein the conformational rearrangement in the receptor/KCTD12 complex may reduce the rate of G-protein activation [19,27,31–33]. Because KCTD12-induced desensitization is activity-dependent [19], a reduction in the G-protein activation rate would slow fast desensitization.
Conversely, assembly of GABAB receptors with KCTD12 promotes S892 phosphorylation, which may induce a conformational rearrangement in the cytoplasmic domain of GB2 that makes S892 more accessible for phosphorylation by PKA. This agrees with earlier findings that show that receptors with KCTD12 are more stable at the cell surface [21].
Overall, the interplay of fast and slow mechanisms of homologous desensitization will have several effects on GABAB receptor-activated K+ current responses. Assembly of receptors with KCTD12 will increase basal S892 phosphorylation and stabilize receptors at the cell surface [21]. Increased tonic S892 phosphorylation will attenuate KCTD12-induced fast desensitization. Conversely, prolonged exposure to agonists will reduce PKA activity and S892 phosphorylation. This will accelerate KCTD12-induced fast desensitization and promote slow desensitization due to receptor degradation. The interplay of the two mechanisms of desensitization underlies the sharpening of the K+ current response observed in heterologous cells during repeated receptor activation. In addition to this homologous regulation of desensitization, it is possible that other GPCRs regulating cAMP/PKA signaling modulate GABAB receptor desensitization in a heterologous manner. Moreover, GABAB receptor desensitization may be regulated at S892 during neuronal processes in which PKA activity is altered, such as NMDA-mediated synaptic plasticity, learning and memory, seizures or during aging [34–37].
Acknowledgments
This work was supported by grants of the National Center for Competences in Research (NCCR) ‘Synapsy, Synaptic Bases of Mental Health Disease’ and the Swiss National Science Foundation (31003A_152970) to B.B., and the Lundbeck Foundation to A.A.J. We thank V. Besseyrias for technical help.
References
- 1.Gassmann M, Bettler B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- 2.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 4.Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- 5.Calver AR, Robbins MJ, Cosio C, Rice SQ, Babbs AJ, Hirst WD, et al. The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J Neurosci. 2001;21:1203–1210. doi: 10.1523/JNEUROSCI.21-04-01203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, et al. Activation mechanism of the heterodimeric GABAB receptor. Biochem Pharmacol. 2004;68:1565–72. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Chalifoux JR, Carter AG. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sickmann T, Alzheimer C. Short-term desensitization of G-protein-activated, inwardly rectifying K+ (GIRK) currents in pyramidal neurons of rat neocortex. J Neurophysiol. 2003;90:2494–2503. doi: 10.1152/jn.00112.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetherington JP, Lambert NA. GABAB receptor activation desensitizes post-synaptic GABAB and A1 adenosine responses in rat hippocampal neurones. J Physiol. 2002;544:459–467. doi: 10.1113/jphysiol.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benke D, Zemoura K, Maier PJ. Modulation of cell surface GABAB receptors by desensitization, trafficking and regulated degradation. World J Biol Chem. 2012;3:61–72. doi: 10.4331/wjbc.v3.i4.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couve A, Thomas P, Calver AR, Hirst WD, Pangalos MN, Walsh FS, et al. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABAB receptor-effector coupling. Nat Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- 14.Fairfax BP, Pitcher JA, Scott MG, Calver AR, Pangalos MN, Moss SJ, et al. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J Biol Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- 15.Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABAB receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol. 2007;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutneja M, Berton F, Suen KF, Luscher C, Slesinger PA. Endogenous RGS proteins enhance acute desensitization of GABAB receptor-activated GIRK currents in HEK-293T cells. Pflugers Arch. 2005;450:61–73. doi: 10.1007/s00424-004-1367-1. [DOI] [PubMed] [Google Scholar]
- 17.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, et al. Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. doi: 10.1038/nature08964. [DOI] [PubMed] [Google Scholar]
- 19.Turecek R, Schwenk J, Fritzius T, Ivankova K, Zolles G, Adelfinger L, et al. Auxiliary GABAB receptor subunits uncouple G protein βγ subunits from effector channels to induce desensitization. Neuron. 2014;82:1032–1044. doi: 10.1016/j.neuron.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Correale S, Esposito C, Pirone L, Vitagliano L, Gaetano SD, Pedone E. A biophysical characterization of the folded domains of KCTD12: insights into interaction with the GABAB2 receptor. J Mol Recognit. 2013;26:488–495. doi: 10.1002/jmr.2291. [DOI] [PubMed] [Google Scholar]
- 21.Ivankova K, Turecek R, Fritzius T, Seddik R, Prezeau L, Comps-Agrar L, et al. Up-regulation of GABAB receptor signaling by constitutive assembly with the K+ channel tetramerization domain-containing protein 12 (KCTD12) J Biol Chem. 2013;288:24848–24856. doi: 10.1074/jbc.M113.476770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acidB receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol. 2001;60:963–971. [PubMed] [Google Scholar]
- 23.Metz M, Gassmann M, Fakler B, Schaeren-Wiemers N, Bettler B. Distribution of the auxiliary GABAB receptor subunits KCTD8, 12, 12b, and 16 in the mouse brain. J Comp Neurol. 2011;519:1435–1454. doi: 10.1002/cne.22610. [DOI] [PubMed] [Google Scholar]
- 24.Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, et al. The sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucl Acid Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engle MP, Gassman M, Sykes KT, Bettler B, Hammond DL. Spinal nerve ligation does not alter the expression or function of GABAB receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience. 2006;138:1277–1287. doi: 10.1016/j.neuroscience.2005.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binet V, Duthey B, Lecaillon J, Vol C, Quoyer J, Labesse G, et al. Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J Biol Chem. 2007;282:12154–12163. doi: 10.1074/jbc.M611071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turecek R, Vlcek K, Petrovic M, Horak M, Vlachova V, Vyklicky L., Jr Intracellular spermine decreases open probability of N-methyl-d-aspartate receptor channels. Neuroscience. 2004;125:879–887. doi: 10.1016/j.neuroscience.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Castro LRV, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, et al. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J Neurosci. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havlickova M, Prezeau L, Duthey B, Bettler B, Pin JP, Blahos J. The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric γ-aminobutyrate B receptor. Mol Pharmacol. 2002;62:343–350. doi: 10.1124/mol.62.2.343. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Maurel D, Etzol S, Brabet I, Ansanay H, Pin JP, et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J Biol Chem. 2004;279:15824–15830. doi: 10.1074/jbc.M313639200. [DOI] [PubMed] [Google Scholar]
- 34.Vázquez SI, Vázquez A, Peña de Ortiz S. Different hippocampal activity profiles for PKA and PKC in spatial discrimination learning. Behav Neurosci. 2000;114:1109–1118. doi: 10.1037//0735-7044.114.6.1109. [DOI] [PubMed] [Google Scholar]
- 35.Jay TM, Gurden H, Yamaguchi T. Rapid increase in PKA activity during long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in vivo. Eur J Neurosci. 1998;10:3302–3306. doi: 10.1046/j.1460-9568.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu JX, Tang YC, Liu Y, Tang FR. Status epilepticus alters hippocampal PKAβ and PKAγ expression in mice. Seizure. 2010;19:414–420. doi: 10.1016/j.seizure.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, et al. Redistribution of GABAB1 protein and atypical GABAB responses in GABAB2-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]