Abstract
Background
Isolated limb infusion with melphalan (ILIM) corrected for ideal body weight (IBW) is a well-tolerated treatment for patients with in-transit extremity melanoma with an approximate 29 % complete response (CR) rate. Sorafenib, a multi-kinase inhibitor, has been shown to augment tumor response to chemotherapy in preclinical studies.
Methods
A multi-institutional, dose-escalation, phase I study was performed to evaluate the safety and antitumor activity of sorafenib in combination with ILI-M. Patients with AJCC stage IIIB/IIIC/IV melanoma were treated with sorafenib starting at 400 mg daily for 7 days before and 7 days after ILI-M corrected for IBW. Toxicity, drug pharmacokinetics, and tumor protein expression changes were measured and correlated with clinical response at 3 months.
Results
A total of 20 patients were enrolled at two institutions. The maximum tolerated dose (MTD) of sorafenib in combination with ILI-M was 400 mg. Four dose-limiting toxicities occurred, including soft tissue ulcerations and compartment syndrome. There were three CRs (15 %) and four partial responses (20 %). Of patients with the Braf mutation, 83 % (n = 6) progressed compared with only 33 % without (n = 12). Short-term sorafenib treatment did alter protein expression as measured with reverse phase protein array (RPPA) analysis, but did not inhibit protein expression in the MAP kinase pathway. Sorafenib did not alter melphalan pharmacokinetics.
Conclusion
This trial defined the MTD of systemically administered sorafenib in combination with ILI-M. Although some responses were seen, the addition of sorafenib to ILI-M did not appear to augment the effects of melphalan but did increase regional toxicity.
Despite initial therapy, 2–10 % of extremity melanoma lesions will recur as in-transit metastases, representing melanoma tumor deposits in the dermal or subcutaneous lymphatic vessels located between the primary tumor and regional lymph nodes.1,2 Regional chemotherapy, frequently with melphalan (L-phenylalanine mustard) delivered via either hyperthermic isolated limb perfusion (HILP) or isolated limb infusion (ILI-M), has emerged as the treatment of choice for unresectable recurrent extremity melanoma.2–7 ILI-M has been found to be associated with significantly less major morbidity compared with HILP, and a recent multi-institutional study found a complete response (CR) rate to ILI-M of 29 % in patients who had their melphalan dose corrected for ideal body weight (IBW).7–10
Recent research has focused on improving response rates to ILI-M through the use of targeted agents directed against proliferation and chemoresistance pathways important in melanoma.10 One of the most common genetic alterations found in approximately 40–50 % of melanomas is the constitutively activating mutation of the Braf serine/threonine kinase, which leads to unchecked stimulation of the mitogen-activated kinase/extracellular signal-regulated kinase (MEK) and extracellular signal-regulated kinase (ERK) pathway.11,12 Increased activity of this pathway leads to proliferative, survival/antiapoptotic, and angiogenic pathway activation that enhances growth and progression of melanoma tumors.12 The high prevalence of activating Braf mutations in melanoma makes this pathway an attractive target for therapeutic intervention.
Sorafenib (Nexavar) is an oral multikinase inhibitor that has been shown to antagonize both Raf serine/threonine kinases and receptor tyrosine kinases associated with tumor proliferation and survival.13 Trials in which sorafenib was combined with systemic cytotoxic chemotherapy have suggested that sorafenib can augment the antitumor activity of some chemotherapeutic agents.14–16 In preclinical studies, sorafenib in combination with melphalan led to significantly improved responses in vitro, and the combination was more effective than either treatment alone in slowing tumor growth in a rat regional-therapy xenograft model of extremity melanoma.17,18 Here, we report results from a multicenter, phase I dose escalation study to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of systemic sorafenib in combination with ILI-M in patients with locally advanced in-transit melanoma of the extremity.
MATERIALS AND METHODS
Patient Eligibility
Major inclusion criteria included: age 18 years or older, histologically confirmed recurrent American Joint Commission on Cancer (AJCC) stage IIIB or IIIC melanoma limited to a single extremity or stage IV melanoma limited to a single extremity, tumor tissue available for pre-ILI biopsy, measurable cutaneous disease distal to the location of tourniquet placement, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. We did not mandate that patients have their tumors tested for the Braf mutation as our preclinical data suggested that sorafenib may augment the efficacy of melphalan independent of tumor mutational status.18
Patients previously exposed to sorafenib were excluded, though patients having undergone prior ILI-M or HILP were allowed. All patients were required to give informed consent. The institutional review boards of the participating institutions approved the study.
Study Design
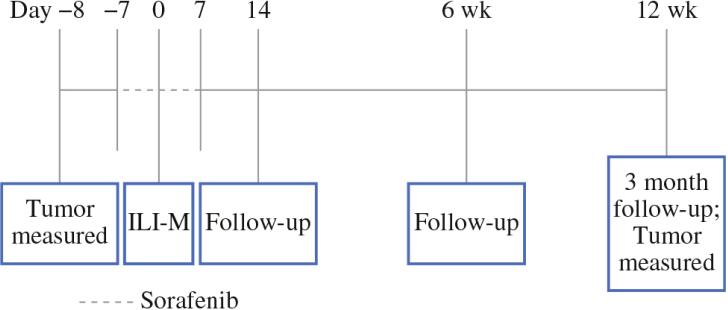
This trial was an open-label, multicenter, phase 1 dose escalation study of systemic sorafenib in conjunction with ILI-M. Human data suggested that sorafenib reaches steady-state concentrations after 7 days.19 A comparison of melanoma biopsies in another study showed that the percentage of cell nuclei staining positively for phosphorylated ERK decreased with sorafenib treatment after 7 days.20 Our own preclinical work also suggested that sorafenib could significantly decrease phosphorylated ERK levels after 7 days of treatment in a rat model of regionally advanced melanoma.18 Patients thus received sorafenib for 7 days prior to and 7 days following ILI-M (Fig. 1). We continued sorafenib after ILI based in part on our animal model trying to maintain suppression of phosphorylated Erk activity and in an attempt to simulate trials in metastatic melanoma that found the best response rates were achieved when sorafenib was administered concurrently and continuously with chemotherapy.18,21 This study was designed to treat three patient cohorts at sorafenib daily doses of 400, 600, and 800 mg (given in divided doses twice daily). These doses were chosen based on prior studies that established the maximum tolerated dose (MTD) of sorafenib as a single agent to be 800 mg daily; thus we chose to start the dose escalation for the combination therapy at 50 % (400 mg) of the sorafenib MTD in an attempt to define and minimize any potential interactions with melphalan.22 Melphalan was administered regionally via ILI at a dose of 10 mg/L for the upper extremity and 7.5 mg/L for the lower extremity corrected for ideal body weight (IBW).7 ILI was performed as previously described using an arterial injection of papaverine just prior to rapid infusion of melphalan into the arterial catheter (over the course of 2–5 min) after achieving a limb temperature of at least 37.0 °C.7 This study was initially designed as a standard phase I dose escalation study in which each cohort included a minimum of two patients or a maximum of six patients depending on the observed dose limiting toxicities (DLTs).
Figure. 1.
Trial design is depicted. Sorafenib was administered daily for seven days prior to and seven days following isolated limb infusion with melphalan (ILI-M). Follow-up occurred at 14 days and 6 weeks. Follow-up and tumor assessment occurred at 12 weeks
Toxicity Evaluation
Adverse events were graded using the National Cancer Institute's Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0. Adverse events were classified as DLTs if they occurred within 30 days from the last dose of sorafenib, were judged to be possibly, probably, or definitely related to sorafenib or its combination with ILI-M, and met one of the following parameters: absolute granulocyte count <0.5 × 109/L lasting ≥7 days, CTCAE grade ≥ 3 neutropenic infection or febrile neutropenia, platelets <50 × 109/L lasting ≥7 days, symptomatic bleeding sufficient to require therapeutic intervention, nonhematological toxicity of CTCAE grade ≥ 3 severity, CTCAE grade ≥ 4 drug-related regional toxicities as defined by specified areas pertinent to limb toxicity, or inability to take two planned doses of sorafenib during the 14-day regimen. The maximum tolerated dose (MTD) was defined as the highest dose at which <2 of six patients or ≤3 of ten patients experienced a DLT. The MTD cohort was then expanded to ten patients.
During the study we obtained IRB approval for a modification of the definition of DLT to allow for isolated creatine phosphokinase (CPK) elevation in the absence of compartment syndrome to be considered a non-DLT. This change affected patient accrual to dose cohorts as explained in the results section.
Assessment of Tumor Response
Tumor response was evaluated at 3 months posttreatment using the Response Evaluation Criteria in Solid Tumors (RECIST) modified for cutaneous lesions.10 Patients were classified as having a CR, partial response (PR), stable disease (SD), or progressive disease (PD).7 We chose the 3-month posttreatment time point to evaluate response as a way to standardize response evaluation and because we have used this benchmark in our previous work.7,9,10 However, we acknowledge that this method may not capture an individual patient's best response, which may vary among patients.6 New disease in the field of treatment was automatically classified as having PD regardless of target lesion response.
Pharmacokinetic Evaluations
Just before ILI and during the 30-min circulation of melphalan through the infusion circuit, blood samples (5 mL) were obtained from the venous stopcock of the circuit to assess melphalan and sorafenib levels. Melphalan concentrations were measured using high-performance liquid chromatography (HPLC) fluorescence as described previously.23 Sorafenib levels from a plasma sample collected just before ILI were measured by liquid chromatography electrospray ionization tandem mass spectrometry as previously described with modifications to accommodate equipment and internal standard material available.24
Braf and Nras Mutation Analysis
Tumor tissue was obtained prior to initiation of sorafenib and again prior to ILI. The samples were snap frozen and stored at -133 °C until analysis. Tissue samples were homogenized using a mini bead-beater (Biospec Products) and lysing matrix A (MP Biomedicals), total RNA isolated (Qiagen RNeasy), and cDNA synthesized (Roche Firststrand cDNA synthesis). PCR amplification for Braf and Nras sequencing was performed on a Stratagene Robocycler 96 using HotStart Taq DNA polymerase (Qiagen) in a 50 μL reaction volume (primer sequences and reaction settings available upon request). Purified PCR products (Qiaquick PCR purification kit; Qiagen) were sequenced by the Duke University DNA Analysis Facility using the Applied Biosystems Dye Terminator Cycle Sequencing system with AmpliTaq DNA Polymerase and ABI 377 PRISM DNA sequencing instruments and analysis software.
Reverse Phase Protein Array and Analysis
Tumor samples from five patients were analyzed for protein expression using reverse phase protein array (RPPA). Prior to homogenization, tumor biopsy samples were evaluated for percent tumor content. All samples harbored a minimum of 70 % tumor. Proteins were isolated from the tumor samples as previously described, and protein lysates were prepared using standard methods.25 After normalizing the samples to the same concentration and denaturing the sample, reverse phase protein array (RPPA) analysis was performed by the Functional Proteomics Core Facility at the MD Anderson Cancer Center (Houston, TX). Relative protein expression levels were determined for 194 protein markers (data available upon request). Hierarchical cluster analysis was done using Cluster v3.0 and visualized with Treeview 1.6.
Statistical Considerations
Descriptive statistics were used to describe the distribution of baseline demographics, perioperative variables, response, and toxicity. In-field response was defined as CR or PR and served as the main outcome variable of interest with no response defined as either SD or PD. For the RPPA analysis, the reproducibility and correlation of results were tested by calculating Pearson Correlation coefficients. The associations between protein levels and sorafenib dose level were determined by χ2 and Fisher exact testing as previously described.26 Proteins significantly correlated with sorafenib dose level were selected based on Pearson Correlation Coefficients using an alpha level of 0.90 (P <0.10; unpaired t test) because of the small number of patient samples (n = 5).
RESULTS
A total of 20 patients received sorafenib plus lower extremity ILI-M at two institutions over 22 months. Of these patients, 70 % (14 of 20) had received a prior melphalan-based regional therapy. Other patient characteristics are shown in Table 1. Hypoxia and acidosis during ILI-M were achieved with a median ischemic time of 68 min (range 44–94 min) and median peak temperature 39.3 °C (range 37.0–41.3 °C). The median values of other procedural variables at 30 min were: pH 7.14 (range 6.99–7.29), base excess 11.0 (range −16.0 to -2.4), and pO2 5.5 mm Hg (range 2–37).
TABLE 1.
Patient characteristics; Bruf and Nras mutational status established using PCR and DNA sequencing
| Subject no. | Sex | AJCC stage | Disease burden | Previous regional therapy | Response | B-Raf mutational status | N-Ras mutation status | Toxicity grade |
|---|---|---|---|---|---|---|---|---|
| 1a | F | IIIC | High | ILI-M (2) | PD | Mutant | wt | II |
| 2a | M | IIIC | High | ILI-M (2) | SD | wt | wt | III |
| 3a | F | IIIC | High | PD | Mutant | wt | II | |
| 4b | SI | IIIC | High | ILI-M | PD | Mutant | wt | II |
| 5b | F | IIIB | High | ILI-M | PR | Mutant | wt | IV |
| 6b | F | IIIB | High | ILI-M | PD | wt | wt | III |
| 7b | F | IIIC | High | ILI-M (2) | PD | Mutant | wt | II |
| 8b | M | IV | High | ILI-M | PD | * | * | II |
| 9b | F | IIIB | High | ILI-M | PD | wt | Mutant | IV* |
| 10a | F | IIIC | High | PD | Mutant | wt | II | |
| 11a | M | IIIC | Low | PR | wt | wt | III | |
| 12a | M | IV | Low | CR | wt | wt | II | |
| 13b | M | IIIC | High | ILI-M | CR | wt | Mutant | I |
| 14b | F | IIIIB | Low | ILI-M | PR | wt | Mutant | II |
| 15b | M | IIIB | Low | PD | wt | wt | IV | |
| 16b | M | IIIC | High | HILP-M | SD | wt | wt | IV |
| 17a | F | IV | Low | ILI-M, HILP-M | NE | * | * | II |
| 18a | F | IIIC | High | PD | wt | Mutant | IV | |
| 19a | F | IIIB | Low | ILI-M | CR | wt | wt | III |
| 20a | M | IIIB | Low | ILI-M | PR | wt | Mutant | III |
ILI-M isolated limb infusion willi melphalan, HILP-M hyperthermic isolated limb perfusion with melphalan, CR complete response, PR partial response. SD stable disease, PD progressive disease
Subjects in the 400 mg cohort
Subjects in the 600 mg cohort
Tumor biopsies from subject 8 and 17 were unavailable
Patient 9 had an elevation in creatine kinase that was initially considered a grade IV toxicity but was later changed to a lower grade after a protocol amendment
Safety Profile
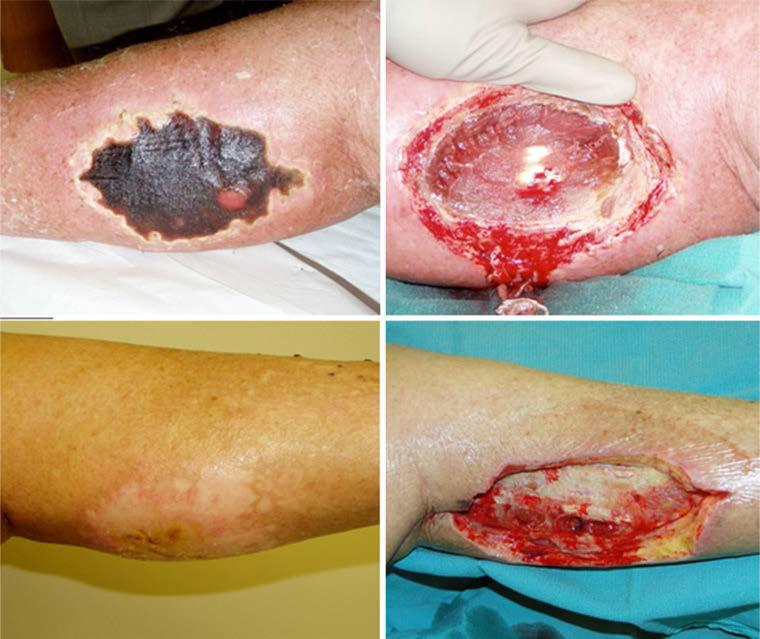
Grade 1 and 2 toxicities in all cohorts included edema (85 %, 17 of 20), pain (80 %, 16 of 20), rash/erythema (70 %, 14 of 20), and nausea (55 %, 11 of 20), all of which are associated with ILI-M alone.7 After none of the initial three patients enrolled in the first cohort (400 mg dose of sorafenib) experienced a DLT, dose escalation proceeded to 600 mg sorafenib. In this cohort, one of the first three patients experienced a DLT (grade 4 ulceration), and the cohort was expanded by an additional three patients. The sixth patient enrolled in this cohort experienced an isolated grade 4 CPK elevation ([10 times the upper limit of normal), which at that time was considered a DLT. As transient spikes in CPK are frequently observed following ILI, an IRB-approved amendment was instituted for the remainder of the study, which stated “...isolated CPK elevations in the absence of compartment syndrome should be considered a NON-dose limiting toxicity.”7 The IRB-approved amendment had the effect of restarting dose escalation at the lowest dose (400 mg sorafenib) and resetting the DLT count. Three more patients were enrolled at the 400-mg dose without serious toxicity, leading to dose escalation back to the 600-mg level. Of the next four patients, two enrolled in the second cohort experienced a DLT (compartment syndrome requiring surgical decompression; grade 4 ulceration requiring surgical debridement, see Fig. 2), establishing this dose level as the maximum administered dose (MAD). The first cohort (400 mg sorafenib) was subsequently expanded by four patients to a total of ten patients, one of whom experienced a DLT (grade 4 ulceration requiring surgical debridement; see Fig. 2) and one of whom experienced a delayed complication (7 months after ILI-M the patient was found to have an abscess of the posterior leg compartment in the infused leg and was treated with surgical debridement). With just the one DLT among the ten patients in the low-dose cohort, 400 mg was declared the MTD of sorafenib when used in combination with ILI-M.
Figure 2.
Top left: grade 4 ulceration of the infused extremity 2 weeks after isolated limb infusion (ILI-M) which demonstrated evidence of muscle necrosis and required surgical debridement (top right). Bottom left: fat necrosis and non-healing ulceration of the infused leg 12 weeks after ILI-M which required surgical debridement (bottom right).
Clinical Response
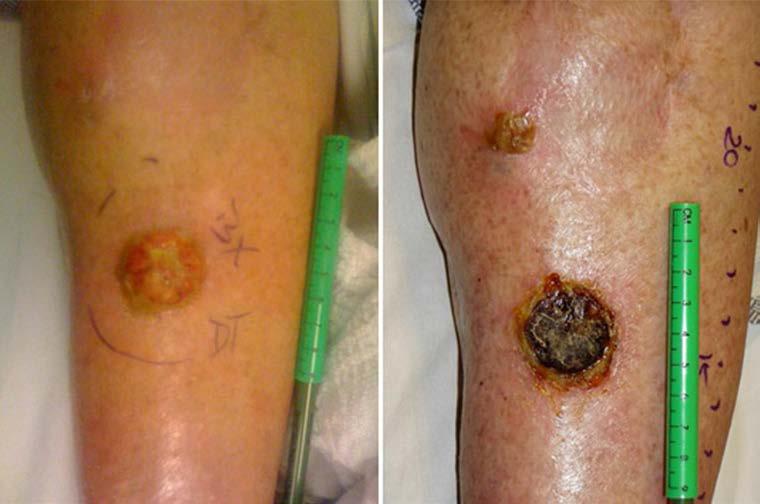
Of the 20 patients, 19 were assessed for in-field tumor response at 3 months following ILI (one patient progressed out of field prior to the 3-month endpoint and was treated with additional systemic therapy). Of the 20 patients, three achieved a CR (15 %), four achieved a PR (20 %), 2 had SD (10 %), and 10 had PD in field (50 %). Interestingly, regression of at least 1 lesion occurred after 1 week of sorafenib therapy as measured just prior to ILI-M in 50 % (ten of 20) of the patients. In some of these lesions, necrosis and involution was marked, as shown for one patient (Fig. 3). Of the patients who had received previous melphalan-based therapy (14 of 20), there were three CRs, three PRs, two SDs, and six PDs. Among the three CRs in this pretreated group, all had also achieved a CR after prior ILI with melphalan alone. Of the chemonaive patients (six of 20) in this study, one experienced a PR, four experienced PD, and one was nonevaluable as mentioned previously.
Figure 3.
Clinical response to sorafenib. Photographs obtained from a patient before (left) and after (right) treatment with sorafenib before ILI-M was performed.
Of the patients who did not progress in field at 3 months (nine of 20), eight were available for 6-month follow-up (one of the patients with SD at 3 months was treated with systemic chemotherapy following metastatic recurrence prior to the 6-month follow-up time point). Of the eight evaluable patients, four maintained their in-field response at the 6-month time point and four progressed in field, necessitating further treatment for their PD.
Among all 20 patients, six experienced disease progression outside the region infused prior to the 3-month time point (subcutaneous tissue, n = 2; brain, n = 1; mediastinum and peritoneum, n = 1, regional LN, n = 1, and widely metastatic, n = 1). At the 3-month time point, three more patients were found to have out-of-field progression (subcutaneous tissue, n = 1; liver, n = 1; inguinal lymph nodes, n = 1). Two patients developed out-of-field disease discovered prior to the 6-month time point (inguinal lymph nodes, n = 1; and subcutaneous tissue n = 1), and one patient was found to have out-of-field progression at the 6-month time point (liver). Two patients could not be evaluated for out-of-field recurrence as mentioned previously, and six patients (two CRs, three PRs, and one SD at 3 months) had no evidence of out-of-field disease at the 6-month time point. Finally, one patient developed squamous cell carcinoma in the field of treatment approximately 7 weeks after ILI-M.27
Pharmacokinetics
We have previously demonstrated that melphalan pharmacokinetics correlate with toxicity and have optimized melphalan dosing to reduce toxicity by correcting the dose for IBW.28 The rate of grade ≥ 3 limb toxicity in this study was 45 % (nine of 20) compared with rates of 21 % (14 of 66) in a multicenter study of ILI-M and 20 % (nine of 45) in a multicenter study of ILI-M plus systemic ADH-1 (an N-cadherin antagonist).11,29 All patients in these studies had a melphalan dose corrected for IBW. In the present study, none of the measured pharmacokinetic parameters differed between the 11 patients with grade <3 limb toxicities and the nine patients with grade ≥ 3 toxicities: the mean measured melphalan maximum concentrations (Cmax) in these subgroups were 29.01 and 28.15 μg/mL, respectively; the melphalan area under the plasma concentration time curves (AUC) were 6.76 and 6.88, respectively; and the mean sorafenib plasma concentrations immediately prior to ILI were 7.46 and 6.47 μg/mL, respectively. When compared with the ADH-1 phase 1 study, melphalan pharmacokinetic parameters observed in this trial did not differ appreciably. The mean postoperative CPK value observed in the group of patients treated with ILI-M in combination with systemic sorafenib was 2,280 U/L (range 42–14,459 U/L). This value was higher than that from our database of patients treated with ILI-M alone (mean 1,879 U/L, range 38–11,674 U/L) and patients treated with ILI-M in combination with systemic ADH-1 (mean 1,075 U/L; range 38–8,705 U/L), an N-cadherin antagonist we investigated in phase I and II trials.10,29
Correlative Data
Tumor tissue from each patient was collected just prior to beginning sorafenib therapy and following the completion of the first week of sorafenib just prior to ILI-M. Of the 19 patients evaluable at 3 months, 18 were successfully genotyped for Braf and Nras mutational status using PCR amplification and DNA sequencing (one patient's samples yielded inadequate tumor tissue, precluding mutational status assessment). Also, 33 % of patient tumors (six of 18) were found to have the Braf mutation while 28 % of patient tumors (five of 18) had the Nras mutation (Table 1). The response rate was similar between patients with and without the Braf and Nras mutations. Interestingly, the patient who had marked involution of tumor after treatment with sorafenib (Fig. 3) did not exhibit the Braf or Nras mutation. Progression occurred in 54 % of patients with wild-type Nras (seven of 13) and 40 % of patients with the Nras mutation (two of five). With respect to Braf, 33 % of patients expressing wild-type Braf (four of 12) progressed in field at 3-months compared with 83 % of patients expressing mutant Braf (five of six).
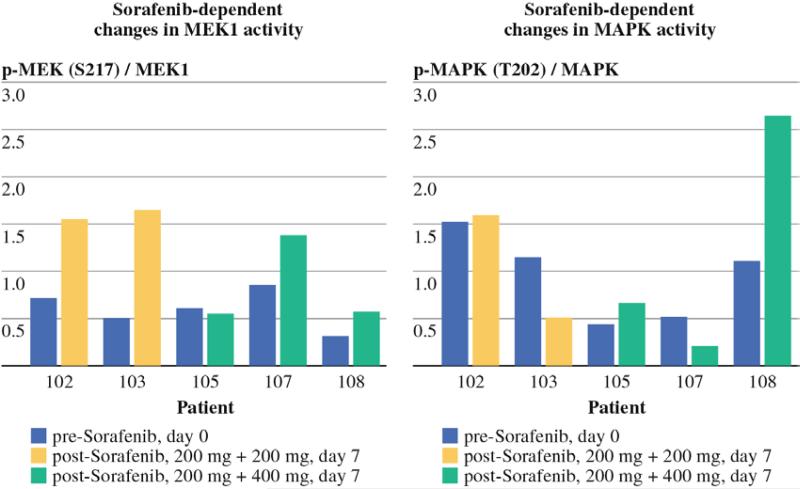
In order to determine the effects of sorafenib on protein signaling networks, remaining tumor tissue was analyzed using RPPA in five patients. After confirming that the biopsies had >70 % tumor content and lacked significant necrosis, RPPA analysis was performed on proteins isolated from the tumor samples. The relative expression of a total of 194 protein markers, including many activationspecific epitopes, was determined for the samples. Previous work in preclinical models demonstrated that the maximal in vitro antitumor activity of sorafenib correlates with the inhibition of the RAF-MEK-ERK signaling pathway.30 Analysis of the expression of phosphorylated MEK (PMEK) relative to total MEK and P44/42 MAPK (PMAPK) relative to total MAPK failed to demonstrate a notable decrease in either of these activation-specific protein markers of the Raf-MEK-ERK pathway with sorafenib treatment (Fig. 4) despite the pronounced clinical responses observed in some patients after 1 week of systemic sorafenib treatment (Fig. 3).
Figure 4.
Reverse phase protein array (RPPA) analysis. Using linear load-corrected measurements of protein expression, the ratio of phospho-protein to total protein is plotted for 5 patients before and after treatment with sorafenib for MEK (left) and MAPK (right). Tumor tissue was analyzed from two patients from the low-dose (400 mg) sorafenib cohort and 3 patients from the high-dose (600 mg) sorafenib cohort.
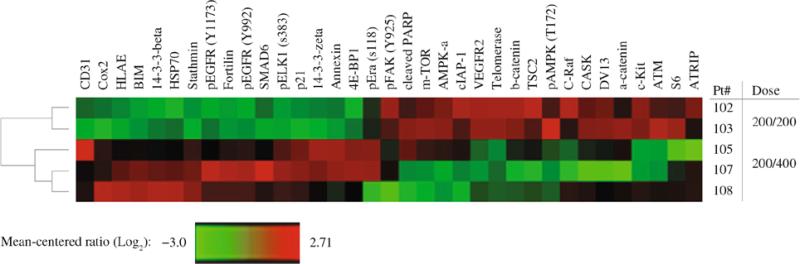
Unsupervised clustering analysis of protein expression changes in the tumor tissue of these five patients failed to identify patterns associated with the mutational status of the tumors or with clinical response at 3 months (data not shown). Comparison of changes in protein expression patterns for patients treated with low (400 mg) versus high (600 mg) doses of sorafenib showed a more striking pattern (Fig. 5). Overall, 35 proteins showed significant differences between the two doses (P <.10; see Fig. 5). In patients receiving 600 mg of sorafenib daily, 16 proteins showed increased expression and 19 proteins showed decreased expression. The pro-apoptotic Bcl-2 family protein Bim and the cyclin-dependent kinase inhibitor p21 both showed marked treatment-induced increases in expression (P = 0.025 and P = 0.017, respectively) in patients receiving 600 mg sorafenib compared with patients receiving 400 mg sorafenib. Among the 19 proteins with decreased expression in the 600-mg dose cohort are three protein kinases that are known targets of sorafenib: VEGFR2 (or KDR; P = 0.045), CRaf (or RAF1; P = 0.075), and cKit (P = 0.062).28 Also of note, several mTOR pathway proteins showed altered expression patterns in the high sorafenib dose patients, including decreased expression of mTOR (P = 0.059), TSC2 (P = 0.068), AMPK (P = 0.081), and S6 ribosomal proteins (P = 0.055) and increased expression of the translation repressor protein 4E-BP1 (P = 0.093). These results suggest that sorafenib has a dose-dependent effect on important apoptotic and proliferative pathways.
Figure 5.
Dose-dependent changes in protein expression. Unsupervised hierarchical clustering was performed on linear load-corrected measurements of protein expression and a heatmap generated where relative protein expression is color-coded such that red indicates higher expression and green indicates lower expression. Rows represent patients and columns represent the ratio of post-treatment to pre-treatment expression for each protein. Patients 102 and 103 received a lower dose (400 mg) of sorafenib while patients 105, 107, 108 received a higher dose (600 mg) of sorafenib. Expression ratios are shown for the 35 proteins with P\0.10 in an unpaired t-test comparing low dose to high dose patients.
DISCUSSION
In this study, the MTD of systemic sorafenib in combination with ILI-M was established as 400 mg daily for 1 week prior to and 1 week following ILI-M. This dose, half that used in the setting of RCC and HCC, was chosen as the starting dose in an attempt to avoid any serious additive or synergistic toxicity between sorafenib and melphalan.31–33 Overall, we observed sorafenib-related systemic adverse events and regional toxicity from ILI-M similar to those that have previously been reported.16 Importantly, we also observed significant regional toxicity primarily involving the posterior compartment of the limb that included three instances of grade 4 ulceration, one grade 3 ulceration, one compartment syndrome, and one delayed complication (an abscess that ultimately required surgical debridement) that was potentially associated with the combination treatment. Also of note was the significant degree of CPK elevation, which we have shown correlates with regional toxicity.7 The postoperative CPK values from this study were higher than we have previously seen. This suggests that sorafenib may have exacerbated melphalan-induced muscle toxicity, although certainly in this small study the toxicity could have been related only to ILI-M. Preclinical studies of combination sorafenib and ILI-M in a regional melanoma rat model did not demonstrate exacerbation of ILI-M toxicity, signifying that preclinical animal studies may underplay clinical toxicity seen in humans.18
One patient in the 400 mg cohort developed squamous cell carcinoma (SCC) approximately 7 weeks after treatment. At least 13 episodes of SCC associated with sorafenib treatment have been reported with an average treatment interval (time between sorafenib treatment and diagnosis of skin neoplasm) of 33 weeks.32 In a recent report, 31 % of melanoma patients (ten of 32) with a Braf mutation treated with the Braf inhibitor, PLX4032, developed SCC.34 The underlying mechanism leading to the development of SCC in melanoma patients treated with Braf inhibitors is potentially related to the ability of these inhibitors to activate the MAP kinase pathway in cells lacking the Braf mutations.35–37 That the patient in this study developed squamous cell cancer in the infused limb considerably more quickly than has previously been reported, and was taking a relatively low daily dose of sorafenib, suggests that melphalan may augment this specific sorafenib-associated toxicity as well.
The CR rate of 15 % and overall response (OR) rate (CR rate + PR rate) of 35 % in this trial are lower than our previously reported CR rate of 31 % and OR rate of 64 % in patients undergoing ILI-M corrected for IBW.9 Moreover, all of the patients who experienced a CR (three of three) and 75 % of the patients who experienced a PR (three of four) had responded to previous melphalan-based regional therapy. Sorafenib thus did not appear to help overcome melphalan resistance in this trial. Our preclinical data suggested that sorafenib could augment melphalanbased cytotoxicity independent of mutational status, but that maximal cytotoxicity was seen when MAPK signaling pathways were inhibited.18 Although previous studies have demonstrated that sorafenib is a potent inhibitor of MEK and MAPK in vitro as well as in our rat extremity melanoma model, we did not observe a substantial decrease in P-MAPK or P-MEK expression levels in any of the 5 patients for whom matched tumor tissues were available for RPPA analysis (Fig. 4) with 1 week dosing of sorafenib.18,37 Recent studies have demonstrated that clinical benefit from a more selective Braf inhibitor PLX4032 occurs only at a dose sufficient to inhibit P-MAPK levels to about 80 %.38 While this trial only included 20 patients, the lack of clinical benefit from sorafenib in patients with mutant versus wild-type Braf suggests that a 1-week course of sorafenib failed to significantly inhibit the signaling mediated by the Braf protein. Of note, a previous analysis of candidate biomarkers corresponding with clinical benefit in metastatic melanoma patients treated with sorafenib, paclitaxel, and carboplatin also failed to identify an association with Braf mutation status and clinical response.39
Although we did not observe significant inhibition of the RAF-MEK-MAPK signaling pathway by sorafenib, there were both clinical and biochemical indications that the drug did affect the tumors in this group of patients (Fig. 3). Sorafenib may also exert significant growth-inhibitory effects via its antagonism of the VEGF/PDGFR signaling pathway, leading to tumor vasculature compromise.37 In the prior analysis of patients treated with sorafenib, paclitaxel, and carboplatin, the pretreatment expression levels of the VEGF-R2 did significantly correlate with clinical responsiveness.39 In our study, patients treated with 600 mg of sorafenib achieved a greater reduction in VEGF-R2 expression than patients treated with 400 mg daily (P = 0.04). We also identified 34 other proteins known to regulate cellular proliferation and apoptosis, which exhibited marked dose-dependent expression in response to sorafenib (Fig. 5). Unfortunately, the theoretical benefits of reduced CRaf, cKit, and mTOR expression and increased Bim and p21 expression were not borne out in improved clinical responses.
This trial defined the MTD of systemically administered sorafenib in combination with ILI-M. The addition of sorafenib appeared to increase the regional toxicity of ILI-M in a manner not explained by pharmacokinetic analysis. Although sorafenib did demonstrate dose-dependent changes in protein expression, these changes did not inhibit Braf signaling or augment response to ILI-M. Future directions include elucidating the mechanism of melphalan toxicity exacerbation by sorafenib, determining if a more selective and potent inhibitor of Braf signaling pathways might augment regionally delivered chemotherapy in patients whose tumors harbor the Braf mutation, and establishing the optimal dosing and timing of therapy.
ACKNOWLEDGMENT
Bayer provided drug only (sorafenib, Nexavar) for the phase I trial of systemic sorafenib and regional melphalan. This paper was supported in part by Duke University's CTSA grant TL1RR024126 from NCRR/NIH (Coleman/Raymond), T32 grant CA093245-10 from NIH (Beasley), and the Duke Melanoma Research Fund (Tyler).
REFERENCES
- 1.Balch CM, Peters LJ. Cutaneous melanoma. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 4 ed. JB Lippincott; Philadelphia: 1993. pp. 1612–61. [Google Scholar]
- 2.Skene AI, Bulman AS, Williams TR, Thomas JM, Westbury G. Hyperthermic isolated perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J Surg. 1990;77:765–7. doi: 10.1002/bjs.1800770716. [DOI] [PubMed] [Google Scholar]
- 3.Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American college of surgeons oncology group trial Z0020. J Clin Oncol. 2006;24:4196–201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 4.Taber SW, Polk HC., Jr. Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol. 1997;4:440–5. doi: 10.1007/BF02305559. [DOI] [PubMed] [Google Scholar]
- 5.Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: indications and results. Br J Surg. 1996;83:1319–28. doi: 10.1002/bjs.1800831004. [DOI] [PubMed] [Google Scholar]
- 6.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 7.Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 8.Coleman A, Augustine CK, Beasley G, Sanders G, Tyler D. Optimizing regional infusion treatment strategies for melanoma of the extremities. Expert Rev Anticancer Ther. 2009;9:1599–609. doi: 10.1586/era.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beasley GM, Caudle A, Petersen RP, McMahon NS, Padussis J, Mosca PJ, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–15. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715–17. [DOI] [PubMed] [Google Scholar]
- 10.Beasley GM, McMahon N, Sanders G, Augustine CK, Selim MA, Peterson B, et al. A phase 1 study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. Cancer. 2009;115:4766–74. doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88. doi: 10.1002/humu.20481. [DOI] [PubMed] [Google Scholar]
- 12.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 13.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 14.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, et al. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13:1801–9. doi: 10.1158/1078-0432.CCR-06-1432. [DOI] [PubMed] [Google Scholar]
- 16.McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 study group. J Clin Oncol. 2008;26:2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 18.Augustine CK, Toshimitsu H, Jung SH, Zipfel PA, Yoo JS, Yoshimoto Y, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. 2010;9:2090–101. doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 19.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 20.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–61. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaravadi RK, Schuchter LM, McDermott DF, Kramer A, Giles L, Gramlich K, et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res. 2009;15:7711–8. doi: 10.1158/1078-0432.CCR-09-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 23.Ehrsson H, Eksborg S, Lindfors A. Quantitative determination of melphalan in plasma by liquid chromatography after derivatization with N-acetylcysteine. J Chromatogr. 1986;380:222–8. doi: 10.1016/s0378-4347(00)83648-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, et al. A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, et al. Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res. 2009;15:7538–46. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9:257–67. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond A, Puri PK, Selim MA, Tyler DS, Nelson KC. Regional squamous cell carcinomas following systemic sorafenib and isolated limb infusion for regionally advanced metastatic melanoma of the limb. Arch Dermatol. 2010;146:1438–9. doi: 10.1001/archdermatol.2010.367. [DOI] [PubMed] [Google Scholar]
- 28.McMahon N, Cheng TY, Beasley GM, Spasojevic I, Petros W, Augustine CK, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–61. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beasley G, Riboh JC, Augustine CK, Zager JS, Hochwald SN, Grobmyer SR, et al. A prospective multi-center phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion (M-ILI) in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–5. doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 32.Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 33.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;20:3960–7. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 38.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009;15:1076–85. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]