Abstract
We recently reported an amelogenin-chitosan (CS-AMEL) hydrogel as a promising biomimetic material for future in situ human enamel regrowth. To further optimize the necessary conditions for clinical applicability of CS-AMEL hydrogel, herein we studied the effects of viscosity and supersaturation degree on the size and orientation of synthetic crystals by means of scanning electron microscopy (SEM) and X-ray diffraction (XRD). Raising the hydrogel viscosity by increasing chitosan concentration from 1% to 2% (w/v) improved the orientation of the crystals, while a higher supersaturation (σ(HAp) >10.06, [Ca2+] >5 mM) resulted in the formation of random crystals with larger sizes and irregular structures. We conclude that optimal conditions to produce organized enamel-like crystals in a CS-AMEL hydrogel are: 2% (w/v) chitosan, 2.5 mM calcium, and 1.5 mM phosphate (degree of supersaturation = 8.23) and 200 μg/ml of amelogenin.
Keywords: Apatite crystals, amelogenin, chitosan hydrogel, enamel biomimetic, supersaturation
Introduction
Due to the irreversibility of enamel damage under natural conditions, developing an artificial strategy to repair enamel defects is of great importance. Although amorphous substitutes such as amalgam, metal alloys and ceramics are widely used to refill enamel defects in a clinical setting, they are not ideal therapies since these restorative materials have relatively poor adhesion and weak mechanical strength. A primary goal of modern dentistry is therefore early prevention of tooth decay rather than invasive restorative therapy. In recent years, biomimetic synthesis of enamel-mimicking material has been touted as a highly promising approach in preventive dentistry. To date, a few biomimetic systems containing nano-apatites or different organic additives have been developed for remineralization of enamel (1–4). Despite all these tremendous in vitro efforts, the clinical application of these biomimetic systems for treatment of early carious lesions is not yet conceivable.
Recently, we have developed a new amelogenin-chitosan (CS-AMEL) hydrogel as a promising biomimetic material for in situ enamel regrowth on an acid-etched enamel surface (5). Amelogenin is the major structural protein in developing dental enamel, constituting more than 90% of the extracellular organic matrix. Many in vitro studies have shown that amelogenin plays a key role in controlling the oriented and elongated growth of the apatite crystals (3). Indeed, we recently found that amelogenin assemblies carried in chitosan hydrogel could stabilize Ca-P clusters and arrange them into linear chains, which fused with enamel crystals and then developed into enamel-like co-aligned crystals (5). The organized enamel-like layer formed in the CS-AMEL hydrogel significantly improved the hardness and elastic modulus of etched enamel. Importantly, this biomimetic in situ regrowth of apatite crystals generated a robust enamel-restoration interface, which is important for ensuring the efficacy and durability of restorations. Chitosan which was used as an amelogenin carrier did not affect crystal orientation but has the potential to protect repaired enamel from secondary caries and erosion due to its apparent antimicrobial and pH-responsive properties (5).
In the present study, in order to further optimize the CS-AMEL hydrogel to meet the necessary conditions for clinical applicability (i.e. hydrogel stability, shorter preparation and crystallization times), we investigated the effects of viscosity and supersaturation degree in CS-AMEL hydrogel on apatite crystal growth on an acid-etched enamel surface. The size, orientation and composition of newly-grown crystals were studied by scanning electron microscopy (SEM) and X-ray diffraction (XRD). Our long-term objective is to develop strategies for the application of CS-AMEL hydrogel in reconstructing human tooth enamel.
Materials and methods
Recombinant full-length porcine amelogenin rP172 was expressed and purified as described previously (5,6). Human third molars free of cavities were collected following an Institutional Review Board protocol and acid-etched tooth slices were prepared as described previously (5,6). CS-AMEL hydrogel was prepared by mixing chitosan (1, 2 and 3 % m/v), Ca2+ (2.5, 5.0, 7.5 and 10 mM) and PO4 (1.5, 3.0, 4.5 and 6 mM) solutions with amelogenin rP172 (200 μg/ml), followed by stirring at room temperature overnight, and the pH value was adjusted to 6.5 with 1 M NaOH. The initial molar ratio of Ca2+ to PO4 ions was kept steady at 1.67; for simplicity, we only report the Ca2+ concentration in the text that follows. The relative supersaturation degree with respect to hydroxyapatite σ(HAp) was calculated using previously described methods (7). Twenty microliters of chitosan-based hydrogel were carefully applied onto the enamel surface and dried for 15 min in air at room temperature. This is a shorter time than in our previous protocol (5,6). The tooth slices were then immersed in 30 ml of artificial saliva solution with 1 mg/l of NaF at 37 °C (5). After 7 days, the tooth slices were removed from the solution, rinsed with running deionized water for 50 s and air-dried.
The morphologies of newly grown crystals were observed by SEM, which was performed on a field emission scanning electron microscope (JEOL JSM-7001F), operating at an accelerating voltage of 15 kV. The diameter of crystals in the digital SEM images was measured using Photoshop (n>45) and expressed as mean ± standard deviation. Student’s t-test was applied to identify differences in the sizes of crystals formed in different CS-AMEL hydrogels. The differences were considered statistically significant at p≤0.05 and highly significant at p<0.001. All the statistical analyses were carried out using Origin 8.0 (Origin Lab, Northampton, MA) and Microsoft Office Excel 2007.
The composition and orientation of the newly grown crystals were studied by XRD. XRD patterns were recorded on a Rigaku Diffractometer with Cu Kr radiation (λ = 1.542Å) operating at 70 kV and 50 mA with a step size of 0.02°, at a scanning rate of 0.1° s−1 in a 2θ range from 10° to 60°. To estimate the orientation degree of the apatite crystals (JCPDS 09-0432), the intensity (I) of diffraction peaks at 2θ = 25.8° for (002) and 31.8° for (211) were used in a quantitative evaluation. The (002)-(211) intensity ratios in the XRD patterns ware calculated after fitting the peak profiles in a MDI Jade 6.5.
The viscosity of CS-AMEL hydrogel was measured using a dynamic rheometer (TA Instrument, Delaware company, New Castle, DE, AR2000ex). The settings for viscosity measurement were as follows: parallel plate apparatus = 40 mm, gap size = 0.5 mm, temperature = 37 °C, strain = 1%, frequency sweep range = 0.1–10 Hz. Rheological parameter of storage modulus (G’) was determined and the average storage modulus () was calculated in order to provide a better comparison.
Results
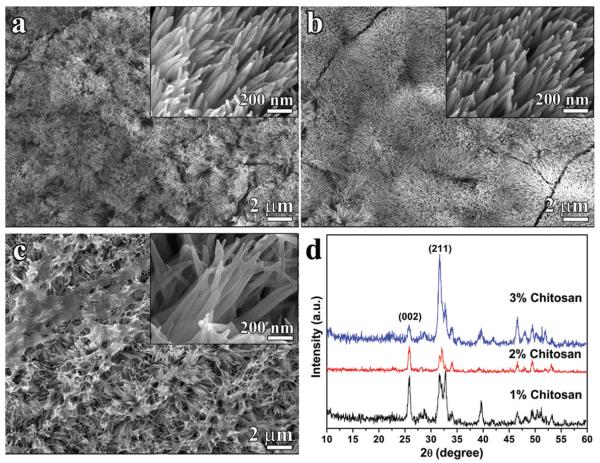
The effect of viscosity on crystal growth was studied in the presence of 1%, 2% and 3% (m/v) chitosan in CS-AMEL hydrogel containing a constant level of [Ca2+]=2.5 mM, and 200 μg/ml amelogenin. With increasing chitosan concentration, the average storage modulus of CS-AMEL hydrogel raised from 9.92 to 227.59 Pa, indicating a dramatic increase in viscosity (Table 1). For all the etched tooth samples, after treatment with the different CS-AMEL hydrogels, a synthetic layer composed of hydroxyapatite crystals with diameters of ~ 50 nm was observed on the enamel surface (Figure 1 and Table 1). The majority of the crystals grown in CS-AMEL hydrogel with 1% chitosan had a needle-like shape and were arranged roughly parallel to each other (Figure 1a). At 2% chitosan, a denser layer composed of more organized crystals was formed on the etched enamel surface (Figure 1b). When the chitosan concentration increased to 3%, the newly grown layer became a loose aggregate of crystals with a porous structure (Figure 1c). SEM observations showed an obvious change in the crystal orientation, which was further confirmed by XRD analyses. The ratio of diffraction intensity of c axis (002) to another direction (211) has been widely used to evaluate the orientation degree of the apatite crystals (2). The increased value of (002)-(211) intensity ratio usually indicates an improved preferential orientation of apatite crystals parallel to the c axis (2). The (002)-(211) intensity ratios of the synthetic crystals formed in different CS-AMEL hydrogels increased from 0.9 to 1.71 and then dropped to 0.17 when the chitosan concentration was increased from 1% to 2% and then 3%, respectively (Table 1). Based on the SEM and XRD results, it can be seen that the orientation of the newly grown crystals was affected by the hydrogel viscosity. The best crystal orientation was achieved in the sample remineralized in CS-AMEL hydrogel with 2% chitosan.
Table 1.
Size and orientation degree (I002:I211) of the newly grown crystals formed in CS-AMEL hydrogel with different chitosan concentrations and viscosities represented by the average storage modulus.
| [Chitosan] (m/v) |
[Ca2+] (mM) |
[PO4] (mM) |
Average storage modulus (Pa) |
σ(HAp) | Crystal Diameter (nm) |
I002 : I211 |
|---|---|---|---|---|---|---|
| 0% [7] | 2.8 | 1.67 | 0 | 9.65 | 54.08±8.24 | – |
| 1% | 2.5 | 1.5 | 9.92 | 8.23 | 51.82±13.84 | 0.90 |
| 2% | 2.5 | 1.5 | 17.55 | 8.23 | 52.77±10.78 | 1.71 |
| 3% | 2.5 | 1.5 | 227.59 | 8.23 | 53.33±14.86 | 0.17 |
Figure 1.
(a–c) SEM images of the newly grown layer after remineralization in CS-AMEL hydrogel with 1% m/v (a), 2% m/v (b) and 3% m/v (c) chitosan. Insets show the crystal morphology at high magnification. (d) XRD patterns of the newly grown layer after remineralization in CS-AMEL hydrogel with different concentrations of chitosan.
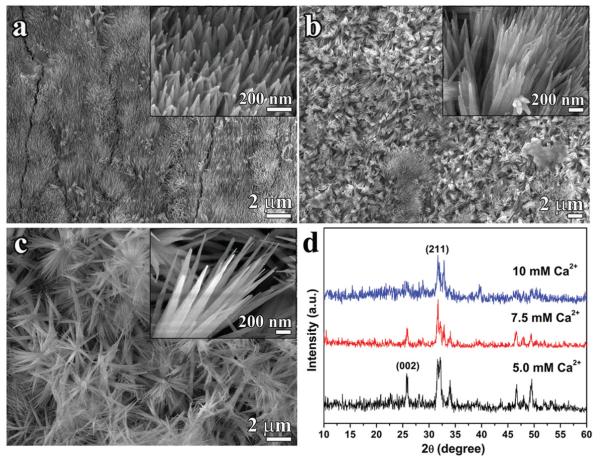
With the optimal chitosan concentration determined to be 2% m/v, with an average storage modulus of 17.55 (Pa) the effects of supersaturation degree on the crystal morphology and organization were assayed for [Ca2+] values ranging from 2.5 mM to 10.0 mM in the CS-AMEL hydrogel with 2% (m/v) of chitosan and 200 μg/ml of amelogenin. The increase in supersaturation degree resulted in an obvious decrease in the crystal length (based on the decreasing intensities of the (002) peaks in the XRD results, Figure 2d) and a significant increase in crystal diameter from 52.77 ± 10.78 nm to 95.03 ± 26.30 nm (Table 2), and also dramatically disrupted the organization of crystals formed in the hydrogel. At low supersaturation (σ(HAp) <10.06, [Ca2+] <5.0 mM), organized apatite crystals with enamel-like structure formed on the etched enamel surface (Figures 1b and 2a). At a σ(HAp) of 11.07 ([Ca2+]=7.5 mM), the newly grown layer was loose despite the appearance of some enamel-like crystal bundles. Increasing the σ(HAp) to 11.76 ([Ca2+] 10.0 mM) resulted in a porous coating with irregularly packed crystals. From the XRD results, the (002)-(211) intensity ratios dropped from 1.71 to 0.45 with increasing σ(HAp) from 8.23 to 11.07, revealing a significant reduction in the crystal orientation degree. There was no detectable (002) peak observed in the XRD pattern of the newly grown layer formed in CS-AMEL hydrogel with σ(HAp) of 11.76 ([Ca2+] 10.0 mM). The SEM and XRD results indicated σ=(HAp) at 10.06 ([Ca2+]=5.0 mM) was the upper limit for preserving the enamel-like oriented crystal structure.
Figure 2.
(a–c) SEM images of the newly grown layer after remineralization in CS-AMEL hydrogel with 5.0 mM (a), 7.5 mM (b) and 10 mM (c) of Ca2+. Insets show the crystal morphology at high magnification. (d) XRD patterns of the newly grown layer remineralized in CS-AMEL hydrogel with different concentrations of Ca2+.
Table 2.
Size and orientation degree (I002:I211) of the newly grown crystals formed in CS-AMEL hydrogel with different supersaturation degree σ(HAp).
| [Chitosan] (m/v) |
Ca2+] (mM) |
[PO4] (mM) |
Average storage modulus (Pa) |
σ (HAp) |
Crystal diameter (nm) |
I002 : I211 |
|---|---|---|---|---|---|---|
| 2% | 2.5 | 1.5 | 17.55 | 8.23 | 52.77±10.78 | 1.71 |
| 2% | 5.0 | 3.0 | 39.72 | 10.06 | 63.26±16.47a | 0.63 |
| 2% | 7.5 | 4.5 | 58.21 | 11.07 | 71.15±14.37b | 0.45 |
| 2% | 10.0 | 6.0 | 74.70 | 11.76 | 95.03±26.30c | – |
p<0.001 compared with size of crystals formed in CS-AMEL hydrogel with 2.5mM Ca2+.
p<0.05 compared with size of crystals formed in CS-AMEL hydrogel with 5.0mM Ca2+.
p<0.001 compared with size of crystals formed in CS-AMEL hydrogel with 10.0mM Ca2+.
Discussion
CS-AMEL hydrogel has been reported as a promising material for superficial enamel repair. During enamel remineralization in CS-AMEL hydrogel, the amelogenin assemblies promote the formation of an organized enamel-like microstructure which is crucial for successful enamel reconstruction (5).
Due to the need to keep the amelogenin on the enamel surface for effective repair, the stability of the hydrogel carrier is a necessary prerequisite for clinical application. Since chitosan is the major component of the CS-AMEL hydrogel, one way to improve stability is to raise the viscosity of the hydrogel by increasing the chitosan concentration. In our previous study, the CS-AMEL hydrogel with low chitosan concentration (1% m/v) needed a relatively long time (2 h) to be stabilized on the enamel surface (5). This highly flexible hydrogel () had difficulty stabilizing on the etched enamel surface in a short time (15 min), leading to the loss of a small amount of amelogenin. For this reason, the new layer grown after 15 min in the CS-AMEL hydrogel with 1% chitosan did not present the best crystal orientation. Increasing chitosan concentration to 2% (m/v) increased its viscosity () and improved the stability of the CS-AMEL hydrogel. During the enamel remineralization experiments in the artificial saliva solution, we observed a transparent layer of CS-AMEL hydrogel covering the enamel surface. As a result, with this concentration of chitosan (2%), the amelogenin assemblies were protected inside the hydrogel and were able to produce a dense layer composed of organized crystals. However, amelogenin residues in the newly formed crystals, which could potentially be improved by removal of the protein material using proteolytic enzymes. When the chitosan concentration increased to 3%, the viscosity of CS-AMEL hydrogel was increased dramatically (), however a relatively loose structure was observed. It has been reported that chitosan is able to form hydrophobic aggregation at a high concentration (8). This association between macromolecular chains appears to have decreased the mobility of the chains towards others and hence sterically hindered the oriented growth of apatite crystals.
The optimal size and orientation of the synthetic nanocrystals will provide a newly formed repaired layer with better mechanical properties (9). Therefore, it is important to study the factors that affect the size and orientation of crystals grown in CS-AMEL matrix. Recent research has shown that the nanocrystal structures were sensitive to the degree of supersaturation and that dense nanorod crystals only formed in the supersaturation degree range from 8 to 12 (7). In a CS-AMEL system, the region of supersaturation degree for forming the oriented crystals is narrower. A supersaturation degree higher than 10.06 ([Ca2+] >5 mM) will result in random crystals with a larger size and irregular structure due to the disruption of the balance between the Ca-P prenucleation cluster and its stabilizer, amelogenin. Unlike the classical nucleation process, the formation of enamel-like apatite crystals in a CS-AMEL matrix proceeds through a continuous growth process that involves the amelogenin-mediated formation, aggregation and transformation of Ca-P prenucleation clusters. It is noteworthy that the regulatory effects of amelogenin on the growth of calcium phosphate crystals are dose-dependent (10). A higher supersaturation degree will thermodynamically accelerate the crystal nucleation, resulting in numerous excessive Ca-P clusters, which cannot be stabilized by the limited amount of protein, leading to the random growth of crystals with a larger diameter and irregular structure. As a result, in our experiment, it was difficult to find an organized enamel-like microstructure when the [Ca2+] was higher than 5 mM.
Conclusion
In a CS-AMEL system, enamel-like organized apatite crystals can only be formed under certain conditions, which are determined by the synergetic effects of hydrogel viscosity and supersaturation degree with respect to apatite. A suitable concentration of chitosan will provide the CS-AMEL hydrogel with optimal stability, ensuring the amelogenin-mediated growth of oriented crystals. On the other hand, a higher degree of supersaturation will thermodynamically promote the formation of random crystals with a larger size and irregular structure. The optimal conditions to produce organized enamel-like crystals in a CS-AMEL hydrogel are: 2% (w/v) chitosan, 2.5 mM calcium, and 1.5 mM phosphate (supersaturation degree = 8.23), with 200 μg/ml of full-length amelogenin.
Acknowledgments
The authors would like to thank the Center for Electron Microscopy and Microanalysis (CEMMA) at USC for electron microscopy.
This research was supported by NIH-NIDCR grants DE-13414 and DE-020099 to J.M.O. We would like to acknowledge the NSF grant 02-024 and Windsong Trust for their support of high school student Nadia Siddiqah.
Footnotes
Declaration of interest The authors report no conflicts of interest.
References
- 1.Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nature Nanotech. 2010;5:565–9. doi: 10.1038/nnano.2010.83. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Mao C, Wang J, Xu X, Pan H, Deng Y, Gu X, Tang R. Bio-inspired enamel repair via glu-directed assembly of apatite nanoparticles: an approach to biomaterials with optimal characteristics. Adv Mater. 2011;23:4695–701. doi: 10.1002/adma.201102773. [DOI] [PubMed] [Google Scholar]
- 3.Moradian-Oldak J. Protein-mediated enamel mineralization. Front Biosci. 2012;17:1996–2023. doi: 10.2741/4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Yang JJ, Li JY, Chen L, Tang B, Chen XY, Wu W, Li JS. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials. 2013;34:5036–47. doi: 10.1016/j.biomaterials.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 5.Ruan QC, Zhang YZ, Yang XD, Nutt S, Moradian-Oldak J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013;9:7289–97. doi: 10.1016/j.actbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q, Moradian-Oldak J. Development of amelogenin-chitosan hydrogel for in vitro enamel regrowth with a dense interface. J Vis Exp. 2014:e51606. doi: 10.3791/51606. In press. doi:10.3791/51606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan YW, Nelson JR, Alvarez JR, Hagan J, Berrier A, Xu XM. Amelogenin-assisted ex vivo remineralization of human enamel: effects of supersaturation degree and fluoride concentration. Acta Biomater. 2011;7:2293–302. doi: 10.1016/j.actbio.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desbrieres J. Viscosity of semiflexible chitosan solutions: influence of concentration, temperature, and role of intermolecular interactions. Biomacromolecules. 2002;3:342–9. doi: 10.1021/bm010151+. [DOI] [PubMed] [Google Scholar]
- 9.Eimar H, Ghadimi E, Marelli B, Vali H, Nazhat SN, Amin WM, Torres J, Ciobanu O, Albuquerque RF, Tamimi F. Regulation of enamel hardness by its crystallographic dimensions. Acta Biomater. 2012;8:3400–10. doi: 10.1016/j.actbio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Iijima M, Moradian-Oldak J. Interactions of amelogenins with octacalcium phosphate crystal faces are dose dependent. Calcif Tissue Int. 2004;74:522–31. doi: 10.1007/s00223-002-0011-3. [DOI] [PubMed] [Google Scholar]