Abstract
Minocycline, a tetracycline-derived compound, mitigates damage caused by ischemia/reperfusion (I/R) injury. Here, 19 tetracycline-derived compounds were screened in comparison to minocycline for their ability to protect hepatocytes against damage from chemical hypoxia and I/R injury. Cultured rat hepatocytes were incubated with 50 μM of each tetracycline-derived compound 20 min prior to exposure to 500 μM iodoacetic acid plus 1 mM KCN (chemical hypoxia). In other experiments, hepatocytes were incubated in anoxic Krebs-Ringer-Hepes buffer (KRH) at pH 6.2 for 4 h prior to reoxygenation at pH 7.4 (simulated I/R). Tetracycline-derived compounds were added 20 min prior to reperfusion. Ca2+ uptake was measured in isolated rat liver mitochondria incubated with Fluo-5N. Cell killing after 120 min of chemical hypoxia measured by propidium iodide (PI) fluorometery was 87%, which decreased to 28% and 42% with minocycline and doxycycline, respectively. After I/R, cell killing at 120 min decreased from 79% with vehicle to 43% and 49% with minocycline and doxycycline. No other tested compound decreased killing. Minocycline and doxycycline also inhibited mitochondrial Ca2+ uptake and suppressed the Ca2+-induced mitochondrial permeability transition (MPT), the penultimate cause of cell death in reperfusion injury. Ru360, a specific inhibitor of the mitochondrial calcium uniporter (MCU), also decreased cell killing after hypoxia and I/R and blocked mitochondrial Ca2+ uptake and the MPT. Other proposed mechanisms, including mitochondrial depolarization and matrix metalloprotease inhibition could not account for cytoprotection. Taken together, these results indicate that minocycline and doxycycline are cytoprotective by way of inhibition of MCU.
Keywords: Calcium, doxycycline, minocycline, mitochondria, MPT, uniporter
INTRODUCTION
Tetracycline antibiotics discovered in 1948 have been used clinically for more than half a century to treat bacterial infections (Dugger 1948; Mainoli and Piccinelli 1955). Compounds in the tetracycline group share a linear fused four-ring nucleus and act antimicrobially by blocking association of aminoacyl-tRNA with bacterial ribosomes to inhibit protein synthesis (Chopra and Roberts 2001). More recently, some tetracyclines were shown to possess therapeutic properties beyond their initial antimicrobial action. Minocycline mitigates damage from reperfusion injury in the spinal cord, kidney, and liver (Kelly et al. 2004; Theruvath et al. 2008a; Wells et al. 2003). Doxycycline has shown similar protective effects for myocardial infarction and cerebral ischemia (Castro et al. 2011; Pires et al. 2011). Chlorotetracycline and demeclocycline appear to inhibit reperfusion injury in neurons through suppression of an intracellular rise in Ca2+ and inhibition of calpains (Jiang et al. 2005).
Reperfusion injury is associated with morbidity and mortality after heart attack, stroke, diabetes, organ transplantation and other clinical situations (Aronowski et al. 1997; King et al. 2000; Mustoe 2004; Yellon and Hausenloy 2007). Reperfusion injury occurs after blood re-flows into an ischemic tissue. The surge of oxygen to oxygen-deprived tissues causes increased production of reactive oxygen species (ROS), leading to onset of the mitochondrial permeability transition (MPT). Opening of MPT pores that nonspecifically conduct low weight molecules up to 1500 Da causes the MPT and leads to mitochondrial depolarization, uncoupling of oxidative phosphorylation, high amplitude mitochondrial swelling and outer membrane rupture (Di Lisa et al. 2003; Di Lisa et al. 2011; Lemasters et al. 2009). Such ruptured mitochondria release proapoptotic factors like cytochrome c into the cytosol that activate caspases and other apoptotic events, culminating in cell death (Lemasters et al. 2002). Apoptosis requires ATP. When the MPT is severe and widespread, profound ATP depletion occurs that inhibits apoptosis and instead causes cell death via necrosis (Kim et al. 2003a). The MPT causes both apoptotic and necrotic cell death and organ destruction during ischemia/reperfusion (I/R) injury of liver, heart, and kidney (Jaeschke and Lemasters 2003; Linkermann et al. 2012; Weiss et al. 2003). Prevention of the MPT inhibits cell death after reperfusion (Halestrap 2009; Kim et al. 2003b; Lemasters et al. 1997). The tetracycline derivative minocycline inhibits the MPT by diminishing mitochondrial Ca2+ uptake, a trigger of the MPT (Theruvath et al. 2008a). The aim of this study was to evaluate cytoprotection by tetracycline derivatives against injury from hypoxia and I/R and to determine whether cytoprotection was mediated by inhibition of the mitochondrial calcium uniporter (MCU) and onset of the MPT.
MATERIALS AND METHODS
Chemicals and reagents
Cyclosporin A (CsA) and Ru360 were purchased from Calbiochem (La Jolla, CA). Fluo-5N was obtained from Life Technologies (Grand Island, NY). Other reagents, including propidium iodide (PI) and tetracycline-derived compounds anhydrochlortetracycline, anhydrotetracycline, α-apo-oxytetracycline, β-apo-oxytetracycline, chlorotetracycline, demeclocycline, doxorubicin, 4-epianhydrotetracycline, 4-epichlorotetracycline, 4-epioxytetracycline, 4-epitetracycline, meclocycline, metacycline, minocycline, oxytetracycline, penimepicycline, rolitetracycline, tetracycline, and tigecycline, were obtained from Sigma-Aldrich (St. Louis, MO) and VWR (Randor, PA).
Isolation of hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats (200–300 g), as described previously (Herman et al. 1988). Rat livers were perfused with 0.8 mg/mL collagenase (Type I; Worthington Biochemical Corporation, Lakewood, NJ, USA) through the portal vein. Hepatocytes were separated from non-parenchymal cells by centrifugation at 50 g for 2 min at 4°C. Viability of isolated hepatocytes was ≥90%, as determined by trypan blue exclusion or by using a Cellometer Vision Cell Profiler (Nexcelom Biosciences, Lawrence, MA). Hepatocytes were resuspended in Waymouth’s medium MB-752/1 containing 27 mM NaHCO3, 2 mM L-glutamine, 5% fetal calf serum, 100 nM insulin and 10 nM dexamethasone at pH 7.4. Hepatocytes were plated in acid-soluble rat tail tendon collagen (20 μg/well)-coated 24-well microtiter plates (Corning Incorporated, Corning, NY) at 150,000 cells/well in 1 ml of medium (Bissell et al. 1987). Hepatocytes were cultured overnight in 5% CO2/air at 37°C.
Chemical hypoxia
After overnight culture, hepatocytes were washed 3 times with air-saturated Krebs-Ringer-Hepes buffer (KRH) containing (in mM): 115 NaCl, 5 KCI, 1 KH2PO4, 1 CaCl2, 1.2 MgSO4, and 25 Na-Hepes buffer, pH 7.4. PI (30 μM) was added to the last wash and the plates were placed in an air incubator at 37°C for 20 minutes. Tetracycline derivatives, CsA, Ru360, MMP2/MMP9 Inhibitor 1, or cis-9-octadeconyl-N-hydroxylamide (OA-Hy) were then added 30–60 min prior to induction of chemical hypoxia. Chemical hypoxia was induced with iodoacetic acid (500 μM, IAA), a glycolytic inhibitor, plus KCN (1 mM), a respiratory inhibitor, to cause rapid and profound depletion of ATP (Gores et al. 1988).
Ischemia/reperfusion injury
To simulate the anoxia and acidosis of ischemia, overnight-cultured rat hepatocytes were incubated in anoxic KRH buffer at pH 6.2 in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI) for 4 h at 37°C. Anoxia in the anaerobic chamber was maintained under an atmosphere of 90% N2-10% H2 in the presence of a heated palladium catalyst to convert residual oxygen to water vapor. Oxygen tension in the chamber was <0.001 Torr. To simulate the reoxygenation and return to physiological pH after reperfusion, anaerobic KRH at pH 6.2 was replaced with aerobic KRH at pH 7.4. In some experiments, hepatocytes were reoxygenated with the same medium at pH 6.2. This model is widely used to study mechanisms of I/R injury in cells isolated from liver and other tissues (Kim et al. 2003c; Qian et al. 1997). For simplicity and directness of expression, we refer to “simulated I/R” simply as I/R. Some hepatocytes were treated 20 min before and then continuously after reperfusion with tetracycline-derived compounds or CsA. In other experiments, hepatocytes were treated with Ru360 (1 μM) 45 minutes before ischemia and then continuously after reperfusion.
Assay for cell death
Cell death was assessed by PI fluorometery using a NovoStar multiwell plate reader (BMG LABTECH GmbH, Offenburg, Germany), as described (Nieminen et al. 1992). Briefly, hepatocytes during all phases of I/R were incubated with 30 μM PI. Fluorescence from each well was measured at excitation and emission wavelengths of 530 nm and 590 nm, respectively. An initial measurement (A) of fluorescence was made 20 min after PI addition with subsequent measurements (X) during the course of I/R or chemical hypoxia. Experiments were terminated by permeabilizing plasma membranes with 200 μM digitonin, and a final fluorescence measurement (B) was collected after 20 more min. The percentage of nonviable cells (D) was calculated as D = 100(X − A)/(B − A). PI fluorometery reflects necrotic cell death and correlates closely with trypan blue uptake (Kim et al. 2003c; Nieminen et al. 1992). To prevent oxygen back diffusion during ischemia, plates were sealed with vacuum tape (3M, Minneapolis, MN) inside the anaerobic chamber.
Assay for mitochondrial depolarization
Hepatocytes during I/R were incubated 500 nM rhodamine 123 (Rh123), an indicator of mitochondrial polarization. Rh123 fluorescence (495-nm excitation, 520-nm emission) was then measured in a multiwell plate reader. Cationic Rh123 accumulates electrophoretically into negatively polarized mitochondria, leading to quenching of fluorescence (Emaus et al. 1986). Thus, a decrease in Rh123 fluorescence measured with a fluorescence plate reader indicated an increase of mitochondrial polarization. Rh123 and PI are respectively red- and green-fluorescing indicators, and their fluorescence could be measured simultaneously without crosstalk.
Isolation of rat liver mitochondria
Rat liver mitochondria were isolated by differential centrifugation in ice cold 0.25 M sucrose, 0.5 mM EGTA, 2 mM K-Hepes buffer, pH 7.4 from fasted male Sprague Dawley rats (250–350 g), as previously described (Lemasters et al. 1984). Mitochondrial protein concentration was determined using a biuret procedure with bovine serum albumin as standard (Gornall et al. 1949). Oxygen consumption was assessed using a Clark electrode in 150 mM sucrose, 5 mM MgCl2, 5 mM succinate, 1 μM rotenone, and 10 mM NaPi buffer, pH 7.4. Only mitochondria with respiratory control ratios greater than 5 after addition of 250 μM ADP were used for experiments.
Ca2+ uptake in isolated mitochondria
To assess Ca2+ uptake, isolated mitochondria were incubated in 1 mM KH2PO4, 200 mM sucrose, 20 mM HEPES, 5 mM succinate, 5mM Mg2+, 1 μM rotenone, 2 μM CsA, and 1 μM Fluo-5N (pH 7.2) with and without 5 – 50 μM tetracycline-derived compounds or 100 nM Ru360. After 3 min, aliquots of 50 μM CaCl2 were added at 5 min intervals for 30 min. Fluorescence of Ca2+-indicating Fluo-5N (excitation 495 nm and emission 520 nm) was determined using a multiwell plate reader.
Plate reader assays of the mitochondrial permeability transition
Mitochondrial swelling was assessed from the decrease of absorbance at 540 nm of 0.5 mg/mL rat liver mitochondria suspended in an MPT swelling medium consisting of 0.2 M sucrose, 20 mM Tris, 20 mM HEPES, 5 mM succinate, 1 mM KH2PO4, 1 μM rotenone, and 1 μg/ml oligomycin at pH 7.2, 25°C using a NovoStar multiwell plate reader, as described (He and Lemasters 2002). After 3 min of incubation with various compounds, 250 μM CaCl2 was added to induce the MPT. Decreased absorbance at 540 nm indicated mitochondrial swelling.
Fe2+ and Ca2+ uptake in isolated mitochondrial
To assess mitochondrial Fe2+ and Ca2+ uptake, Fe(NH4)2(SO4)2 and CaCl2, respectively, were added to air-saturated MPT swelling medium containing 5 mM MgCl2 and 0.5 mg/mL rat liver mitochondria. Compounds, mitochondria, and Fe2+ or Ca2+ were added sequentially at 2 minutes intervals. Fe2+- and Ca2+-stimulated oxygen uptake was measured using a Clark oxygen electrode.
Statistics
Data are presented as means ± SEM. Images shown are representative of three or more experiments. Statistical analysis was performed by the Student’s t-test or analysis of variance using P ≤ 0.05 as the criterion of significance.
RESULTS
Minocycline and doxycycline protect against cell death from chemical hypoxia
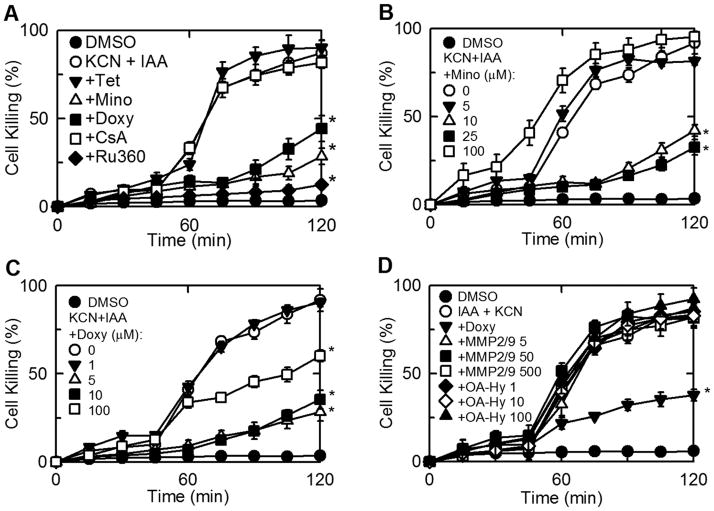
Cultured rat hepatocytes were treated with each of 19 tetracycline-derived compounds (Suppl. Fig. 1) for 20 min and then subjected to chemical hypoxia with KCN plus IAA. After 2 h, loss of cell viability increased to 87% with vehicle (DMSO) pretreatment (Fig. 1A). By contrast after pretreatment with minocycline and doxycycline (50 μM), cell death increased to only 28% and 42%, respectively (Fig. 1A). No other tetracycline-derived compound tested showed protection (Suppl. Fig. 2 and Suppl. Table 1).
Fig. 1. Chemical hypoxia-induced killing of hepatocytes: protection by minocycline, doxycycline and Ru360 but not by other tetracycline derivatives, cyclosporin A, or matrix mettaloprotease inhibitors.
Cultured rat hepatocytes were treated with (A) tetracycline (Tet, 50 μM), minocycline (Mino, 50 μM), doxycycline (Doxy, 50 μM), CsA (2 μM), Ru360 (100 nM), vehicle (DMSO), (B) minocycline (0–100 μM), (C) doxycycline (0–100 μM), or (D) matrix metalloproteases MMP2/MMP9 Inhibitor 1 (5–500 μM) and cis-9-octadecenoyl-N-hydroxylamide (OA-Hy, 1–100 μM) 30–60 min prior to addition of 500 μM iodoacetic acid (IAA) plus 1 mM KCN (chemical hypoxia). Cell killing was determined by PI fluorometery. Filled circles are DMSO-treated cells not exposed to IAA and KCN. Values are means ± SE from 4 or more experiments. *p ≤ 0.05 versus KCN + IAA.
Dose-response experiments were performed for minocycline and doxycycline. Minocycline showed greatest protection at 25 μM, but became toxic at 100 μM, whereas doxycycline showed greatest protection at 5 μM and did not become toxic at concentrations up to 100 μM (Fig. 1B and C). Experiments were also performed with the remaining tetracycline-derived compounds at concentrations of 5 μM and 25 μM to evaluate cytoprotection at lower concentrations. However, none of the other tetracycline-derived compounds decreased cell killing during chemical hypoxia at any concentration examined (Suppl. Table 1).
Minocycline and doxycycline protect hepatocytes against cell death after ischemia/reperfusion
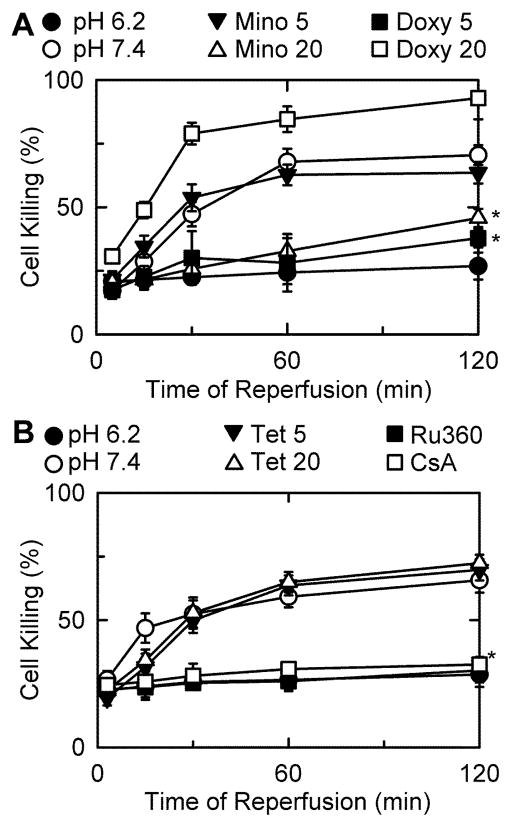
As an additional test for cytoprotection, minocycline, tetracycline, and the 17 other tetracycline-derived compounds were assessed for their ability to decrease cell death from I/R injury to hepatocytes. Overnight-cultured hepatocytes were subjected to simulated ischemia for 4 h followed by reperfusion with normoxic KRH at pH 7.4 in the presence of compound. After vehicle treatment, cell death increased progressively to 79% after 2 h of reperfusion (Fig. 2A). After treatment with 20 μM minocycline and 5 μM doxycycline, cell death was diminished and increased to only 49% and 43%, respectively (Fig. 2A). Tetracycline and all other compounds tested failed to decrease cell death at 5 or 20 μM (Fig. 2B and Suppl. Table 1).
Fig. 2. Protection against ischemia/reperfusion injury by Ru360, minocycline and doxycycline but not tetracycline.
Hepatocytes were subjected to simulated I/R as described in MATERIALS AND METHODS. At 20 min prior to reperfusion, 5 and 20 μM minocycline, doxycycline (A) or tetracycline (B) were added. Ru360 (100 nM) was added 45 min prior to reperfusion. Cell killing was assessed by PI fluorometery. Values are means ± SE from experiments with 3 independent cell isolations. *p ≤ 0.05 versus pH 7.4.
Minocycline and doxycycline inhibit the MPT
Minocycline, but not tetracycline, inhibits the MPT and prevents cell killing after both warm and cold I/R (Theruvath et al. 2008b; Zhang et al. 2010). To test the hypothesis that cytoprotection by tetracycline derivatives was related to inhibition of the MPT, we assessed the panel of tetracycline derivatives for their ability to block the MPT in isolated mitochondria. The MPT was identified by Ca2+-induced swelling measured by decreased absorbance at 540 nm. The MPT inhibitor CsA (Fig. 3A), minocycline (Fig. 3B), and doxycycline (Fig. 3C), but no other tetracycline derivative, blocked Ca2+-induced swelling (Fig. 3A and Suppl. Table 1). Moreover, just as doxycycline was more potent for cytoprotection, doxycycline was also more potent than minocycline at inhibiting the MPT (Fig. 3B and C).
Fig. 3. Inhibition of Ca2+-induced swelling of isolated mitochondria by doxycycline and minocycline but not tetracycline.
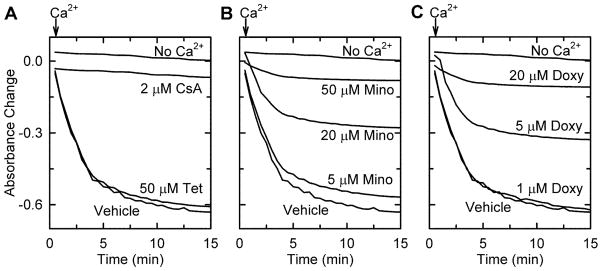
Swelling of rat liver mitochondria was monitored by absorbance after addition CaCl2 (250 μM, arrows), except for the line marked No Ca2+. Three minutes before CaCl2 addition, mitochondria were treated with tetracycline (Tet) or CsA (2 μM) (A), minocycline (Mino) (B), or doxycycline (Doxy) (C) at indicated concentrations.
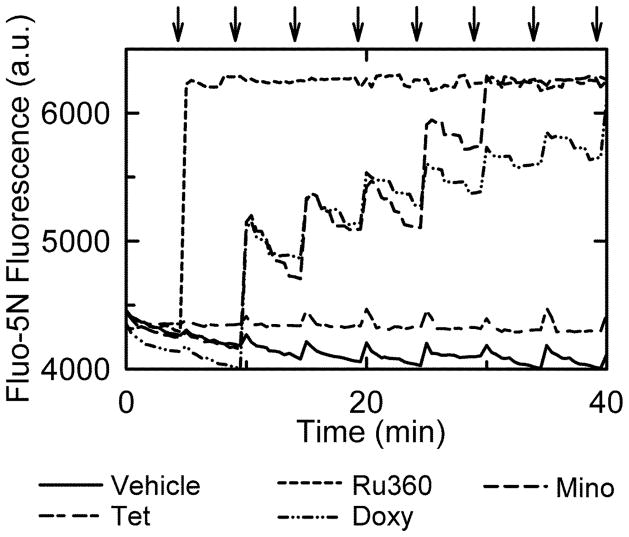
Minocycline and doxycycline block mitochondrial Ca2+ uptake
Previously, minocycline was shown to block MPT onset by inhibition of mitochondrial Ca2+ uptake (Theruvath et al. 2008a). To test whether inhibition of mitochondrial uptake is a unique feature of cytoprotective tetracycline derivatives, doxycycline and the 15 other tetracycline-derived compounds were compared to minocycline, vehicle (DMSO), and Ru360 (100 nM, a high affinity inhibitor of mitochondrial Ca2+ uptake) for their ability to block mitochondrial Ca2+ uptake measured by the extra-mitochondrial Ca2+ indicator Fluo-5N. After each addition of 50 μM CaCl2, Fluo-5N fluorescence rose quickly before decreasing to baseline as mitochondria took up Ca2+ (Fig. 4). Minocycline, doxycycline, and Ru360 inhibited this decrease in Fluo-5N fluorescence, which indicated these compounds were inhibiting mitochondrial Ca2+ uptake. However, minocycline and doxycycline did not inhibit Ca2+ uptake after the first addition of CaCl2 but only after subsequent additions. Ru360, a high affinity MCU inhibitor, showed the greatest inhibition of Ca2+ uptake followed by doxycycline and minocycline. No other tetracycline-derived compound tested inhibited mitochondrial Ca2+ uptake (Fig. 4 and Suppl. Table 1).
Fig. 4. Inhibition of mitochondrial Ca2+ uptake by doxycycline and minocycline but not tetracycline.
Mitochondria were pretreated with tetracycline (18 μM), minocycline (20 μM) doxycycline (10 μM) or Ru360 (100 nM), and extramitochondrial Ca2+ was measured by Fluo-5N fluorescence. CaCl2 (50 μM) was added every 5 minutes (arrows), as described in MATERIALS AND METHODS.
Ru360, a selective inhibitor of the mitochondrial Ca2+ uniporter, protects against chemical hypoxia and I/R
If inhibition of the MCU is the mechanism responsible for cytoprotection by minocycline and doxycycline, the more potent Ca2+ inhibitor Ru360 should also protect against cell killing. Consistent with this expectation, Ru360 was highly cytoprotective after I/R (Fig. 2B). Ru360 was more potent at inhibiting mitochondrial Ca2+ uptake than minocycline or doxycycline and was also more strongly cytoprotective (Fig. 2A). After I/R, protection by CsA confirmed the role of the MPT in reperfusion injury (Fig. 2B). Ru360 also protected against cell death during chemical hypoxia (Fig. 1A). Again cytoprotection was stronger with Ru360 than the less potent MCU inhibitors, minocycline and doxycycline (Fig. 1A). During chemical hypoxia, CsA was not protective (Fig. 1A). Thus, the benefit of MCU inhibition was not always via inhibition of the MPT.
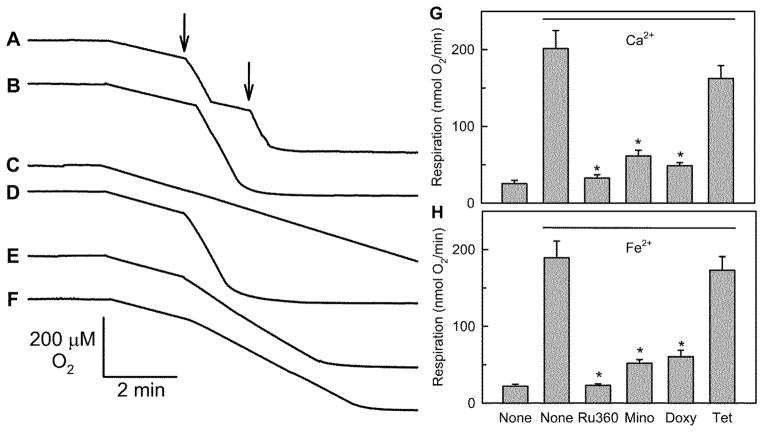
Minocycline, doxycycline and Ru360 inhibit Fe2+-stimulated mitochondrial respiration
MCU also transports Fe2+ (Flatmark and Romslo 1975). Accordingly, the panel of tetracycline derivatives was assessed for the ability to inhibit mitochondrial uptake of Fe2+. Fe2+ as Fe(NH4)2(SO4)2 was added to isolated mitochondria, and respiratory stimulation was measured with a Clark electrode as an indicator of electrogenic ion uptake. After addition of 50 μM Fe2+, mitochondrial oxygen respiration increased 8-fold and then returned to baseline after about 40 sec (Fig. 5A). A second Fe2+ addition stimulated respiration again. The duration of the respiratory stimulation was proportional to the amount of Fe2+ added. Consequently after addition of 250 μM Fe2+, stimulated respiration was sustained until oxygen was exhausted (Fig. 5B).
Fig. 5. Inhibition of mitochondrial iron uptake by doxycycline, minocycline and Ru360 but not tetracycline.
Oxygen consumption by isolated rat liver mitochondria was measured after addition of 50 μM (A, arrows) or 250 μM Fe(NH4)2(SO4)2 (B–F), as described in MATERIALS AND METHODS. Ru360 (100 nM) (C), tetracycline (20 μM) (D), minocycline (20 μM) (E), or doxycycline (10 μM) (F) were added 4 min prior to addition of Fe(NH4)2(SO4)2. Rates of respiration after addition as indicated of 250 μM CaCl2 or Fe(NH4)2(SO4)2 plus various compounds are shown in G and H, respectively. *p ≤ 0.05 versus None with Ca2+ or Fe2+.
Ru360 (100 nM) blocked Fe2+-stimulated respiration completely (Fig. 5C). Minocycline (20 μM) and doxycycline (10 μM) also inhibited Fe2+-stimulated respiration by 82% and 78%, respectively (Fig. 5E and F). Tetracycline and other tetracycline derivatives had no effect (Fig. 5D and Suppl. Table 1) on Fe2+-stimulated respiration. Mitochondrial Ca2+ uptake was also evaluated in a similar manner to Fe2+ uptake using a Clark electrode. Similar to Fe2+, Ru360 (100 nM), minocycline (20 μM), and doxycycline (10 μM) inhibited Ca2+-stimulated respiration by 96%, 79%, and 87%, respectively (Fig. 5G). Remarkably, rates of Ru360-sensitive Fe2+ and Ca2+ uptake as measured by stimulated respiration were very similar (Fig. 5G and H).
Minocycline and doxycycline do not cytoprotect by depolarizing mitochondria
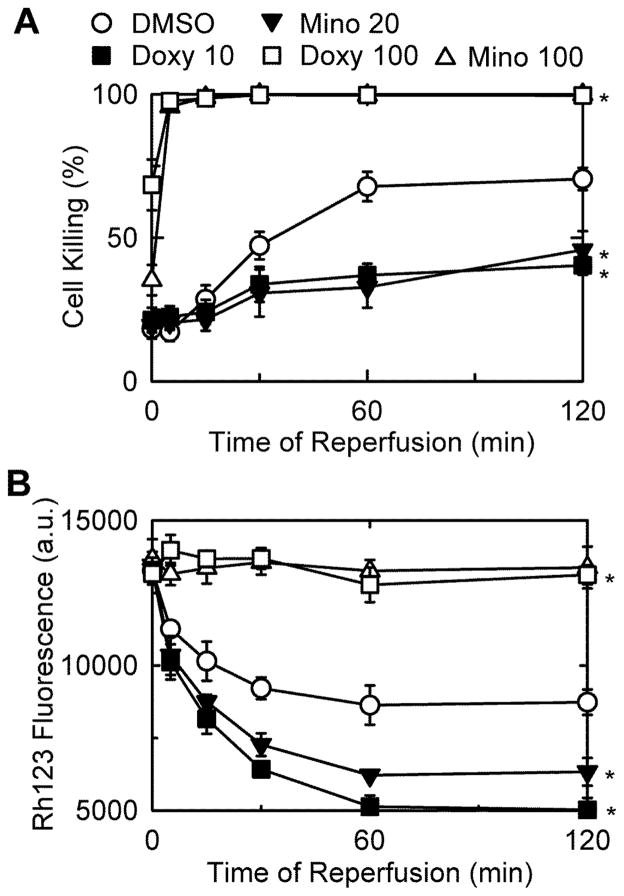
One proposal for the mechanism by which minocycline cytoprotects is that minocycline creates ion channels that depolarize mitochondria leading to less ROS formation, which indirectly prevents onset of the mitochondrial permeability transition (Antonenko et al. 2010). To test this hypothesis, rat hepatocytes were incubated with PI and Rh123, fluorogenic indicators of cell death and mitochondrial polarization, respectively, during I/R to determine if minocycline and doxycycline depolarize mitochondria at cytoprotective concentrations. By PI fluorometry, minocycline and doxycycline inhibited cell death at 20 and 10 μM (Fig. 6A), respectively, but did not prevent mitochondria repolarization after reperfusion, as indicated by Rh123 quenching (Fig. 6B). By contrast, minocycline and doxycycline at 100 μM each blocked mitochondria repolarization during reperfusion, an event associated with cell killing (Fig. 6A and B). Thus, depolarization was associated with enhanced cell killing.
Fig. 6. Cytoprotection by minocycline and doxycycline is not associated with mitochondrial depolarization after I/R.
Primary rat hepatocytes were subjected to simulated I/R, as described in MATERIALS AND METHODS. At 20 min prior to reperfusion, minocycline (20 or 100 μM) or doxycycline (10 or 100 μM) were added. Cell killing (A) and mitochondrial depolarization (B) were assessed by PI and Rh123 fluorometery, respectively. Values are means ± SE from experiments with 3 independent cell isolations. *p ≤ 0.05 versus pH 7.4.
Matrix metalloprotease 2/9 inhibition does not protect
Another proposal is that the mechanism of cytoprotection by tetracycline derivatives is by inhibition of matrix metalloproteases (MMP) 2 and/or 9 (Castro et al. 2011). MMPs are responsible for tissue remodeling, including breakdown of extracellular matrix (Page-McCaw et al. 2007). Accordingly, potent non-tetracycline inhibitors of MMP2 and MMP9 were tested for protection against chemical hypoxia. MMP2/MMP9 Inhibitor 1 and cis-9-octadecenoyl-N-hydroxylamide (OA-Hy) were added 60 minutes prior to induction of chemical hypoxia to rat hepatocytes.In comparison to vehicle treatment, MMP2/MMP9 Inhibitor 1 and OA-Hy did not prevent cell killing, whereas doxycycline serving as a positive control did protect (Fig. 1D).
DISCUSSION
Hypoxia and ischemia/reperfusion injury (I/R) are implicated in the pathophysiology of numerous disease states in organ systems throughout the body. The aim of this study was to determine which of several available tetracycline-derived compounds protect against damage to hepatocytes caused by chemical hypoxia and I/R and to characterize the relationship of cytoprotection to inhibition of MPT onset and MCU activity. Only minocycline and doxycycline protected hepatocytes against chemical hypoxia and I/R injury (Fig. 1 and 2 and Suppl. Table 1). In isolated mitochondria, minocycline and doxycycline inhibited Ca2+ and Fe2+ uptake and the MPT, whereas non-cytoprotective tetracycline derivatives did not (Fig. 3–5 and Suppl. Table 1). Since the MCU blocker, Ru360, also protected against chemical hypoxia and I/R, and since MCU inhibition prevented the Ca2+-induced MPT, the most likely mechanism of minocycline and doxycycline cytoprotection is MCU inhibition. However, during chemical hypoxia, protection by minocycline and doxycycline appeared to be independent of the MPT, since CsA, a blocker of the MPT, protected against I/R injury (Fig. 2B) but not against chemical hypoxia (Fig. 1A). Nonetheless, iron chelators also protect against chemical hypoxia-induced cell death (Kim et al. 2002). Thus, minocycline and doxycycline likely protected during chemical hypoxia by blocking MCU-mediated mitochondrial iron uptake.
Previous work indicates that minocycline forms a complex with Ca2+ (Antonenko et al. 2010). Our results are consistent with complex formation, since minocycline and doxycycline did not inhibit Ca2+ uptake until after the second injection of 50 μM CaCl2 (Fig. 4). By contrast, the MCU inhibitor Ru360 inhibited mitochondrial Ca2+ uptake upon the first injection of CaCl2. This may indicate that a minocycline- and doxycycline-Ca2+ (or Fe2+) complex, not minocycline or doxycycline alone, is the MCU-inhibiting species. In a cellular environment, however, a delay of inhibition of Ca2+ uptake by minocycline and doxycycline may not occur, since much loosely bound Ca2+ (~1 mM) is already present in the intracellular milieu, and a Ca2+ complex would form as soon as minocycline and doxycycline enter the cells.
During chemical hypoxia, minocycline and doxycycline, but not CsA, decreased cell killing (Fig. 1A). Thus, the MPT is not the determining factor for cell death during chemical hypoxia, which indicates that cytoprotection of minocycline and doxycycline is not due to direct MPT inhibition. Necrotic cell death is downstream of ATP depletion (Jeong et al. 2003; Nieminen et al. 1994). During I/R, MPT onset prevents recovery of ATP, whereas during chemical hypoxia ATP generation is directly blocked and ATP depletion occurs independently of the MPT. Protection of minocycline and doxycycline against chemical hypoxia may still be through a similar mechanism as protection against I/R injury, namely by inhibition of MCU. Lysosomes maintain a pH of 4–5 through the action of the proton-pumping V-ATPase. When V-ATPase becomes inhibited, as occurs from ATP depletion during hypoxia/anoxia, lysosomal pH increases, and lysosomes release iron into the cytosol (Uchiyama et al. 2008; Yoshimori et al. 1991; Zhang and Lemasters 2013). Even in the absence of a mitochondrial membrane potential, cytosolic iron which increases to hundreds of micromolar in concentration can equilibrate into mitochondria via the MCU to promote Fenton-type reactions and ROS formation leading cell death (Kon et al. 2010). Future studies will be needed to characterize intracellular iron translocation during chemical hypoxia in relation to cytoprotection by minocycline and doxycycline.
One proposal for cytoprotection is that cytoprotective tetracyclines cause mitochondrial depolarization, which decreases mitochondrial ROS formation and indirectly prevents MPT onset (Antonenko et al. 2010). However at cytoprotective concentrations, minocycline and doxycycline did not prevent mitochondrial repolarization after reperfusion. Rather, depolarization only occurred at higher cytotoxic concentrations of minocycline and doxycycline. Chelation of iron has also been suggested as a mechanism of inhibiting mitochondrial iron uptake and cytoprotection (Chen-Roetling et al. 2009), but we observed inhibition of iron uptake at iron concentrations far in excess of the concentration of minocycline or doxycycline. Thus, MCU inhibition by minocycline and doxycycline was a direct effect rather than an indirect effect due to chelation Fe2+ and/or Ca2+. Indeed, minocycline and doxycycline would have to chelate Fe2+ or Ca2+ at ratios of 12 or more, which is inconsistent with the 1 to 1 binding stoichiometry of tetracycline derivatives with cations (M.Nelson et al. 2002). Moreover, tetracycline also binds divalent metals but does not inhibit MCU and is not cytoprotective. Inhibition of MMPs has also been proposed to be the basis for cytoprotection by minocycline and doxycycline. However, other well characterized MMP inhibitors showed no cytoprotection against chemical hypoxia at concentrations that inhibit MMPs (Fig. 1D) (Ben-Yosef et al. 2005; Ulrich et al. 2005). A previous study demonstrated that chlorotetracycline and demeclocycline, like minocycline, are protective during cerebral ischemia. However, chlorotetracycline and demeclocycline conferred neuroprotection through a unique mechanism compared with minocycline, namely by inhibiting calpain I and II, which minocycline does not inhibit (Jiang et al. 2005). Calpain I and II are well recognized to promote neuronal injury (Huang and Wang 2001), and protection by minocycline and doxycycline but not by chlorotetracycline or demeclocycline may indicate that calpain I/II activation does not play an important role in our models of hepatocellular injury.
In clinical situations where I/R is unavoidable, such as organ preservation for transplantation and hepatic surgery requiring the Pringle maneuver, minocycline and doxycycline could be effective at reducing injury. Although Ru360 also inhibits MCU and protected against cell killing (Fig. 4, 5 and 1D), Ru360 is chemically unstable, making it unsuitable for clinical use. Both minocycline and doxycycline are safe and effective for long term treatment of diseases like acne (Goulden et al. 1996; Valentin et al. 2009). Moreover, toxicity associated with use of minocycline or doxycycline at doses required to prevent I/R injury occurs after months of use rather than the days of use needed in the context of liver preservation and surgery.
Other than the discovery of the mechanism of cytoprotection, which enhances our understanding of mitochondrial ion uptake in hypoxic and I/R injury, the uniqueness of minocycline and doxycycline as tetracycline cytoprotectants in liver is the major relevance of this study. Future studies by computer modeling will be directed to developing a pharmacophore for cytoprotection and MCU inhibition from comparison of the structures of minocycline and doxycycline with those of non-protective tetracyclines. Such a pharmacophore could be used to synthesize more potent tetracycline derivatives for cytoprotection and MCU inhibition.
In conclusion, minocycline and doxycycline were unique among tetracyclines for the ability to protect hepatocytes against chemical hypoxia and I/R injury. Although minocycline and doxycycline can depolarize mitochondria at high concentration, chelate Ca2+ and Fe2+, and inhibit MMP, these effects did not account for cytoprotection. Rather, inhibition of MCU by minocycline and doxycycline best explained cytoprotection. Further studies will be needed to determine if these tetracycline derivatives protect against I/R injury in vivo in clinical settings.
Supplementary Material
Highlights.
Minocycline and doxycycline are cytoprotective
Minocycline and doxycycline are the only cytoprotective tetracyclines
Cytoprotective tetracyclines inhibit MPT and mitochondrial calcium and iron uptake
Cytoprotective tetracyclines protect by inhibiting the MCU
Abbreviations used
- CsA
cyclosporin A
- IAA
iodoacetic acid
- I/R
ischemia/reperfusion
- KRH
Krebs-Hepes-Ringer
- MMP
matrix metalloprotease
- MCU
mitochondrial calcium uniporter
- MPT
mitochondrial permeability transition
- OA-Hy
cis-9-octadeconyl-N-hydroxylamide
- PI
propidium iodide
- Rh123
rhodamine 123
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonenko YN, Rokitskaya TI, Cooper AJ, Krasnikov BF. Minocycline chelates Ca2+, binds to membranes, and depolarizes mitochondria by formation of Ca2+-dependent ion channels. J Bioenerg Biomembr. 2010;42(2):151–163. doi: 10.1007/s10863-010-9271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17(10):1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Yosef Y, Miller A, Shapiro S, Lahat N. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol. 2005;289(5):C1321–C1331. doi: 10.1152/ajpcell.00079.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64(6):551–560. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009;386(2):322–326. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lisa F, Canton M, Menabo R, Dodoni G, Bernardi P. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res Cardiol. 2003;98(4):235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- 9.Di Lisa F, Carpi A, Giorgio V, Bernardi P. The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim Biophys Acta. 2011;1813(7):1316–1322. doi: 10.1016/j.bbamcr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- 11.Flatmark T, Romslo I. Energy-dependent accumulation of iron by isolated rat liver mitochondria. Requirement of reducing equivalents and evidence for a unidirectional flux of Fe(II) across the inner membrane. J Biol Chem. 1975;250(16):6433–6438. [PubMed] [Google Scholar]
- 12.Gores GJ, Nieminen AL, Fleishman KE, Dawson TL, Herman B, Lemasters JJ. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988;255(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- 13.Gornall A, Bardawill C, David M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 14.Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693–695. doi: 10.1111/j.1365-2133.1996.tb06972.x. [DOI] [PubMed] [Google Scholar]
- 15.Halestrap AP. Mitochondria and reperfusion injury of the heart--a holey death but not beyond salvation. J Bioenerg Biomembr. 2009;41(2):113–121. doi: 10.1007/s10863-009-9206-x. [DOI] [PubMed] [Google Scholar]
- 16.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512(1–3):1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 17.Herman B, Nieminen AL, Gores GJ, Lemasters JJ. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7(8):355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125(4):1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 20.Jeong JI, Lee YW, Kim YK. Chemical hypoxia-induced cell death in human glioma cells: role of reactive oxygen species, ATP depletion, mitochondrial damage and Ca2+ Neurochem Res. 2003;28(8):1201–1211. doi: 10.1023/a:1024280429036. [DOI] [PubMed] [Google Scholar]
- 21.Jiang SX, Lertvorachon J, Hou ST, Konishi Y, Webster J, Mealing G, Brunette E, Tauskela J, Preston E. Chlortetracycline and demeclocycline inhibit calpains and protect mouse neurons against glutamate toxicity and cerebral ischemia. J Biol Chem. 2005;280(40):33811–33818. doi: 10.1074/jbc.M503113200. [DOI] [PubMed] [Google Scholar]
- 22.Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2004;287(4):F760–F766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003a;304(3):463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003b;3(6):527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003c;124(2):494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Lee SK, Ha MS, Woo JS, Jung JS. Differential role of reactive oxygen species in chemical hypoxia-induced cell injury in opossum kidney cells and rabbit renal cortical slices. Exp Nephrol. 2002;10(4):275–284. doi: 10.1159/000063702. [DOI] [PubMed] [Google Scholar]
- 27.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69(6):1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 28.Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. Lysosomal Iron Mobilization and Induction of the Mitochondrial Permeability Transition in Acetaminophen-Induced Toxicity to Mouse Hepatocytes. Toxicol Sci. 2010 doi: 10.1093/toxsci/kfq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemasters JJ, Grunwald R, Emaus RK. Thermodynamic limits to the ATP/site stoichiometries of oxidative phosphorylation by rat liver mitochondria. J Biol Chem. 1984;259(5):3058–3063. [PubMed] [Google Scholar]
- 30.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B. The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem. 1997;174(1–2):159–165. [PubMed] [Google Scholar]
- 31.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4(5):769–781. doi: 10.1089/152308602760598918. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787(11):1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linkermann A, De ZF, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27 (9):3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 34.Nelson M, Hillen W, Greenwald RA. Tetracyclines in Biology, Chemistry and Medicine. Birkhauser; 2002. [Google Scholar]
- 35.Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187(5A):65S–70S. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 36.Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol. 1992;115(2):147–155. doi: 10.1016/0041-008x(92)90317-l. [DOI] [PubMed] [Google Scholar]
- 37.Nieminen AL, Saylor AK, Herman B, Lemasters JJ. ATP depletion rather than mitochondrial depolarization mediates hepatocyte killing after metabolic inhibition. Am J Physiol. 1994;267(1 Pt 1):C67–C74. doi: 10.1152/ajpcell.1994.267.1.C67. [DOI] [PubMed] [Google Scholar]
- 38.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301(1):H87–H97. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273(6 Pt 1):C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 41.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008a;47(1):236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008b;47(1):236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48 (5):1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116(2):539–545. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 45.Valentin S, Morales A, Sanchez JL, Rivera A. Safety and efficacy of doxycycline in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2009;2:129–40. 129–140. doi: 10.2147/ccid.s4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93(4):292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 47.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126(Pt 7):1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 48.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266(26):17707–17712. [PubMed] [Google Scholar]
- 50.Zhang X, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic Biol Med. 2013;63C:243–253. 243–253. doi: 10.1016/j.freeradbiomed.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Schwartz J, Ramshesh VK, Pediaditakis P, Holmuhamedov E, Zhong Z, Theruvath TP, Lemasters JJ. Minocycline protects against the mitochondria permeability transition after both warm and cold ischemia-reperfusion. Hepatology. 2010;51:349–350. Abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.