Abstract
The characterization of the T-cell receptor (TCR) repertoire of CD4+ regulatory T cells (TR) have been limited due to the RNA degradation that results following permeabilization and fixation as routinely used for intracellular staining of Foxp3. In the present study the clonal composition of human umbilical cord blood (UCB) and adult peripheral blood mononuclear cells (PBMC) CD4+ TR and non-TR was characterized by a DNA-based multiplex PCR which allowed for the consistent clonotypic characterization of cells that have undergone fixation and permeabilization. To validate this method, CD8+ T cells from two HLA A*0201 individuals were sorted and compared clonotypically based upon their ability either to secrete interferon-γ in response to a CMV pp65 epitope or to bind to the corresponding pMHC I tetramer. In the UCB and PBMCs clonotypes shared between the CD4+CD25+Foxp3+ and CD4+CD25+Foxp3− was observed in all 3 UCB and in one adult PBMCs, suggesting that naïve and memory CD4+ TR can share the same clonotypes as CD4+ non-TR in humans.
Keywords: T cells, human, T cell receptors, repertoire development, regulatory T cells
1. Introduction
In recent years CD4+CD25+ T cells have been recognized as an integral part of the cellular immune system, providing regulatory functions in tumor-specific, autoreactive and alloimmune responses in animal models and in humans (Edinger et al., 2003; Trenado et al., 2003; Curiel et al., 2004; Ehrenstein et al., 2004; Viglietta et al., 2004; Viguier et al., 2004). Expression of the high affinity IL-2 receptor α chain (CD25) has served as a phenotypic surface marker for these CD4+ regulatory T cells (TR) (Sakaguchi et al., 1995). However, in isolation, this discriminator has proven insufficient because CD25 is expressed as a consequence of activation in non-regulatory CD4+ T cells. More recently, the identification of TR cells has been better defined by expression of the forkhead family transcription factor P3 (Foxp3), which is required for the development and function of these cells (Hori et al., 2003; Ramsdell, 2003). Foxp3 identification, either by real time PCR or by flow cytometry using Foxp3-specific monoclonal antibodies (Roncador et al., 2005) has permitted a more accurate segregation of regulatory and non-regulatory T cells within the CD4+CD25+ subset.
Such analyses do not however define the origin and antigen specificity of human TR cells which remains obscure as does the diversity of their T-cell receptor (TCR) repertoire. To examine these issues in more detail in humans, we aimed to assess and compare the clonal composition of CD4+CD25+ TR and non-TR cell populations identified by flow cytometry.
The identification of CD4+CD25+Foxp3+ cells by flow cytometry requires permeabilization and fixation for intracellular staining (ICS) of Foxp3. Under these conditions RNA is extremely vulnerable to degradation; thus, the integrity of the RNA template is compromised and cannot be used reliably for RNA-based molecular analysis. Thus, a genomic DNA-based PCR that allows for the characterization of T-cell clonotypes in non-viable cell populations is a pre-requisite for the study of TR cell clonality.
As the combinatorial diversity of the T-cell receptor β-chain (TCRB) gene is extensive (Arstila et al., 2000), the design of primers to include all possible rearrangements has been challenging. Towards this goal, the European BIOMED-2 collaborative study developed DNA-based multiplex PCR assays for the detection of rearranged TCRB genes in lymphoproliferative disorders (van Dongen et al., 2003); consensus primers including all known functional TCRBV and TCRBJ regions were designed. Based on the experience of the BIOMED-2 collaborative study investigators and others (van Dongen et al., 2003; Du et al., 2006), we designed and optimized a heminested multiplex PCR assay that allows for consistent characterization of TCRB complementarity determining region 3 (CDR3) sequences in cells isolated on the basis of parameters that are incompatible with cellular viability, such as intracellular cytokine production. This approach was then used to study the baseline clonality of naïve and memory TR and non-TR CD4+ T cells in unstimulated adult peripheral blood mononuclear cells (PBMCs) and umbilical cord blood cells (UCBCs).
2. Materials and methods
2.1. Samples
Adult PBMCs were prepared from venous blood by density gradient centrifugation. UCBCs was provided by the New York Blood Center and were enriched for T cells by negative selection with the MIDI-magnetic cell sorting (Miltenyi Biotec, Auburn, CA, USA) protocol provided by the manufacturer. Frozen PBMCs and UCBC were thawed and washed prior to staining. All PBMC donors gave written permission for their blood to be used for research under Institutional Review Board–approved National Heart, Lung, and Blood Institute stem cell allotransplantation protocols.
2.2. Peptide-major histocompatibility complex class I (pMHCI) tetrameric complexes
Tetrameric recombinant pMHCI antigens for the HLA A*0201-restricted CMV pp65-derived epitope (NLVPMVATV; residues 495–503) used in this study were produced as described previously (Hutchinson et al., 2003). Once prepared, tetramers were stored in the dark at 4°C.
2.3. Cell stimulation for CMV responses
HLA A*0201-restricted CD8+ T cells specific for CMV pp65495–503 were identified directly ex vivo with cognate fluorescent pMHCI tetramers (Price et al., 2005) and by antigen-induced interferon-γ (IFN-γ) expression in parallel experiments, then sorted by flow cytometry. Tetramer stains were performed at 37°C for 20 min as described previously (Whelan et al., 1999). Antigenic peptide stimulation was performed with PBMCs as previously described (Pitcher et al., 1999; Betts et al., 2000). Briefly, CMV pp65495–503 peptide was used to stimulate cognate T cells in the presence of costimulatory mAbs (anti-CD28 and anti-CD49d; 1 μg/ml final concentration) and brefeldin A (10 μg/ml; Sigma) overnight at 37°C. A negative control (costimulatory mAbs alone) was included in all experiments to quantify spontaneous production of effector cytokines.
2.4. Immunofluorescence staining
PBMCs were stained with directly conjugated mAbs specific for surface and intracellular markers (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) for 30 min at 4°C. Surface stains were performed prior to, and intracellular stains subsequent to, fixation/permeabilization for 10 min. Cells were washed and resuspended in 1% paraformaldehyde (PFA) in phosphate-buffered saline on completion of the staining procedure except in the case of tetramer-based viable cell sorts. PBMCs stimulated overnight with the pp65495–503 peptide were stained with conjugated mAbs specific for CD3, CD4, CD8 and interferon-γ. CMV-specific CD8+ T cells were identified by surface staining with CMV pp65495–503 tetramer, CD3 and CD8. Unstimulated adult PBMCs and UCBCs were stained with conjugated mAbs specific for CD3, CD4, CD25, and Foxp3 (BioLegend, San Jose, CA). Fluorescein isothiocyanate (FITC), Alexa 488, phycoerythrin (PE), peridinin chlorophyll protein (PerCP), allophycocyanin (APC) and phycoerythrin-Cy5 (PE-Cy5) were used as the fluorophores. FITC, PE and APC were used as fluorophores. BDIS solution was used for the detection of intracellular cytokine and the Biolegend fixation/permeabilization kit was used for the Foxp3 intracellular staining.
2.5 Flow cytometric analysis
Four-parameter flow cytometric analysis was performed using a FACSCalibur flow cytometer (BDIS). At least 100,000 live CD3+ lymphocytes were collected for each experimental condition. The list mode data files were analyzed using FlowJo software (Tree Star, Inc., San Carlos, CA).
2.6. Flow cytometric cell sorting
All sorts were performed on stained cells fixed with 1% PFA using a FACS Aria (BDIS) or a FACSVantage SE Diva (BDIS) with the exception of tetramer sorted cells which were not fixed. Tetramer binding CMV-specific CD8+ T cells were sorted into RNAlater (Ambion, Austin, TX, USA); CD8+ T cells expressing IFN-γ after overnight incubation with the pp65495–503 peptide were sorted into a dry collection tube. Unstimulated adult PBMCs and UCBCs were sorted based upon expression of CD25+ and/or Foxp3+ on CD3+CD4+ gated cells. Three populations were studied in each sample: CD25+Foxp3−; CD25+Foxp3+; and CD25−Foxp3+. A 3-way sort into a dry collection tube was performed and the cell pellets were then frozen at −80°C. Instrument set-up in all cases was performed according to the manufacturer’s instructions. Instrument compensation was performed with antibody capture beads (BD Pharmingen) stained singly with individual antibodies used in the test samples.
2.7 RNA-based clonotypic assay
Clonotypes in the tetramer-sorted populations were identified using a strand-switch–anchored reverse transcriptase polymerase chain reaction (RT-PCR) that amplifies all expressed TCRB gene products without bias (Douek et al., 2002; Price et al., 2004). Briefly, mRNA was extracted from the sorted antigen-specific T cells (Oligotex kit, Qiagen, Valencia, CA, USA). Amplification of TCRB CDR3 sequences was performed by using a modified version of the SMART procedure (switching mechanism at the 5′ end of RNA transcript) to generate cDNA followed by a template-switch anchored RT-PCR with a 3′ C-region primer. The PCR product was gel purified and ligated into the pGEMT Easy vector (Promega, Madison, WI, USA) and used to transform Escherichia coli. Individual colonies were selected, amplified by PCR with M13 primers, and then sequenced to obtain TCRB CDR3 sequences.
2.8. Multiplex DNA-based clonotypic assay
The consensus primers for the multiplex PCR were based on the BIOMED-2 Concerted Action and were designed to cover all functional TCRBV and TCRBJ gene segments and to be compatible with multiplex PCR reactions (van Dongen et al., 2003). In order to minimize primer dimerization and allow for amplification of CDR3 sequences from low cell numbers, we designed an external set of TCRBJ primers and performed a heminested touchdown PCR. These primers were tested with all 23 TCRBV and internal TCRBJ primer set to ensure that primer dimerization or cross-priming did not occur. The final primer set was divided into 3 tubes: tube A contained all 23 TCRBV and 6 TCRBJ primers (1.1 – 1.6); tube B contained all 23 TCRBV and 4 TCRBJ primers (2.1, 2.3, 2.4 and 2.5); and tube C contained all 23 TCRBV and 3 TCRBJ primers (2.2, 2.6 and 2.7). The TCRBV and TCRBJ primers used for each PCR reaction are shown in Table 1. This approach allowed for a significant reduction in primer dimerization and crosspriming, as well as a substantial decrease in nonspecific amplification. Specific bands of 250–300 bp in size that contained TCRB CDR3 sequences were consistently obtained. Sorted T cells were lysed in 100 μg/mL proteinase K (Boehringer, Indianapolis, IN) for 1 hr at 56°C and then 10 min at 95°C. PCR conditions were as follows: 1 × HiFi Buffer, 3mM MgSO4, 200 μM dNTPs, 0.056 U platinum Taq Hi-Fi DNA polymerase (Invitrogen, Carlsbad, CA) and 10 pmol of each primer in a final volume of 50 μL. Touchdown PCR was run with the following cycling conditions: 95°C for 30 sec, 68°C for 30 sec for 2 cycles; 95°C for 30 sec, 65°C for 30 sec and 68°C for 30 sec for 3 cycles; 95°C for 30 sec, 60°C for 30 sec and 68°C for 30 sec for 30 cycles. All samples were preactivated for 5 min at 95°C. The product of the first PCR was purified (Qiagen, Valencia, CA) and used as a template in the second PCR. The final PCR product was purified, ligated and transformed as detailed above for the RNA-based clonotype assay. PCR conditions and cycling parameters were identical for both PCR reactions. For clonotype classification the International Immunogenetics Information System (IMGT) nomenclature is used (Lefranc, 2004).
Table 1.
TCRBV and TCRBJ primers used in multiplex PCR
| Primer name | Sequence | Primer name | Internal TCRBJ primers | External TCRBJ primers |
|---|---|---|---|---|
| BV2 | ATACTTCTATTGGTACAGACAAATCT | BJ 1.1 | CTTACCTACAACTGTGAGTCTGGTG | AAAAATGTCTTACCTACAACTG |
| BV3 | CGCTATGTATTGGTATAAACAG | BJ 1.2 | CTTACCTACAACGGTTAACCTGGTC | CCCAGCCTTACCTACAAC |
| BV4 | CGCTATGTATTGGTACAAGCA | BJ 1.3 | CTCACCTACAACAGTGAGCCAACTT | TGACTTACTCACCTACAAC |
| BV5 | CAGTGTGTCCTGGTACCAACAG | BJ 1.4 | CATACCCAAGACAGAGAGCTGGGTTC | TCTTTTACATACCCAAGAC |
| BV6a | ATACATGTACTGGTATCGACAAGAC | BJ 1.5 | CTTACCTAGGATGGAGAGTCGAGTC | TTCTGCAACTTACCTAGGAT |
| BV6b | GGCCATGTACTGGTATAGACAAG | BJ 1.6 | CATACCTGTCACAGTGAGCCTG | GAGCCCCCATACCTGTCAC |
| BV6c | GTATATGTCCTGGTATCGACAAGA | BJ 2.1 | CCTTCTTACCTAGCACGGTGA | CCTGGAGCCCCCTTCTTAC |
| BV7a | AACCCTTTATTGGTACCGACA | BJ 2.2 | CTTACCCAGTACGGTCAGCCT | CCGCCTCCTTACCCAGTAC |
| BV7b | AACCCTTTATTGGTATCAACAG | BJ 2.3 | CCCGCTTACCGAGCACTGTCA | AGCCCCCGCTTACCGAGCAC |
| BV7c | ATCCCTTTTTTGGTACCAACAG | BJ 2.4 | CCAGCTTACCCAGCACTGAGA | GCCCCAGCTTACCCAGCAC |
| BV11 | TACCCTTTACTGGTACCGGCAG | BJ 2.5 | CGCGCTCACCGAGCAC | AGCCCGCGCTCACCGAGCAC |
| BV12a | CTCCCGTTTTCTGGTACAGACAGAC | BJ 2.6 | CTCACCCAGCACGGTCAGCCT | GCGAAAACTCACCCAGCAC |
| BV12b | CACGGTCTACTGGTACCAGCA | BJ 2.7 | CTCACCTGTGACCGTGAGCCTG | GCCCGAATCTCACCTGTGAC |
| BV14 | TAACCTTTATTGGTATCGACGTGT | |||
| BV15 | CGTCATGTACTGGTACCAGCA | |||
| BV18 | TCATGTTTACTGGTATCGGCAG | |||
| BV19 | GGCCATGTACTGGTACCGACA | |||
| BV20 | AACTATGTTTTGGTATCGTCA | |||
| BV21 | TTATGTTTACTGGTATCGTAAGAAGC | |||
| BV23 | TTATGTTTATTGGTATCAACAGAATCA | |||
| BV25 | CAAAATGTACTGGTATCAACAA | |||
| BV29 | CACGATGTTCTGGTACCGTCAGCA | |||
| BV30 | CAACCTATACTGGTACCGACA |
3. Results
3.1. Validation of the multiplex PCR
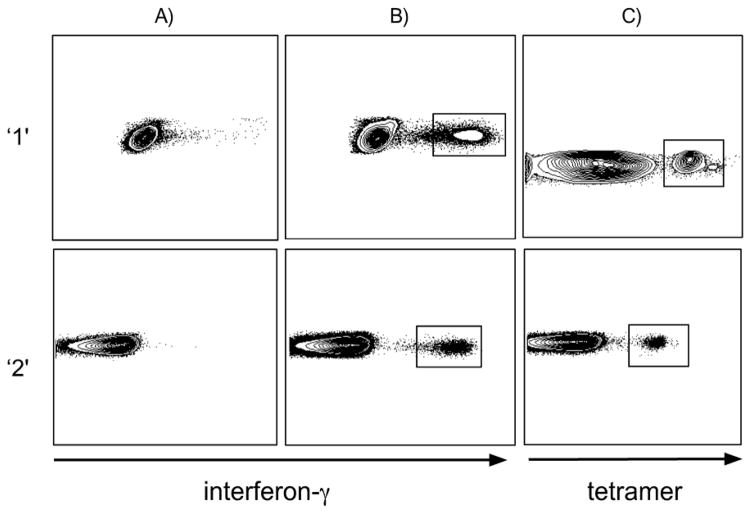
The development of a reliable multiplex PCR for TCRB gene rearrangements would enable clonotypic analysis of T cell populations where the integrity of the RNA is compromised such as in cells subjected to intracellular staining protocols or when amplicon size is small as in archived formalin-fixed tissue. Towards this goal, we developed a heminested multiplex DNA-based PCR as described in Materials and Methods. To validate this assay, we examined the functional antigen-specific CD8+ T-cell repertoire recruited in two CMV seropositive HLA A*0201+ individuals after stimulation with the CMV pp65495–503 peptide and flow cytometric sorting on the basis of IFN-γ expression using the multiplex PCR. In tandem, we sorted viable CD8+ T cells of the same specificity identified physically with the corresponding pMHCI tetramer and examined clonality according to the well established RNA-based strand-switch RT-PCR protocol (Figure 1). In our experience, CD8+ T cell responses to this pMHCI antigen are generally oligoclonal (Price et al., 2005) and this system thus provides a rigorous test of assay specificity. Within each individual, the clonotypes identified in the CD8+ T cell populations specific for CMV pp65495–503 using these two distinct approaches were highly comparable (Table 2). In separate experiments, unstimulated PBMCs were analyzed using the multiplex PCR and a polyclonal repertoire was observed as expected (data not shown). Furthermore, the TCRBJ genes identified in tubes A, B and C corresponded only to the respective TCRBJ primers added to each PCR reaction; thus, only TCRBJ 1.1 – 1.6 were present in the PCR product from tube A, only TCRBJ 2.1, 2.3, 2.4 and 2.5 were present in tube B, and only TCRBJ 2.2, 2.6 and 2.7 were present in tube C.
Figure 1.
CD8+ T cell populations specific for the HLA A*0201-restricted CMV pp65495–503 antigen. A) Negative control (no peptide + anti-CD28/CD49d; 1 μg/ml final). B) Specific CD8+ T cell response following overnight stimulation with the CMV pp65495–503 peptide. C) Specific CMV pp65495–503/HLA A*0201 tetramer binding CD8+ T cells. All plots are gated on CD3+CD8+ cells and the sorted populations are indicated. ‘1’ and ‘2’ refer to separate individuals seropositive for CMV.
Table 2.
Comparison of CD8+ T cell clonotypes specific for CMV pp65495–503 obtained by mRNA-based PCR and DNA-based multiplex PCR
| ID | mRNA clonotyping | Multiplex PCR | ||||
|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | |
| 1 | 6.5 | CASSYSTGTPGIYT* | 1.2 | 6.5 | CASSYSTGTPGIYT | 1.2 |
| 27 | CASTPAGGAPGELF** | 2.2 | 27 | CASTPAGGAPGELF | 2.2 | |
|
| ||||||
| 2 | 6.5 | CASSPQTGAGRYGYT* | 1.2 | 6.5 | CASSPQTGAGRYGYT | 1.2 |
| 6.5 | CASSVQTGTGNYGYT** | 1.2 | 6.5 | CASSVQTGTGNYGYT | 1.2 | |
| 5.4 | CASRVGGDTEAF | 1.1 | 24.1 | CATSPGLAGAYQETQY | 2.5 | |
| 4.2 | CASSQESGNTEAF | 1.1 | 29.1 | CSVPGIGNIYNEQF | 2.1 | |
BV, variable region of the T-cell receptor β-chain; BJ, J-region of the T-cell receptor β-chain; CDR3, complementarity determining region 3. ID 1 and 2 represent two separate CMV-seropositive HLA A*0201 individuals.
dominant clonotype
sub-dominant clonotype
3.2. Repertoire of Foxp3 CD4+ T cells in adult PBMCs and UCBCs
Three separate sets of unstimulated adult PBMCs and three UCBCs were analyzed for Foxp3 and/or CD25+ expression in CD4+ T cells by flow cytometry (Figure 2). At least 1,000 cells were sorted from each of the following CD4+ T cell populations: CD25+Foxp3−, CD25+Foxp3+ and CD25−Foxp3+. A 250–300 bp band was obtained for all sorted populations following the heminested PCR, as illustrated in Figure 3. Repertoire analysis of all 3 sorted populations both in the UCBCs and adult PBMCs demonstrated substantial diversity and polyclonality. Molecular clonotyping data from each of the sorted populations from UCBCs are shown on tables 3–5 and of PBMC are shown on Tables 6–8. Although all the populations analyzed were polyclonal, the same clonotype was identified at least once in the CD25+Foxp3− and CD25+Foxp3+ sorted cells from all 3 UCB samples and in one of the 3 adult PBMC samples (Table 9).
Figure 2.

Umbilical cord blood cells (left) and adult PBMCs (right) are shown gated on CD3+CD4+ T cells. CD25+Foxp3− (upper left gate), CD25+Foxp3+ (upper right gate) and CD25−Foxp3+ (lower right gate) CD3+CD4+ cells were sorted into a dry pellet and frozen at −80°C prior to multiplex PCR.
Figure 3.

A 250–300 bp band is obtained in tubes A, B and C (right 3 lanes) following heminested multiplex PCR of CD4+CD25+Foxp3+ sorted cells. The 3 lanes on the left are the negative controls for tubes A, B and C, respectively.
Table 3.
T-cell receptor repertoire of UCB #1
| CD4+CD25+FOXP3− | CD4+CD25+FOXP3+ | CD4+CD25−FOXP3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 5.4 | CASSLDSPNEQY | 2.7 | 5.4 | CASSLDRASNTQY | 2.3 | 5.4 | CASSFATNSQEAQY | 2.5 |
| 5.4 | CASSLGEGYYGYT | 1.2 | 5.4 | CASSWGTNTGELF | 2.2 | 5.4 | CASSFGAHYTQN | 2.5 |
| 5.4 | CASSLGISEETQY | 2.5 | 5.5 | CASEGTVRSPLH | 1.6 | 5.4 | CASSFGAQETQY | 2.5 |
| 5.4 | CASSLGSSNTGELF | 2.2 | 5.5 | CASSLEITGKGSPLH | 1.6 | 5.4 | CASSLGQSPTGELF | 2.2 |
| 5.4 | CASSLMGLAGAAQY | 2.3 | 5.6 | CASRSGPLRRGLYEQY | 2.7 | 5.4 | CASSSAGLEEETQY | 2.5 |
| 5.4 | CASSLTPTENSPLH | 1.6 | 5.6 | CASSHGGTGGYT | 1.2 | 5.6 | CASRRQEYQETQY | 2.5 |
| 5.4 | CASSLVPETQY | 2.5 | 5.6 | CASSLEAGGTLGTDTQY | 2.3 | 5.6 | CASSLRGTLTDTQY | 2.3 |
| 5.4 | CASSPDRGEGYGYT | 1.2 | 5.6 | CASSLEITGKGSPLH | 1.6 | 5.6 | CASSPSPASSYNEQF | 2.1 |
| 5.5 | CASEGTVRSPLH | 1.6 | 5.6 | CASSLWGKGYT | 1.2 | 6.1 | CASKETGRNEQY | 2.7 |
| 5.5 | CASSEAGQRTETQY | 2.5 | 6.1 | CASRGLTDSRRSYNSPLH | 1.6 | 6.1 | CASRVNYEQY | 2.7 |
| 5.5 | CASSLDSPNEQY | 2.7 | 6.1 | CASRGTSAYNEQF | 2.1 | 6.1 | CASSAGWGSSYNSPLH | 1.6 |
| 5.5 | CASSLEITGKGSPLH | 1.6 | 6.1 | CASSASWGGDTQY | 2.3 | 6.1 | CASSGAPGQGQPQH | 1.5 |
| 5.5 | CASSLKGTGGGYGYT | 1.2 | 6.2 | CASRALYGYT | 1.2 | 6.1 | CASSPRGTSGITDTQY | 2.3 |
| 5.5 | CASSQQGAGELF | 2.2 | 6.2 | CASSLGWGGTNYGYT | 1.2 | 6.1 | CASSYSTNGSSSDEQY | 2.7 |
| 5.5 | CASSSPARDRRLSPLH | 1.6 | 6.2 | CASSSIIEQF | 2.1 | 6.2 | CASGSSLNTEAF | 1.1 |
| 5.6 | CASSFDRGFTDTHY | 2.3 | 6.5 | CASNDLTAKYEQY | 2.7 | 6.2 | CASSEAGNGYT | 1.2 |
| 5.6 | CASSLGGTANSPLH | 1.6 | 6.5 | CASSERGASTDTHY | 2.3 | 6.2 | CASSPGQGVINEQY | 2.7 |
| 5.6 | CASSLRTGNNEQF | 2.1 | 6.5 | CASSFPIRAGGRLTYEQY | 2.7 | 6.2 | CASSPGRTETQY | 2.5 |
| 5.6 | CASSLRTGNNEQF | 2.1 | 6.5 | CASSLDRVVTGELF | 2.2 | 6.2 | CASSYSARGVQY | 2.3 |
| 5.6 | CASSRDRGREQY | 2.7 | 6.5 | CASSYSFSRVETQY | 2.3 | 6.2 | CASSYSTSGSSSDEQY | 2.7 |
| 5.6 | CASSRRDSSGNTIY | 1.3 | 6.5 | CASSYSFTYWGSYEQY | 2.7 | 6.4 | CASSATGTGRIQY | 2.4 |
| 5.6 | CASSSPSGGTDTQY | 2.3 | 6.5 | CASTRQGHGYT | 1.2 | 6.5 | CASSRHHGGGAGELF | 2.2 |
| 5.6 | CASSYRGLDGYTF | 1.2 | 6.9 | CASSYSFTYWGSYEQY | 2.7 | 6.6 | CASRVNYEQY | 2.7 |
| 5.8 | CASKRSVGRNSPLH | 1.6 | 11 | CASRASWGEQF | 2.1 | 6.6 | CASRVNYEQY | 2.7 |
| 6.1 | CASSFSSGTDEQY | 2.7 | 11 | CASSLDGDRGTDTQY | 2.3 | 6.6 | CASSAGANVLT | 2.6 |
| 6.2 | CAGSYGYGGQNIQY | 2.4 | 11 | CASSQNIRGLAGGISDTQY | 2.3 | 6.6 | CASSPGQGVINEQY | 2.7 |
| 6.2 | CASSYSPFSGNYEQY | 2.7 | 12 | CASRPGGGYEQY | 2.7 | 6.6 | CASSRGWGAF | 1.1 |
| 6.2 | CASSYSRFSTYSYEQY | 2.7 | 12 | CASRSGGVNYGYT | 1.2 | 11 | CASSFSRDYEQY | 2.7 |
| 6.4 | CASSAPDGTEAF | 1.1 | 12 | CASSFLAGSNTGELF | 2.2 | 14 | CASSQTGNSPLH | 1.6 |
| 6.5 | CASRTIDLETQY | 2.5 | 12 | CASSLDRVVTGELF | 2.2 | 18 | CASSKTLGGAENSPLH | 1.6 |
| 6.5 | CASSAIRTGGGYGYT | 1.2 | 19 | CASGQGGYNSPLH | 1.6 | 19 | CASSPTHTDTQY | 2.3 |
| 6.5 | CASSEAGQRTETQY | 2.5 | 19 | CASSGTWLQPQH | 1.5 | 19 | CASSTQGLYNEQF | 2.1 |
| 6.5 | CASSFRLRTATDTQY | 2.3 | 19 | CASSLDYGQWETQY | 2.5 | 19 | CASTKRGVPYNEQF | 2.1 |
| 6.5 | CASSPRGLAGHTGELF | 2.2 | 19 | CASSRFEGQETQY | 2.3 | 19 | CASTPGRGDPNYGYT | 1.1 |
| 6.5 | CASSYSISRVGEQY | 2.7 | 19 | CASSRTGQGDSPLH | 1.6 | 19 | CATSDHRASDTGELF | 2.2 |
| 6.5 | CASSYSISRVGEQY | 2.7 | 19 | CVSSGTWLQPQH | 1.5 | 24 | CATSDHRASDTGELF | 2.2 |
| 6.5 | CASSYSSGFRSSYNSPLH | 1.6 | 27 | CASSFSLIYNSPLH | 1.6 | 27 | CASSIQGRNQPQH | 1.5 |
| 7.2 | CASSLRVGPYEQY | 2.7 | 27 | CASSSQGAGANVLT | 2.6 | 27 | CASSSRGEQY | 2.7 |
| 13 | CASSLDGRGSREQY | 2.7 | 29 | CSLGRAGGFTDTQY | 2.3 | 29 | CSVARGNTEAF | 1.1 |
| 19 | CASEPGQGNSPLH | 1.6 | 29 | CSVSQTGGYGYT | 1.2 | 30 | CAWSVGLWDSPLH | 1.6 |
| 19 | CASRTRQGQETQY | 2.5 | 30 | CAWSGGQGPQETQY | 2.5 | |||
| 19 | CASSITPQMDQYF | 2.5 | ||||||
| 19 | CASSPPGPSTDTQY | 2.3 | ||||||
| 19 | CASTSRPGRPDTQY | 2.3 | ||||||
| 24 | CATSDGAGNTYNEQF | 2.1 | ||||||
| 27 | CASQLDYSNTGELF | 2.2 | ||||||
| 27 | CASSGLTYNSPLH | 1.6 | ||||||
| 29 | CSDGQLNTEAF | 1.1 | ||||||
| 29 | CSTGPRGEGRTDTQY | 2.3 | ||||||
| 29 | CSVAVAGKLRGDTQY | 2.3 | ||||||
| 29 | CSVEFLGWGGETQY | 2.5 | ||||||
| 30 | CAWSGGLGETQY | 2.5 | ||||||
Bolded clonotypes are present on CD4+CD25+FOXP3− and CD4+CD25+FOXP3+ populations
Table 5.
T-cell receptor repertoire of UCB #3
| CD4+CD25+FOXP3− | CD4+CD25+FOXP3+ | CD4+CD25−FOXP3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 4.1 | CASSQWAVSSGSYEQY | 2.3 | 5.4 | CASSLSRDRGDEQF | 2.1 | 5.3 | CARSLGEQY | 2.7 |
| 5.4 | CASSLDSYGYT | 1.2 | 5.4 | CASSHLGETQY | 2.5 | 5.3 | CARSLTGGKQY | 2.7 |
| 5.4 | CASSPGHLYNSPLH | 1.6 | 5.4 | CASSLTSGGEETQY | 2.5 | 5.4 | CASSLGGGYT | 1.2 |
| 5.4 | CASSLIWGTDTQY | 2.3 | 5.4 | CASSGYRGINYEQY | 2.7 | 5.4 | CASSLGTDSPLH | 1.6 |
| 5.4 | CASSLTSSYEQY | 2.3 | 5.4 | CASSLRPAGHYEQY | 2.7 | 5.4 | CASSPTEYNEQF | 2.1 |
| 5.4 | CASSHLGETQY | 2.5 | 5.4 | CASSLSGSSYEQY | 2.7 | 5.4 | CASSLATGKNIQY | 2.4 |
| 5.4 | CASSLDRAETQY | 2.5 | 5.4 | CASSLTGGSYEQY | 2.7 | 5.4 | CASSLGRDWETQY | 2.5 |
| 5.4 | CASSLGGSYEQY | 2.7 | 5.4 | CASSYRGYEQY | 2.7 | 5.4 | CASSLVGAGETQY | 2.5 |
| 5.4 | CASSLGTSSGEQY | 2.7 | 5.5 | CASSLQGNTEAF | 1.1 | 5.4 | CASSLGEQY | 2.7 |
| 5.4 | CASSLTSSYEQY | 2.7 | 5.6 | CASSELAGSYNEQF | 2.1 | 5.4 | CASSPRTGGYEQH | 2.7 |
| 5.5 | CASSLLPVETQY | 2.5 | 5.6 | CASSPWAGAGGRRSYNEQF | 2.1 | 5.5 | CASSWLFAGPQETQY | 2.5 |
| 5.6 | CASSLGTSSGEQY | 2.3 | 5.6 | CASSLGSETQY | 2.5 | 5.5 | CASSPPSGGAETQY | 2.7 |
| 5.6 | CASSLWSGSRVSDTQY | 2.3 | 5.6 | CASSFLQYEQY | 2.7 | 6.2 | CASTDNRTPNSPLH | 1.6 |
| 5.6 | CASSLLPVETQY | 2.5 | 6.1 | CAGRPRVGETQY | 2.5 | 6.4 | CASTRLAGGYNEQF | 2.1 |
| 6.5 | CASSELDYEQY | 2.7 | 6.2 | CATSTSGNEQF | 2.1 | 6.5 | CASSQGYGYT | 1.2 |
| 10.2 | CASKTILYNEQF | 2.1 | 6.2 | CASSPDRGQWAGGTQY | 2.3 | 6.5 | CASSRDTNYGYT | 1.2 |
| 11.2 | CASRLAGVRDNEQF | 2.1 | 6.2 | CASSYSTTDINTDAQY | 2.3 | 6.5 | CASSYSSRDNYGYT | 1.2 |
| 12.5 | CASGLVLQGKVFGYT | 1.2 | 6.2 | CASSYEYRGGYEQY | 2.7 | 6.5 | CASRQGMQPQH | 1.5 |
| 19 | CASTEQGAIWNSPLH | 1.6 | 6.2 | CASSYSTGVRYEQY | 2.7 | 6.5 | CASSLNLLAITYNEQF | 2.1 |
| 24.1 | CATKPGQGANSPLH | 1.6 | 6.5 | CASSYGGLYGYT | 1.2 | 6.5 | CASVRDNYNEQF | 2.1 |
| 27 | CASSFSSYNSPLH | 1.6 | 6.5 | CASIEQTVGGLNQPQH | 1.5 | 6.5 | CASRDAVPDTQY | 2.3 |
| 27 | CASSLYNSPLH | 1.6 | 6.9 | CASSYQPRHLAKNIQY | 2.4 | 6.5 | CASSYLAGASEQY | 2.7 |
| 29.1 | CSVGGNTEAF | 1.1 | 11.2 | CASSLGTGGEQY | 2.7 | 6.5 | CASSYQGQRSYEQY | 2.7 |
| 29.1 | CSVVVRQNRTALYGYT | 1.2 | 12.3 | CASSLERQGARYEQY | 2.7 | 6.5 | CASSYRGRRSYEQY | 2.7 |
| 29.1 | CSVEEGTGGYEQY | 2.7 | 19 | CASSILQDNSPLH | 1.6 | 6.5 | CASSYSLKLTSAYEQY | 2.7 |
| 19 | CASSIRYNSPLH | 1.6 | 19 | CASSIGSKETQY | 2.5 | |||
| 19 | CASSRDRGSPLH | 1.6 | 29.1 | CSARPYATDTQY | 2.3 | |||
| 19 | CASSRTGGADTQY | 2.3 | 29.1 | CSVESPGGYEQY | 2.7 | |||
| 19 | CASSIDRTGGAETQY | 2.5 | 29.1 | CSVQGQLYEQY | 2.7 | |||
| 19 | CASSIGKTQY | 2.5 | ||||||
| 19 | CASSMDRGTYEQY | 2.7 | ||||||
| 27 | CASSLSGQGYNSPLH | 1.6 | ||||||
| 27 | CASSGGGRRGDTQY | 2.3 | ||||||
| 29.1 | CSVRGTTRPNYGYT | 1.2 | ||||||
| 29.1 | CSVDFTGEVTDTQY | 2.3 | ||||||
| 29.1 | CSVEGLSGIGTDTQY | 2.3 | ||||||
| 29.1 | CSGTGITYEQY | 2.7 | ||||||
| 29.1 | CSVSTGGYEQY | 2.7 | ||||||
Bolded clonotype is present on CD4+CD25+FOXP3− and CD4+CD25+FOXP3+ populations
Table 6.
T-cell repertoire of PBMCs #1
| CD4+CD25+Foxp3− | CD4+CD25+Foxp3+ | CD4+CD25−Foxp3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 5.3 | CARRPGQGSYEQY | 2.7 | 5.4 | CASSLGGVGTEAF | 1.1 | 5.4 | CASSANLRDRGNTGELF | 2.2 |
| 5.4 | CASSATISNEQF | 2.1 | 5.4 | CASSPTGTGGYGDTEAF | 1.1 | 5.4 | CASSFIEDTGELF | 2.2 |
| 5.4 | CASSFPRGVNTGELF | 2.2 | 5.4 | CASSVRLAGGTTDTQY | 2.3 | 5.4 | CAAGTSQETQY | 2.3 |
| 5.5 | CASSTGYEQY | 2.7 | 5.4 | CASSWTTWSDYGYT | 1.2 | 5.4 | CASSLGTGANVLT | 2.6 |
| 5.6 | CASSLGGRPDTQY | 2.3 | 5.5 | CASSLYSFYEQY | 2.7 | 5.4 | CASSLGSYEQY | 2.7 |
| 5.6 | CASSLVGGHTEAF | 1.1 | 5.5 | CASSRLYEQY | 2.7 | 5.6 | CASSQTGTAYEQY | 2.7 |
| 5.6 | CASSRGPVNTEAF | 1.1 | 5.5 | CASSWDRDEQY | 2.7 | 6.5 | CASSETGTRRSPLH | 1.6 |
| 5.6 | CASSRTGTSGSHEQY | 2.7 | 5.6 | CASSAWSSYNSPLH | 1.6 | 6.5 | CASSYAHPGQANSPLH | 1.6 |
| 5.6 | CASSSPGQVNSPLH | 1.6 | 5.6 | CASSLGGTYTQY | 2.3 | 6.5 | CASSYPSLAGGQGSYNEQF | 2.1 |
| 5.6 | CASSSVGVKTQY | 2.5 | 5.6 | CASSLYSFYEQY | 2.7 | 6.5 | CASSYSGLAGGTDTQY | 2.3 |
| 6.5 | CASSLGGRPDTQY | 2.3 | 5.6 | CASSPGVAGRNQETQY | 2.5 | 6.5 | CASSYSHGVSLL | 2.3 |
| 6.5 | CASSQTSGRRGNEQF | 2.1 | 5.6 | CASSPLSSMNTEAF | 1.1 | 6.5 | CASSYSMLGRSTDTQY | 2.3 |
| 6.5 | CASSRGREPTYEQY | 2.7 | 5.6 | CASSRLYEQY | 2.7 | 6.5 | CASSQPRVRRRY | 2.7 |
| 6.5 | CASSSGLAGLQETQY | 2.5 | 6.1 | CASSDRSGSKYEQF | 2.1 | 6.6 | CACSLRGDYGYT | 1.2 |
| 6.5 | CASSSPGQVNPPLH | 1.6 | 6.1 | CASSEGVANTGELF | 2.2 | 11.3 | CASSTRGRLNSPLH | 1.6 |
| 6.5 | CASSSQVNNSPLH | 1.6 | 6.1 | CASSLLGKGEQF | 2.1 | 11.3 | CASSLGTGANVLT | 2.6 |
| 6.5 | CASSYGLAGADTQY | 2.3 | 6.2 | CASSDRSGSKYEQF | 2.1 | 11.3 | CASSLRDSSYEQY | 2.7 |
| 6.9 | CASNKRDSTYEQY | 2.7 | 6.2 | CASSYSSGTSGRNEQY | 2.7 | 24.1 | CATSDRTGNGYEQY | 2.7 |
| 7.9 | CATPDVTGESGANVLT | 2.6 | 6.4 | CASRKTVNTEAF | 1.1 | 28 | CASSPWGFTLH | 1.6 |
| 11.2 | CASSLVRLARGDTQY | 2.1 | 6.5 | CASSLLGKGEQF | 2.1 | 29.1 | CSVEGWVTEAF | 1.1 |
| 11.3 | CASRRSGTRRSQETQY | 2.5 | 6.5 | CASSPPQGTGGYT | 1.2 | 29.1 | CSVGLGGVASEAF | 1.1 |
| 11.3 | CASSAGLAGGLSSYEQY | 2.7 | 6.5 | CASSYSIHADTQY | 2.3 | 29.1 | CSVEQGRQPQH | 1.5 |
| 11.3 | CASSLGAGGGETQY | 2.5 | 6.5 | CASTSQGIYEQY | 2.7 | 29.1 | CSVDYRALYNEQF | 2.1 |
| 11.3 | CASSLGCTSGICEETQY | 2.5 | 10 | CASSVWTSGRLYEQY | 2.7 | 29.1 | CSVEMVGGRETQY | 2.3 |
| 11.3 | CASSLRWQIEQF | 2.1 | 10 | CAIAPRSLRYNEQF | 2.1 | 29.1 | CSVKQLAAETQY | 2.3 |
| 11.3 | CASSLTGGFSPLH | 1.6 | 10 | CAISGSGVTDTQY | 2.3 | 29.1 | CSIALGSDNQETQY | 2.5 |
| 11.3 | CASSLVRLARGDTQY | 2.3 | 11 | CASSLRWDRVYEQY | 2.7 | 29.1 | CSVALGSDNQETQY | 2.5 |
| 12.3 | CASSLGWQGPLH | 1.6 | 11 | CASSLEWTGGTYEQY | 2.7 | 29.1 | CSVEMVGGRETQY | 2.5 |
| 27 | CASKTGRGGANVLT | 2.6 | 12 | CASSLTSGSPLYPSSYEQY | 2.7 | 29.1 | CSVKQLAAETQY | 2.5 |
| 27 | CASSFLPRLGNSPLH | 1.6 | 19 | CASSRKPRSGVVSYEQY | 2.7 | 29.1 | CSGVTASSGEAYEQY | 2.7 |
| 29.1 | CSVDRGLMETQY | 2.5 | 19 | CATSESGTGITGELF | 2.2 | 29.1 | CSVGQGAVYEQY | 2.7 |
| 29.1 | CSVEKGSYEQY | 2.7 | 19 | CVSSRKPRSGVVSYEQY | 2.7 | |||
| 29.1 | CSVGKGLSNTEAF | 1.1 | 24 | CATSDASGSYTDTQY | 2.3 | |||
| 29.1 | CSVGYRPNTEAF | 1.1 | 24 | CATSEAGPLDTQY | 2.3 | |||
| 29.1 | CSVIWGGYEQY | 2.7 | 27 | ASRPQGLLSTDTQY | 2.3 | |||
| 29.1 | CSVRLQSGETQY | 2.5 | 27 | CASRPQGLLSTDTQY | 2.3 | |||
| 29.1 | CSVSGTGGPVRSEQY | 2.7 | 27 | CASRPQGLVSTDTQY | 2.3 | |||
| 29.1 | CSVVPGNQETQY | 2.5 | 27 | CASSPTAGLEAF | 1.1 | |||
| 27 | CASSSLREGSDTQY | 2.3 | ||||||
| 29 | CSSIGGTTSGISYNEQF | 2.1 | ||||||
| 29 | CSVETVSIWAADRANYGYT | 1.2 | ||||||
| 29 | CSVGGTRRNYGYT | 1.2 | ||||||
| 29 | CSVKRGGTGGFEQY | 2.7 | ||||||
| 29 | CSVVGQGLSEQY | 2.7 | ||||||
Table 8.
T-cell repertoire of PBMC #3
| CD4+CD25+Foxp3− | CD4+CD25+Foxp3+ | CD4+CD25−Foxp3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 5.3 | CASTLDSSYNEQF | 2.1 | 5.4 | CASSAEGTSGYT | 1.2 | 5.1 | CASSLESGSGRD | 2.7 |
| 5.4 | CARIRTRGQGDRCEQY | 2.7 | 5.4 | CASSIGQAAASPLH | 1.6 | 5.4 | CASSLALGRNPPYNEQF | 2.1 |
| 5.4 | CASSLDISGANVLT | 2.6 | 5.4 | CASSLAGQRPEY | 2.7 | 5.4 | CASSPSLAVAQDTQY | 2.3 |
| 5.4 | CASSLGQLSYNEQF | 2.1 | 5.4 | CASSLEPRTGEAGYT | 1.2 | 5.5 | CASSLVGRGEEGYT | 1.2 |
| 5.4 | CASSLRTRSVGEQY | 2.7 | 5.4 | CASSPGGLAGYEQY | 2.7 | 6.2 | CAIRGFSSNYGYT | 1.2 |
| 5.4 | CASSPGFLAGGRETQY | 2.5 | 5.4 | CASSSITNGYT | 1.2 | 6.2 | CASNQALAGADTQY | 2.3 |
| 5.4 | CASSPPDDGQETQY | 2.5 | 5.4 | CASTLEPRTGEAGYS | 1.2 | 6.2 | CASQLGGTPSNYGYT | 1.2 |
| 5.4 | CASTPRGGSDTQY | 2.3 | 5.5 | CASRSTQETQY | 2.5 | 6.5 | CASGLGGLASPLH | 1.6 |
| 5.4 | CASTPRGGSDTQY | 2.3 | 5.5 | CASSLEPRTGEAGYT | 1.2 | 6.5 | CASSGSQTGKGDEQY | 2.7 |
| 5.5 | CASSFGDTSGANVLT | 2.6 | 5.5 | CASSLRGQYEQY | 2.7 | 6.5 | CASSRGLAGVPETQY | 2.5 |
| 5.5 | CASSLDGQYSGNTIY | 1.6 | 5.5 | CASSLSGTLSSYNSPLH | 1.6 | 6.5 | CASSYDRGINSPLH | 1.6 |
| 5.5 | CASSLRTRSVGEQY | 2.7 | 5.5 | CASSRRQGGENSPLH | 1.6 | 6.5 | CASSYQGVGRYEQY | 2.7 |
| 5.6 | CAGSFGDTSGANVLT | 2.6 | 5.5 | CASSSLGTAINSPLH | 1.6 | 6.5 | CASSYRRGGSYEQY | 2.7 |
| 5.6 | CASRRVGGIYEQY | 2.7 | 5.5 | CASSWDGNYGYT | 1.2 | 6.5 | CASSYSFYNGYT | 1.2 |
| 5.6 | CASSFGDTSGANVLT | 2.6 | 5.6 | CASSERQARRGYT | 1.2 | 6.6 | CASSYSQGAGADTQY | 2.3 |
| 5.6 | CASSPPSGASYEQY | 2.7 | 5.6 | CASSLEQTYEQY | 2.7 | 7.9 | CASSSTRTQY | 2.5 |
| 5.8 | CASSLVGLTYEQY | 2.7 | 5.6 | CASSLERGLYNEQF | 2.1 | 11.3 | CASSLGTSGILRGETQY | 2.5 |
| 6.1 | CAGSSSGGSNSPLH | 1.6 | 5.6 | CASSRPQVNNEQF | 2.1 | 11.3 | CASSSGTSGILRGETQY | 2.5 |
| 6.1 | CASRWDGSYSPLH | 1.6 | 5.6 | CASSRRQGGENSPLH | 1.6 | 12.3 | CASSRDMLPQPQH | 1.5 |
| 6.1 | CASSESVRGGRYNEQF | 2.1 | 5.6 | CASSRTGLETQY | 2.5 | 18 | CASSDREGSPLH | 1.6 |
| 6.1 | CASSSSGGSNSPLH | 1.6 | 6.1 | CAISERDPSSYNSPLH | 1.6 | 18 | CASSPTGSRDSPLH | 1.6 |
| 6.5 | CASRTSGWAYNEQF | 2.1 | 6.1 | CASGSTGVSYNSPLH | 1.6 | 24.1 | CATSDPSPLTGGAETQY | 2.5 |
| 6.5 | CASSYLRTGGGYGYT | 1.2 | 6.1 | CASRGTGLSPLH | 1.6 | 24.1 | CATSDSSGGNNEQF | 2.5 |
| 11.2 | CASSLEMGDGDYEQY | 2.7 | 6.1 | CASRPGTGFSWDSPLH | 1.6 | 27 | CAGSDREGSPLH | 1.6 |
| 18 | CASSLAGGREETQY | 2.5 | 6.1 | CASRTTSGRKRNEQF | 2.1 | 27 | CASGVRGNSPLH | 1.6 |
| 19 | CAAGQSSYNSPLH | 1.6 | 6.1 | CASSPLVGVYNEQF | 2.1 | 27 | CASRTSGSGFSGANVLT | 2.6 |
| 19 | CASRPGQRERGYT | 1.2 | 6.4 | CASSDVPDRARNTEAF | 1.1 | 27 | CASSDREGSPLH | 1.6 |
| 19 | CASRQGLGTGELF | 2.2 | 6.5 | CASRGTTFEQF | 2.1 | 27 | CASSLEQYNSPLH | 1.6 |
| 19 | CASSLGLAGNYEQY | 2.7 | 6.5 | CASSPLRGRVFYEQY | 2.7 | 27 | CASSLWDRITDTQY | 2.3 |
| 19 | CASSNLAGEGETQY | 2.5 | 6.5 | CASSPSYSPDNEQF | 2.1 | 27 | CASSPTGSRDSPLH | 1.6 |
| 19 | CASSVMTGVGNSPLH | 1.6 | 6.5 | CASSSPTRLVYEQY | 2.7 | 29.1 | CSVSTSLDRVKEQY | 2.7 |
| 24.1 | CATSDAAEVGETQY | 2.5 | 6.5 | CASSYSPRGPPYEQY | 2.7 | 29.1 | CSVTQGLYGYT | 1.2 |
| 24.1 | CATSDRAGLGDEQF | 2.1 | 6.5 | CASTHRQGANYHY | 2.7 | 29.1 | CSVVWGDGYT | 1.2 |
| 24.1 | CATSGNSGSQNIQY | 2.4 | 6.9 | CASTTPGLAGGSSYNEQF | 2.1 | 30 | CAWSGLARDGELF | 2.2 |
| 24.1 | CATSGSSGSQNIQY | 2.4 | 11.1 | CASSLSGTLSSYNSPLH | 1.6 | |||
| 27 | CASSYSRGTGGDSPLH | 1.6 | 11.1 | CASSSRAQGHEQY | 2.7 | |||
| 29.1 | CSPSGELF | 2.2 | 11.3 | CASRPGLGRGTQY | 2.5 | |||
| 29.1 | CSVDQTSGATDTQY | 2.3 | 11.3 | CASSLILGRETQY | 2.5 | |||
| 29.1 | CSVEGRTSGSTRTQY | 2.3 | 11.3 | CASSLRGSGTYEQY | 2.7 | |||
| 29.1 | CSVENRGDPGANVLT | 2.6 | 11.3 | CASSSYYNSPLH | 1.6 | |||
| 29.1 | CSVGFSEDSPLH | 1.6 | 19 | CASSELAGGLGNEQF | 2.1 | |||
| 29.1 | CSVGTDTQY | 2.3 | 19 | CASSIAFRGYQAGGANVLT | 2.6 | |||
| 19 | CASSIDLSRTSARTDTQY | 2.3 | ||||||
| 19 | CASSLPTGTGLNSPLH | 1.6 | ||||||
| 19 | CASSPGLVAGVGETQY | 2.5 | ||||||
| 19 | CASSPGTFLPNSPLH | 1.6 | ||||||
| 19 | CASSPGTGLGRNEQF | 2.1 | ||||||
| 19 | CATRTQSLTRANTGELF | 2.2 | ||||||
| 24.1 | CATSDLGGYT | 1.2 | ||||||
| 24.1 | CATSDSPRTSLVRETQY | 2.5 | ||||||
| 24.1 | CATSDTGHPQETQY | 2.5 | ||||||
| 27 | CASSLGLGGTDTQY | 2.3 | ||||||
| 27 | CASSPQRSYGYT | 1.2 | ||||||
| 29.1 | CSATGQLNTEAF | 1.1 | ||||||
| 29.1 | CSGGRTVLSGEAF | 1.1 | ||||||
| 29.1 | CSGRMGQATEAF | 1.1 | ||||||
| 29.1 | CSVDEGNTGELF | 2.2 | ||||||
| 29.1 | CSVDGTRGDTDTHY | 2.3 | ||||||
| 29.1 | CSVDRGKGNYGYT | 1.2 | ||||||
| 29.1 | CSVEGRGRGVTEAF | 1.1 | ||||||
| 29.1 | CSVEPVGGSGAYEQY | 2.7 | ||||||
| 29.1 | CSVLGQAPSSYEQY | 2.7 | ||||||
| 29.1 | CSVQPVGGSGAYEQY | 2.7 | ||||||
| 29.1 | CSVVQPSGTSGKETQY | 2.5 | ||||||
Table 9.
Shared clonotypes between CD4+CD25+Foxp3+, CD4+CD25+Foxp3− and CD4+CD25−Foxp3+ populations
| CD4+CD25+Foxp3− | CD4+CD25+Foxp3+ | CD4+CD25−Foxp3+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ | |
| PBMC 1 | ---------- | ---------- | ---------- | ||||||
|
| |||||||||
| PBMC 2 | 5.4 | CASSFPLLEQY | 2.7 | 5.4 29.1 |
CASSFPLLEQY CSVEDLGPF |
2.7 2.1 |
29.1 | CSVEDLGPF | 2.1 |
|
| |||||||||
| PBMC 3 | ---------- | ---------- | ---------- | ||||||
|
| |||||||||
| UCBC 1 | 5.5 5.5 |
CASEGTVRSPLH CASSLEITGKGSPLH |
1.6 1.6 |
5.5 5.5 |
CASEGTVRSPLH CASSLEITGKGSPLH |
1.6 1.6 |
---------- | ||
|
| |||||||||
| UCBC 2 | 19 | CASSIATGSNTGELF | 2.2 | 19 | CASSIATGSNTGELF | 2.2 | ---------- | ||
|
| |||||||||
| UCBC 3 | 5.4 | CASSHLGETQY | 2.5 | 5.4 | CASSHLGETQY | 2.5 | ---------- | ||
PBMC, peripheral blood mononuclear cells; UCBC, umbilical cord blood cells, BV, variable region of the T-cell receptor β-chain; BJ, J-region of the T-cell receptor β-chain; CDR3, complementarity determining region 3
4. Discussion
The mechanism of immune suppression by CD4+ TR cells is poorly understood but it appears to be antigen-independent with a requirement for cell-cell contact (Sakaguchi, 2004). However, the clonal composition of CD4+ TR cells and their corresponding antigen specificity remains obscure. The basis for the identification CD4+ TR cells by flow cytometry has been the high level of CD25 expression by these cells. Although the CD4+CD25hi T cell subset include the majority of Foxp3+ cells, CD4+ TR cells can express no or low levels of CD25 and recent studies have suggested that the downregulation of the IL-7 receptor (CD127) or the isolation of CD45RA+ naïve CD4+CD25high may serve as a discriminator between TR and non-TR CD4+ T cells (Hoffmann et al., 2006; Liu et al., 2006; Seddiki et al., 2006) without the need for ICS. However, the CD127 molecule may be downregulated upon T-cell activation and therefore, a consensus in which surface marker(s) can reliably and consistently identify human TR is lacking. Thus, the expression of Foxp3 by ICS remains the most precise method for the flow cytometric identification of CD4+ TR cells.
The study of the clonal composition of human CD4+ TR has been hampered by unreliable methods for the identification of these cells and by methodological limitations which prevented the reliable characterization of TCRB CDR3 region of permeabilized and fixed cells. In mice the repertoire of CD25+ and CD25−CD4+ T cells has been shown to be equally diverse with minimal overlap of TCRα sequences between the two populations (Hsieh et al., 2004; Hsieh et al., 2006) suggesting that regulatory and non-regulatory CD4+ T cells in the mouse originate in the thymus as separate lineages. In humans the clonal composition of CD4+ TR has not been characterized clonotypically, but clonal homology between TR and non-TR CD4+ T cell subsets was addressed indirectly in a recent study where similar expansions in Vβ2 was observed in CD4+CD25− and CD4+CD25hi subsets and in the CD4+Foxp3− and CD4+Foxp3+ subsets after stimulation with CMV lysate (Vukmanovic-Stejic et al., 2006).
Previously published methods for DNA-based PCR approaches are time-consuming, require multiple tubes with panels of family-specific primers or use highly degenerate consensus primers which limit the number of detectable rearrangements (Rosenberg et al., 1992; Kneba et al., 1995; Assaf et al., 2000; Du et al., 2006). Our initial experience with the method proposed by the BIOMED-2 investigators was confounded by frequent primer dimerization and amplification of non-specific products, some of which were observed within the expected size range for true TCR amplicons. These problems occurred particularly in cases where the number of analyzed cells was low, posing additional limitations for certain applications. To overcome these issues, we developed a heminested approach and resolved problematic primer combinations with the addition of a third PCR tube; these modifications significantly improved the methodology allowing for a more efficient PCR reaction with elimination of primer dimerization and non-specific bands.
As reliable antibodies for the identification of Foxp3 expression by flow cytometry became available recently, we chose to use our modified DNA-based multiplex PCR to analyze the TCR repertoire of CD4+CD25+Foxp3+ cells in UCB and adult PBMC; CD4+CD25+Foxp3− and CD4+CD25−Foxp3+ populations were analyzed in parallel as comparators. Our data show that the repertoire of unstimulated CD4+ TR cells in UCB and PBMCs is highly polyclonal and that, on occasion, the same TCRs can be expressed in both CD4+CD25+Foxp3+ and CD4+CD25+Foxp3− populations. The latter observation suggests an ontogenetic relationship between the CD4+CD25+Foxp3+ and CD4+CD25+Foxp3− populations that might relate to separate differentiation pathways after encounter with the same cognate antigen or to a common origin in the thymus. Further studies of antigen-specific CD4+ T cells are required to distinguish between these and other possibilities.
In summary, we have developed a practical and reliable multiplex DNA-based PCR for TCRB gene rearrangements that can be used to examine the clonal composition of T cell populations in which the RNA has been degraded. The power of this technique was illustrated with a comprehensive assessment of unmanipulated CD4+ TR cell clonality directly ex vivo both in adult PBMCs and in UCBCs. While the resultant data reveal marked polyclonality in these CD4+ TR cell populations, potential relationships to other peripheral blood T cell subsets were also revealed.
Table 4.
T cell receptor repertoire of UCBC #2.
| CD4+CD25+Foxp3− | CD4+CD25+Foxp3+ | CD4+CD25−Foxp3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 5.4 | CASTSPGSNYGYT | 1.2 | 5.4 | CASSPDRGSRGYT | 1.2 | 5.4 | CASSTGQGNYGYT | 1.1 |
| 5.4 | CASTPGGGANNSPLH | 1.6 | 5.4 | CASSQAQSNYGYT | 1.2 | 5.4 | CASSLVHTEDGYT | 1.2 |
| 5.4 | CASSLDFSFEQF | 2.1 | 5.4 | CASSVSTGAHGYT | 1.2 | 5.4 | CASSLRLAGHTDTQY | 2.3 |
| 5.4 | CASSLGAGQGTQETQY | 2.5 | 5.4 | CASSLDHNEQF | 2.1 | 5.4 | CASSLASFGEQY | 2.7 |
| 5.4 | CASSFGGLYEQY | 2.7 | 5.4 | CASSLKTGGGRETQY | 2.5 | 5.4 | CASSLGLFYEQY | 2.7 |
| 5.4 | CASSLDQYEQY | 2.7 | 5.4 | CASRPNPTSGSRYEQY | 2.7 | 5.4 | CASSLGQVHEQY | 2.7 |
| 5.4 | CASSLDYSYEQY | 2.7 | 5.4 | CASSALGPGKGRVAEQY | 2.7 | 5.4 | CASSWDRGDEQY | 2.7 |
| 5.4 | CASSLRTAYEQY | 2.7 | 5.4 | CASSLQGREQY | 2.7 | 5.5 | CASSKDRHLIGELF | 2.2 |
| 5.4 | CASSSRGRAYEQY | 2.7 | 5.4 | CASSWGLAGSKQY | 2.7 | 5.5 | CASSLGDTQY | 2.3 |
| 5.4 | CASSTKGGAGDEQY | 2.7 | 5.5 | CASSSQGTGYGYT | 1.2 | 5.6 | CASSLGPSYNSPLH | 1.6 |
| 5.5 | CASIKTGERTGELF | 2.2 | 5.6 | CASSLVLVNTEAF | 1.1 | 5.6 | CASSPRDTNTGELF | 2.2 |
| 5.5 | CASSLYGELF | 2.2 | 5.6 | CASSLEGDRDHYGYT | 1.2 | 5.6 | CASSFRVDPSTDTQY | 2.3 |
| 5.5 | CASSPGQGAPKTQY | 2.5 | 5.6 | CASSLVGRGNYGYT | 1.2 | 5.6 | CASSLTPGAKNIQY | 2.4 |
| 5.5 | CASRLGLAGVQETQY | 2.5 | 5.6 | CASRPNPTSGSRYEQY | 2.3 | 5.6 | CASSWARSGANALT | 2.6 |
| 5.5 | CASSPGQGAPETQY | 2.5 | 5.6 | CASSWNPDTQY | 2.3 | 5.6 | CASSLGGARVMQY | 2.7 |
| 5.5 | CASSLPGAGGQETQY | 2.5 | 5.6 | CASSLPGAYEQY | 2.7 | 6.2 | CASSHAGSGANVLT | 2.6 |
| 5.5 | CASSPGQGAPETQY | 2.5 | 5.8 | CASSFRQGANYGYT | 1.2 | 6.2 | CASSYRAVQGANVLT | 2.6 |
| 5.5 | CASSFSSGGLSSYEQY | 2.7 | 6.2 | CASSRDRGASGNQPQH | 1.5 | 6.5 | CASSYSEGLEAF | 1.1 |
| 5.5 | CASSLRTAYEQY | 2.7 | 6.2 | CASSTRSPLH | 1.6 | 6.5 | CASSFGTGWRSYGYT | 1.2 |
| 5.5 | CASSPGQGAPETQY | 2.7 | 6.2 | CASRGTSTDTQY | 2.3 | 6.5 | CASSYTRGIYGYT | 1.2 |
| 5.6 | CASSLVGTNTEAF | 1.1 | 6.2 | CASSAIDTQY | 2.3 | 6.5 | CASKLTGANSPLH | 1.6 |
| 5.6 | CASSFFRVEETQY | 2.5 | 6.5 | CASSVGTGGTEAF | 1.1 | 6.5 | CASSYGRQSSYNSPLH | 1.6 |
| 5.6 | CASRPGQGRRKETQY | 2.5 | 6.5 | CASSPGTGNYGYT | 1.2 | 6.5 | CASSYRAPLWSSPLH | 1.6 |
| 5.6 | CASRPGQGRRKETQY | 2.7 | 6.5 | CASSYRGGNYGYT | 1.2 | 6.5 | CASSYRQGATLH | 1.6 |
| 6.1 | CASISTAYNSPLH | 1.6 | 6.5 | CASRAGHPNSPLH | 1.6 | 6.5 | CASSYHYGGFQQNTH | 2.5 |
| 6.2 | CASSYLWEAWTVNPSNQPQH | 1.5 | 6.5 | CASSHPGQAVPLH | 1.6 | 6.9 | CASSCTEQGNSPLH | 1.6 |
| 6.2 | CASSYDGHQPQH | 1.5 | 6.5 | CASSTRSPLH | 1.6 | 6.9 | CASSYTEHANSPLH | 1.6 |
| 6.2 | CASRPGVSQGEQF | 2.1 | 6.5 | CASSWGDNSPLH | 1.6 | 6.9 | CASSYTGQGNSPLH | 1.6 |
| 6.2 | CASSAPRGNGYEQY | 2.7 | 6.5 | CASSLKHYNEQF | 2.1 | 6.9 | CASRVGGPDEQY | 2.7 |
| 6.2 | CASSFGTSGSEQY | 2.7 | 6.5 | CASSNIRASMNEQF | 2.1 | 7.3 | CASSRDLGPYEQY | 2.7 |
| 6.4 | CASISTAYNSPLH | 1.6 | 6.5 | CASSYSTASTDTQY | 2.3 | 7.9 | CASSLKDGREY | 2.4 |
| 6.5 | CASSTRPANYGYT | 1.2 | 6.5 | CASSSLAERWETQY | 2.5 | 10 | CASSERRLGLRSPPH | 1.6 |
| 6.5 | CASSRDRGANQPQH | 1.5 | 6.9 | CASSPGGRGLTGANVLT | 2.6 | 11 | CASSPTAGGETQY | 2.5 |
| 6.5 | CASRRDRGWNSPLH | 1.6 | 6.9 | CASSTSGEQY | 2.7 | 19 | CASSARQGVTPLH | 1.6 |
| 6.5 | CASSYSSGISGAGELF | 2.2 | 10 | CAISAAVSTSGSPLH | 1.6 | 19 | CASSTSNRGGSPLH | 1.6 |
| 7.2 | CASSFLGDSTDTQY | 2.3 | 19 | CASRGRTVRNSPLH | 1.6 | 19 | CASSLRGRSDNEQF | 2.1 |
| 11 | CASSDNRDYEQY | 2.7 | 19 | CASSQGRTNSPLH | 1.6 | 19 | CATSDHRLAGEDEQF | 2.1 |
| 11 | CASSLGGDSYEQY | 2.7 | 19 | CAGRRTDTGELF | 2.2 | 19 | CASSIAGGDIQY | 2.4 |
| 11 | CASSSRTGYEQY | 2.7 | 19 | CASSIATGSNTGELF | 2.2 | 19 | CASSVGPSAYEQY | 2.7 |
| 11 | CASSLISRLAGQDYEQY | 2.7 | 19 | CASSTGGKTQY | 2.5 | 27 | CASSLVGLGPLH | 1.6 |
| 12 | CASSSLGTGNSPLH | 1.6 | 27 | CASSLLAWGADTQY | 2.3 | 29 | CSVEITGRGGYT | 1.2 |
| 19 | CASRSPPNYGYT | 1.2 | 27 | CASSLYGKETQY | 2.5 | 29 | CSAQTGQGAYGELF | 2.2 |
| 19 | CASSSRAENSPLH | 1.6 | 27 | CASRNSGANVLT | 2.6 | 29 | CSVEEVARGGEDTQY | 2.3 |
| 19 | CASSIATGSNTGELF | 2.2 | 27 | CASRNSGANVLT | 2.6 | 29 | CSVPGSSGSYEQY | 2.7 |
| 19 | CASSIGGTQTQY | 2.5 | 27 | CASSSLGGRANVLT | 2.6 | |||
| 24 | CATSDLRRQGCYEQY | 2.7 | 29 | CSVVRGDLIEAF | 1.1 | |||
| 24 | CATSDPGAGYGEQY | 2.7 | 29 | CSVGGAIYGYT | 1.2 | |||
| 27 | CASSLLGTGDSPLH | 1.6 | 29 | CSVGLSDRGTQY | 2.5 | |||
| 27 | CASSRRWQETQY | 2.5 | 30 | CAWSFNQYNSPLH | 1.6 | |||
| 29 | CSVAGQGASNQPQH | 1.5 | ||||||
| 29 | CSVEKGRTGNEQF | 2.1 | ||||||
| 29 | CSVEQGPGETQY | 2.5 | ||||||
| 29 | CSVVFFSGGLGANVLT | 2.6 | ||||||
| 30 | CAWSEQMSYT | 1.2 | ||||||
| 30 | CAFIPRGSPYNSPLH | 1.6 | ||||||
| 30 | CAWRSQGSYNSPLH | 1.6 | ||||||
| 30 | CAFIPRGSPYNSPLH | 1.6 | ||||||
| 30 | CAWSVPRVYQETQY | 2.7 | ||||||
Bolded clonotype is present in CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ populations
Table 7.
T cell receptor repertoire of PBMC #2.
| CD4+CD25+Foxp3− | CD4+CD25+Foxp3+ | CD4+CD25−Foxp3+ | ||||||
|---|---|---|---|---|---|---|---|---|
| BV | CDR3 | BJ | BV | CDR3 | BJ | BV | CDR3 | BJ |
| 5.4 | CASSAQGRSPLH | 1.6 | 5.4 | CASSLARGRAHNEQF | 2.1 | 5.4 | CASSLRALSFGYT | 1.2 |
| 5.4 | CASSLGSVSGANVLT | 2.6 | 5.4 | CASSLGQGVYNEQF | 2.1 | 5.4 | CASSQLTDGYT | 1.2 |
| 5.4 | CASSFPLLEQY | 2.7 | 5.4 | CASSLALAGVTGELF | 2.2 | 5.4 | CASSLKGHYSPLH | 1.6 |
| 5.5 | CASSLEREQF | 2.1 | 5.4 | CASSLGLAGGADMQY | 2.3 | 5.4 | CASLVQGAETQY | 2.5 |
| 5.6 | CASTQNTEAF | 1.1 | 5.4 | CASSLGLAGGADTQY | 2.3 | 5.4 | CASTSRGPETQY | 2.5 |
| 5.6 | CASSWGGGRHSSYEQY | 2.7 | 5.4 | CASGFTQETQY | 2.5 | 5.4 | CASSLDEGQGWSYEQY | 2.7 |
| 6.1 | CASDAWDEQF | 2.1 | 5.4 | CASSFPLLEQY | 2.7 | 5.5 | CASSLQGSYGYT | 1.2 |
| 6.1 | CASSELIPGLPDGNEQF | 2.1 | 5.4 | CASSLDIAGFYEQY | 2.7 | 5.6 | CASSFVGRDSPLH | 1.6 |
| 6.1 | CASSPSTSGSNEQF | 2.1 | 5.5 | CASSFGRINQPQH | 1.5 | 5.6 | CASSLKGHYSPLH | 1.6 |
| 6.1 | CASSYRRRTSPGELF | 2.2 | 5.6 | CASSLQFGQAYEQY | 2.7 | 5.6 | CASSLRTSGKSDTQY | 2.3 |
| 6.2 | CASRDRGLRDEQF | 2.1 | 6.5 | CASSLTGSNYGYT | 1.2 | 5.6 | CASSLWGSETQY | 2.5 |
| 6.4 | CASSRPFPYY | 2.7 | 6.5 | CASSYNFGNTGELF | 2.2 | 5.6 | CASSPDRGETQY | 2.5 |
| 6.5 | CASSLGDGANTIY | 1.3 | 6.5 | CASSYNFGNTGKLF | 2.2 | 6.5 | CASSYGGGGRPQH | 1.5 |
| 6.5 | CASSYSTRTGLGNTIY | 1.3 | 6.5 | CASSYTGAANTGELF | 2.2 | 6.5 | CASSYSGARYNSPLH | 1.6 |
| 6.5 | CASRQAHYNSPLH | 1.6 | 6.5 | CASGFTQETQY | 2.5 | 6.5 | CASSRRLGATSRIHEQY | 2.7 |
| 6.5 | CASSELIPGLPDGNEQF | 2.1 | 6.5 | CASRNQREPCEQY | 2.7 | 6.5 | CASSYWDSPYEQY | 2.7 |
| 6.5 | CASSPPQRDRADTQY | 2.3 | 6.5 | CASSYSRLAGYEQY | 2.7 | 6.5 | CASTHRVGHEQY | 2.7 |
| 6.5 | CASKLAGAGETQY | 2.5 | 7.8 | CASSSSAGNEQF | 2.1 | 6.7 | CASTGSTDTQY | 2.3 |
| 11.1 | CASSDSELAGGKGMEF | 2.1 | 12.3 | CASSHRTGLLNSPLH | 1.6 | 7.3 | CASSPGGPNEQF | 2.1 |
| 11.2 | CASSTGLQETQY | 2.5 | 18 | CASSREEGEQY | 2.7 | 7.9 | CASREMGNSPLH | 1.6 |
| 19 | CASIGRAGVLQPQH | 1.5 | 19 | CASSTRNSPLH | 1.6 | 13 | CASSFVGRDSPLH | 1.6 |
| 19 | CATSETHGNTGELF | 2.2 | 19 | CASSAPRQGSYNEQF | 2.1 | 19 | CASSINTGTSCGYT | 1.2 |
| 19 | CASSILGTEETQY | 2.5 | 19 | CASSSQGSTDTQY | 2.3 | 19 | CASSGQLNQPQH | 1.5 |
| 27 | CASSLSLVGAGGPNTQY | 2.3 | 19 | CASSILQGWETQY | 2.5 | 24.1 | CATSDSGQGGNSPLH | 1.6 |
| 29.1 | CSVEGQAFDISYNSPLH | 1.6 | 19 | CASSWDKIRGETQY | 2.5 | 27 | CASSDSQTSGSNEQF | 2.1 |
| 29.1 | CSGRLAGVNEQF | 2.1 | 24.1 | CATSDSYGYT | 1.2 | 27 | CASRPQGRETQY | 2.5 |
| 29.1 | CSVLGLAGVKQF | 2.1 | 29.1 | CSVGGEKLF | 1.4 | 29.1 | CSVGEGGGYT | 1.2 |
| 29.1 | CGVVPLGGMGETQY | 2.5 | 29.1 | CSVEDLGPF | 2.1 | 29.1 | CSVDPTGGSEKLF | 1.4 |
| 29.1 | CSVVTDSYEQY | 2.7 | 29.1 | CSVVWTGLTGELF | 2.2 | 29.1 | CSVEDLGPF | 2.1 |
| 30 | CASRPRSSYNSPLH | 1.6 | 29.1 | CSVEVGAGKTQY | 2.5 | 29.1 | CSVERQGRAGELF | 2.2 |
| 30 | CAWEGTVNSPLH | 1.6 | 30 | CAWSAGTGVNSPLH | 1.6 | 29.1 | CSVIRGSGANVLT | 2.6 |
| 30 | CAWGRGGYNSPLH | 1.6 | 29.1 | CSVGVGQGAYEQY | 2.7 | |||
| 30 | CAWGEWEQF | 2.1 | ||||||
| 30 | CACRDRQETQY | 2.5 | ||||||
| 30 | CAWNSPARSQETQY | 2.5 | ||||||
| 30 | CAWRDRDIVNSGANVLT | 2.6 | ||||||
Bolded clonotype is present on CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ populations
Underlined clonotype is present on CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ populations
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung and Blood Institute and National Institute of Allergy and Infectious Diseases. DAP is a Medical Research Council (UK) Senior Clinical Fellow.
We are thankfully grateful to Dr. Pablo Rubinstein from the New York Blood Center for providing the umbilical cord cells for the naïve CD4+ regulatory T cell analysis and David Ambrozak for performing the sort of CMV-specific T cells.
Abbreviations
- TR
regulatory T cells
- UCB
umbilical cord blood
- PBMC
peripheral blood mononuclear cells
- TCR
T-cell receptor
- Foxp3
forkhead family transcription factor P3
- ICS
intracellular staining
- CDR3
complementarity determining region 3
- TCRB
the T-cell receptor β-chain
- BV
variable region of the T-cell receptor β-chain
- BJ
J-region of the T-cell receptor β-chain
- IFN-γ
interferon-γ
Footnotes
P Scheinberg provided primary conception, development of assay, execution, data analysis and drafted the manuscript. J Melenhorst contributed to primary conception, execution, sorting and cell processing, and manuscript preparation. B Hill was involved in assay development and interim discussions. K Keyvanfar conducted the cell sorting. D Price, AJ Barrett and D Douek were involved in primary conception, interpretation of results, interim discussions and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. Diversity of human alpha beta T cell receptors. Science. 2000;288:1135. doi: 10.1126/science.288.5469.1135a. [DOI] [PubMed] [Google Scholar]
- Assaf C, Hummel M, Dippel E, Goerdt S, Muller HH, Anagnostopoulos I, Orfanos CE, Stein H. High detection rate of T-cell receptor beta chain rearrangements in T-cell lymphoproliferations by family specific polymerase chain reaction in combination with the GeneScan technique and DNA sequencing. Blood. 2000;96:640–6. [PubMed] [Google Scholar]
- Betts MR, Casazza JP, Patterson BA, Waldrop S, Trigona W, Fu T-M, Kern F, Picker LJ, Koup RA. Putative Immunodominant Human Immunodeficiency Virus-Specific CD8+ T-Cell Responses Cannot Be Predicted by Major Histocompatibility Complex Class I Haplotype. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Douek DC, Betts MR, Brenchley JM, Hill BJ, Ambrozak DR, Ngai KL, Karandikar NJ, Casazza JP, Koup RA. A Novel Approach to the Analysis of Specificity, Clonality, and Frequency of HIV-Specific T Cell Responses Reveals a Potential Mechanism for Control of Viral Escape. J Immunol. 2002;168:3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- Du G, Qiu L, Shen L, Sehgal P, Shen Y, Huang D, Letvin NL, Chen ZW. Combined megaplex TCR isolation and SMART-based real-time quantitation methods for quantitating antigen-specific T cell clones in mycobacterial infection. J Immunol Methods. 2006;308:19–35. doi: 10.1016/j.jim.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T cell lines upon in vitro expansion. Blood. 2006 doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Hutchinson SL, Wooldridge L, Tafuro S, Laugel B, Glick M, Boulter JM, Jakobsen BK, Price DA, Sewell AK. The CD8 T Cell Coreceptor Exhibits Disproportionate Biological Activity at Extremely Low Binding Affinities. J Biol Chem. 2003;278:24285–24293. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- Kneba M, Bolz I, Linke B, Hiddemann W. Analysis of rearranged T-cell receptor beta-chain genes by polymerase chain reaction (PCR) DNA sequencing and automated high resolution PCR fragment analysis. Blood. 1995;86:3930–7. [PubMed] [Google Scholar]
- Lefranc MP. IMGT, the international ImMunoGenetics information system®. In: Lo BKC, editor. Antibody Engineering Methods and Protocols. 2. Vol. 248. Humana Press; Totowa, NJ: 2004. pp. 27–49. http://imgt.cines.fr. Methods in Molecular Biology. [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with Foxp3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–61. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, West SM, Betts MR, Ruff LE, Brenchley JM, Ambrozak DR, Edghill-Smith Y, Kuroda MJ, Bogdan D, Kunstman K. T Cell Receptor Recognition Motifs Govern Immune Escape Patterns in Acute SIV Infection. Immunity. 2004;21:793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–8. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of Foxp3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- Rosenberg WM, Moss PA, Bell JI. Variation in human T cell receptor V beta and J beta repertoire: analysis using anchor polymerase chain reaction. Eur J Immunol. 1992;22:541–9. doi: 10.1002/eji.1830220237. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–53. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4 CD25 Foxp3 regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan JA, Dunbar PR, Price DA, Purbhoo MA, Lechner F, Ogg GS, Griffiths G, Phillips RE, Cerundolo V, Sewell AK. Specificity of CTL Interactions with Peptide-MHC Class I Tetrameric Complexes Is Temperature Dependent. J Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]