Abstract
Background
Although exercise impacts quality of life in patients with inflammatory bowel disease (IBD), little is known about its role in disease activity. Among IBD patients in remission, we aimed to evaluate the association between exercise and subsequent active disease.
Methods
We performed a prospective study using the Crohn's and Colitis Foundation of America (CCFA) Partners Internet-based cohort of individuals with self-reported IBD. We identified participants in remission, defined as short Crohn's disease activity index (sCDAI) <150 or simple clinical colitis activity index (SCCAI) ≤2. The primary exposure was exercise status, measured using the validated Godin leisure time activity index. The primary study outcome, assessed after six months, was active disease defined using the above disease activity index thresholds. We used bivariate and multivariate analyses to describe the independent association between exercise and risk of active disease.
Results
We identified 1308 patients with Crohn's Disease (CD) and 549 with ulcerative or indeterminate colitis (UC/IC) in remission, of whom 227(17.4%) with CD and 135 (24.6%) with UC/IC developed active disease after 6 months. Higher exercise level was associated with decreased risk of active disease for CD (adjusted RR 0.72, 95% CI 0.55-0.94) and UC/IC (adjusted RR 0.78, 95% CI 0.54-1.13).
Conclusions
In patients with CD in remission, those with higher exercise levels were significantly less likely to develop active disease at six months. In patients with UC/IC in remission, patients with higher exercise levels were less likely to develop active disease at six months, however this was not statistically significant.
Keywords: Exercise, IBD, Crohn's disease, Ulcerative Colitis, Godin
Introduction
Inflammatory bowel disease (IBD), including Crohn's disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC) are chronic, immune-mediated diseases with a clinical course often marked by periods of relapse alternating with periods of quiescence. Environmental factors, such as exercise, may impact the pathogenesis and course of IBD. Prior studies have shown an association between physical activity and development of IBD. Sonnenberg et al. 1 demonstrated an increased prevalence of IBD in patients with sedentary or less physically demanding occupations compared to those with physically demanding jobs. A Swedish case-control study reported that patients who exercised regularly in the previous five years were at decreased risk for developing CD, especially if they exercised daily.2 More recently, Khalili et al. found that physical activity was inversely associated with the risk of CD, but not UC.3
Less is known about the impact of physical activity on the course of IBD. One population-based study4 demonstrated that only 25% of patients with IBD exercised with moderate intensity and that patients with IBD were less likely to report active lifestyles compared to unaffected individuals. Although the effect of exercise on quality of life and stress has been studied in patients with IBD,5 there are no large prospective studies examining the association between exercise and disease activity. Exercise is associated with variable effects on the gastrointestinal system.6 Some studies in humans and rodents have suggested that exercise increases pro-inflammatory cytokines7, 8 while other studies have shown decreases in these cytokines. 9, 10 Athletes are noted to have increased gut microbiota diversity than controls,11 however it is unclear how these physiologic changes might impact symptoms of inflammatory bowel disease.
Exercise is thought to lead to improved mood, decreased stress and increased quality of life. There are other physiologic benefits to exercise such as improved bone density12 and decreased risk of colon cancer, 13 both of particular importance to patients with IBD. Exercise is important for prevention and treatment of obesity, and obesity is an increasing problem in patients with IBD.14-16 Obesity may complicate the clinical course of IBD leading to increased rates of hospitalization, perianal complications17 and decreased time to first surgery.18 While the benefits of exercise are likely to outweigh theoretical risks in patients with mild and moderate IBD, little data exist on this topic. We therefore sought to evaluate the association between exercise patterns and subsequent disease activity in a large cohort of patients with IBD.
MATERIALS AND METHODS
Study Cohort
CCFA Partners is an Internet-based cohort of adult patients (>= 18 years of age) with self-reported IBD (Crohn's disease (CD), ulcerative colitis (UC) or indeterminate colitis (IC)) sponsored by the Crohn's and Colitis Foundation of America (CCFA). The development of this cohort has been described elsewhere.19 In brief, patients are recruited through CCFA email rosters, the CCFA website, word of mouth and social media websites. Those who agree to participate complete a baseline survey which collects demographic information as well as information about disease activity, medication use, physical activity, quality of life, and overall health status. Participants are invited, through email, to complete follow-up surveys on a semi-annual basis. A prior validation study of CCFA Partners has shown high levels of accuracy for presence and type of IBD (97% for both).20
From within this cohort, we identified a population of patients who were in remission, defined as having a short Crohn's disease activity index (sCDAI) <15021 or simple clinical colitis activity index (SCCAI)22 ≤2.23 We investigated associations between baseline exercise and active disease 6 months later. Active disease was defined as disease activity index above these thresholds.
Inclusion/Exclusion Criteria
All patients in clinical remission during the time of survey completion were included in this analysis. For patients who completed surveys at multiple time points, their first survey indicating clinical remission was used as the baseline for this analysis. We excluded those with pregnancy or recent (within the past month) surgery, fracture, or myocardial infarction as we expected that these conditions would limit ability to exercise. All individuals with an ostomy were excluded, because these individuals could not complete disease activity indices that rely upon reports of bowel movement frequency. Similarly, individuals with a pouch were excluded, because sCDAI and SCCAI may not accurately reflect disease activity in this population.
Assessment of Exercise
The Godin Leisure Time Activity Index was developed to assess exercise behavior24 and has been validated in different populations.25, 26 Weekly leisure activity score is calculated by the following formula: Weekly leisure activity score = (9 × Strenuous) + (5 × Moderate) + (3 × Light).24 For example, a Godin score of 30 could be achieved by vigorous exercise three times per week, moderate exercise six times per week or light exercise ten times per week performed for at least fifteen minutes each time. Baseline exercise status was dichotomized at the median score into categories of higher and lower exercise activity. Baseline exercise status was also stratified into quartiles for secondary analyses.
Outcomes
The primary outcome of interest was the presence of active disease at 6 months, defined as sCDAI>150 for CD or SCCAI >2 for UC/IC on the follow-up survey.
Statistical Analysis
Continuous variables were analyzed using means and standard deviations. Categorical variables were expressed using proportions. Comparisons were made using Student's t-test or the Kruskal-Wallis rank-sum test for continuous variables, and Pearson's chi-square test for categorical variables. We estimated crude and adjusted risk ratios (RR) for active disease at follow-up with respect to baseline physical activity using multivariate log-binomial or Poisson regression modeling. We used stratified analysis and logistic regression modeling with multiple degree-of-freedom likelihood ratio tests to assess for effect modification between physical activity and all variables. Potential confounders, age, gender, body mass index (BMI), global health status and cigarette smoking, were identified a priori through a directed acyclic graph (DAG) based on clinical reasoning. Physical activity tends to decrease with age, which is associated with both incidence and prevalence of IBD27. There is not a strong gender predilection in IBD, 27 however gender is associated with physical activity and has been shown to affect subjective measures of disease activity. 28 BMI may impact disease activity 16, 17 and is associated with physical activity. Health status is expected to be associated with physical activity as individuals who feel they are in excellent health are more likely to exercise than those who feel they are in poor health. Also, we hypothesize that one's perception of their health status might be affected by disease activity. There is an inverse relationship between smoking and physical activity29 and smoking has variable effects on disease activity in patients with IBD. 30, 31 Covariates were also assessed for multicollinearity. We constructed a full model using all potential confounders and eliminated these potential confounders via a backwards elimination strategy using a change in estimate approach (threshold of <10% change). For all analyses, p-values were two-sided, and a p-value of 0.05 or less was considered statistically significant. All statistical analyses were performed using Stata version 12.0 (College Station, TX, USA).
ETHICAL CONSIDERATIONS
The study protocol was approved by the Institutional Review Board at University of North Carolina.
RESULTS
Study Cohort
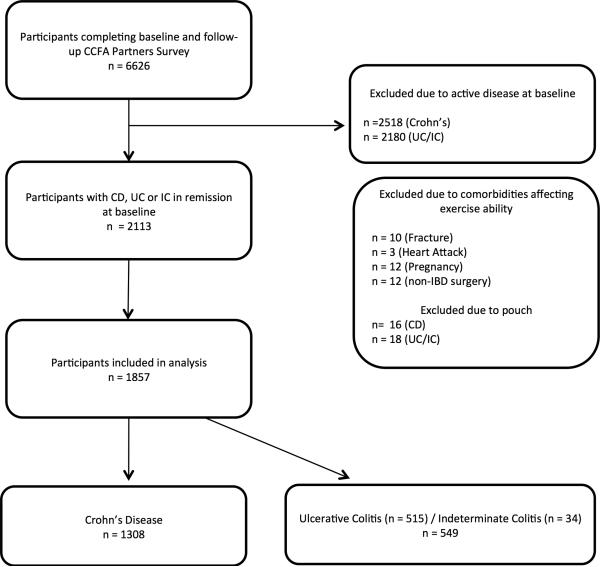
A total of 6,626 participants completed both baseline and follow-up surveys and did not have an ostomy. After excluding those not in clinical remission or who had comorbidities affecting ability to exercise, 1,857 participants were eligible for inclusion (Figure 1); 1,308 participants had Crohn's disease and 549 had ulcerative colitis or indeterminate colitis. The median age was 45 years for CD and 43 for UC/IC and the median disease duration was 12 years and 9 years for CD and UC/IC respectively. (Tables 1 and 2). The majority of participants were women. Most patients were of normal body mass index (BMI), however both groups had overweight and obese participants, defined as BMI from 25-29.9 and greater than 30, respectively. More patients with CD had been hospitalized and had bowel surgery previously compared to participants with UC/IC. More patients with UC/IC were treated with either oral or rectal 5-aminosalicylate (5-ASA) than CD patients. More patients with CD were treated with biologics, which included adalimumab, infliximab, certolizumab pegol or natalizumab. Less than 1% of patients were taking medications as part of a clinical trial.
Figure 1.
Flow Diagram of Study Participants
Table 1.
Baseline Characteristics of the Crohn's Disease Population in Remission, Overall and Stratified by Activity Level within CCFA^ Partners Cohort
| Crohn's Disease N = 1,308 Median (IQR) or Percent | ||||
|---|---|---|---|---|
| Characteristic | Overall | Low Activity Level† Godin <28 | High Activity Level† Godin ≥ 28 | p-value |
| Age (years) | 45 (32-58) | 48 (34-59) | 42 (29-56) | <0.01 |
| Disease duration (years) | 12 (5-24) | 14 (6-26) | 10 (5-21) | <0.01 |
| BMI (kg/m2) | 24 (22-28) | 25 (20-29) | 23 (21-27) | <0.01 |
| sCDAI | 79 (58-111) | 86 (58-121) | 76 (44-107) | <0.01 |
| Gender (% women) | 71 | 75 | 67 | <0.01 |
| Ever smokers, % | 34 | 36 | 33 | 0.30 |
| Current smokers, % | 5 | 7 | 4 | 0.02 |
| Education (% with ≤ High School) | 7 | 8 | 5 | <0.01 |
| Global Health Status, % | ||||
| Excellent/very good | 45 | 35 | 55 | <0.01 |
| Good | 44 | 50 | 39 | |
| Fair/Poor | 11 | 15 | 6 | |
| Prior bowel surgery, % | 47 | 50 | 43 | <0.01 |
| Current fistula, % | 20 | 21 | 18 | 0.17 |
| Ever hospitalized for IBD, % | 70 | 71 | 68 | 0.18 |
| 5-ASA, Oral, % | 38 | 38 | 38 | 0.89 |
| 5-ASA, Rectal, % | 2 | 2 | 2 | 0.79 |
| Corticosteroids, % | ||||
| Oral | 6 | 7 | 5 | 0.05 |
| Rectal | 1 | |||
| Budesonide, % | 4 | 4 | 4 | 0.42 |
| Thiopurines , % | 31 | 31 | 31 | 1 |
| Biologics*, % | 37 | 38 | 36 | 0.47 |
N = total participants, IQR = interquartile range, BMI = body mass index sCDAI = short Crohn's disease activity index, IBD = inflammatory bowel disease, 5-ASA= 5-aminosalicylic acid
Crohn's and Colitis Foundation of America
Low activity level is below median Godin index; high is above median Godin index
adalimumab, infliximab, certolizumab pegol or natalizumab.
Table 2.
Baseline Characteristics of the Ulcerative Colitis/Indeterminate Colitis Population in Remission, Overall and Stratified by Activity Level within CCFA^ Partners Cohort
| Ulcerative Colitis / Indeterminate Colitis N = 549 Median (IQR) or Percent | ||||
|---|---|---|---|---|
| Characteristic | Overall | Low Activity Level† Godin <34 | High Activity Level† Godin ≥ 34 | p-value |
| Age (years) | 43 (31-56) | 43 (31-57) | 43 (31-55) | 0.55 |
| Disease duration (years) | 9 (4-16) | 10 (4-16) | 8.5 (4-15) | 0.36 |
| SCCAI | 1(0-2) | 1 (1-2) | 1 (0-2) | 0.35 |
| BMI (kg/m2) | 24 (22-27) | 24 (22-28) | 23 (21-26) | <0.01 |
| Gender (% women) | 69 | 72 | 66 | 0.12 |
| Ever Smokers, % | 30 | 29 | 31 | 0.66 |
| Current smokers, % | 3 | 3 | 3 | 0.79 |
| Education (% with ≤ High School) | 6 | 10 | 2 | <0.01 |
| Global Health Status, % | ||||
| Excellent/very good | 67 | 56 | 77 | <0.01 |
| Good | 28 | 36 | 20 | |
| Fair/Poor | 5 | 8 | 3 | |
| Prior bowel surgery, % | 3 | 2 | 4 | 0.22 |
| Ever hospitalized for IBD, % | 38 | 38 | 38 | 0.98 |
| 5-ASA, Oral, % | 69 | 70 | 69 | 0.62 |
| 5-ASA, Rectal, % | 13 | 11 | 14 | 0.46 |
| Corticosteroids, % | ||||
| Oral | 5 | 7 | 5 | 0.35 |
| Rectal | 3 | 2 | 3 | 0.59 |
| Budesonide, % | 1 | 1 | 0 | 0.04 |
| Thiopurines , % | 24 | 26 | 23 | 0.38 |
| Biologics*, % | 17 | 19 | 16 | 0.36 |
N = total participants, IQR = interquartile range, SCCAI = simple clinical colitis activity index, BMI = body mass index, IBD = inflammatory bowel disease, 5-ASA= 5-aminosalicylic acid
Crohn's and Colitis Foundation of America
Low activity level is below median Godin index; high is above median Godin index
adalimumab, infliximab, certolizumab pegol or natalizumab.
Baseline Characteristics by Exercise
The median Godin score was 28 for CD and 34 for UC/IC. Bivariate analyses were performed using dichotomous categories of exercise (high vs. low) (Tables 1 and 2) as well as quartiles of exercise. CD patients in the lower category of exercise were significantly older than participants in the higher category of exercise. More women were in the less active category compared to the more active category for CD and UC/IC. BMI was higher for the less active category of participants. For CD participants in the lower exercise category, a higher proportion were current smokers, had previous bowel surgery, and were treated with oral steroids compared to the higher exercise category. For all IBD patients, the less active category had the highest proportion of participants with high school education or less and the more active category had the highest proportion of participants who rated their health status as excellent or very good. There were no significant differences in age at diagnosis, disease duration, last hospitalization or other IBD medications, when stratified by two categories of exercise, dichotomized at the median. These findings were echoed when baseline characteristics were stratified by quartiles of exercise.
Association Between Exercise and the Risk of Active Disease at Six Months
Exercise status, gender, oral steroids, timing since the last IBD hospitalization, and global health status were all associated with active disease at 6 months for both CD and UC/IC (Table 3). Education level was found to be associated with active disease at 6 months for CD. As reported in other studies,30, 31 individuals with CD who currently smoke had increased risk for active disease at six months. For patients with UC/IC, those with active disease had a shorter duration of disease, were younger and a higher proportion were using rectal 5-ASAs. BMI was not associated with active disease at six months.
Table 3.
Patient Characteristics by Disease Activity at 6 Months for Crohn's Disease and Ulcerative Colitis / Indeterminate Colitis
| Crohn's Disease | Ulcerative Colitis/Indeterminate Colitis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Remission | Active Disease | p-value | Remission | Active Disease | p-value |
| Physical Activity, % < median Godin | 48 | 57 | 0.01 | 47 | 58 | 0.04 |
| Age (years) Median(IQR) | 45 (32-58) | 43 (31-55) | 0.22 | 44 (32-57) | 41 (29-53) | 0.03 |
| Duration (years) Median (IQR) | 12 (5-24) | 12 (5-24) | 0.59 | 10 (5-16) | 7 (3-14) | <0.01 |
| Gender (% women) | 70 | 77 | 0.05 | 67 | 76 | 0.06 |
| BMI (kg/m2) Median (IQR) | 24 (22-27) | 25 (22-28) | 0.22 | 24 (22-27) | 23 (21-28) | 0.74 |
| Ever Smokers, % | 33 | 39 | 0.08 | 30 | 29 | 0.81 |
| Current Smokers, % | 5 | 8 | 0.02 | 3 | 1 | 0.31 |
| Prior bowel surgery, % | 46 | 51 | 0.18 | 2 | 5 | 0.11 |
| Ever Hospitalized, % | 69 | 70 | 0.68 | 38 | 36 | 0.59 |
| Last IBD hospitalization, % | ||||||
| <1 year ago | 79 | 21 | <0.01 | 56 | 44 | 0.09 |
| 1-2 years ago | 76 | 24 | 72 | 28 | ||
| 3-10 years ago | 83 | 17 | 79 | 21 | ||
| >10 years ago | 89 | 11 | 84 | 16 | ||
| Oral 5-ASA, % | 38 | 39 | 0.64 | 69 | 72 | 0.44 |
| Rectal 5-ASA, % | 2 | 3 | 0.10 | 11 | 18 | 0.03 |
| Oral Prednisone, % | 5 | 9 | 0.03 | 4 | 11 | <0.01 |
| Thiopurines, % | 31 | 29 | 0.55 | 25 | 23 | 0.78 |
| Biologics, % | 36 | 40 | 0.23 | 18 | 15 | 0.41 |
| Methotrexate, % | 3 | 6 | 0.06 | 0.8 | 0 | 0.32 |
| Certolizumab pegol, % | 4 | 7 | 0.02 | 0.5 | 0 | 0.41 |
| Education (% ≤ High School) | 6 | 9 | <0.01 | 6 | 6 | 0.61 |
| Global Health, % | <0.01 | <0.01 | ||||
| Excellent/ Very Good | 50 | 20 | 71 | 53 | ||
| Good | 41 | 61 | 25 | 38 | ||
| Fair/Poor | 9 | 16 | 4 | 9 | ||
IQR = interquartile range, IBD = inflammatory bowel disease, 5-ASA= 5-aminosalicylic acid
Crohn's Disease
Among those with CD, 20% of those in low exercise category experienced relapse or developed active disease at 6 months, compared to 15% of those in the high exercise category and this was statistically significant, p = 0.01. There was significant interaction between steroids and physical activity found using stratified analysis. For patients using steroids, those in the higher exercise class (n=30) had increased risk of active disease of (RR 1.87) compared to those in the lower exercise class (n=46). However, for patients who were not on steroids, those in the higher exercise class (n=627) had decreased risk of active disease (RR 0.70) compared to those in the lower exercise class (n=603), p 0.01. When adjusted for other covariates, this interaction remained statistically significant using logistic regression modeling with multiple degree-of-freedom likelihood ratio tests, p 0.05, and all multivariate analyses included an interaction term.
We found that increased physical activity was associated with a reduced risk of active disease at 6 months; crude RR 0.68, 95% confidence interval (CI) 0.53-0.89 (Table 4). Global health status was a candidate confounder based on a priori reasoning and bivariate analysis. However, global health status was highly correlated with quartiles of physical activity (p <0.001for Spearman's rank correlation), leading to multicollinearity in the models. Thus, global health status was excluded as a potential confounder to avoid overfitting and instability in estimated coefficients. When adjusted for age, education, steroids, gender, BMI, current smoking status and disease duration, the product term of physical activity and steroids, the risk ratio was 0.72, 95% CI 0.55-0.94. Results of the fully adjusted model are also in Table 4.
Table 4.
Risks Ratios for the Development of Active Disease for Participants with Godin Lesiure Time Activity Index Above Median Compared to Those Below Median and Adjusted Risk Ratios for Covariates.
| Crohn's Disease§,^ | Ulcerative Colitis/Indeterminate Colitis† | |||||
|---|---|---|---|---|---|---|
| Covariate | Risk Ratio | 95% Confidence Interval | p value | Risk Ratio | 95% Confidence Interval | p value |
| Physical Activity-Crude* | 0.68 | 0.53-0.89 | <0.01 | 0.76 | 0.53-1.09 | 0.13 |
| Physical Activity-Adjusted | 0.72 | 0.55-0.94 | 0.02 | 0.78 | 0.54-1.13 | 0.18 |
| Age, years | 0.99 | 0.98-1 | 0.08 | 1 | 0.98-1 | 0.64 |
| BMI <18.5 | 1 | 1 | ||||
| BMI 18.5-24.99 | 1.15 | 0.50-2.63 | 0.49 | 0.98 | 0.47-2.06 | 0.96 |
| BMI 25-29.99 | 1.41 | 0.61-3.28 | 0.61 | 1.05 | 0.48-2.27 | 0.91 |
| BMI ≥ 30 | 1.19 | 0.50-2.84 | 0.53 | 1.28 | 0.58-2.83 | 0.54 |
| Current Smoker | 1.65 | 1.11-2.47 | 0.01 | 0.67 | 0.16-2.07 | 0.48 |
| Disease Duration, years | 1 | 0.99-1 | 0.69 | 0.98 | 0.95-1 | 0.02 |
| Male Gender | 0.80 | 0.59-1.07 | 0.13 | 0.79 | 0.55-1.14 | 0.21 |
| Steroids | 1.21 | 0.63-2.29 | 0.57 | 2.33 | 1.57-3.46 | <0.01 |
| Greater than High School Education | 0.70 | 0.47-1.06 | 0.09 | 1.20 | 0.58-2.48 | 0.62 |
| Physical Activity × Steroids | 2.20 | 0.98-4.97 | 0.06 | - | - | - |
Risks ratios were adjusted for age, education, steroids, gender, BMI, current smoking status and disease duration and calculated using the Log Binomial method.
All models for Crohn's disease include the product term of physical activity and steroids as significant interaction was noted.
Risks ratios were adjusted for age, education, steroids, gender, BMI, current smoking status and disease duration and calculated using the Poisson method.
Unadjusted risk ratios
We conducted sensitivity analyses using alternative definitions of active disease. Studies have used a decrease in sCDAI of 70 or greater from the baseline to define remission.32 When we defined active disease as an increase in sCDAI of 70 and the adjusted RR was 0.74 (0.56-0.99) and the adjusted RR was 0.74 (0.56-0.99). We also conducted a sensitivity analysis, which excluded individuals taking steroids as these individuals might have active disease not captured by the sCDAI. The results were nearly identical to those obtained with our original models with a crude RR of 0.68 (95% CI 0.58-0.89) and adjusted risk ratio of 0.72 (95% CI 0.55-0.94).
Ulcerative Colitis/Indeterminate Colitis
Among those with UC/IC, 28% of those in the lower exercise category had active disease at six months compared to 21% of those in the high-exercise category, p 0.04. We found that higher exercise was associated with decreased risk of active disease at six months crude RR 0.76, 95% CI 0.53-1.09. (Table 4). There were no significant interactions noted. As in building models for CD, global health status was excluded as a potential confounder due to multicollinearity with physical activity. When adjusted for age, education, steroids, gender, BMI, current smoking status and disease duration, adjusted risk ratio was 0.78, 95% CI 0.54-1.13 (Table 4.) We conducted a sensitivity analysis where active disease was defined as an increase in SCCAI of at least 2 points and the results were similar.
DISCUSSION
There is considerable interest in the impact of exercise on individuals with IBD. Our study is the largest prospective analysis of exercise in patients with IBD to date with 1,857 participants included, 1,308 with CD and 549 with UC/IC. We found that increased levels of exercise may decrease the risk active disease at 6 months by 32% in individuals with CD and by 24% in individuals with UC/IC. This decrease in risk persisted when exercise status was adjusted for age, education, steroids, gender, BMI, current smoking status and disease duration. Studies have demonstrated that patients who exercise more are at decreased risk to develop CD2, 3 however there is a paucity of published data about the effects of exercise in patients with established IBD. Much of what is published about the effects of exercise on the gastrointestinal system is incongruent and has to be extrapolated to patients with IBD.
Exercise is believed to have variable effects on the gastrointestinal system and may lead to gastrointestinal symptoms, such as increased urge to defecate33, 34 through unclear mechanisms. Previously postulated mechanisms for gastrointestinal symptoms include decreased gastrointestinal blood flow, increased gastrointestinal motility, increased mechanical bouncing and altered neuroendocrine modulation.6 Decreases in splanchnic blood flow during exercise can lead to ischemia-induced inflammation and increased intestinal permeability, which could impact the disease course in patients with IBD. Some studies suggest that exercise may worsen disease activity by increasing levels of interleukin (IL)-6,7, 35 IL-17, IL-10, tumor necrosis factor-alpha (TNF-α),8 neutrophils, lymphocytes and monocytes.7 Extreme exercise may increase the number of CD4 and CD8 lymphocytes, natural killer (NK) cells as well as the level of free radicals, reactive oxygen species and reactive nitrogen species, which could contribute to intestinal inflammation.36 In contrast, other studies have demonstrated decreased levels of IL-6 and TNF-α in exercised mice.10 Regular exercise attenuated the microscopic and macroscopic effects of dextran sodium sulfate (DSS)-induced colitis in exercised mice compared to sedentary mice,37 but it is unclear how physiologic changes noted in exercised rodents correlate with disease activity in humans with IBD.
Light and moderate exercise is believed to be safe in patients with IBD33, 34 and several studies support this belief. An evaluation of moderate-intensity exercise in six males with ileal Crohn's disease demonstrated a decrease in total transit time similar to that seen in six age-matched healthy controls, but there was no increase in intestinal permeability, lipid peroxidation or gastrointestinal symptoms.38 In a prospective evaluation of patients with inactive or mildly active Crohn's disease,5 participants walked three times per week in a structured group or individually and 42% of participants reported a decrease in disease-related symptoms, 58% of participants reported improved body image and life satisfaction. This study did not include a control group and the beneficial effects could be due to group interaction, however a later study randomized participants to an independent walking program vs. no exercise 39 and found that the control group had a statistically significant worsening of disease activity, while the exercise group reported a significant reduction in symptoms.
Despite the uncertainty in understanding how exercise impacts IBD, there are known physiologic benefits to exercise, which may be important for patients with IBD. Unfortunately, patients with CD and UC are more likely to be inactive compared to those without IBD4, 40 and were found to have decreased aerobic and anaerobic exercise capacity when compared with healthy patients.41 Patients who have had surgical resection may have diminished exercise capacity commensurate with the amount of intestine resected.42, 43 These factors might prevent patients with IBD from reaping the known benefits of exercise. Exercise may improve skeletal health and has been shown to improve bone mineral density in patients with IBD.12 Many patients with CD have some degree of bone loss 44 and are at increased risk to develop fracture.45 Physical activity has been shown to decrease the risk of colon cancer13 and this association might have implications in the IBD population already at increased risk for colon cancer. Exercise may also be beneficial for extra-intestinal manifestations of IBD as well as related conditions such as ankylosing spondylitis. Even if exercise does not exert its effect directly on disease activity, it may improve perception of pain, decrease fatigue, and improve body composition, sleep and quality of life.35, 39 Experts have recommended a prescription of exercise for IBD patients that consists of walking 20-30 min at 60% of maximal heart rate 3 days per week along with resistance training 2-3 times per week for its impact on bone mineral density,35 however this has not been tested prospectively. Our study suggests that this level of activity is not only safe, but may potentially decrease the risk of active disease at six months. It is unknown if exercise is protective against flare or if persons who are healthier have a decreased risk of flare and increased ability to participate in exercise.
There are several strengths to this study. Individuals within the CCFA Partners cohort come from a geographically diverse area, with every US state and territory represented. Diagnoses within CCFA Partners have been validated within a subset, with 97% accuracy for the diagnosis of IBD.20 Validated scales for exposures such as exercise and disease activity, previously used in self-report, were used in this study. The large number of participants allows for adequate power to investigate the effects of exposures, while taking into account confounding factors and interactions. There was a significant interaction between oral corticosteroids and physical activity in patients with CD. Participants on steroids who were in the higher exercise category had increased risk of active disease at 6 months, however these patients make up a small percentage, 4.3%, of patients in the higher exercise category. It is possible that despite a sCDAI ≤150, these patients were not truly in remission and differ from the remainder of the study population.
This study does have limitations. One limitation of this study is that the patients who participate in CCFA Partners likely represent a highly motivated subset of patients who might not reflect the majority of patients with IBD, impacting external generalizability. Physical activity is self-reported using the Godin Leisure Time Activity Index and patients could exaggerate or underestimate physical activity. With the current study design, it was not possible to assess exercise activity objectively and this is a limitation. Also, the same score on the Godin leisure time activity index could be obtained by many different exercise regimens, introducing variability into the exposure. While the CCFA Partners Core Baseline Adult Survey asks about surgeries within the month prior to survey completion, surgeries performed within the past year could impact exercise ability. We were unable to exclude such individuals and this is a limitation of the study. Although global health status and physical activity do not measure the same thing in the general population, they were highly correlated and therefore we were unable to control for the effect of global health on disease activity. Another limitation of this study is that sCDAI46 and SCCAI are subjective markers of disease activity. Despite these limitations, this is the largest study to date that examines the effect of exercise on disease of IBD patients to date. Previously, the largest study of physical activity and IBD course included only thirty-two subjects,39 compared to our study which includes nearly 1900 persons.
In conclusion, we found that higher levels of exercise at baseline were inversely associated with the presence of active disease after six months in patients with CD. This study highlights the need for assessment of exercise status as a potential environmental factor in disease activity. If these results were replicated, exercise could be prescribed to prolong, and possibly promote, remission in patients with IBD.
ACKNOWLEDGEMENT
This research was supported, in part, by grants from the NIH T32DK07634 and P30 DK034987 and by the Crohn's and Colitis Foundation of America.
Footnotes
Disclosures: None
Author contributions:
Jones- study concept and design, analysis and interpretation, drafting of the manuscript; Kappelman - study design, data collection, interpretation of results, final approval of manuscript; Martin - study design, data collection, analysis and interpretation of results, final approval of manuscript; Chen- study design, data collection, interpretation of results, final approval of manuscript; Sandler - data collection, interpretation of results, final approval of manuscript; Long - study concept and design, data collection, analysis and interpretation of results, final approval of manuscript.
REFERENCES
- 1.Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31(9):1037–40. doi: 10.1136/gut.31.9.1037. Epub 1990/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson PG, Leijonmarck CE, Bernell O, et al. Risk indicators for inflammatory boweldisease. Int J Epidemiol. 1993;22(2):268–72. doi: 10.1093/ije/22.2.268. Epub 1993/04/01. [DOI] [PubMed] [Google Scholar]
- 3.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ. 2013;347:f6633. doi: 10.1136/bmj.f6633. Epub 2013/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack DE, Wilson PM, Gilmore JC, et al. Leisure-time physical activity in Canadians living with Crohn disease and ulcerative colitis: population-based estimates. Gastroenterol Nurs. 2011;34(4):288–94. doi: 10.1097/SGA.0b013e3182248732. Epub 2011/08/05. [DOI] [PubMed] [Google Scholar]
- 5.Loudon CP, Corroll V, Butcher J, et al. The effects of physical exercise on patients with Crohn's disease. Am J Gastroenterol. 1999;94(3):697–703. doi: 10.1111/j.1572-0241.1999.00939.x. Epub 1999/03/23. [DOI] [PubMed] [Google Scholar]
- 6.Peters HP, De Vries WR, Vanberge-Henegouwen GP, et al. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–9. doi: 10.1136/gut.48.3.435. Epub 2001/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploeger H, Obeid J, Nguyen T, et al. Exercise and inflammation in pediatric Crohn's disease. Int J Sports Med. 2012;33(8):671–9. doi: 10.1055/s-0032-1304323. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman-Goetz L, Spagnuolo PA, Guan J. Repeated exercise in mice alters expression of IL-10 and TNF-alpha in intestinal lymphocytes. Brain Behav Immun. 2008;22(2):195–9. doi: 10.1016/j.bbi.2007.07.002. Epub 2007/08/28. [DOI] [PubMed] [Google Scholar]
- 9.Saxena A, Fletcher E, Larsen B, et al. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J Inflamm (Lond) 2012;9(1):30. doi: 10.1186/1476-9255-9-30. Epub 2012/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer N, Hoffman-Goetz L. Exercise training reduces inflammatory mediators in the intestinal tract of healthy older adult mice. Can J Aging. 2012;31(2):161–71. doi: 10.1017/S0714980812000104. Epub 2012/06/01. [DOI] [PubMed] [Google Scholar]
- 11.Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014 doi: 10.1136/gutjnl-2013-306541. Epub 2014/07/16. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RJ, Krzywicki T, Almond L, et al. Effect of a low-impact exercise program on bone mineral density in Crohn's disease: a randomized controlled trial. Gastroenterology. 1998;115(1):36–41. doi: 10.1016/s0016-5085(98)70362-2. Epub 1998/07/03. [DOI] [PubMed] [Google Scholar]
- 13.Friedenreich C, Norat T, Steindorf K, et al. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2398–407. doi: 10.1158/1055-9965.EPI-06-0595. Epub 2006/12/14. [DOI] [PubMed] [Google Scholar]
- 14.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2(6):370–2. doi: 10.1159/000262276. Epub 2010/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geerling BJ, Badart-Smook A, Stockbrugger RW, et al. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67(5):919–26. doi: 10.1093/ajcn/67.5.919. Epub 1998/05/16. [DOI] [PubMed] [Google Scholar]
- 16.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(10):2162–8. doi: 10.1002/ibd.21585. Epub 2011/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blain A, Cattan S, Beaugerie L, et al. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21(1):51–7. doi: 10.1054/clnu.2001.0503. Epub 2002/03/09. [DOI] [PubMed] [Google Scholar]
- 18.Hass DJ, Brensinger CM, Lewis JD, et al. The impact of increased body mass index on the clinical course of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4(4):482–8. doi: 10.1016/j.cgh.2005.12.015. Epub 2006/04/18. [DOI] [PubMed] [Google Scholar]
- 19.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18(11):2099–106. doi: 10.1002/ibd.22895. Epub 2012/01/31. [DOI] [PubMed] [Google Scholar]
- 20.Randell RL, Long MD, Cook SF, et al. Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners). Inflamm Bowel Dis. 2014;20(3):541–4. doi: 10.1097/01.MIB.0000441348.32570.34. Epub 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thia K, Faubion WA, Jr., Loftus EV, Jr., et al. Short CDAI: development and validation of a shortened and simplified Crohn's disease activity index. Inflamm Bowel Dis. 2011;17(1):105–11. doi: 10.1002/ibd.21400. Epub 2010/07/16. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. doi: 10.1136/gut.43.1.29. Epub 1998/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(10):1081–8. doi: 10.1016/j.cgh.2009.06.024. Epub 2009/07/07. [DOI] [PubMed] [Google Scholar]
- 24.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6. Epub 1985/09/01. [PubMed] [Google Scholar]
- 25.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77(5):359–62. Epub 1986/09/01. [PubMed] [Google Scholar]
- 26.Gionet NJ, Godin G. Self-reported exercise behavior of employees: a validity study. J Occup Med. 1989;31(12):969–73. doi: 10.1097/00043764-198912000-00007. Epub 1989/12/01. [DOI] [PubMed] [Google Scholar]
- 27.Loftus CG, Loftus EV, Jr., Harmsen WS, et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13(3):254–61. doi: 10.1002/ibd.20029. Epub 2007/01/09. [DOI] [PubMed] [Google Scholar]
- 28.Lesuis N, Befrits R, Nyberg F, et al. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med. 2012;10:82. doi: 10.1186/1741-7015-10-82. Epub 2012/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadell MJ, Mermelstein RJ, Hedeker D, et al. Work and Non-work Physical Activity Predict Real-time Smoking Level and Urges in Young Adults. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu244. Epub 2014/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a metaanalysis. Mayo Clin Proc. 2006;81(11):1462–71. doi: 10.4065/81.11.1462. Epub 2006/11/24. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–406. doi: 10.1038/ajg.2012.196. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9. doi: 10.1016/S0140-6736(02)08512-4. Epub 2002/06/06. [DOI] [PubMed] [Google Scholar]
- 33.Ng V, Millard W, Lebrun C, et al. Exercise and Crohn's disease: speculations on potential benefits. Can J Gastroenterol. 2006;20(10):657–60. doi: 10.1155/2006/462495. Epub 2006/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi L, Triadafilopoulos G. Exercise and gastrointestinal function and disease: an evidencebased review of risks and benefits. Clin Gastroenterol Hepatol. 2003;1(5):345–55. doi: 10.1053/s1542-3565(03)00178-2. Epub 2004/03/16. [DOI] [PubMed] [Google Scholar]
- 35.Perez CA. Prescription of physical exercise in Crohn's disease. J Crohns Colitis. 2009;3(4):225–31. doi: 10.1016/j.crohns.2009.08.006. Epub 2010/12/22. [DOI] [PubMed] [Google Scholar]
- 36.Packer N, Hoffman-Goetz L, Ward G. Does physical activity affect quality of life, disease symptoms and immune measures in patients with inflammatory bowel disease? A systematic review. J Sports Med Phys Fitness. 2010;50(1):1–18. Epub 2010/03/24. [PubMed] [Google Scholar]
- 37.Kasimay O, Guzel E, Gemici A, et al. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol. 2006;91(5):897–906. doi: 10.1113/expphysiol.2006.034439. Epub 2006/06/10. [DOI] [PubMed] [Google Scholar]
- 38.D'Inca R, Varnier M, Mestriner C, et al. Effect of moderate exercise on Crohn's disease patients in remission. Ital J Gastroenterol Hepatol. 1999;31(3):205–10. Epub 1999/06/24. [PubMed] [Google Scholar]
- 39.Ng V, Millard W, Lebrun C, et al. Low-intensity exercise improves quality of life in patients with Crohn's disease. Clin J Sport Med. 2007;17(5):384–8. doi: 10.1097/JSM.0b013e31802b4fda. Epub 2007/09/18. [DOI] [PubMed] [Google Scholar]
- 40.Werkstetter KJ, Ullrich J, Schatz SB, et al. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J Crohns Colitis. 2012;6(6):665–73. doi: 10.1016/j.crohns.2011.11.017. Epub 2012/03/09. [DOI] [PubMed] [Google Scholar]
- 41.Ploeger HE, Takken T, Wilk B, et al. Exercise capacity in pediatric patients with inflammatory bowel disease. J Pediatr. 2011;158(5):814–9. doi: 10.1016/j.jpeds.2010.10.020. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 42.Brevinge H, Berglund B, Bosaeus I, et al. Exercise capacity in patients undergoing proctocolectomy and small bowel resection for Crohn's disease. Br J Surg. 1995;82(8):1040–5. doi: 10.1002/bjs.1800820813. Epub 1995/08/01. [DOI] [PubMed] [Google Scholar]
- 43.Narula N, Fedorak RN. Exercise and inflammatory bowel disease. Can J Gastroenterol. 2008;22(5):497–504. doi: 10.1155/2008/785953. Epub 2008/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee N, Radford-Smith G, Taaffe DR. Bone loss in Crohn's disease: exercise as a potential countermeasure. Inflamm Bowel Dis. 2005;11(12):1108–18. doi: 10.1097/01.mib.0000192325.28168.08. Epub 2005/11/25. [DOI] [PubMed] [Google Scholar]
- 45.Mauro M, Armstrong D. Evaluation of densitometric bone-muscle relationships in Crohn's disease. Bone. 2007;40(6):1610–4. doi: 10.1016/j.bone.2007.02.026. Epub 2007/04/17. [DOI] [PubMed] [Google Scholar]
- 46.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122(2):512–30. doi: 10.1053/gast.2002.31072. Epub 2002/02/08. [DOI] [PubMed] [Google Scholar]