Abstract
The pervasive and persistent nature of depressive symptoms has made resting-state functional magnetic resonance imaging (rs-fMRI) an appropriate approach for understanding the underlying mechanisms of major depressive disorder. The majority of rs-fMRI research has focused on depression-related alterations in the interregional coordination of brain baseline low frequency oscillations (LFOs). However, alteration of the regional amplitude of LFOs in depression, particularly its clinical, cognitive and network implications have not been examined comprehensively yet. rs-fMRI amplitudes of low-frequency fluctuation (ALFF/fALFF) mediated by two LFOs bands of 0.01-0.08 Hz (LF-ALFF/fALFF) and 0.1-0.25 Hz (HF-ALFF/fALFF) were measured in unmedicated subjects with major depressive disorder (n=20) and a healthy control group (n=25). A novel method of “ALFF-based functional connectivity” analysis was developed to test regional/network interaction abnormalities in depression. Our results revealed abnormal alterations in ALFF for both lower and higher frequency bands of LFOs in regions that participate in affective networks, corticostriatal circuits and motor/somatosensory networks. A strong positive correlation was detected between depressive symptom severity and fALFF in the anterior cingulate cortex. Functional connectivity of the thalamus and postcentral area with altered ALFF were found to be decreased with other interacting regions of their involved networks. Major depressive disorder relates to the alterations of regional properties of intrinsic neural activity with meaningful clinical and cognitive correlations. This study also proposes an integrating regional/network dysfunction in MDD.
Keywords: Major Depressive Disorder, Neuroimaging, Resting-state fMRI, ALFF/fALFF, Functional Connectivity
Introduction
Major Depressive Disorder (MDD) has been reported as the leading cause of disability in the US and the second cause of disability worldwide (Ferrari et al., 2013). Despite the availability of different treatment options, the remission rate from MDD is estimated not to be more than 50%, even after one year of treatment (Whiteford et al., 2013). Better understanding of the underlying mechanisms of MDD and development of more effective treatment options are critical for reducing the huge burden of this mental illness for affected patients, their families and society in general.
Neuroimaging has been one of the principal research modalities for investigating the neuropathology of depression. Structural brain imaging studies in MDD have pointed to cortico-striato-limbic neurocircuitries as the anatomical substrates of depression (Ballmaier et al., 2008; Bora et al., 2012; Koolschijn et al., 2009; Kumar et al., 2014; Liao et al., 2013; Sheline, 2003; Tadayonnejad and Ajilore, 2014). Another major line of research in the neuroimaging of depression uses functional magnetic resonance imaging (fMRI) to explore abnormal alterations of brain function in depressed subjects. In those studies, abnormal activity in terms of the blood-oxygen-level dependent (BOLD) signal are examined in patients with MDD during the performance of a cognitive, emotional or reward processing task (Diener et al., 2012; Hamilton et al., 2012; Hasler et al., 2009; Heller et al., 2009; Pizzagalli, 2011).
Recent advances in fMRI have led to the development of resting-state fMRI (rs-fMRI) (Biswal et al., 1995; Fox and Raichle, 2007). In rs-fMRI, brain baseline fluctuations in the BOLD signal are measured when a subject with open or closed eyes is not doing anything in the scanner. Considering the pervasive nature of several depressive symptoms like depressed mood, negative rumination or lack of motivation, rs-fMRI actually might be even a better approach for investigating the abnormal neural mechanisms of depression.
In rs-fMRI, two measures are used commonly to examine the network-related and regional characteristics of low frequency oscillations (LFOs): Functional Connectivity (FC) and Amplitude of Low-Frequency Fluctuation (ALFF). In FC analysis, the temporal correlation (synchronicity) of resting-state BOLD signals of spatially distributed brain areas is calculated as a measure of brain region resting-state functional interaction (Fox and Greicius, 2010; Fox et al., 2005). In ALFF analysis, the baseline intensity or the amplitude of LFOs is quantified as a regional characteristic of resting-state intrinsic neural activity (Zang et al., 2007; Zou et al., 2008; Zuo et al., 2010). Although FC or ALFF analysis have commonly focused on the 0.01 to 0.08 Hz frequency range, some reports have suggested the involvement of higher frequencies range in LFOs characteristics under normal physiological conditions in regions like brain stem, basal ganglia, or amygdala (Salvador et al., 2008; Zuo et al., 2010), during pathological conditions such as pain (Baliki et al., 2011) or after noninvasive cortical stimulation (Chen et al., 2013).
Alterations in ALFF values in depression have been the subject of a few recent rs-fMRI studies. Those studies mainly focused on the pattern of ALFF changes in depression and reported MDD-related ALFF alterations in several brain areas like the frontal cortex, parietal cortex, temporal cortex, limbic system, visual network and cerebellum (Guo et al., 2013; Liu et al., 2014; Wang et al., 2012; Zhang et al., 2014). Clinical and cognitive correlations of ALFF alterations in MDD have not been examined comprehensively. The possible contribution of higher frequency ALFF (0.1-0.25 Hz) changes has also not been tested yet. Furthermore, it has not been investigated that how regions with ALFF changes in depression behave differently in their interactions with other elements of involved networks.
In this study, we aimed to test three hypotheses. First, MDD is related to alterations in ALFF calculated for traditional lower frequency (0.01-0.08 Hz) as well as untested higher frequency (0.1-0.25Hz) ranges in areas belong to cortico-striato-limbic circuits. Second, changes in ALFF values in MDD are correlated with depression symptoms severity and cognitive performance. Third, FC between regions with ALFF alterations and other nodes in the related networks are altered in depression.
Methods
Participants
For this study, we recruited 45 subjects. Of these, 20 were unmedicated subjects with unipolar major depression (MDD) and 25 were nondepressed comparison subjects (HC). All study subjects were recruited from the local community through advertisements in flyers, newspapers, and radio. The inclusion criteria for all subjects were 30 years of age and older, antidepressant-naive or free of antidepressant use for at least two weeks and no history of unstable cardiac or neurological diseases. The exclusion criteria included: schizophrenia, bipolar or any psychotic disorders; history of anxiety disorder outside of major depressive episodes; history of head trauma or loss of consciousness; history of substance abuse; contraindications to MRI such as metal implants; Mini Mental Status Exam (MMSE) Score fl 24. This study was approved by the University of Illinois-Chicago Institutional Review Board, and written informed consent was obtained from each participant.
All eligible subjects were assessed by a trained research assistant with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (First et al., 2012). The severity of depression was quantified by a board-certified/board-eligible psychiatrist (AK or OA) using the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960). At the time of enrollment, depressed subjects met DSM-IV criteria for MDD and required a score of 15 or greater on the HAM-D. Subjects were also administered the Center for Epidemiological Studies-Depression (CES-D) scale as an independent measure of depression severity (Yesavage et al., 1982). The CES-D was used for correlation analyses as the HAM-D was the measure used in the determination of subject eligibility for depression.
Neuropsychological Battery
All subjects underwent a comprehensive neuropsychological battery covering domains of attention/information processing (AIP: Stroop Word Score, Stroop Color Score, Trail Making Test Part A, WAIS-III Digit Symbol Coding), learning and memory (LM: California Verbal Learning Test (CVLT), Wechsler’s Memory Scale 3rd Edition Logical Memory and Visual Reproduction), and executive function (EF: Category Switching Accuracy, Trail Making Test Part B, Digit Span Backwards, Stroop Interference Score, Self-Ordered Pointing Task)(Delis et al., 2011; Golden et al., 1978; Wechsler, 1997; Woods et al., 2006). Individual test scores for each domain were averaged and converted to z-scores. Cronbach’s alpha calculated for each domain were 0.87 for AIP, .84 for LM, and 0.77 for EF demonstrating high internal consistencies for each domain.
MRI Acquisition
Brain MRI data were acquired on a Philips Achieva 3.0T scanner (Philips Medical Systems, Best, The Netherlands) using an 8-channel SENSE (Sensitivity Encoding) head coil. Participants were positioned comfortably on the scanner bed and fitted with soft ear plugs; foam pads were used to minimize head movement. Participants were instructed to remain still throughout the scan. High resolution three-dimensional T1-weighted images were acquired with a MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) sequence (field of view (FOV)=240mm; 134 contiguous axial slices; TR/TE=8.4/3.9ms; flip angle=8°; voxel size=1.1 × 1.1 × 1.1 mm3). rs-fMRI data were acquired with the following parameters: Single-shot gradient-echo EPI sequence, TR/TE = 2000/30 ms, Flip angle = 80 degree, EPI factor = 47, FOV = 23 × 23 × 15 cm3, in-plane resolution = 3x3 mm2, slice thickness/gap = 5/0 mm, slice number = 30, SENSE reduction factor = 1.8, NEX = 200, total scan time = 6:52’. Subjects were instructed to keep their eyes close and “not think of anything in particular”.
fMRI data preprocessing
All preprocessing were conducted using statistical parametric mapping software (SPM8, http//www.fil.ion.uvl.ac.uk/spm). The first 10 volumes of the functional images were discarded for obtaining signal equilibrium and allowing participants adaptation to scanning noise. The artifact detection tool (ART: http://www.nitrc.org/projects/artifact_detect) was used to measure motion artifacts in all subjects. None of the subjects used in this study had more than 2 mm maximum displacement in x, y or z axis or 2° angular motion during fMRI scanning. Furthermore, there was no significant difference in composite motion between groups (HC: 0.24 ± 0.12; MDD: 0.24 ± 0.11; p =0.98). Raw EPI images were subsequently realigned, coregistered, normalized, and smoothed with a smoothing kernel of 8 mm before analyses. Confound effects from motion artifact, white matter, and CSF were regressed out of the signal. Finally BOLD signal data was passed through two band-pass filters (lower frequency band: 0.01 to 0.08 Hz and higher frequency band: 0.1 to 0.25 Hz) for further ALFF and FC analyses.
ALFF/fALFF Calculation
ALFF and fALFF analysis were performed using Resting-State fMRI Data Analysis Toolkit (REST, http://www.rest.restfmri.net). For each voxel, the filtered time series were transformed to the frequency domain using a fast Fourier transformation (FFT) analysis, and the power spectrum was then measured. The average square root of power in the 0.01-0.08 Hz (lower frequency) or 0.1-0.25 Hz (higher frequency) bands were calculated and taken as lower frequency ALFF (LF-ALFF) and higher frequency ALFF (HF-ALFF). For fractional ALFF (fALFF) analysis, the average square root of power in the 0.01-0.08 Hz (lower frequency) or 0.1-0.25 Hz (higher frequency) bands for each voxel was normalized by total power across all available frequencies for that voxel (LF-fALFF and HF-fALFF). We applied a brain mask on subject-level voxel-wise ALFF and fALFF maps for removing non-brain tissues. Finally, all ALFF and fALFF maps were standardized into subject-level Z-score maps for improving statistical analyses and test-retest reliability (Chen et al., 2013; Zuo et al., 2010).
Functional connectivity analysis
Functional connectivity analysis was performed with REST software. We applied an integrated ALFF- and seed-based FC analysis: In brief, we predefined 2 clusters that showed up in group differences and clinical and cognitive analyses as preselected seeds for the FC study. After 0.01-0.08 Hz band pass filtering and linear regression removal of ventricular, white matter, and global changes, the time series of voxels within each seed region were averaged as the seed reference time course. For each subject, FC of each seed reference time course with the rest of the brain gray matter voxels (extracted by using a mask) were calculated separately for obtaining correlation coefficient maps. Finally, all correlation maps were transformed to z-value FC maps by applying Fisher’s r-to-z conversion for performing subsequent FC group comparison.
Statistical analysis
Demographic, clinical and cognitive variables were analyzed for between-group differences using an independent sample t-test for continuous variables and chi-squared test for categorical variables. ALFF and FC group differences were analyzed using univariate analysis of covariance with age, sex, and education as covariates. Pearson’s correlations were used to analyze the relationship between LF- and HF-fALFF values and depression severity or cognitive performance scores in depressed subjects. For all of the above analyses, Monte Carlo simulation was applied for multiple comparisons correction using the REST AlphaSim program (Ledberg et al., 1998). In this study, a corrected significant level of p<0.05 was obtained by using a combination of individual voxel probability threshold p < 0.009 and a minimum cluster size of 38 voxels (or 304 mm3).
Results
Demographics
Demographic, clinical and cognitive data of both healthy and depressed subjects are summarized in Table 1. Compared to the healthy controls, patients with MDD had a significantly lower mean age. There were no significant differences between the two groups in gender, education, or MMSE. As expected, depressed subjects scored significantly higher on both measures of depression severity. There were no significant differences in cognitive performance across three domains of EF, AIP and LM.
Table 1.
Demographic, clinical and cognitive characteristics.
| HC(n=25) | MDD(n=20) | P value | |
|---|---|---|---|
| Age | 63.3 ± 12.7 | 54.5 ± 10.9 | 0.018a |
| Sex (M/F) | 15/10 | 9/11 | 0.316b |
| Education | 14.3 ± 2.0 | 15.4 ±2.1 | 0.08a |
| MMSE | 28.8 ± 1.15 | 28.8 ±1.2 | 0.89a |
| HAM-D | 1.8 ± 1.4 | 18.7 ± 3.2 | < 0.001a |
| CES-D | 5.8 ± 4.0 | 32.0 ± 7.9 | < 0.001a |
| EF | 0.0 ± 0.57 | −0.13 ± 0.24 | 0.09a |
| AIP | 0.0 ± 0.85 | 0.23 ± 0.95 | 0.44a |
| LM | 0.0 ± 0.80 | 0.54 ± 0.82 | 0.26a |
The P value were obtained by sample t-test.
The P value was obtained by chi-square root.
Abbreviation: HC: healthy control; MDD: major depressive disorder; EF: Executive Function, AIP: Attention/Information processing; LM: Learning and Memory; HAM-D: Hamilton Rating Scale for Depression; CES-D: Center for Epidemiological Studies-Depression Rating Scale MMSE: mini mental status examination.
ALFF group differences
First, we examined the gray matter distributions of LF- and HF-ALFF/fALFF in our healthy subjects. Consistent with ALFF and fALFF literature specifically the comprehensive report of Zuo et al. (Zuo et al., 2010), we found higher LF-ALFF and LF-fALFF in visual and auditory cortex, thalamus, and default mode network (DMN) regions including posterior cingulate cortex/precuneus and medial prefrontal cortex (mPFC) (Supplementary Figure 1a,b). For HF-ALFF and HF-fALFF, significant maximal signals were found in brain stem, basal ganglia and parahippocampal areas (Supplementary Figure 1c,d).
We chose ALFF as a more reliable and sensitive measure compared to fALFF for detecting group differences for our group comparison in this study (Zuo et al., 2010). Compared with healthy controls, depressed subjects showed clustered with significantly smaller LF-ALFF in the left hippocampus and left superior temporal gyrus but significant larger LF-ALFF in the right middle orbitofrontal cortex, left dorsomedial prefrontal cortex, right putamen, right caudate and right middle frontal gyrus (Figure 1). Patients with MDD were found to have clusters with significant larger HF-ALFF in the right pons and left thalamus but significant smaller HF-ALFF in the left postcentral, precentral and paracentral areas when compared to control group (Supplementary Figure 2). ALFF group differences results are summarized in Table 2.
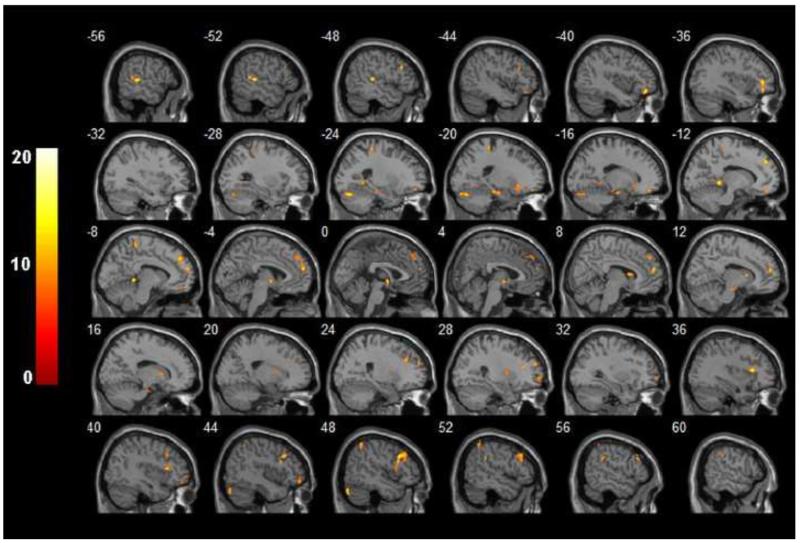
Figure 1.
F-statistical maps showing LF-ALFF differences between patients with MDD and healthy subjects. Significant clusters of bigger than 304 mm3 were shown after controlling for age, sex and years of education and correction for multiple comparison (p < 0.05). The color bar on the left side refers to the range of F values.
Table 2.
Brain regions showing statistically significant altered LF/HF-ALFF in ANCOVA between HC and MDD groups.
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates |
F values | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| LF-ALFF | ||||||
| Left hippocampus (BA34) | 50 | −18 | −12 | −20 | HC>MDD | 12.74 |
| Left superior temporal gyrus (BA22) | 60 | −50 | −32 | 8 | HC>MDD | 16.56 |
| Right middle orbitofrontal cortex (BA10) | 51 | 42 | 50 | −14 | MDD>HC | 10.66 |
| Left dorsomedial prefrontal cortex (BA10,9) | 58 | −6 | 60 | 20 | MDD>HC | 15.90 |
| Right putamen | 39 | 26 | 1 | 16 | MDD>HC | 10.27 |
| Right caudate | 53 | 8 | 10 | 10 | MDD>HC | 12.53 |
| Right middle frontal gyrus (BA9) | 90 | 46 | 22 | 36 | MDD>HC | 17.12 |
| HF-ALFF | ||||||
| Left postcentral (BA 40,2) | 72 | −52 | −36 | 32 | HC>MDD | 21.19 |
| Left precentral (BA 6) | 41 | −28 | −12 | 62 | HC>MDD | 12.85 |
| Left paracentral (BA 5) | 87 | −4 | −42 | 68 | HC>MDD | 17.10 |
| Right pons | 46 | 16 | −38 | −32 | MDD>HC | 18.06 |
| Left thalamus | 58 | −2 | −22 | −16 | MDD>HC | 17.12 |
Abbreviations: ANCOVA: analysis of covariance; BA: Brodmann area; HC: healthy control; LF- and HF-ALFF: low frequency and high frequency amplitude of low-frequency fluctuation; MNI: Montreal Neurologic Institute; MDD: major depressive disorder.
Clinical and cognitive correlation
We focused on fALFF, as the more specific measure compared to ALFF in our clinical and cognitive correlation analysis (Zuo et al., 2010). In the MDD group, CES-D scores were positively associated with LF-fALFF in both the right anterior cingulate cortex and right superior temporal pole (Figure 2) in addition to being negatively correlated in other brain regions (Table 3).
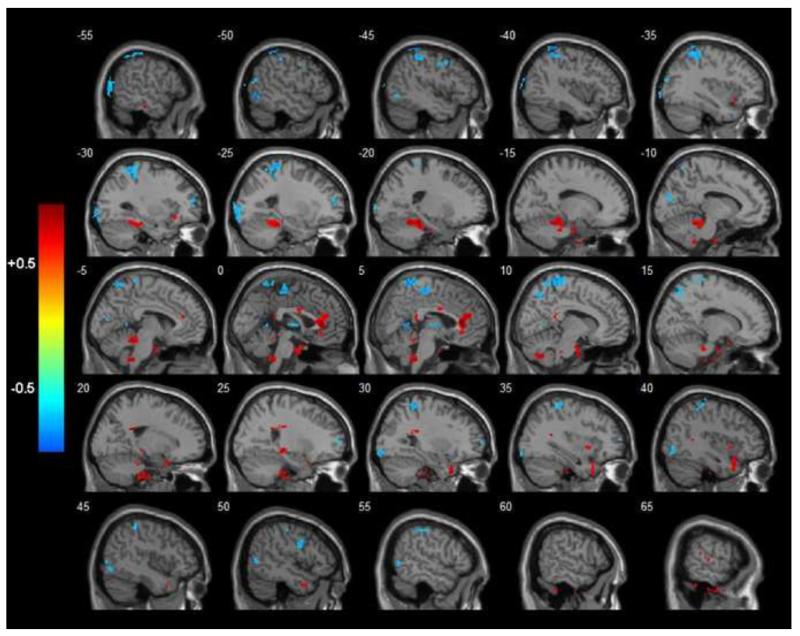
Figure 2.
R-statistical maps illustrating correlation between LF-fALFF and CES-D values in depressed subjects. Significant clusters of bigger than 304 mm3 were shown after correction for multiple comparison (p < 0.05). The color bar on the left side refers to the range of R values. Hotter and colder colors display positive and negative correlations respectively.
Table 3.
Brain regions showing statistically significant correlation between LF/HF-fALFF and depression severity in terms of CES-D scores in subjects with MDD.
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates |
R values | ||
|---|---|---|---|---|---|
| X Y | Z | ||||
| LF-ALFF | |||||
| Left mid occipital lobe (BA18, 19) | 191 | −60 | −72 | 10 | −0.73 |
| Left thalamus | 53 | −4 | −6 | 4 | −0.73 |
| Right precuneus (BA7) | 42 | 12 | −66 | 50 | −0.67 |
| Left postcentral (BA40) | 228 | −40 | −46 | 58 | −0.77 |
| Right postcentral (BA40) | 93 | 36 | −34 | 64 | −0.69 |
| Right anterior cingulate cortex (BA24,32) | 155 | 2 | 32 | 8 | +0.83 |
| Right superior temporal pole (BA38) | 93 | 30 | 16 | −38 | +0.72 |
| HF-ALFF | |||||
| Left postcentral (BA 40,2) | 182 | −44 | −40 | 52 | +0.77 |
| Right thalamus | 58 | 2 | −14 | 14 | +0.66 |
Abbreviations: BA: Brodmann area; CES-D: Center for Epidemiological Studies-Depression; LF- and HF-fALFF: low frequency and high frequency fractional of amplitude of low-frequency fluctuation; MNI: Montreal Neurologic Institute; MDD: major depressive disorder.
A positive correlation was detected between CES-D scores and HF-fALFF values in right thalamus and left postcentral area (Supplementary Figure 3 and Table 3).
Cognitive correlation analyses were performed across the total sample for all three domains (EF (Figure 3), AIP (Supplementary Figure 4) and LM). There was a general pattern of cognitive performance being negatively associated with LF-fALFF while being positively associated with HF-fALFF. Cognitive correlation results are summarized in Table 4.
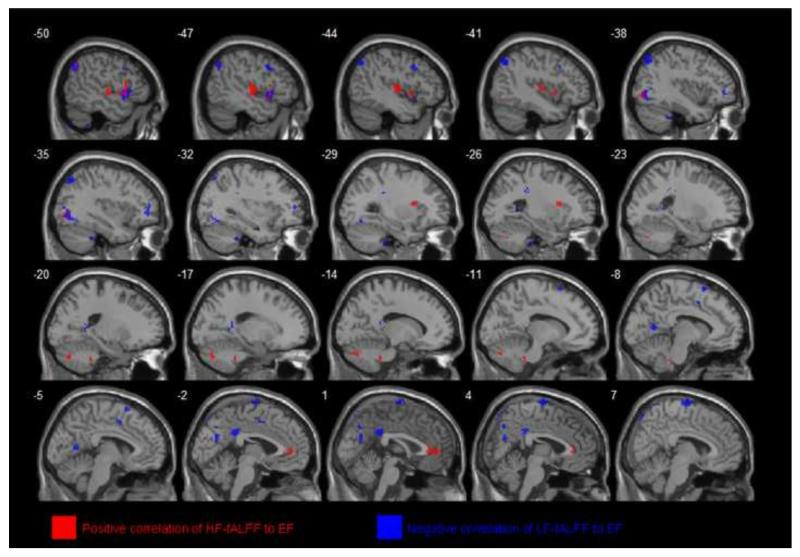
Figure 3.
R-statistical maps illustrating correlation between LF- and HF-fALFF and Executive Function (EF) scores in total subjects. Significant clusters of bigger than 304 mm3 were shown after correction for multiple comparison (p < 0.05). Clusters with red color show areas with positive correlation between HF-fALFF and EF scores but blue colored clusters demonstrate regions with negative correlation between LF-fALFF and EF scores.
Table 4.
Brain regions showing statistically significant correlation between LF/HF-fALFF and different cognitive performances Z-scores in total sample.
| Brain regions (Brodmann areas) | Cluster size | MNI coordinates |
R values | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| LF-ALFF in EF | |||||
| Left inferior frontal gyrus (B22) | 82 | −50 | 12 | −4 | −0.54 |
| Left posterior cingulate (BA31) | 66 | −4 | −38 | 30 | −0.52 |
| Left angular (BA18, 19) | 130 | −40 | −70 | 64 | −0.60 |
| Left supplementary motor area (B24) | 39 | −6 | 4 | 48 | −0.45 |
| Right supplementary motor area (BA6) | 111 | 10 | −10 | 80 | −0.56 |
| HF-ALFF in EF | |||||
| Right inferior temporal gyrus (B37) | 47 | 52 | −54 | −16 | −0.56 |
| Left superior temporal gyrus (BA22) | 101 | −54 | 12 | −6 | +0.51 |
| Left insula (BA13) | 87 | −46 | −14 | 10 | +0.54 |
| Right anterior cingulate cortex (B32) | 51 | 2 | 34 | 2 | +0.45 |
| LF-ALFF in AIP | |||||
| Left Thalamus | 60 | −20 | −20 | 12 | −0.44 |
| Left posterior cingulate (BA31) | 252 | −4 | −38 | 28 | −0.55 |
| Right postcentral (BA4) | 38 | 64 | −12 | 30 | −0.50 |
| HF-ALFF in AIP | |||||
| Right lingual (BA19) | 46 | 12 | −70 | 2 | −0.52 |
| Left posterior temporal (BA21) | 45 | −48 | −14 | 6 | +0.50 |
| Right postcentral (BA4) | 41 | 64 | −12 | 30 | +0.58 |
| LF-ALFF in LM | |||||
| Right supramarginal (BA2) | 50 | 60 | −36 | 42 | −0.51 |
Abbreviations: BA: Brodmann area; AIP: attention/information processing; EX: executive function; LF- and HF-fALFF: low frequency and high frequency fractional of amplitude of low-frequency fluctuation; LM: learning and memory; MNI: Montreal Neurologic Institute.
Functional connectivity analysis
Finally, we examined how the FC of significant clusters in group differences and clinical/cognitive correlation analyses differ between healthy controls and the depressed group (ALFF/fALFF-based FC). We preselected two seeds that appeared in both group differences and clinical/cognitive correlation results for FC analysis: the left thalamus and left postcentral gyrus. Depressed subjects showed significantly lower FC between the left thalamus seed and the left orbitofrontal cortex, right superior frontal gyrus, right precuneus and left middle frontal gyrus (Figure 4) and between the left postcentral gyrus seed and the left superior frontal gyrus, right middle frontal gyrus and left mid cingulum (Supplementary Figure 5). We then calculated correlation between LF/HF fALFF values extracted from seeds used for FC analysis and values of FC between those seeds and functionally connected clusters. Only an uncorrected positive correlation was found between LF-fALFF values in the left thalamus and its FC with the left middle frontal gyrus in this analysis.
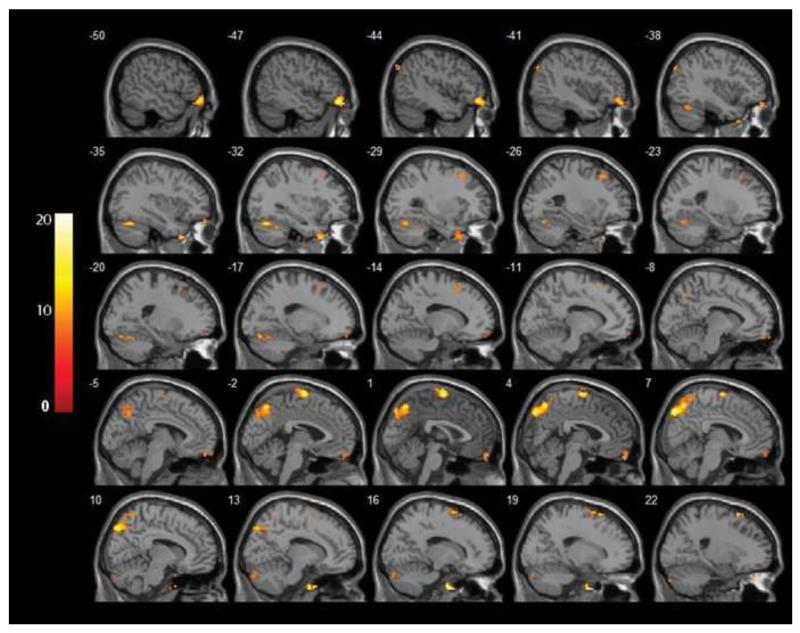
Figure 4.
F-statistical maps demonstrating clusters with different functional connectivity with the left thalamus seed in depressed subjects compared to controls. Significant clusters of bigger than 304 mm3 were shown after controlling for age, sex and years of education and correction for multiple comparison (p < 0.05). The color bar on the left side refers to the range of F values.
Discussion
This study revealed alterations of both lower frequency (0.01-0.08 Hz) ALFF in regions belonging to affective networks and corticostriatal neurocircuitry and changes in higher frequency (0.1-0.25 Hz) ALFF in the left thalamus, left motor and somatosensory networks and the right pons. We found that depressive symptoms severity is positively correlated with the LF-fALFF in the right anterior cingulate cortex as well as higher frequency fALFF in the left somatosensory region and right thalamus in unmedicated depression. Cognitive performance in executive function and attention/information processing domains were shown to be positively correlated with HF-fALFF in the left superior temporal gyrus and negatively correlated with LF-fALFF in the left post cingulate. The present study extends ALFF analysis in depression by examining regional alterations of resting-state intrinsic neural activity in both traditional lower frequency and also higher frequency bands in unmedicated depression. We complemented the ALFF group comparison analysis with clinical and cognitive correlations of fALFF in depressed subjects. We also developed a new analytical strategy for combining ALFF and FC analyses to test how interaction of clusters with ALFF/fALFF based regional alterations and clinical/cognitive correlations changes in terms of resting-state functional connectivity.
Patterns of lower and higher frequency baseline intrinsic activity changes in unmedicated depression
Consistent with recent ALFF studies in depression, our results showed regional alteration of ALFF in clusters belonging to cortico-striato-limbic neurocircuitries (Guo et al., 2013; Liu et al., 2014; Wang et al., 2012; Zhang et al., 2014). In all of those studies the focus was on the lower resting-state frequency band of 0.01-0.08 Hz for group comparisons. The possible role of higher resting-state frequencies (0.1-0.25) ALFF abnormalities in depression has not been evaluated yet. As it was mentioned above, recent studies in healthy subjects have shown that higher frequencies oscillations are present and participate in the dynamics of LFOs in several gray matter regions, particularly in brain stem, basal ganglia and the amygdala (Salvador et al., 2008; Zuo et al., 2010). In a recent study, Chen et al, showed that inhibitory rTMS stimulation of the posterior middle frontal gyrus can increase the magnitude of higher frequency fALFF in the DMN in healthy individuals (Chen et al., 2013). Baliki et al, reported an increase in high-frequency BOLD oscillations (0.12-0.20 Hz) in mPFC and some areas of DMN in subjects suffering from chronic back pain (Baliki et al., 2011). Finding the depression-related significant increases in high-frequency ALFF in the right thalamus in our study has an important implication regarding the strategic anatomical location of thalamus in corticostriatal networks and the well-reported involvement of thalamic dysfunction in the pathophysiology of depression (Hamilton et al., 2012).
Another notable finding from our results is the lateralization of ALFF alterations in our study. In line with several previous PET and task-based fMRI studies, we also found regional baseline intrinsic right hemispheric hyperactivity and left hemispheric hypoactivity in terms of lower frequency ALFF in our depressed subjects compared to controls (Grimm et al., 2008; Kennedy et al., 2001; Mayberg, 2003).
Clinical correlations of lower and higher frequency fALFF in depression
Finding the biomarkers of disease mechanisms, diagnosis, symptom severity, treatment selection or prognosis has been one of the major aims in the neuroimaging of depression (McGrath et al., 2013). In the current study, we tested the hypothesis that symptom severity in unmedicated depression is correlated with the regional resting-state lower or higher frequency amplitude of LFOs in the brain. Significant correlations were found between lower frequency fALFF values and depressive severity in terms of CES-D scores in several areas such as left thalamus, right precuneus and left somatosensory cortex. The most robust positive correlations were detected in the right rostral anterior cingulate cortex (rACC). This is not surprising regarding the extensive accumulating evidence of rACC involvement, particularly its subgenual part in the pathophysiology of depression (de Asis et al., 2001; Drevets et al., 1997; Mayberg, 1997). Another interesting related finding in our study was the significant positive correlation of higher frequency fALFF values in the right thalamus and left somatosensory network and depression severity. In Baliki et al, study noted above, a positive correlation was reported between higher frequency LFOs in mPFC and subjective reports of pain perception in subjects with chronic back pain (Baliki et al., 2011). Their findings in conjunction with our results suggest that higher frequency resting-state BOLD signal alterations can be tested and used as a regional imaging biomarker of disease and symptom severity in neuropsychiatric disorders.
Cognitive correlations of lower and higher frequency fALFF in depression
Cognitive impairments are a widely recognized aspect of the symptom complex seen in depression. Attention difficulties, memory impairments, negatively distorted thinking and problems in decision making are among the common symptoms in depressed patients even after remission. Several task-based fMRI studies have shown an abnormal pattern of functioning in brain structures involve in frontoparietal, frontolimbic and corticostriatal networks in depressed individual during emotional and non-emotional cognitive task performances (Thomas and Elliott, 2009). Resting-state and task-based connectivity analysis studies have also found network dysregulation in terms of increased or diminished coupling within and between related cortico-striato-limbic networks (Alexopoulos et al., 2012; Chen et al., 2008; Hamilton and Gotlib, 2008). Interestingly, our results revealed that cognitive performance is negatively correlated with LF-fALFF and positively correlated with HF-fALFF suggesting a frequency-dependent association between resting-state amplitude of LFOs and cognitive performance.
Anatomically, two areas showed up consistently in our cognitive correlation analyses: the left posterior cingulate cortex and the left superior temporal gyrus. The posterior cingulate cortex is one the key nodes of “task-negative” DMN (Buckner et al., 2008; Leech et al., 2011). Reactive and fast deactivation of posterior cingulate is necessarily needed for effective cognitive task performance (Singh and Fawcett, 2008). Failure in time-sensitive deactivation of that area has been shown to be associated with ineffective cognitive function in healthy subjects and also in patients with neuropsychiatric disorders (Bonnelle et al., 2012; Weissman et al., 2006). Negative correlations between LF-fALFF and EF or AIP performance scores may be explained by a potential difficulty in required deactivation of posterior cingulate region during task when it has higher prior resting-state activity.
The superior temporal gyrus has functional role in auditory processing and sort-term memory, facial related emotional perception and also social cognition processing through its connection with the prefrontal cortex and amygdala (Bigler et al., 2007; Leff et al., 2009; Radua et al., 2010). Our results in showing a positive correlation between HF-fALFF in superior temporal gyrus and cognitive performing scores can suggest an association between higher frequency resting-states activity in that area and its better readiness for being involved in successful task execution.
It is important to mention that rs-fMRI scans were not done immediately before or at the time of cognitive testing in our study. Therefore, the results of cognitive correlation of fALFF should be interpreted with caution. An interesting experimental design would be to do the rs-fMRI scan just before task execution in healthy and MDD subjects to test the relationship between the baseline amplitude of LFOs and subsequent patterns of activation in task-related regions associated with cognitive performance. This would address the hypothesis that prior-task resting-state amplitudes of LFOs generated from different frequency bands in specific related regions predict success rates of task execution mediated by networks involved those regions.
ALFF-based FC analysis in depression
Methodologically, two main techniques are used for FC analysis: hypothesis-driven region of interest (ROI) analysis and data-driven independent component analysis (ICA). In ROI approach, a “seed” region of interest is pre-defined and then temporal correlation of activity of that seed with pre-selected region(s) or all brain voxels is measured during the resting state (Van Dijk et al., 2010). In contrast, ICA approach of FC analysis place no emphasis on a specific brain region and the whole brain is investigated for detecting significant correlation patterns and extracting functional networks (Beckmann et al., 2005). Here, we developed a new approach we called “ALFF-based FC analysis” to investigate interaction and association between regional resting-state amplitude of LFOs and their network-based temporal correlations with other brain areas. Specifically, we tried to answer this question that how FC of a region that shows ALFF alteration differs in depression. We pre-defined ALFF altered clusters with clinical and cognitive correlations using the left thalamus and left postcentral gyrus as our ROIs and measured FC of each cluster/seed with the rest of the brain in depressed and control subjects.
The thalamus has a strategic anatomical position and significant functional role in corticostriatal system (Alexander and Crutcher, 1990; Leh et al., 2007); it also plays an important role in reward processing due to its anatomical direct connections with main nodes of the reward network including nucleus accumbens and orbitofrontal cortex (OFC) (Cauda et al., 2011; Kringelbach, 2005). Altered FC of thalamus with the superior and middle frontal gyrus that include dorsolateral prefrontal cortex (DLPFC) and also with OFC suggest dysregulation in functional connectivity of corticostriatal and reward networks respectively.
The postcentral gyrus (primary somatosensory cortex) along with pain-related thalamic nuclei, the middle cingulate cortex (MCC) (particularly the anterior MCC) and amygdala form the main neurocircuitry of pain perception processing (Gasquoine, 2013; Vogt, 2005). Decrease in the FC between postcentral gyrus and MCC in MDD subjects can point to an impairment functional interaction between nodes participating in pain perception network suggesting a potential network-based underlying mechanism of somatic complains in depression. Several studies have shown the gating/modulatory effect of prefrontal regions (like DLPFC) on somatosensory processing in the postcentral region (Bolton and Staines, 2011; Yamaguchi and Knight, 1990). The postcentral gyrus was also found to have decreased FC with superior and middle frontal gyrus in MDD patients which subsequently suggests another network-based mechanism of possible somatosensory dysregulation in MDD.
An important point that should be discussed here is that the direction of causality is not clear between depressions related regional ALFF changes and alterations in FCs. In other words, we do not know if an ALFF altered region causes connectivity disruption in its involved network or the network with dysregulated connectivity causes ALFF change in its containing node(s).
Methodological consideration and future direction
The current work has some limitations that must be discussed here. First, the age of our control group is significantly higher than subjects with MDD. To address this issue, all group difference analyses were controlled for age. Second, in this study we used a cross-sectional design for examining ALFF alterations in unmedicated and symptomatic patients with depression. It is not clear if our results exclusively show state-dependent ALFF changes or we are observing trait-dependent findings. Third, our ALFF/fALFF findings could be interpreted differently as the exact underlying neural pathologies or as compensatory mechanisms that have been developed during the course of depression, or even a mixture of both. Selectively examining ALFF changes in susceptible individuals at high risk for developing depression, treatment naïve patients in their first episode of disease, or remitted subjects and comparing the results would be helpful in resolving the issue. Furthermore, having longitudinal studies in which ALFF/fALFF patterns can be tested before and after psychotherapy, psychopharmacological or neurostimulation would also provide us a better understanding of treatment selection and remission mechanisms. Fourth, we could not find strong significant linear correlations between FCs and fALFF values in our total sample or in the depressed group. Possible explanations might be the existence of a nonlinear FC/fALFF correlation or the involvement of a third factor like structural connectivity mediating interaction between ALFF and FC. More advanced correlation analyses and using a multimodal approach to test the association between DTI-based structural connectivity and ALFF or FC measured can shed light on this subject (Tadayonnejad et al., 2014). Lastly, the exact neural basis of fMRI BOLD-based ALFF has not been comprehensively investigated. Simultaneous EEG/fMRI will be a useful way to elucidate the exact underlying neural mechanisms of ALFF/fALFF and also explore their temporal dynamics.
Conclusion
In conclusion, we used the ALFF measure to test the difference in the baseline amplitude of intrinsic brain activity mediated in both lower (0.01-0.08 Hz) and higher (0.1-0.25 Hz) frequency bands of BOLD-based resting-state LFOs in non-medicated patients with depression relative to control subjects. ALFF alterations were found in clusters belonging to affective networks, the corticostriatal system and motor/somatosensory networks. We detected significant correlations between depressive symptom severity and fALFF extracted from the anterior cingulate cortex. By using a novel approach of combining ALFF and FC analyses, we found that the temporal coordination of resting-state activity of regions with ALFF changes decreased with other interacting nodes of the involved networks, pointing to the network implications of regional ALFF abnormalities in depression.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (R01 MH-073989 to AK; K23 MH-081175 to OA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Financial Disclosures:
The authors report no biomedical financial interests or potential conflicts of interest.
Contributors:
Authors SY, OA and AK designed the study and wrote the protocol. Authors SY and OA collected the data. Author RT and OA analyzed the data, discussed the results and draw a conclusion. Author RT wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
The funder (National Institute of Mental Health) had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE. Superior temporal gyrus, language function, and autism. Developmental neuropsychology. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bolton DA, Staines WR. Transient inhibition of the dorsolateral prefrontal cortex disrupts attention-based modulation of tactile stimuli at early stages of somatosensory processing. Neuropsychologia. 2011;49:1928–1937. doi: 10.1016/j.neuropsychologia.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- de Asis JM, Stern E, Alexopoulos GS, Pan H, Van Gorp W, Blumberg H, Kalayam B, Eidelberg D, Kiosses D, Silbersweig DA. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158:1321–1323. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E. The Delis-Kaplan Executive Function System. Psychological Corporation; San Antonio, TX: 2011. [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicine. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. 2012 [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neuroscience and biobehavioral reviews. 2013;37:340–348. doi: 10.1016/j.neubiorev.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Hammeke TA, Purisch AD. Diagnostic validity of a standardized neuropsychological battery derived from Luria’s neuropsychological tests. Journal of consulting and clinical psychology. 1978;46:1258–1265. [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Guo WB, Liu F, Xun GL, Hu MR, Guo XF, Xiao CQ, Chen HF, Wooderson SC, Chen JD, Zhao JP. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:153–159. doi: 10.1016/j.pnpbp.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry. 2009;66:201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kumar A, Yang S, Ajilore O, Wu M, Charlton R, Lamar M. Subcortical biophysical abnormalities in patients with mood disorders. Mol Psychiatry. 2014;19:710–716. doi: 10.1038/mp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain: a journal of neurology. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, Lui S, Yue Q, Chan RC, Kemp GJ, Gong Q. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ren L, Womer FY, Wang J, Fan G, Jiang W, Blumberg HP, Tang Y, Xu K, Wang F. Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Phillips ML, Russell T, Lawrence N, Marshall N, Kalidindi S, El-Hage W, McDonald C, Giampietro V, Brammer MJ, David AS, Surguladze SA. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49:939–946. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, Capdevila A, Bullmore E. A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage. 2008;39:279–289. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol. 2014;27:5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Yang S, Kumar A, Ajilore O. Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS One. 2014;9:e96033. doi: 10.1371/journal.pone.0096033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R. Brain imaging correlates of cognitive impairment in depression. Frontiers in human neuroscience. 2009;3:30. doi: 10.3389/neuro.09.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai W, Su Y, Wang G, Tan Y, Jin Z, Zeng Y, Yu X, Chen W, Wang X, Si T. Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS One. 2012;7:e48658. doi: 10.1371/journal.pone.0048658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd edition Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Harris MG, McKeon G, Baxter A, Pennell C, Barendregt JJ, Wang J. Estimating remission from untreated major depression: a systematic review and meta-analysis. Psychological medicine. 2013;43:1569–1585. doi: 10.1017/S0033291712001717. [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Gating of somatosensory input by human prefrontal cortex. Brain Res. 1990;521:281–288. doi: 10.1016/0006-8993(90)91553-s. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu X, Wang X, Zhong M, Yi J, Rao H, Yao S. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One. 2014;9:e85241. doi: 10.1371/journal.pone.0085241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.